Abstract
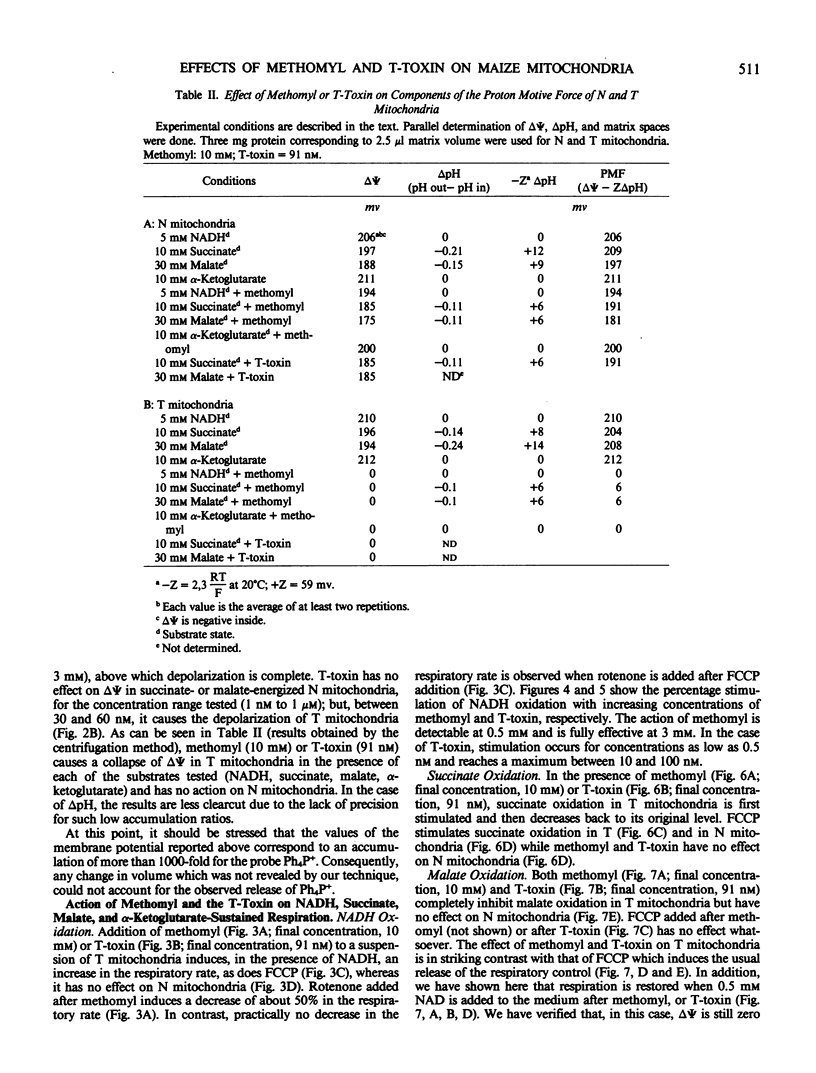
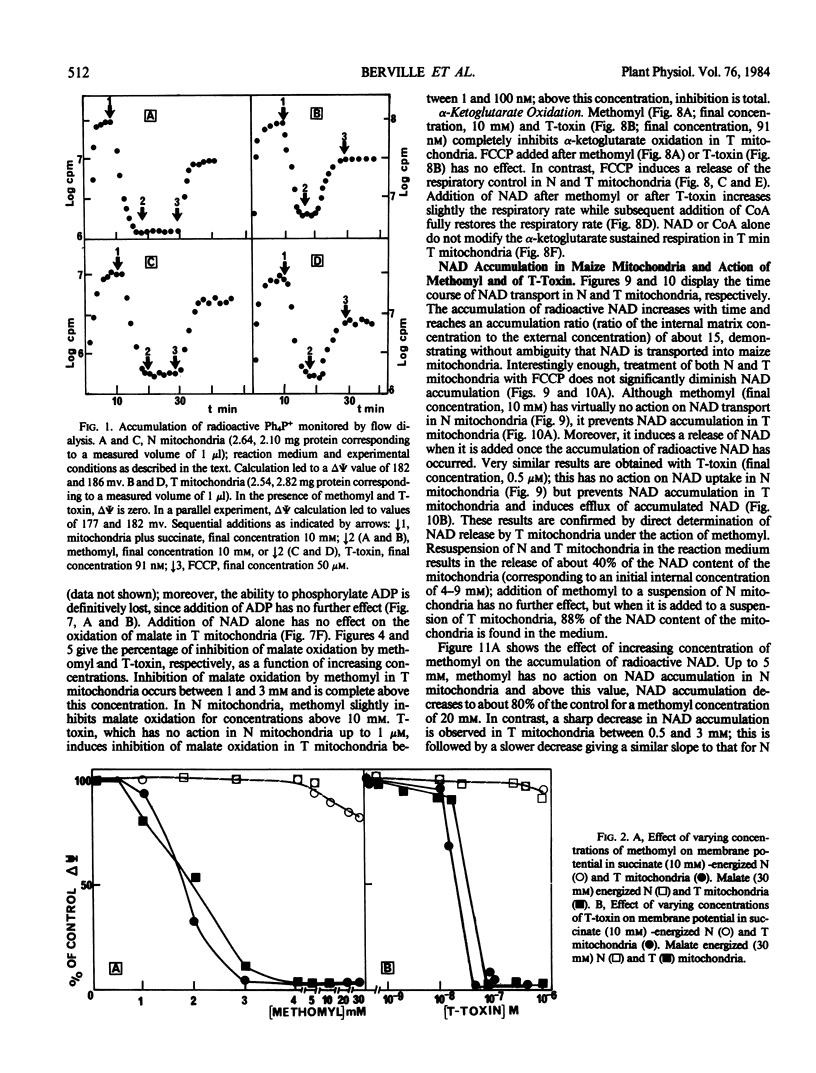
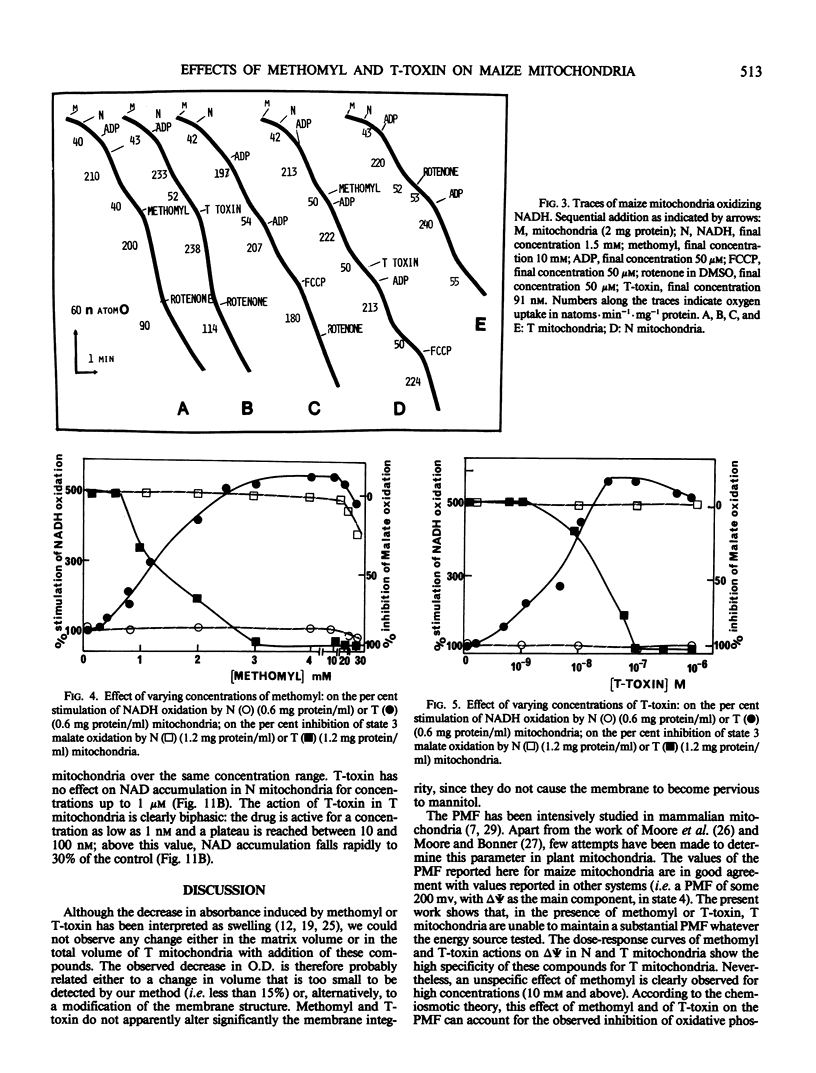
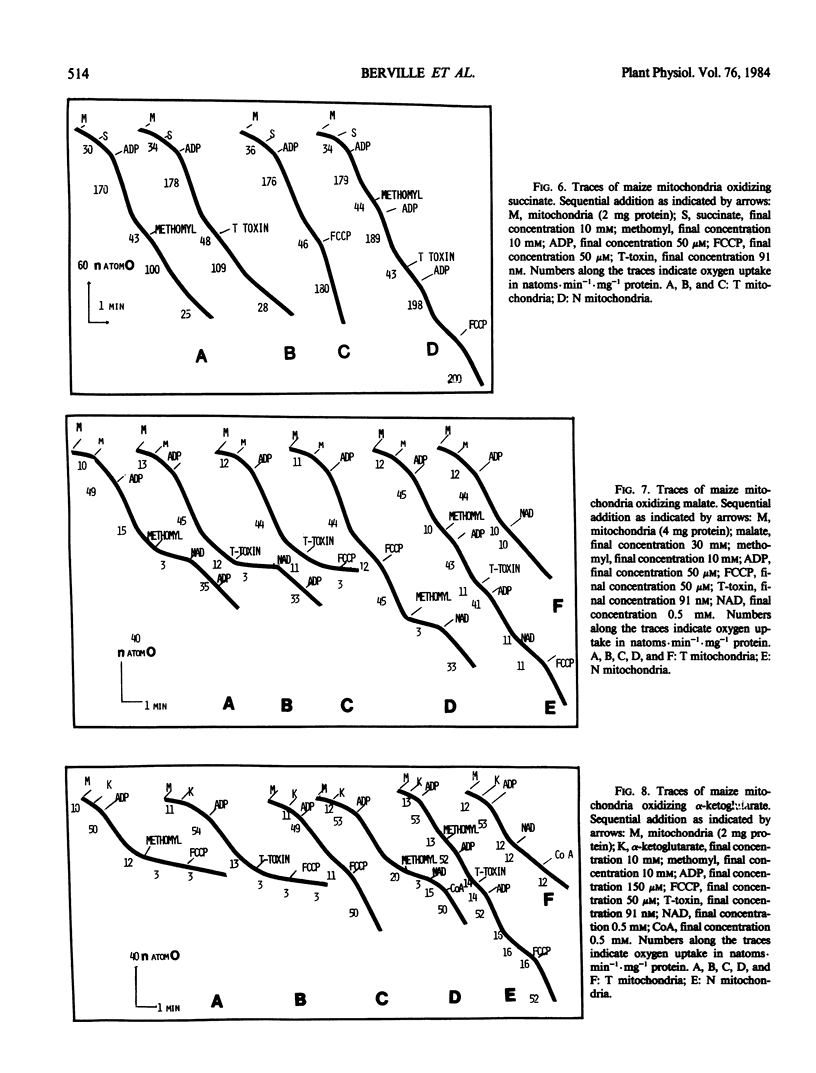
Methomyl and Helminthosporium maydis race T toxin block oxidative phosphorylation in mitochondria isolated from maize plants with Texas male sterile cytoplasm (T) but not in mitochondria isolated from those with Normal cytoplasm (N) (Bednarski, Izawa, Scheffer 1977 Plant Physiol 59: 540-545). Moreover, they have been reported to cause specific swelling in T mitochondria (Miller, Koeppe 1971 Science 173: 67-69; Koeppe, Cox, Malone 1978 Science 201: 1227-1229). We could not detect, by direct volume measurements, any change induced by these compounds in the mitochondrial matrix space. We show here that the proton motive force, which in maize mitochondria is composed of a large transmembrane potential and of a low transmembrane pH difference, is absent in T mitochondria incubated in the presence of methomyl or of Helminthosporium maydis race T toxin, while it is unchanged in N mitochondria. Methomyl and Helminthosporium maydis race T toxin induce, independently of the collapse of the proton motive force, a release of the cofactors NAD and coenzyme A from the mitochondrial matrix space. In particular, we show that NAD is transported in maize mitochondria, and that this transport, which is not dependent on the proton motive force, is inhibited by methomyl or Helminthosporium maydis race T toxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Pozzan T., Massari S., Bragadin M. Proton electrochemical gradient and rate of controlled respiration in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):296–306. doi: 10.1016/0005-2728(78)90035-x. [DOI] [PubMed] [Google Scholar]
- Bednarski M. A., Izawa S., Scheffer R. P. Reversible Effects of Toxin from Helminthosporium maydis Race T on Oxidative Phosphorylation by Mitochondria from Maize. Plant Physiol. 1977 Apr;59(4):540–545. doi: 10.1104/pp.59.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOLL H., TURNER R. A., PIERCE J. G., DU VIGNEAUD V. An investigation of the free amino groups on oxytocin and desulfurized oxytocin preparations. J Biol Chem. 1951 Nov;193(1):363–370. [PubMed] [Google Scholar]
- Gengenbach B. G., Green C. E., Donovan C. M. Inheritance of selected pathotoxin resistance in maize plants regenerated from cell cultures. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5113–5117. doi: 10.1073/pnas.74.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A., Schechter E., Letellier L., Labedan B. Probes of membrane potential in Escherichia coli cells. FEBS Lett. 1981 Mar 23;125(2):197–200. doi: 10.1016/0014-5793(81)80717-x. [DOI] [PubMed] [Google Scholar]
- Koeppe D. E., Cox J. K., Malone C. P. Mitochondrial heredity: a determinant in the toxic response of maize to the insecticide methomyl. Science. 1978 Sep 29;201(4362):1227–1229. doi: 10.1126/science.201.4362.1227. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Gregory P., Gracen V. E. Helminthosporium maydis Race T Toxin Induces Leakage of NAD from T Cytoplasm Corn Mitochondria. Plant Physiol. 1979 Jun;63(6):1149–1153. doi: 10.1104/pp.63.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Electrochemical proton gradient across the cell membrane of Halobacterium halobium: comparison of the light-induced increase with the increase of intracellular adenosine triphosphate under steady-state illumination. Biochemistry. 1980 Sep 30;19(20):4615–4619. doi: 10.1021/bi00561a012. [DOI] [PubMed] [Google Scholar]
- Miller R. J., Koeppe D. E. Southern corn leaf blight: susceptible and resistant mitochondria. Science. 1971 Jul 2;173(3991):67–69. doi: 10.1126/science.173.3991.67. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Bonner W. D., Jr A comparison of the phosphorylation potential and electrochemical proton gradient in mung bean mitochondria and phosphorylating sub-mitochondrial particles. Biochim Biophys Acta. 1981 Jan 14;634(1):117–128. doi: 10.1016/0005-2728(81)90132-8. [DOI] [PubMed] [Google Scholar]
- Payne G., Kono Y., Daly J. M. A Comparison of Purified Host Specific Toxin from Helminthosporium maydis, Race T, and Its Acetate Derivative on Oxidation by Mitochondria from Susceptible and Resistant Plants. Plant Physiol. 1980 May;65(5):785–791. doi: 10.1104/pp.65.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]


