Abstract
Background
Seizures are reported in about one-third of patients with severe liver disease in association with acute or chronic liver failure. The majority of the seizures are of focal type. Occasionally generalized tonic-clonic seizures are seen when there is ethanol withdrawal. Not much is known about ictal blinking (IB) in severe liver disease. IB is the rare form of seizures and was reported in severe liver disease recently from this institute. Oculogyric crisis (OGC) is rarely reported in relation to the severe liver disease. OGC was also noted first time in our intensive care unit.
Methods
At the Institute of Liver and Biliary Sciences (ILBS), data on patients with IB and OGC were analyzed from October 2018 to January 2023 (52 months). All the patients had video electroencephalograph (video-EEG) recording after proper permission/consent. The patients were followed up later for the course of the illness.
Results
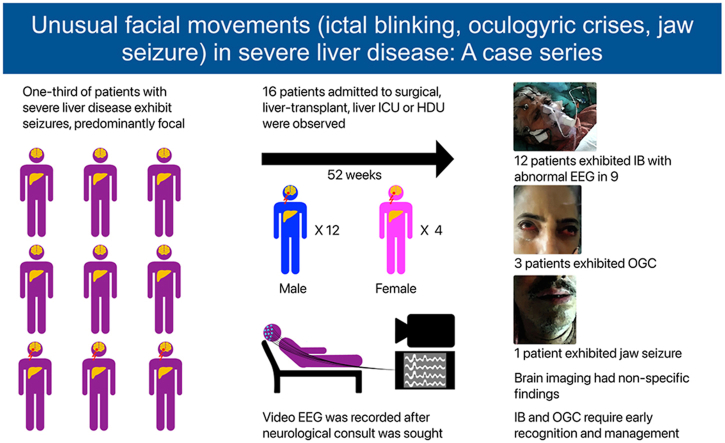
A total of 16 (12M:4F) patients were seen. Majority 12 (75%) were IB and 3 OGC. EEG was abnormal in nine (75.0%) of IB patients. Brain imaging had nonspecific findings. The outcome was based on the severity and recovery of the underlying liver disease.
Conclusions
Unusual facial movements in the form of IB and OGC are reported, which are most of the time missed. This report highlights the importance of recognition of these events and proper in time management to improve the outcome.
Keywords: ictal blinking, oculogyric crises, jaw seizure, severe liver disease, hepatic encephalopathy
Graphical abstract
Seizures are reported in about one-third of patients with severe liver disease in association with acute or chronic liver failure.1 The majority of the seizures are focal and occasionally generalized tonic-clonic when there is ethanol withdrawal.1 Ictal blinking (IB) is a rare type of epilepsy.2 By definition the seizure is confined to the eyelids. There may be eye deviation or involvement of orbicularis oculi without spread to other parts of the body. There is associated loss of consciousness.2 Bilateral blinking has no localizing value; however, unilateral blinking has a localization value.3,4 IB has been seen in patients with lesions in any cortical areas, cerebellum, and even fourth ventricular mass.2,5 IB has not been seen in association with severe liver disease and was reported recently from this center.6 IB mechanism is unknown. Twelve patients with IB were seen in last four years. Brain imaging was nonspecific, and electroencephalograph (EEG) abnormality was seen in about three-fourths of these patients.
Oculogyric literally means pertaining to the rotation of eyeball. When oculogyric crisis (OGC) is used, it pertains to tonic upward gaze of eyeballs with retained consciousness.7,8,9 There may be additional autonomic features or body stiffness. OGC is reported in association with severe liver disease only once before.9 Three patients with OGC and one with jaw seizure were seen.
Material and methods
The patients with liver failure in acute or chronic liver disease with or without hepatic encephalopathy (HE), admitted in various intensive care units (ICUs) like liver ICU, surgical ICU, high dependency unit (HDU), and liver transplant (LT) ICU of the Institute of Liver and Biliary Sciences (ILBS) formed the subjects of the study. Also included were four patients with IB after LT. These four were admitted in LT ICU. All patients had a history of HE in the past. IB and OGC were diagnosed on the defined criteria.2,7 The patients with IB and OGC had detailed neurological examination. Usually, neurological opinion was sought when hepatologists/hepatobiliary surgeons/attending doctors could not explain reasons for unexplained unresponsiveness or obtundation in the patient. During neurological examination when IB or OGC was noted, video recording was done on smartphones after proper permission from the close relatives of the patient. All the patients had EEG, computerized tomography (CT), magnetic resonance imaging (MRI) brain, and baseline blood workup like electrolytes and ammonia. Routine half an hour EEG was recorded usually after managing the IB. Only two patients had EEG at the time of IB. Only three patients had a repeat follow-up EEG. Cerebrospinal fluid (CSF) examination was not done in any patient as it was not indicated clinically, and there was associated coagulopathy in some. All the patients received stat dose of 5 mg midazolam intravenous followed by levetiracetam 1 G intravenous infusion. Levetiracetam was continued in two divided doses 12 h later (usually 750 mg twice a day). All the patients were followed up till discharge or death.
The patients with OGC were not given any particular medications. A drug history and current medications were screened which could result in OGC. They were followed till recovery from OGC. One patient was admitted for the management of chronic liver disease and history of HE and was also diagnosed as jaw seizure. His workup for HE in this admission was negative.
Results
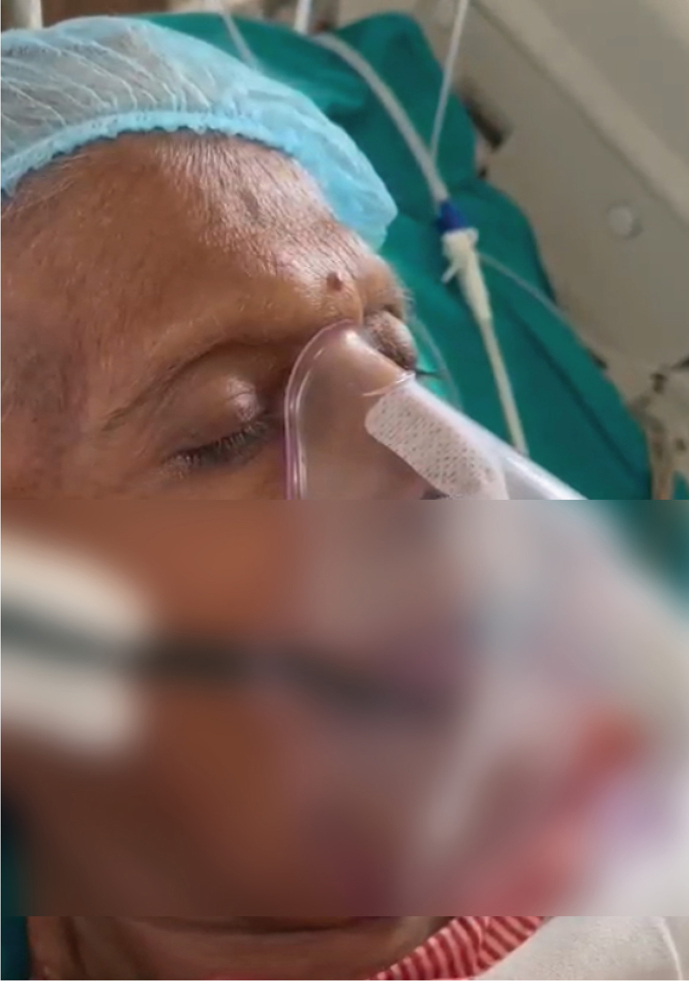
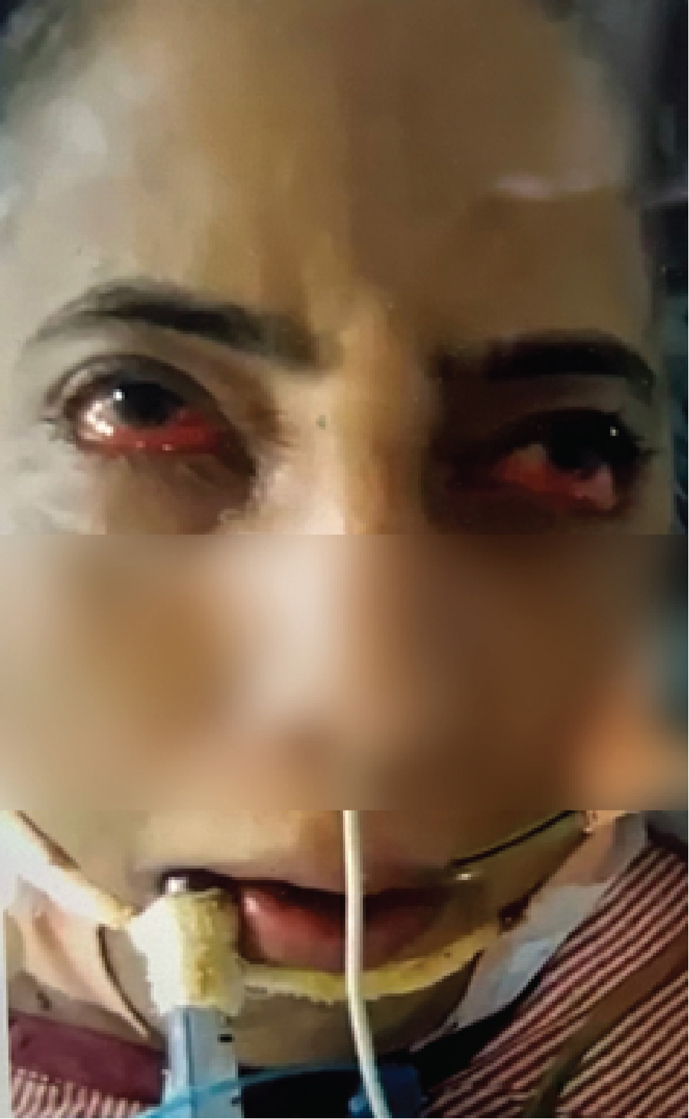
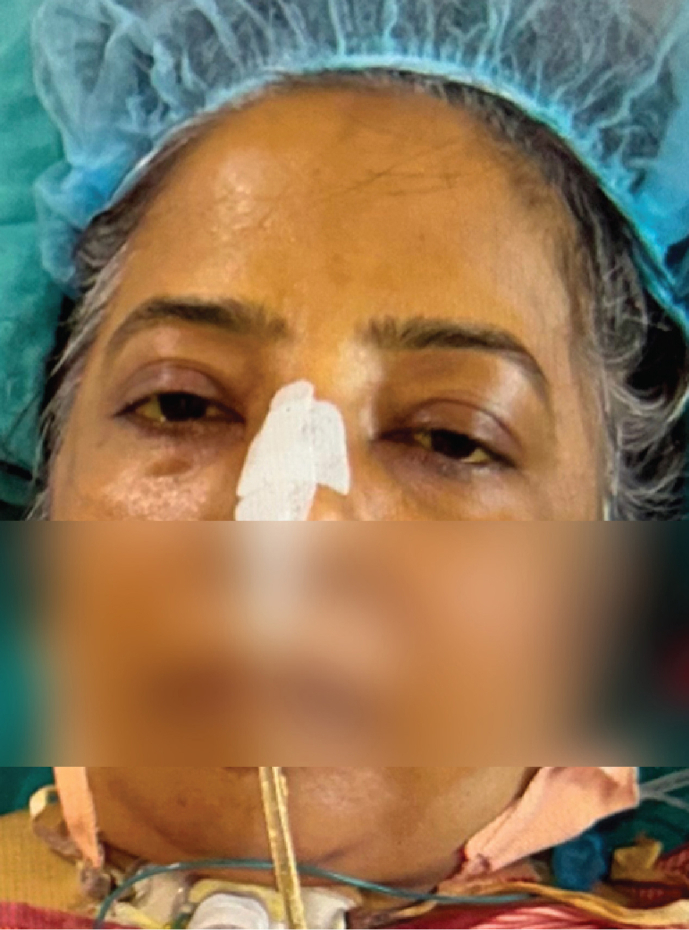
From October 2018 to January 2023 (52 months), 16 patients (12M: 4F) with unusual facial movements were seen at ILBS. IB was the most common and was seen in 12 (75%) patients. IB was seen in severe liver disease patients irrespective of underlying etiology for liver disease, as depicted in Table 1, Table 2. Four patients of IB were post-LT, two in HD, one in SICU, and rest in liver ICU. IB in post-LT patients was seen from day 2 to day 45 (19.2 days mean). At the time of IB in LT patients, serum electrolytes, ammonia, and tacrolimus levels were normal. Typical IB is seen in video 1 (supplementary file). A static photo from video is seen in (a). All 12 IB patients had no previous history of seizures. A total of 9 of the 12 patients with IB had abnormal EEG, as shown in Figure 1. Abnormalities were mainly over bifrontal and frontotemporal regions. Follow-up EEGs were done in three patients only, and the EEGs were normal. Brain imaging had nonspecific findings as seen in Table 1. Only one patient with OGC had microhemorrhages. OGC was seen in three patients (Video 2 supplementary file). A static photo from video (b). In three OGC patients, two had nonalcoholic steatosis hepatitis (NASH) and cirrhosis, while 3rd patient had acute liver failure of unknown etiology (Table 3). These patients were not on drugs known to produce OGC other than propofol anesthesia in two. One of them died due to the liver disease. This patient had NASH with cirrhosis and died of multiorgan failure. However, four days before death, his OGC was not seen in him. OGC lasted from one to seven days in these three patients. EEG did not reveal seizures in any of them. Patient number 1 underwent emergency live liver donor transplant and was discharged from hospital recently.
Table 1.
Clinical Features, Investigations, and Outcome of the Patients with Ictal Blinking with Hepatic Encephalopathy.
| S No | Age/sex | Diagnosis | Seizure semiology | EEG | MRI/CT brain | Treatment | Outcome | Transplant | Other medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | Cirrhosis liver, cryptogenic | Ictal blinking, orbicularis oculi post-LT | Normal | CT volume loss | Mdz., Lev | Seizure free | Deceased LT | Methyl prednisolone, Basiliximab,Tacrolimus |
| 2 | 56/M | Cirrhosis Hep B/C | Ictal blinking post-LT | Frontotemporal spikes, generalization | MRI normal | Mdz., Lev | Seizure free | Live donor LT | Methylprednisolone, tacrolimus |
| 3 | 42/M | Cirrhosis, ethanol related | Ictal blinking | Periodic spikes, burst suppression | CT volume loss | Mdz. Lev., Lac. | Died of sepsis, DIC | No | No |
| 4 | 9 y/M | Acute liver failure, undetermined | Ictal blinking with eyes deviation post-LT | Normal | MRI normal | Mdz. Lev. | Seizure free | Live donor LT | Methylprednisolone, tacrolimus |
| 5 | 50/M | Cirrhosis ethanol related | Blinking | Periodic spikes, focal discharges | CT normal | Mdz. Lev., Lac. | Died of sepsis, DIC | No | No |
| 6 | 55/F | Cirrhosis, scleroderma, disseminated tuberculosis | Ictal blinking, | Focal seizure discharges | CT normal | Mdz., Lev. | Died of sepsis/disseminated tuberculosis | No | No |
| 7 | 52/M | Cirrhosis ethanol related | Ictal blinking and orbicularis oculi | Generalized spike discharges | MRI/CT Subdural hematoma |
Lev | Seizure free | No | Propofol |
| 8 | 51M | Cirrhosis Cryptogenic |
Ictal blinking and right forearm jerks | Generalized spike discharges | MRI/CT normal | Lev | Seizure free | Live donor LT | None |
| 9 | 60 M | Ethanol and cirrhosis | IB and left forearm | Focal seizures | CT volume loss | Lev | Seizure free | No | Liver related |
| 10 | 64 F | NASH & cirrhosis | IB | Ictal EEG | CT normal | Mdz Lev | Seizure free | No | Liver related |
| 11 | 65 M | NASH & cirrhosis | IB | Focal slowing | CT volume loss | Lev | Seizure free | No | Liver related |
| 12 | 54 F | Cholangiocarcinoma with metastasis, secondary biliary cirrhosis | IB | Multifocal seizures | CT/MRI microhemorrhages, no metastases | Lev, phenytoin sodium | Died | No | Anticancer medications |
M, male; F, female, B/C, hepatitis B and C; IB, ictal blinking; NASH, nonalcoholic steatosis hepatitis; CT, computerized tomography; MRI, magnet resonance imaging; Mdz, Midazolam; Lev, Levetiracetam; Lac, Lacosamide; DIC, disseminated intravenous coagulopathy; LT, liver transplant. Please note patient number 1, 2, 4, and 8 had liver transplant.
Table 2.
Type of Underlying Liver Disease in Ictal Blinking.
| Type of liver disease | Acute | Chronic | Total |
|---|---|---|---|
| Ethanol | 4 | 4 | |
| NASH | 2 | 2 | |
| Cryptogenic | 1 | 2 | 3 |
| Autoimmune | 1 | 1 | |
| Hepatitis B C | 1 | 1 | |
| Cholangiocarcinoma with metastases Secondary biliary cirrhosis |
1 | 1 | |
| Total cases | 1 | 11 | 12 |
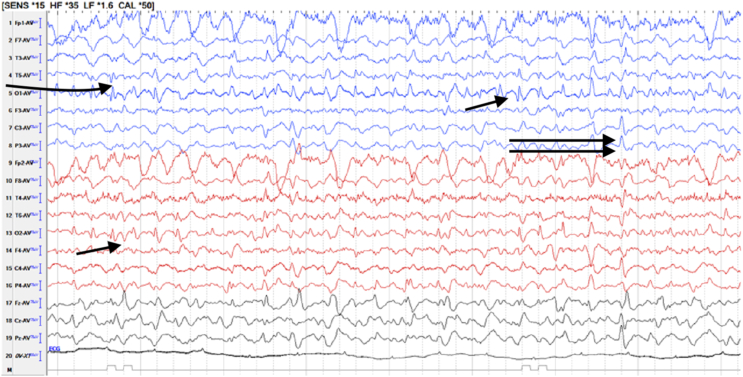
Figure 1.
EEG at the time of blinking in 64-year NASH with cirrhosis lady shows multifocal spikes (single arrows) and generalized seizure discharges (double arrows). NASH, nonalcoholic steatosis hepatitis; EEG, electroencephalograph.
Table 3.
Detailed Features of Oculogyric Patients.
| No | Age/sex | Diagnosis | OGC duration days | EEG | CT/MRI | Medications | Treatment for OGC | Other management | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 52/F | Cryptogenic ALF | 4 | 3 times, mild encephalopathy, no seizures | Both normal | Propofol antibiotics | None | LDLT | Doing well |
| 2 | 64/M | NASH | 7 | 4 EEGs, encephalopathy, no seizures | CT mild atrophy, MRI microhemorrhages | Propofol antibiotics | None | Usual | Died |
| 3 | 78/M | NASH | 1 | 2 EEGs, normal | CT atrophy | Antibiotics | None | Usual | Discharged |
M, male; F, female; ALF, acute liver failure; NASH, nonalcoholic steatosis hepatitis; OGC, oculogyric crises; EEG, electroencephalograph; CT, computerized tomography; MRI, magnetic resonance imaging; LDLT, live liver donor transplant.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.jceh.2023.05.004
The following are the supplementary data related to this article:
Video of 64-year-old lady with NASH and cirrhosis having ictal blinking. EEG electrodes in place while she had ictal blinking.
52-year-old lady with cryptogenic acute liver failure with OGC. There is tonic up-gaze with reduced blinking and contracted frontalis muscle. The patient is conscious, and she closes eyes on seeing the object from left side.
One patient was seen with jaw seizures. He was a 62-year-old male patient with chronic liver disease with NASH and had history of ethanol intake as well. He had history of HE in past. He was admitted four times in past three months at a peripheral hospital with drowsiness thought to be HE. However, his workup was always within normal range (serum ammonia not raised). When he was admitted in this hospital, the laboratory work could not explain his frequent encephalopathy-like illness. The history review suggested he would get involuntary movements of jaw lasting few minutes. At this time, he would become unresponsive without any tonic-clonic movements or loss of posture or tone. The movements would happen even in sleep. His examination was normal. Video recorded by wife was seen (video 3 in supplementary file). MRI brain and EEG were done. His EEG was abnormal (Figure 2), and brain imaging was normal. He was started on levetiracetam and in about half a day his sensorium improved, and he was discharged next day. He was seen on follow-up in the clinic and was doing well.
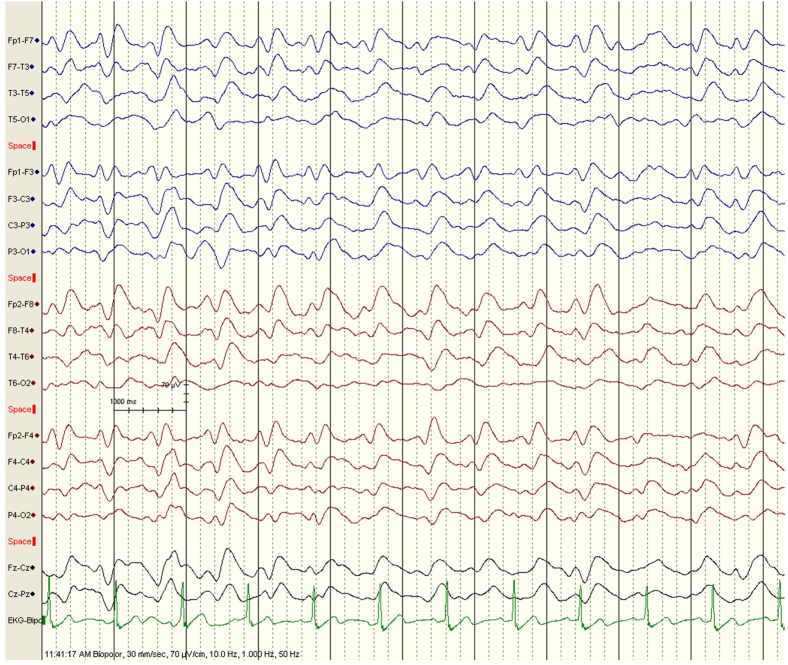
Figure 2.
Interictal EEG of 62-year NASH with cirrhosis patient with jaw seizures shows bifrontal sharp waves, sharp, and slow wave discharges. NASH, nonalcoholic steatosis hepatitis; EEG, electroencephalograph.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.jceh.2023.05.004
The following is/are the supplementary data related to this article:
Video shows 62-year-old male with NASH and cirrhosis having jaw seizures (side-to-side jaw movements).
Discussion
About one-third of patients with severe acute or chronic liver disease with or without HE have seizures.1 Most of the seizures are of focal type. Generalized tonic-clonic seizures are seen in alcohol withdrawal patients.1 These types of seizures are easy to recognize and diagnose. In a previous study reported from this institute, seizures were seen in 20.1% patients.10 IB type of seizures is mostly missed and has not been reported in HE before, except a recent report from this institute.6 IB is a rare form of seizure.2 Seizures in IB are confined to eyelids mainly or spread to orbicularis oculi without spread to rest of the body parts.2,6 Blinking is usually bilateral but can involve only one eye. During blinking, there is impairment to loss of consciousness. Bilateral blinking has less localizing value and has been seen in lesions involving frontal, temporal, occipital lobes, cerebellum, and fourth ventricular mass. 2,3,4,5 Unilateral blinking has more localization value, indicating contralateral cortical lesion.3,11 IB in association with severe liver disease was reported for the first time recently from this institute.6 It constituted 4.4% of all seizures seen in the severe liver disease patients.6 Since the recognition of this entity (IB), more cases are picked up by the treating team, residents, and other staff. Among 12 patients of IB, four were post-LT. IB was seen in them from day 2–45 days (19.2 days mean) after LT. They all were extubated and doing well. The duty doctors in LT ICU noted them in deranged sensorium. All metabolic workup including ammonia was normal. IB was diagnosed on neurological examination. What is the mechanism of IB in severe liver disease with or without HE? It cannot be explained on one factor alone. Underlying reasons seem to be multifactorial like ammonia toxicity, brain edema, neurotransmitter imbalance, toxic aromatic amino acids accumulation, tacrolimus, and anesthetic agents.6,12 There are reports regarding anesthetic agents particularly propofol-producing muscle twitchings.12 Tacrolimus is known to be neurotoxic.13 But only three patients were on tacrolimus post-LT, and the serum levels were in therapeutic range. Fourth LT patient and rest eight patients also did not receive tacrolimus. In nine out of 12 (75%) IB patients, EEG was abnormal. Usually, EEGs were performed after the initial treatment of IB at the time of diagnosis, other than two patients who had EEGs before treatment. Most of the time seizure discharges were from frontotemporal region. However, multifocal and generalized seizure discharges were also seen (Figure 1). Brain imaging was nonspecific in our patients. The abnormalities seen were brain atrophy, subdural hematoma in one, and few microhemorrhages, etc.6,10 The seizures responded very well to levetiracetam alone, though most received midazolam stat dose at the time of diagnosis. Five of 12 patients died due to the underlying serious liver disease and HE and not because of IB. With the features as described in IB in our patients, there is no other diagnosis. However, any person with fast blinking rate, one needs to differentiate from blepharospasms and tics. In both these conditions, consciousness is not lost, and the person can control them at will.
Any part of the central nervous system (cortex, white matter, basal ganglia, brainstem, and cerebellum spinal cord) can be involved in liver disease both acute and chronic type.14, 15, 16 Extrapyramidal (basal ganglia) involvement in form of dystonia, chorea, parkinsonism, and orofaciolingual dyskinesia are seen.9,14 Extraocular muscle involvement in form of OGC in liver disease is a rare condition, reported once before.9 Two of our three OGC patients had NASH with cirrhosis. Third case had acute liver failure of unknown etiology. This patient with acute liver failure underwent emergency LT (Table 3). OGC is seen in association with large number of conditions. These are genetic, neurometabolic, vascular, infective, drugs,dystonia, and dementia.17 Typical features of up rolled eyes, tongue protrusion, lip smacking, blepharospasm, anterocollis, retrocollis, and choreoathetosis may be seen.17 Nigrostriatal pathway malfunction and dopaminergic/cholinergic imbalance are the main mechanism of OGC.17,18 Seizures, paroxysmal tonic up gaze, and ocular dyskinesia have to be considered in the differential diagnosis.19,20 Metallic deposition (Manganese) is suggested to be the mechanism of OGC in liver disease, like copper in Wilson's disease.9,21,22 However in acute liver failure, ammonia toxicity and other aromatic amino acids are supposedly disturbing the dopaminergic pathway resulting OGC.17,18 One can consider liver disease as the cause of OGC when a large number of causes which could result OGC have been excluded.9 Most of these causes are the drugs.7,8 Propofol comes in the drug group and has been associated with dystonic reactions.23,24 Two of our patients (one acute liver failure and second chronic liver disease) were on propofol. However, third patient did not receive propofol. Recovery of OGC on the improvement of ALF could be a point in favor of liver disease as the causative factor in our first patient. In this patient, OGC lasted for four days. Propofol was not used in the third patient. There was no history of other drug intake before hospitalization resulting OGC in any of our patients. Further, OGC developed in hospital in all of them (two after intubation and one without intubation). One has to exclude seizures in OGC. A bedside examination and EEG help to rule this out. These patients are conscious, and a simultaneous EEG is normal other than the effect of underlying encephalopathy. All the three patients had OGC settled, after one to seven days (Photos a1b1).
Jaw seizure in liver disease with HE is not reported before. Since seizures are reported in one-third of HE patients,1 isolated jaw seizure may be seen in them occasionally. Bruxism should be considered in differential diagnosis of jaw seizure. In bruxism, there is continuous teeth grinding due to the excessive use of masseter muscles in sleep. The patient's video did not reveal teeth grinding at all (Video 3 supplementary file). There were no masseter muscle contractions and artifacts noted during EEG recording. The muscle contractions produce a lot of muscle artifacts on EEG which were not seen, and EEG had revealed seizure discharges (Figure 2).
Limitations
The cohort of this case series is quite heterogenous and includes patients with acute liver failure, decompensated cirrhosis, and post-LT patients. Continuous EEG monitoring was not done. Neurotransmitter estimation and CSF study were not done. May be in future study, one can find the triggering mechanism for these unusual facial movements.
IB, OGC, and jaw seizure are reported for the first time in severe liver disease, and sometimes in patients with present or past HE. When there are no clear reasons to explain unresponsiveness on liver disease, one must look for seizures particularly IB in these patients, which can produce obtundation/unresponsiveness. On simple observation of the face for few minutes, one can make the diagnosis.
Credit Authorship Contribution Statement
Roshan Koul: conceptualization, methodology, original draft preparation; Rakhi Maiwall: data curation, resources, validation, editing; Shiv K Sarin: supervision; Vikram Bhatia: resources; Akhil Deshmukh: resources; Chandan Kumar: resources; Rahul Khajuria: resources; Omkar S Rudra: resources; Sangam Papneja: resources; Vineyndra Pamecha: resources, reviewing.
Conflicts of interest
All authors have none to declare.
Acknowledgements
The authors are thankful to Sadam H Bhat, Dept of Molecular and Cellular Medicine, ILBS Hospital Delhi, India, and Dr Shashikant Koul for software work in the manuscript.
Funding
No funding received.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2023.05.004.
Appendix A. Supplementary data
The followings are the supplementary data to this article.
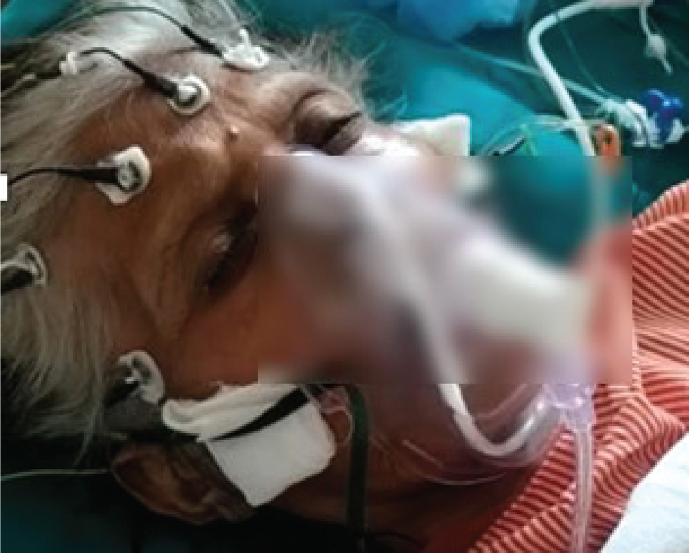
Fig. S1.
Snap photograph of a 52-year-old lady with cryptogenic acute liver failure and oculogyric crisis
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Prabhakar S., Bhatia R. Management of agitation and convulsions in hepatic encephalopathy. Indian J Gastroeneterol. 2003;22:S54–S58. [PubMed] [Google Scholar]
- 2.Saporito M.A.N., Vitaliti G., Pavone P., et al. Ictal blinking, an under-recognized phenomenon: our experience and literature review. Neuropsychiatric Dis Treat. 2017;13:1435–1439. doi: 10.2147/NDT.S135979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benbadis S.R., Kotageal P., Klen G.H. Unilateral blinking: a lateralizing sign in partial seizures. Neurology. 1996;1:45–48. doi: 10.1212/wnl.46.1.45. doe: [DOI] [PubMed] [Google Scholar]
- 4.Ozdag Acarli A.N., Elmali A.D., Sirin N.G., Baykan B., Bebek N. Ictal blinking: reappraisal of the lateralization and localization value in focal seizures. Clin EEG Neurosci. 2022 Jan;5 doi: 10.1177/15500594211070800. [DOI] [PubMed] [Google Scholar]
- 5.Pestana E.M., Gupta A. Ipsilateral blinking seizures during left frontotemporal ictal pattern on scalp EEG. Epileptic Disord. 2007;9:449–452. doi: 10.1684/epd.2007.0130. [DOI] [PubMed] [Google Scholar]
- 6.Koul R., Maiwall R., Alam S., Pamecha V., Tevethia H.V., Sarin S.K. Ictal blinking in hepatic encephalopathy pre and post-liver transplant: report of eight patients roshan. J Neurosci Rural Pract. 2022 Jul;13:476–482. doi: 10.1055/s-0042-1750136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slow E.J., Lang A.E. Oculogyric crises: a review of phenomenology, etiology, pathogenesis and treatment. Mov Diord. 2017;32:193–202. doi: 10.1002/mds.26910. [DOI] [PubMed] [Google Scholar]
- 8.Mahal P., Suthar N., Nebhinani N. Spotlight on oculogyric crisis: a review. Indian J Psychol Med. 2021;43:5–9. doi: 10.1177/0253717620942096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara J., Gupta D., Foster E., Garman K., Stacy M. Extraocular muscle dystonia due to acquired (non-Wilsonian) hepatocerebral degeneration. Mov Disord. 2008;23:875–915. doi: 10.1002/mds.21841. [DOI] [PubMed] [Google Scholar]
- 10.Koul R., Maiwall R., Ramalingam A., et al. Role of EEG in predicting outcome of hepatic encephalopathy patients. Neurdiagn. J. 2020;60:272–288. doi: 10.1080/21646821.2020.1824959. [DOI] [PubMed] [Google Scholar]
- 11.Falsaperla R., Perciavalle V., Pavone P. Unilateral eye blinking arising from the ictal ipsilateral occipital area. Clin EEG Neurosci. 2016;47:243–247. doi: 10.1177/1550059414533111. [DOI] [PubMed] [Google Scholar]
- 12.Khan K. Muscle twitching's and hiccups with Propofol. J Anaesthesiol Clin Pharmacol. 2011;27:418. doi: 10.4103/0970-9185.83703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bechstein W.O. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int. 2000;13:313–326. doi: 10.1007/s001470050708. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson M.H., Superville B. Distribution of cerebral lesions in acquired hepatocerebral degeneration. Brain. 1981;104:79–95. doi: 10.1093/brain/104.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal A., Vaidya S., Shah S., sing J., Desai S., Bhatt M. Reversible Parkinsonism and T1W pallidal hyper intensities in acute liver failure. Mov Disord. 2006;67:1984–1989. doi: 10.1002/mds.21096. [DOI] [PubMed] [Google Scholar]
- 16.Pomier –Layrargues G.P. Movement dysfunction and hepatic encephalopathy. Metab Brain Dis. 2001;16:27–35. doi: 10.1023/a:1011658311004. [DOI] [PubMed] [Google Scholar]
- 17.Gold D.R. In: Volpe and Galetta's Neuro-Ophthalmology. 3rd de. Liu G.T., Volpe N.J., (des) Liu Galetta SL., editors. Elsevier; 2019. Eye movement disorders: conjugate gaze abnormalities [Internet] pp. 549–584.https://www.sciencedirect.com/acience/article/pii/B978032334044100016X [cited Mar 26,2020] [Google Scholar]
- 18.Barrow E., Schneider S.A., Bhatia K.P., Ganos C. Oculogryic crises: etiology, pathophysiology and therapeutic approaches. Parkinsonism Relat Disorders. 2017;36:3–9. doi: 10.1016/j.parkreldis.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Salmina C., Taddeo I., Falesi M., et al. Paroxysmal tonic upgaze in normal children. A caser series and a review of the literature. Eur J Paediatr Neurol. 2012;16:683–687. doi: 10.1016/j.ejpn.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Jankovic J., Stone L. Dystonic tics in patients with Tourette's syndrome. Mov Disord. 1991;6:248–252. doi: 10.1002/mds.870060309. [DOI] [PubMed] [Google Scholar]
- 21.Klos K.J., Ahlskog J.E., Kumar N., et al. Brain metal concentrations in chronic liver failure patients with pallidal T1MRI hyper intensity. Neurology. 2006;67:1984–1989. doi: 10.1212/01.wnl.0000247037.37807.76. [DOI] [PubMed] [Google Scholar]
- 22.Dobson A.W., Erikson K.M., Aschner M. Manganese neurotoxicity. Ann NY Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 23.Mathew J.H., Rubin J.S., Patel A. Acute dystonic reaction to general anesthesia with propofol and ondansteron: a graded response. Ear Nose Throat Online J. 2013;92:E16–E18. doi: 10.1177/0145561313200121. [DOI] [PubMed] [Google Scholar]
- 24.Schramm B.M., Orser B.A. Dystoni reaction to propofol attenuated by benztropine (cogentin) Anesth Analg. 2002;94:1237–1240. doi: 10.1097/00000539-200205000-00034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of 64-year-old lady with NASH and cirrhosis having ictal blinking. EEG electrodes in place while she had ictal blinking.
52-year-old lady with cryptogenic acute liver failure with OGC. There is tonic up-gaze with reduced blinking and contracted frontalis muscle. The patient is conscious, and she closes eyes on seeing the object from left side.
Video shows 62-year-old male with NASH and cirrhosis having jaw seizures (side-to-side jaw movements).