Abstract
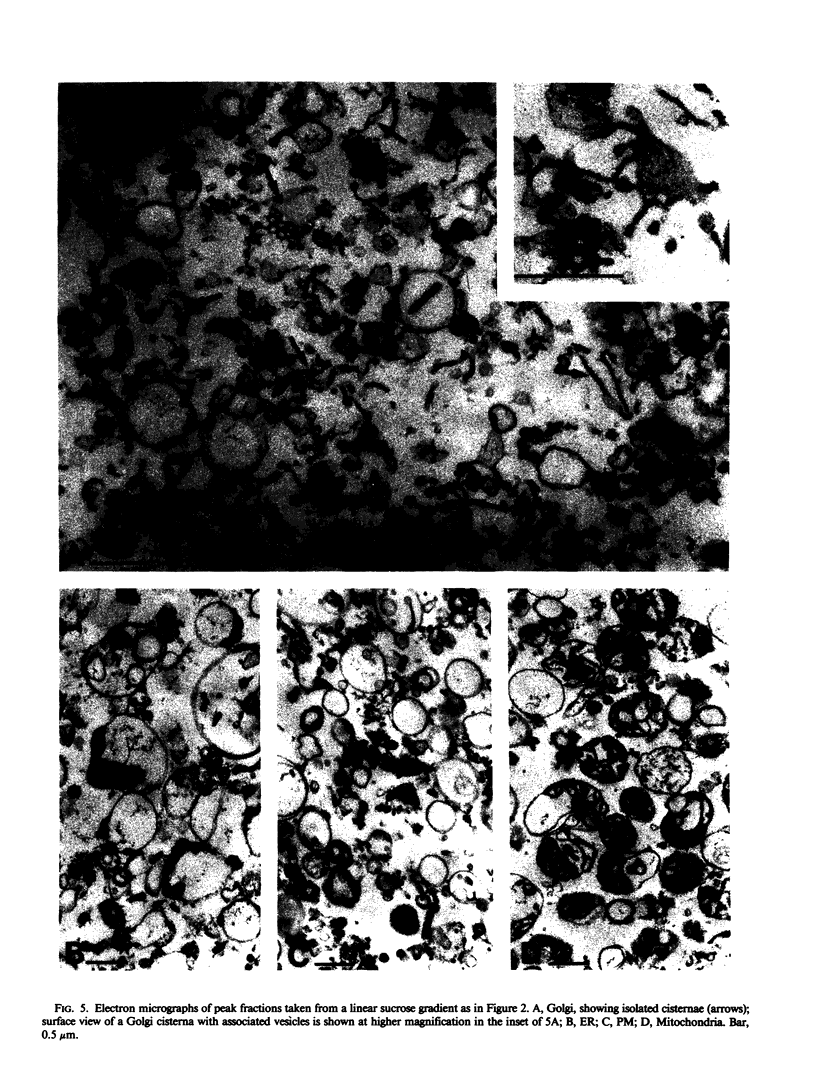
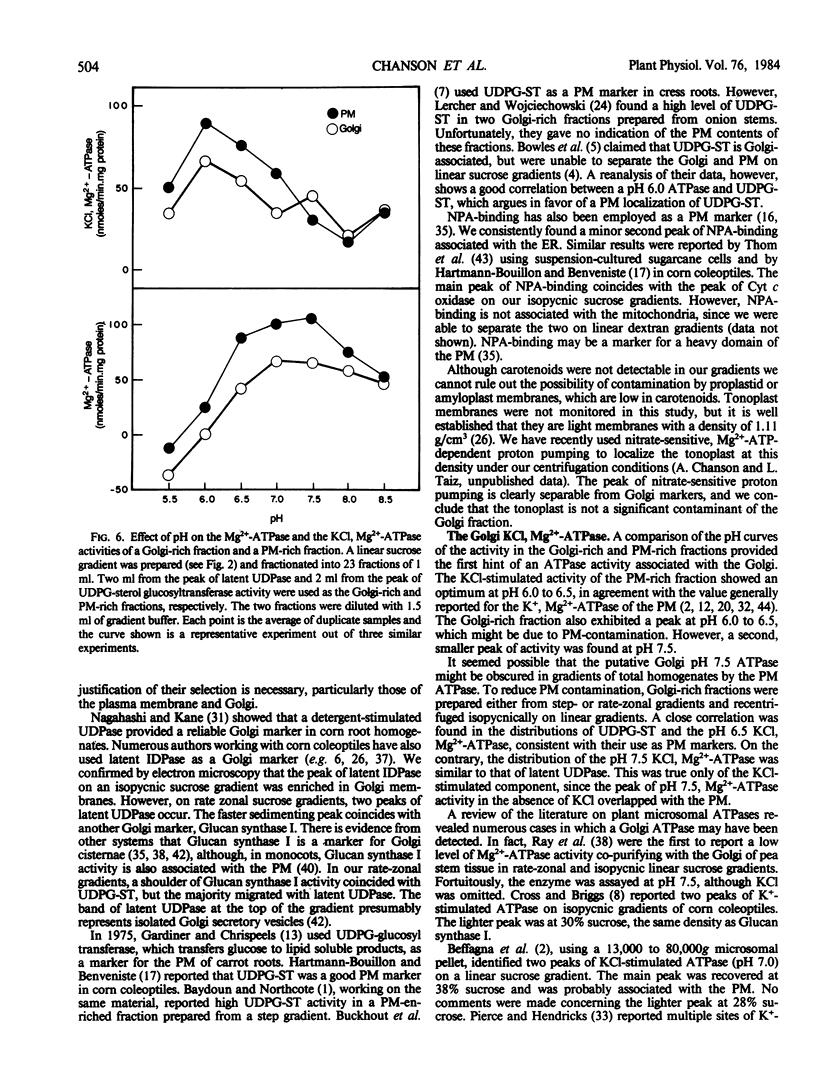
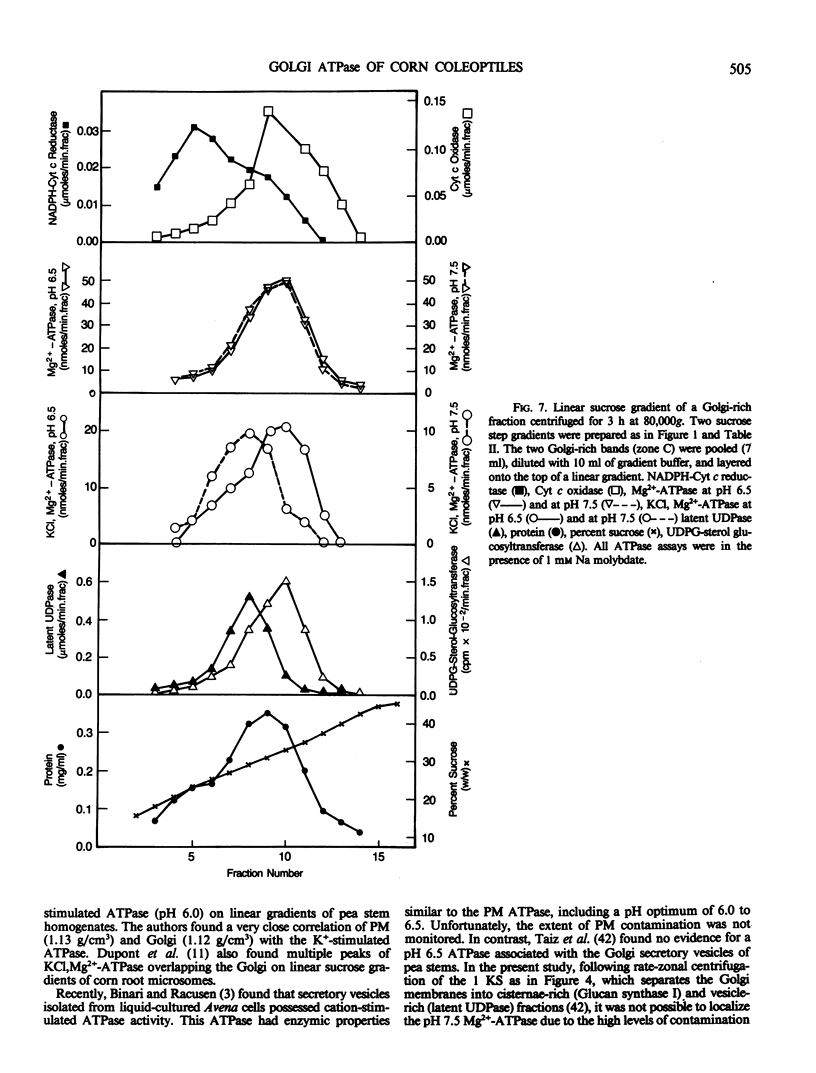
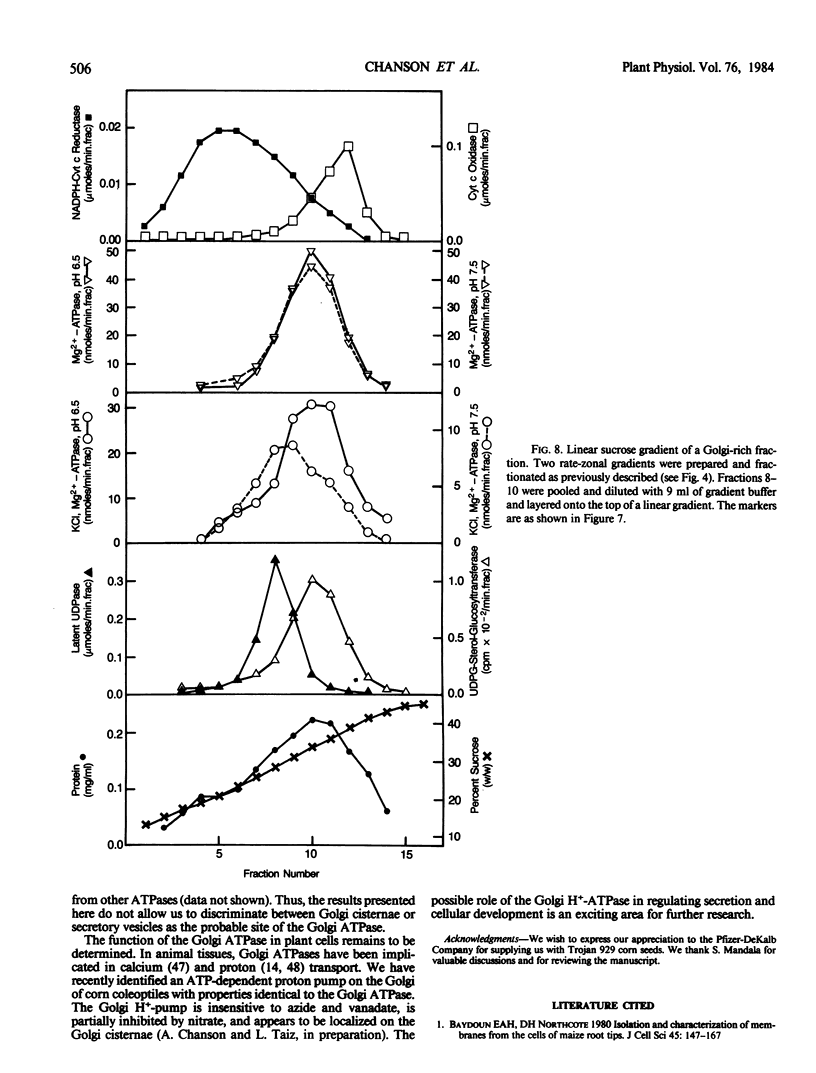
Membranes of corn (Zea mays, cv Trojan 929) coleoptiles were fractionated by sucrose density gradient centrifugation and the locations of organelles were determined using marker enzymes and electron microscopy. Latent IDPase (or UDPase) was selected as the Golgi marker and UDPG-sterol glucosyl transferase was selected as the plasma membrane (PM) marker, because they were clearly separable from markers for the other organelles. Golgi-rich and PM-rich fractions were studied in relation to their ATPase activities. The pH optimum of the KCl, Mg2+-ATPase of the PM-rich fraction from a step gradient was 6.0 to 6.5, while the Golgi-rich fraction had peaks at pH 6.0 to 6.5 and pH 7.5. It is hypothesized that the peak at pH 6.0 to 6.5 for the Golgi-rich fraction is due to PM-contamination, while the peak at pH 7.5 represents the activity of a Golgi ATPase. To reduce PM contamination, Golgi-rich fractions obtained from step or rate-zonal gradients were recentrifuged isopycnically on linear sucrose gradients. The distribution of KCl, Mg2+-ATPase activity was measured at pH 6.5 and 7.5. The pH 6.5 ATPase was coincident with UDPG-sterol glucosyl transferase, a PM marker, while the pH 7.5 ATPase overlapped with latent UDPase, a Golgi marker. These results provide strong evidence for a KCl, Mg2+-ATPase, active at pH 7.5, associated with the Golgi membranes of corn coleoptiles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binari L. L., Racusen R. H. Membrane-associated ATPases in isolated secretory vesicles. Plant Physiol. 1983 Mar;71(3):594–597. doi: 10.1104/pp.71.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman J., Croen K., Kelly S., Al-Awqati Q. Golgi membranes contain an electrogenic H+ pump in parallel to a chloride conductance. J Cell Biol. 1983 Oct;97(4):1303–1308. doi: 10.1083/jcb.97.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A., Frenzel R., Laible D. ATP-dependent proton transport into vesicles of microsomal membranes of Zea mays coleoptiles. Z Naturforsch C. 1980 Sep-Oct;35(9-10):783–793. doi: 10.1515/znc-1980-9-1021. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Jesaitis A. J., Heners P. R., Hertel R. Characterization of a Membrane Fraction Containing a b-type Cytochrome. Plant Physiol. 1977 May;59(5):941–947. doi: 10.1104/pp.59.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi J., Hiraike K. Effects of centrifugal force and centrifugation time on the sedimentation of plant organelles. Plant Physiol. 1982 Feb;69(2):546–548. doi: 10.1104/pp.69.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Labeling and isolation of plasma membranes from corn leaf protoplasts. Plant Physiol. 1980 Jun;65(6):1053–1057. doi: 10.1104/pp.65.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- Zhang F., Schneider D. L. The bioenergetics of Golgi apparatus function: evidence for an ATP-dependent proton pump. Biochem Biophys Res Commun. 1983 Jul 29;114(2):620–625. doi: 10.1016/0006-291x(83)90825-2. [DOI] [PubMed] [Google Scholar]