Abstract
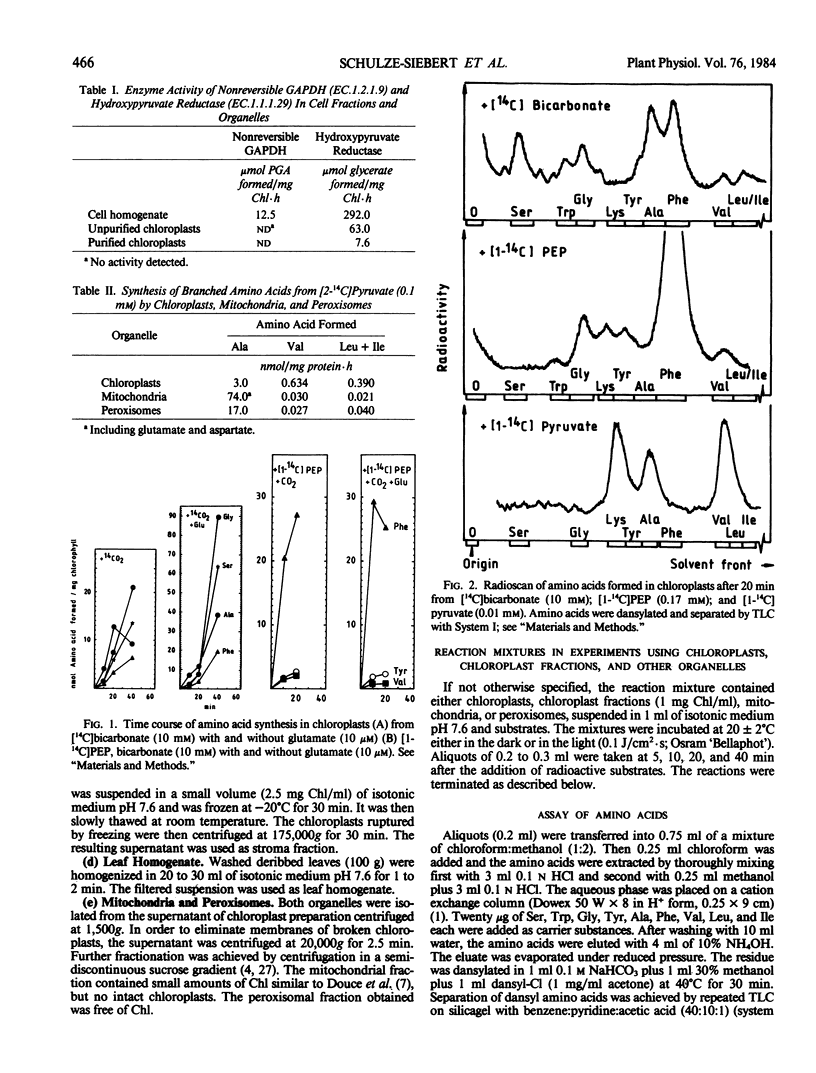
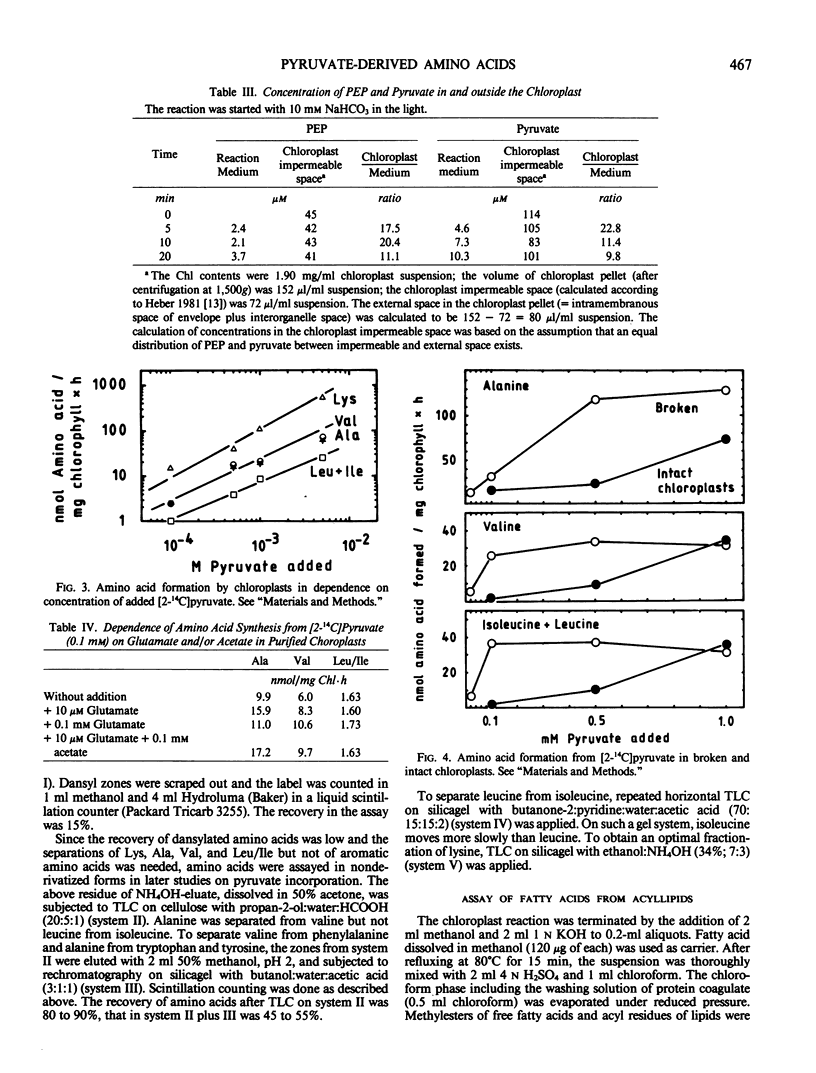
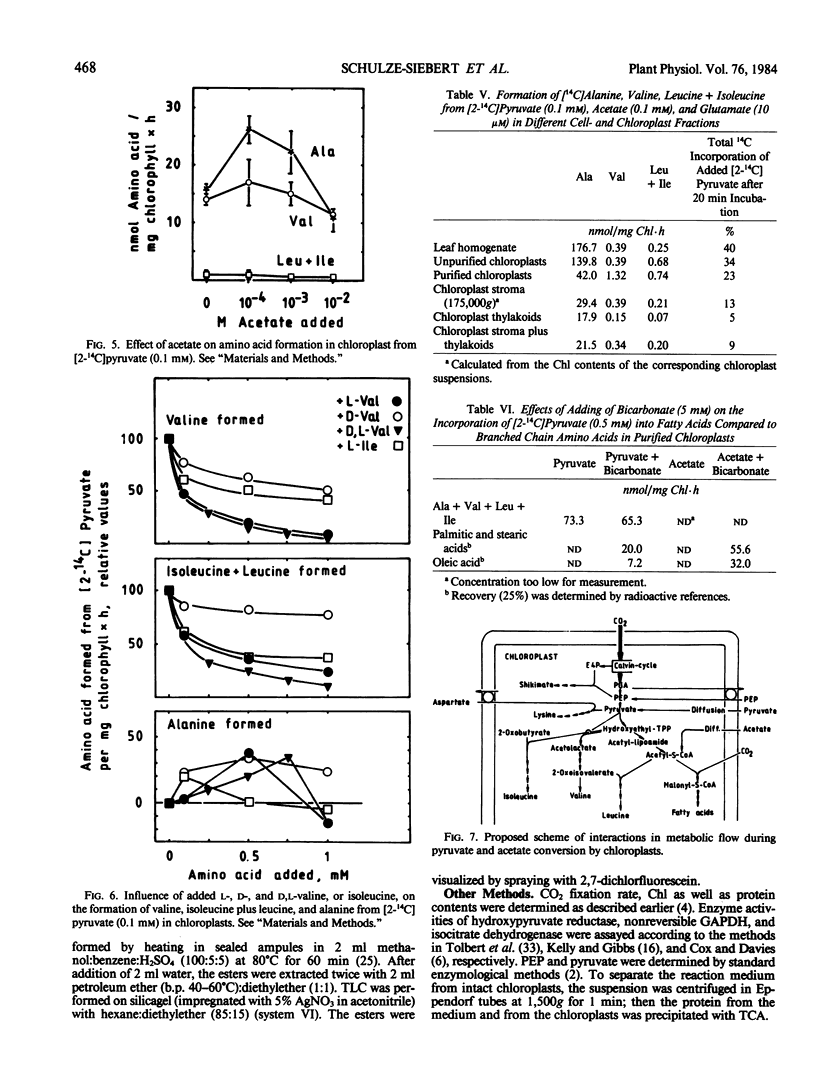
A probable carbon flow from the Calvin cycle to branched chain amino acids and lipids via phosphoenolpyruvate (PEP) and pyruvate was examined in spinach (Spinacia oleracea) chloroplasts. The interpendence of metabolic pathways in and outside chloroplasts as well as product and feedback inhibition were studied. It was shown that alanine, aromatic, and small amounts of branched chain amino acids were formed from bicarbonate in purified intact chloroplasts. Addition of PEP only favored formation of aromatic amino acids. Mechanisms of regulation remained unclear. Concentrations of PEP and pyruvate within the chloroplast impermeable space during photosynthetic carbon fixation were 15 times higher than in the reaction medium. A direct carbon flow to pyruvate was identified (0.1 micromoles per milligram chlorophyll per hour). Pyruvate was taken up by intact chloroplasts slowly, leading to the formation of lysine, alanine, valine, and leucine plus isoleucine (approximate ratios, 100-500:60-100:40-100:2-10). The Km for the formation of valine and leucine plus isoleucine was estimated to be 0.1 millimolar. Ten micromolar glutamate optimized the transamination reaction regardless of whether bicarbonate or pyruvate was being applied. Alanine and valine formation was enhanced by the addition of acetate to the reaction mixture. The enhancement probably resulted from an inhibition of pyruvate dehydrogenase by acetyl-S-coenzyme A formed from acetate, and resulting accumulation of hydroxyethylthiamine diphosphate and pyruvate. High concentrations of valine and isoleucine inhibited their own and each others synthesis and enhanced alanine formation. When pyruvate was applied, only amino acids were formed; when complemented with bicarbonate, fatty acids were formed as well. This is probably the result of a requirement of acetyl-S-coenzyme A-carboxylase for bicarbonate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cox G. F., Davies D. D. Nicotinamide-adenine dinucleotide-specific isocitrate dehydrogenase from pea mitochondria. Purification and properties. Biochem J. 1967 Nov;105(2):729–734. doi: 10.1042/bj1050729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W. Developments in carotenoid biochemistry over 40 years. The third Morton lecture. Biochem Soc Trans. 1983 Oct;11(5):473–483. doi: 10.1042/bst0110473. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Albertsson P. A. Photosynthetic 14CO2 fixation by chloroplast populations isolated by a polymer two-phase technique. Biochim Biophys Acta. 1974 Sep 20;357(3):412–419. doi: 10.1016/0005-2728(74)90031-0. [DOI] [PubMed] [Google Scholar]
- Miflin B. J. The location of nitrite reductase and other enzymes related to amino Acid biosynthesis in the plastids of root and leaves. Plant Physiol. 1974 Oct;54(4):550–555. doi: 10.1104/pp.54.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills W. R. Photosynthetic formation of the aspartate family of amino acids in isolated chloroplasts. Plant Physiol. 1980 Jun;65(6):1166–1172. doi: 10.1104/pp.65.6.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J. An improved method for isolating chloroplasts retaining their outer membranes. Biochim Biophys Acta. 1977 Sep 14;461(3):500–512. [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. Acetate is the preferred substrate for long-chain fatty acid synthesis in isolated spinach chloroplasts. Biochem J. 1979 Dec 15;184(3):565–569. doi: 10.1042/bj1840565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer A., Heise K. P. On the light dependence of Fatty Acid synthesis in spinach chloroplasts. Plant Physiol. 1983 Sep;73(1):11–15. doi: 10.1104/pp.73.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürmann P. Separation of phosphate esters and algal extracts by thin-layer electrophoresis and chromatography. J Chromatogr. 1969 Feb 25;39(4):507–509. [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Williams M., Randall D. D. Pyruvate Dehydrogenase Complex from Chloroplasts of Pisum sativum L. Plant Physiol. 1979 Dec;64(6):1099–1103. doi: 10.1104/pp.64.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]


