Abstract
Inositol monophosphatase (EC 3.1.3.25) plays a pivotal role in the biosynthesis of di-myo-inositol-1,1′-phosphate, an osmolyte found in hyperthermophilic archaea. Given the sequence homology between the MJ109 gene product of Methanococcus jannaschii and human inositol monophosphatase, the MJ109 gene was cloned and expressed in Escherichia coli and examined for inositol monophosphatase activity. The purified MJ109 gene product showed inositol monophosphatase activity with kinetic parameters (Km = 0.091 ± 0.016 mM; Vmax = 9.3 ± 0.45 μmol of Pi min−1 mg of protein−1) comparable to those of mammalian and E. coli enzymes. Its substrate specificity, Mg2+ requirement, Li+ inhibition, subunit association (dimerization), and heat stability were studied and compared to those of other inositol monophosphatases. The lack of inhibition by low concentrations of Li+ and high concentrations of Mg2+ and the high rates of hydrolysis of glucose-1-phosphate and p-nitrophenylphosphate are the most pronounced differences between the archaeal inositol monophosphatase and those from other sources. The possible causes of these kinetic differences are discussed, based on the active site sequence alignment between M. jannaschii and human inositol monophosphatase and the crystal structure of the mammalian enzyme.
The sole pathway for myo-inositol biosynthesis is the cyclization of glucose-6-phosphate to inositol-1-phosphate (I-1-P) by I-1-P synthase (EC 5.5.1.4) and the dephosphorylation of I-1-P by inositol monophosphatase (I-1-Pase; EC 3.1.3.25) (7–9, 12, 16, 24). This de novo pathway provides the ultimate source of free inositol for the cell. It is also a key enzyme involved in second-message signal transduction processes in mammalian and plant cells (2, 24, 28, 37). In phosphoinositide signaling (2, 37), I-1-Pase recycles the water-soluble phospholipase C phospholipid degradation products, inositol phosphates, to myo-inositol and helps to maintain a moderate inositol pool. Its inhibition by millimolar concentrations of lithium (19) has made it a putative target of lithium therapy for manic depression (34).
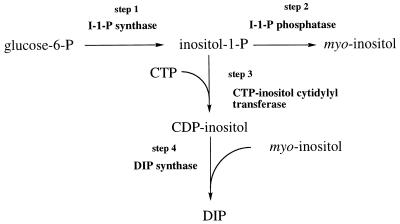
Di-myo-inositol-1,1′-phosphate (DIP), a novel inositol phosphate compound found in hyperthermophilic archaea, including Pyrococcus woesei (43), Pyrococcus furiosus (41), Methanococcus igneus (11), and Thermotoga maritima (36), is used for osmotic balance at high growth temperatures. In order to understand what regulates its accumulation in cells, the DIP biosynthetic pathway must be well characterized in vitro. Based on 13C-labeling studies and assays of crude protein extracts from M. igneus (10), a pathway was proposed that converts glucose-6-phosphate to I-1-P (step 1), hydrolyzes some of the I-1-P to myo-inositol (step 2), and activates I-1-P to CDP-inositol (CDP-I) (step 3) for a final reaction (step 4) whereby CDP-I is coupled to myo-inositol, generating DIP and CMP (Fig. 1). Activities for I-1-P synthase, I-1-Pase, and DIP synthase in the DIP biosynthetic pathway have been detected in crude protein extracts of M. igneus (10). Phosphatase activities are ubiquitous in cells, and the observed activity in M. igneus could be due to a specific I-1-Pase activity or a nonspecific phosphatase. For mammalian and plant cells, I-1-Pases are all lithium sensitive and are inhibited at millimolar concentrations of Li+ (14, 15, 19, 30, 42). The partially purified phosphatase in M. igneus exhibited substrate specificity for dl-I-1-P over other sugar phosphates (10). It had an absolute requirement for Mg2+, a characteristic of all specific I-1-Pases studied thus far, and was also partially inhibited by Li+, though at a much higher concentration (160 mM for 50% activity inhibition) than reported for I-1-Pases from other cells. While this was suggestive of a specific I-1-Pase, the same protein fractions demonstrated considerable activity toward p-nitrophenylphosphate (pNPP), a very poor substrate for mammalian enzymes (1, 14). These preliminary characterizations of phosphatase activity suggested that archaeal I-1-Pases might be different from mammalian and plant enzymes.
FIG. 1.
Proposed DIP biosynthetic pathway. Glucose-6-phosphate is converted to I-1-P (step 1), some of which is hydrolyzed to myo-inositol (step 2), and I-1-P is activated to CDP-I (step 3) for a final reaction in which CDP-I is coupled to myo-inositol (step 4), generating DIP and CMP.
Methanococcus jannaschii was the first archaeon whose complete genomic sequence was determined (6). Of all the archaea with sequenced genomes, it is the closest to M. igneus. MJ109 encodes a 252-amino-acid protein that is highly homologous to both I-1-Pase and extragenic suppressor (the suhB gene product) (6). The latter gene product cloned in E. coli also has I-1-Pase activity (29). The putative identification of the MJ109 gene product as an I-1-Pase prompted us to express the gene product in E. coli and to examine its specific activity toward a variety of phosphate esters. The protein produced in this fashion clearly has I-1-Pase activity and shows several striking differences from plant and mammalian I-1-Pase activities.
MATERIALS AND METHODS
Chemicals.
dl-I-1-P, d-I-1-P, inositol-2-phosphate, 2′-AMP, 5′-AMP, pNPP, β-glycerophosphate, α-d-glucose-1-phosphate, glucose-6-phosphate, fructose-1-phosphate, sodium dodecyl sulfate (SDS)-polyacrylamide gel molecular mass markers, gel filtration molecular mass markers, Sephadex G-150, and Coomassie brilliant blue 250 were obtained from Sigma. The Q-Sepharose fast flow and phenyl-Sepharose were obtained from Pharmacia. Restriction enzymes were obtained from New England Biolabs. The ligation kit and E. coli strains were purchased from Novagene. The PCR kit was obtained from Perkin-Elmer.
Assay of I-1-Pase.
Enzyme activity was measured by colorimetric determination of released inorganic phosphate (Pi) (20). For elution profiles in column chromatography, the reaction mixture contained 1 μl of 10 mM I-1-P (in 50 mM Tris buffer, pH 8.0), 1 μl of 40 mM MgCl2 (in 50 mM Tris buffer, pH 8.0), and 2 μl of diluted protein (0.4 to 0.5 μg/μl). For other assays, the total volume of the reaction mixture was increased to 16 μl to reduce pipetting errors. For the Vmax and Km determinations, the sensitivity range of the colorimetric Pi assay and the lower substrate concentrations needed in the assay required increasing the total assay volumes to 0.5 ml. After incubation at 85 or 100°C (as specified in each experiment) for ∼1 min, the mixtures were quickly chilled on ice. All the substrates were quite stable for at least 5 min at 100°C, as evidenced by the lack of Pi in controls without the enzyme added (data not shown). The liberated Pi was measured by colorimetric phosphate assay with ammonium molybdate malachite green reagents (20). The absorbance at 660 nm of the enzyme sample compared to those of standard Pi concentrations was used to calculate Pi production in micromoles per minute. Specific activity was estimated by normalizing to the protein concentration determined by the Lowry method, which was consistent with the UV absorption and the relative band intensity on the SDS-polyacrylamide gel. For crude cell extracts, bovine serum albumin (Bio-Rad) was used as the standard.
Subcloning the I-1-Pase gene in M. jannaschii.
The pUC18 plasmids harboring AMJER84, a 2,173-bp genomic fragment containing the MJ109 gene in the middle at a unique SmaI site, were obtained from the American Type Culture Collection and linearized by BamHI. The coding region of the I-1-Pase gene (MJ109) was reconstructed to contain a NdeI site at the start codon and a HindIII site right after the stop codon by standard PCR methodology (39). Two oligonucleotide primers, 5′-GCATAGGATGAGCATATGAAATGGGATGAAATTGG-3′ and 5′-GTATCTAAAAATATTTAAGCTTATAAGAGGTCTAAA-3′, were obtained from Operon. The 25-cycle PCR product was cut with NdeI and HindIII and ligated to NdeI-HindIII-digested pET23a(+) (Novagene). The ligation products were transformed into Novablue competent cells (Novagene). Recombinant clones were identified by detailed restriction mapping. The DNA sequences of these clones were confirmed by double-stranded DNA sequencing with the Amersham Life Science thermosequence 33P kit. For the expression of the protein, the recombinant vectors were transformed into BL21(DE3)PLysS cells (Novagene).
Overexpression of the MJ109 gene in E. coli.
A single colony of BL21(DE3)PLysS cells containing the recombinant MJ109-pET23a(+) plasmid was grown in 5 ml of Luria-Bertani medium with 100 mg of ampicillin/ml and 34 mg of chloramphenicol/ml until the optical density at 660 nm reached ∼0.6. Four milliliters of culture cell pellets was used to inoculate 2 liters of fresh Luria-Bertani medium containing 100 μg of ampicillin/ml and 34 μg of chloramphenicol/ml. These cultures were grown to an A660 of ∼0.6 by rapid shaking at 37°C. Production of recombinant protein in the cultures was induced by the addition of 100 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 0.4 mM and continued growth for another 4 h (after which the A660 was ∼1.3). The cells were harvested by centrifugation and stored at −70°C until needed. The time course for expression of protein was monitored by SDS-polyacrylamide gel electrophoresis (PAGE) (a band corresponding to 28 kDa). Dialyzed cell extract had high I-1-Pase activity, whereas the BL21(DE3)/pET23a(+) cell extract, used as a control, did not have the corresponding band on SDS-PAGE and had no detectable I-1-Pase activity (data not shown).
Purification of I-1-Pase.
Frozen cells (∼8.7 g from 4 liters of culture) were thawed and resuspended in 50 ml of buffer A (20 mM Tris-HCl [pH 8.0], 1.0 mM EDTA) and incubated at room temperature for 30 min. The cells were broken by sonication for 10 cycles of 30 s on ice, and the supernatant was separated from cell debris by centrifugation (12,400 × g for 30 min). The supernatants were dialyzed twice against 2 liters of buffer A. The dialyzed crude extracts were then heated at 85°C for 30 min. The precipitated material was removed by centrifugation, and the supernatant was loaded onto a 2.5- by 12-cm Q-Sepharose fast flow column and eluted with a linear gradient of 0 to 0.5 M KCl in buffer A (total, 400 ml). The I-1-Pase was detected by activity, and the purity of each fraction was monitored by SDS-PAGE (22). Fractions (85 to 90% pure) were pooled (total volume, about 32 ml), and aliquots of 5 M NaCl were added to reach a final salt concentration of about 0.5 M. Eight milliliters of this sample was loaded onto a 1.0- by 12-cm phenyl-Sepharose column preequilibrated with buffer B (0.5 M NaCl in 20 mM Tris buffer, 1 mM EDTA, pH 8.0). The column was then washed with the same buffer and eluted with a linear gradient of buffer B and pure water (total volume, ∼200 ml).
Gel filtration.
A column (1.6 by 70 cm) of Sephadex G-150 was equilibrated with buffer A and was used to determine the native molecular mass of pure M. jannaschii I-1-Pase. The void volume was determined with blue dextran, and the column was standardized with β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), albumin (66 kDa), carbonic anhydrase (29 kDa), and cytochrome c (12.4 kDa). Samples (0.5 ml of 2 to 3 A280 U/ml) were applied to the column and eluted at a flow rate of 0.2 ml/min. Fractions of 2 ml each were collected and assayed for I-1-Pase activity.
RESULTS
Purification of M. jannaschii I-1-Pase.
The purification scheme outlined in Materials and Methods yielded ∼20 mg of pure M. jannaschii I-1-Pase. The protein was characterized by a single band on an SDS-polyacrylamide gel with a subunit mass of ∼28 kDa, in agreement with the 27.7 kDa predicted from the DNA sequence (Fig. 2). The overexpressed protein was obtained with an overall 4.3-fold purification and 45% yield. This is consistent with the observation that I-1-Pase is the major band in the crude extracts and represents 20 to 25% of the protein, as estimated from intensities on the SDS-polyacrylamide gel. Activities and yields for each step of the purification are summarized in Table 1. An extinction coefficient, ɛ280 = 29,900 M−1 cm−1 (A0.1% = 1.08), was determined on the basis of a Lowry assay (26) of the protein concentration. According to the peptide sequence, M. jannaschii I-1-Pase contains 2 tryptophans, 13 tyrosines, and 3 cysteines per subunit. The calculated (27) extinction coefficient, ɛ280 = 30,700, is in excellent agreement with the experimental value. Gel filtration on Sephadex G-150 showed the native I-1-Pase molecular mass to be ∼55 kDa. Thus, M. jannaschii I-1-Pase, like all the other I-1-Pases studied thus far, is a dimer (1, 15, 29).
FIG. 2.
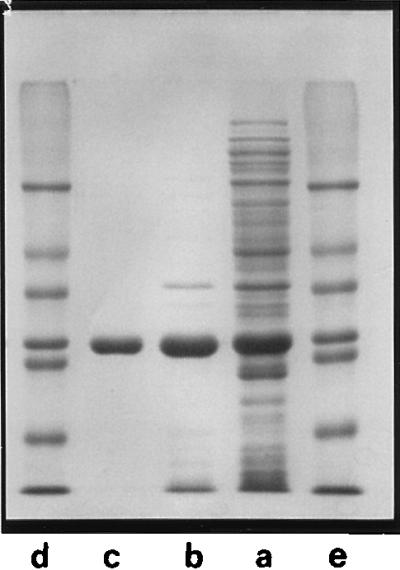
I-1-Pase expression and purification analyzed by SDS–12% PAGE stained with Coomassie brilliant blue. Lane a, crude extract; lane b, heat-treated crude extract; lane c, purified M. jannaschii I-1-Pase; lanes d and e, molecular mass standards (from the top, 66, 45, 36, 29, 24, 21.4, and 14 kDa).
TABLE 1.
Purification of M. jannaschii I-1-Pase
| Step | Total protein (mg) | Sp acta | Total Ub | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude extract | 870 | 2,300 | 2,000 | 100 | 1 |
| Heat treatment | 262 | 7,366 | 1,930 | 96.5 | 3.2 |
| Q-Sepharose fast flow | 128 | 8,632 | 1,150 | 57.5 | 3.75 |
| Phenyl-Sepharose | 91 | 9,876 | 900 | 45 | 4.3 |
In nanomoles per minute per milligram.
One unit is defined as 1 μmol of Pi produced per mg of protein per min at 85°C.
Kinetic characterization of the M. jannaschii I-1-Pase.
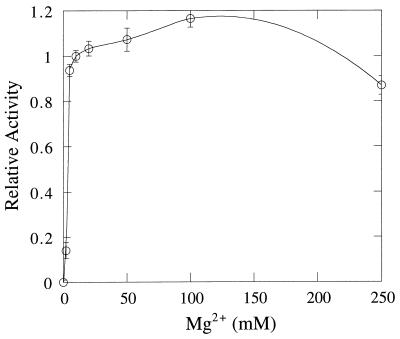
The methanogen I-1-Pase, similar to all other I-1-Pase enzymes known, has an absolute requirement for Mg2+ for activity. At least 10 mM Mg2+ is needed for optimal activity (Fig. 3). This amount of Mg2+ is higher than that needed by the mammalian enzyme (1 to 3 mM) but comparable to that for the E. coli enzyme (10 mM Mg2+ for maximum activity). However, unlike its mammalian and plant counterparts, there was no inhibition of M. jannaschii I-1-Pase when the Mg2+ concentration was increased to 100 mM; instead of inhibition, a slight activation at high Mg2+ concentrations was observed. This activation (∼16%) is outside the error of the I-1-Pase assays (≤5%) and is likely to represent nonspecific activation caused by ionic strength. The only other divalent metal ion that can substitute for Mg2+ is Mn2+ (data not shown). However, for the M. jannaschii I-1-Pase, activation with Mn2+ is only about 64% as effective as that with Mg2+. In the same assay system 10 mM other divalent ions, including Co2+, Ni2+, Cu2+, Zn2+, Ca2+, and Ba2+, showed no significant activation of I-1-Pase in the absence of Mg2+. These cations (at 8 mM) all inhibited the I-1-Pase activity assayed with 8 mM Mg2+. This represents another difference between the M. jannaschii I-1-Pase and the mammalian I-1-Pase (21), since Co2+ was an activator for the mammalian enzyme.
FIG. 3.
Dependence of M. jannaschii I-1-Pase activity (toward 2.5 mM I-1-P in 50 mM Tris HCl [pH 8.0] at 85°C) on Mg2+ concentration. The activities are normalized to the value obtained with 10 mM MgCl2. The error bars indicate standard deviations.
In the presence of 10 mM Mg2+, the I-1-Pase from M. jannaschii displayed a hyperbolic dependence of specific activity on substrate concentration, with Vmax determined to be 9.3 ± 0.45 μmol of Pi min−1 mg of protein−1 and a Km of 0.091 ± 0.016 mM at 85°C. These values are similar to the values reported for mammalian I-1-Pase (notably bovine [15] and human recombinant [30] enzymes) and the E. coli (29) enzyme (Table 2). Thus, under standard assay conditions (2.5 mM I-1-P) the enzyme is saturated.
TABLE 2.
Comparison of kinetic characteristics of M. jannaschii I-1-Pase with those of purified I-1-Pase enzymes from other organisms
| I-1-Pase source | Km (mM) (± SD) | Vmaxa |
|---|---|---|
| M. jannaschii | 0.091 ± 0.016 | 9.3 ± 0.45 |
| Bovine | 0.16 ± 0.02 | 13.3 ± 0.9 |
| Human recombinant | 0.075 ± 0.003 | 36.1 ± 1 |
| E. coli | 0.071 ± 0.008 | 13.3 ± 0.9 |
Shown as micromoles of Pi produced per minute per milligram of protein (± standard deviation).
Substrate specificity.
While both mammalian and plant I-1-Pase enzymes have broad substrate specificities (1, 25, 42), the M. jannaschii I-1-Pase can hydrolyze an even broader range of substrates. A variety of phosphorylated compounds were tested as substrates for M. jannaschii I-1-Pase, and the results are presented in Table 3. The enzyme had essentially the same activity toward d-myo-I-1-P as it had toward dl-myo-I-1-P (the mixture of d and l isomers). Therefore, M. jannaschii I-1-Pase does not discriminate between d and l isomers. Similarly, the mammalian (29) and E. coli (33) enzymes cannot discriminate between d and l isomers. However, it has been reported that the pollen enzyme shows some preference, since it hydrolyzed the d isomer at 80 to 90% of the rate of the l isomer (25). Inositol-2-phosphate was hydrolyzed by the methanogen I-1-Pase, but at a much lower rate than for I-1-P hydrolysis. Both β-glycerol phosphate and 2′-AMP were also good substrates for this enzyme, similar to what is observed for the mammalian and plant I-1-Pases. However, there were three major differences between the methanogen and mammalian enzymes. (i) 2′-AMP was hydrolyzed much faster than I-1-P; the ratio of 2′-AMP activity to I-1-P activity was about 4 to 5 times higher than the highest reported values of 157% (1) for the bovine enzyme and 122% (38) for the chick erythrocyte enzyme. (ii) pNPP, a poor substrate for the mammalian enzymes, with ∼2.6% of the activity they show toward I-1-P (1), was a very good substrate for M. jannaschii I-1-Pase. Although there were some early reports that the yeast enzymes were ∼20% more active toward pNPP than toward I-1-P (13), this activity toward pNPP decreased to 13% of the activity toward I-1-P after one chromatography step (8). Since those results were based on assays with only partially purified enzymes, the possibility of nonspecific phosphatase activity could not be excluded. For the M. jannaschii I-1-Pase, pNPP was clearly a good substrate (the enzyme exhibits ∼20% more activity toward pNPP than toward I-1-P). pNPP was also a good substrate for the partially purified I-1-P phosphatase of M. igneus (10). (iii) Glucose-1-phosphate, which has never been observed to be a good substrate for the other pure I-1-Pases, was significantly hydrolyzed at 68% of the rate of I-1-P by the M. jannaschii I-1-Pase. For comparison, the pure rat enzyme hydrolyzed glucose-1-phosphate at only 4% of its rate toward I-1-P (42). Given the striking sequence homology, there must be some structural variations in this enzyme to accommodate this carbohydrate in the active site.
TABLE 3.
Substrate specificity of M. jannaschii I-1-Pase
| Substratea | Relative activity (%) |
|---|---|
| dl-myo-I-1-P | 100 |
| d-myo-I-1-P | 96 |
| myo-Inositol-2-phosphate | 15 |
| β-Glycerol phosphate | 80 |
| α-d-Glucose-1-phosphate | 68 |
| d-Glucose-6-phosphate | 0 |
| d-Fructose-1-phosphate | 0 |
| 5′-AMP | 0 |
| NAD+ | 0 |
| 2′-AMP | 600 |
| pNPP | 120 |
The assay mixture contained 2.5 mM substrate, 10 mM MgCl2 in 50 mM Tris HCl (pH 8); enzyme (0.4 μg) was added, and the sample was incubated for 1 min at 100°C. Errors in enzyme activity were 3 to 5%.
Li+ inhibition.
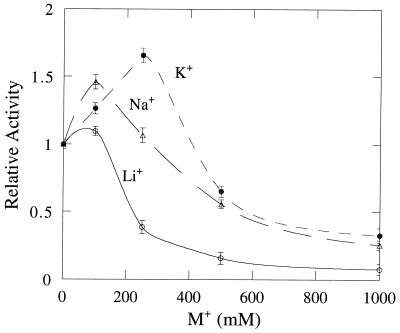
Li+ is a potent inhibitor of mammalian, plant, and E. coli I-1-Pase enzymes (Ki = 0.95, 0.30, and 0.35 mM for bovine [23], human [30], and E. coli [29] I-1-Pases, respectively). However, no inhibition was detected when the M. jannaschii enzyme was incubated with 100 mM Li+. Instead, I-1-Pase activity was slightly enhanced (the 110% activation at this Li+ concentration is outside the 3 to 5% errors in assay values [Fig. 4]). Inhibition by Li+ occurred at concentrations much higher than those for mammalian enzymes: 250 mM Li+ inhibited about 62% of M. jannaschii enzyme activity, and 1 M Li+ was needed to inhibit 90% of enzyme activity. This is again extremely similar to what was observed with the partially purified M. igneus phosphatase, which needed 250 mM LiCl to inhibit about 50% of activity (10). To determine if the Li+ effect on M. jannaschii I-1-Pase was specific or nonspecific, LiCl was replaced by NaCl and KCl. The results show that, compared to Na+ and K+, Li+ is the strongest inhibitor of M. jannaschii I-1-Pase, although it is much less potent than for mammalian and plant enzymes. The only other I-1-Pase reported to require relatively high Li+ concentrations for inhibition is the yeast enzyme (32). It exhibited ∼70% residual activity at 50 mM Li+ and 10% activity at 200 mM Li+. In that study, the authors attributed the poor inhibition by Li+ to the fact that the assays were done with a crude protein extract; they suggested that several monophosphatases with different sensitivities to lithium might be present. However, for the M. jannaschii I-1-Pase, a single activity with only moderate Li+ sensitivity is observed.
FIG. 4.
Effect of Li+, Na+, and K+ on the activity of M. jannaschii I-1-Pase toward 2.5 mM I-1-P in 50 mM Tris HCl, pH 8.0, with 10 mM MgCl2 at 85°C for 1 min. The activities are normalized to the value for the assay without the monovalent cation salt added. The error bars indicate standard deviations.
Heat stability.
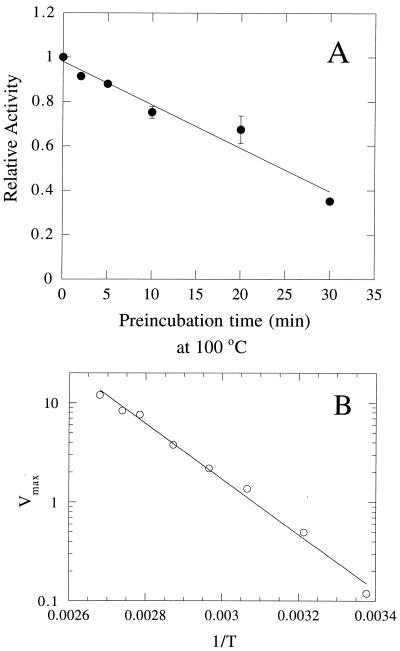
Most plant and mammalian I-1-Pase enzymes, although they have maximum activity at ∼37°C, are very heat stable and can survive long incubation periods at 60 to 70°C (18, 31). This observation led to a critical step in the purification to homogeneity of both recombinant (30) and nonrecombinant (31) mammalian I-1-Pase. M. jannaschii is an extreme thermophile, with an optimum growth temperature of 85°C; hence, the I-1-Pase from this organism is expected to have unusual heat stability. After incubation at 85°C for 30 min, more than 95% of I-1-Pase activity remained. This was a key observation for the purification of the recombinant protein, since it facilitated the removal of the majority of E. coli host cell proteins. Heating at 100°C for 20 min caused a loss of about 30 to 40% of activity; 30 min at that temperature inactivated about 70% of activity (Fig. 5A). With shorter incubation times (∼60 s), the protein can be assayed at 100°C, where it has higher activity than at 85°C. An Arrhenius analysis of I-1-Pase Vmax between 25 and 100°C yields an estimated activation energy of ∼54 kJ/mol (Fig. 5B). On the other end of the temperature scale, no I-1-Pase activity was lost after storage at 4°C for 1 month.
FIG. 5.
(A) Thermal stability of M. jannaschii I-1-Pase after preincubation at 100°C for various times. The enzyme activity was measured after the preincubation time by adding 0.4 μg of protein to the standard assay mixture and incubating the mixture at 100°C for 1 min. (B) Temperature (T) dependence of M. jannaschii I-1-Pase Vmax. The error bars indicate standard deviations.
In the process of cloning the M. jannaschii I-1-Pase, a mutant with three single-amino-acid mutations (D4E, R168K, and L249F) far away from the active site was generated by PCR errors. The specific activity of this mutant was almost the same as that of the wild type. However, both the solubility and heat stability of the mutant I-1-Pase were less than those of the wild type. Most of the mutant protein was packed in inclusion bodies after IPTG induction; only about 20 to 30% of the recombinant activity was soluble. The heat treatment step was also affected by these mutations: incubation at 85°C for 30 min caused a loss of 20% of activity; incubation at 100°C for 10 min caused a loss of 50% of activity.
DISCUSSION
The MJ109 gene product is the first archaeal I-1-Pase to be cloned and studied. Its substrate specificity, Mg2+ requirement, subunit association (dimer), Vmax, Km for I-1-P, and specific activity are very similar to those of the mammalian enzymes. However, the lack of inhibition by low concentrations of Li+ and high concentrations of Mg2+ make it unique in this family of enzymes. The properties of this I-1-Pase from M. jannaschii parallel those detected for partially purified I-1-Pase from M. igneus, an organism where I-1-Pase plays a key role in biosynthesis of the osmolyte DIP (10). I-1-Pase is a highly conserved enzyme, and diverse proteins have been noted to be homologous to it. The bovine I-1-Pase has sequence homologies to the products of the Neurospora crassa qa-x gene (Qa-X), the Aspergillus nidulans qutG gene (QutG), and the E. coli suhB (SuhB) and amtA genes (AmtA). The MJ109 gene product was putatively identified as an extragenic suppressor in the M. jannaschii genome database (6) due to its sequence homology to SuhB. The extragenic suppressor suhB gene was first identified as the locus for the ssyA3(Cs) mutation that was isolated as a extragenic suppressor for the secY24(Ts) mutant of E. coli. The secY gene in E. coli is involved in protein secretion. The secY24 mutant, with a single base change in secY produces an altered SecY protein and is defective in secretion of envelope proteins across the cytoplasmic membrane at a higher temperature (42°C). The mutant accumulates precursors of envelope proteins. Mutant alleles of the suhB gene (designated ssyA3) were isolated as extragenic suppressors for the secY24 mutant. This gene product restores the defect in protein secretion caused by the secY24 mutation, rescues the temperature-sensitive cell growth of secY24 mutant cells, and causes cold-sensitive cell growth (cells form normal colonies at 42°C, small colonies at 37°C, and no colonies at 30°C). While it is not clear if the MJ109 gene product in M. jannaschii really can act as an extragenic suppressor in this organism, it clearly functions as an I-1-Pase, with properties similar to the activity observed in M. igneus.
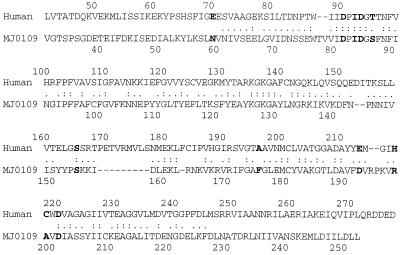
The sequence alignment of MJ109 with human I-1-Pase displayed a 28.7% identity in a 178-amino-acid overlap (Fig. 6). Many of the identical or similar amino acid residues are at the active sites, as determined from the crystal structure of the human enzyme (3–5). Presumably, these residues play a similar role in the structure and catalytic mechanism of the methanogen enzymes. The active site of the human enzyme, based on the X-ray crystallography data, includes the inositol binding site and two catalytic metal binding sites. Residues by D93, A196, E213, S165, and D220 form the inositol binding site for the human enzyme. From the sequence alignment (Fig. 6), the M. jannaschii enzyme has residues D84, F175, D192, S154, and D201 at these positions. Three residues whose side chains form hydrogen bonds with the hydroxyl groups of the inositol ring include D93, E213, and D220. Clearly, in the M. jannaschii enzyme, D84, D192, and D201 could have these functions. A196 of human I-1-Pase uses its main chain amide group to form a hydrogen bond with the substrate. Hence, the substitution of F175 in the M. jannaschii I-1-Pase should not have much effect on the substrate binding. This comparison of the sequences might suggest that there are similar substrate specificities in human and M. jannaschii I-1-Pases. The interaction of S165 with the inositol ring is supposed to occur in the transition state, and mutation of it to alanine or isoleucine lowers the catalytic rate constant, kcat, ∼5-fold (4). In M. jannaschii I-1-Pase this residue was conserved. However, due to the A→F and E→D exchange, the local structure will be affected and could lead to some altered substrate specificities. These changes could facilitate enhanced hydrolysis of 2′-AMP, pNPP, and glucose-1-phosphate by the M. jannaschii I-1-Pase.
FIG. 6.
Sequence alignment between human and M. jannaschii I-1-Pase (MJ109). The residues discussed in the text are in boldface; dashes represent gaps. A single dot indicates similar residues (polar, nonpolar, etc.), whereas two dots indicate conserved residues.
The first metal binding site, with octahedral coordination geometry, in the human enzyme is formed by E70, D90, I92, and T95. The metal binding ligands are provided by active site residues and the solvent water molecules. The proposed catalytic mechanism has Mg2+ binding to this site and activating the ligand water molecule for a nucleophilic attack on the phosphoester bond. The I-1-Pase from M. jannaschii has N59, D81, I83, and S68 that could form this site. If the same metal site is conserved, then there must be some variations in the site because N59 cannot play the same role as E70 does in the human I-1-Pase. In E70, the OE2 atom was supposed to act as one ligand to the catalytic Mg2+. Mutagenesis of this residue in the human enzyme showed a 400-fold drop in kcat for E70N (35).
The second metal binding site of the human enzyme, with tetrahedral coordination geometry, contains residues D90, D93, and D220 and one oxygen of phosphate. Magnesium binding to the second metal binding site is supposed to coordinate the ester oxygen and stabilize the developing negative charge as the phosphate ester is cleaved. This site may also be responsible for the noncompetitive inhibition by Li+ and high concentrations of Mg2+ (40). After phosphate ester hydrolysis, this second Mg2+ must dissociate to allow inorganic phosphate to leave the active site. Therefore, a high concentration of magnesium will prevent the phosphate from diffusing away from the active site. Li+ competes with Mg2+ for this site, forming an enzyme-Mg2+-phosphate-Li+ complex (23, 34), which will trap the enzyme in that state and stop the turnover process. The M. jannaschii enzyme has D81, D84, and D201 in alignment with D90, D93, and D220 of the human enzyme, suggesting that this site may also be conserved. However the inhibition behavior of Li+ and Mg2+ is totally different for the archaeal enzyme. Residues close to the active site could also affect the active site structure. Mutagenesis studies of the mammalian enzyme (17) suggested that C218 and H217, which are close to D220, are also key residues for Li+ and high-concentration Mg2+ inhibition. The 50% inhibition concentration for Mg2+ increased 10-fold for the H217Q enzyme, with no inhibition at all for C218A up to 400 mM Mg2+. The Ki for Li+ increased 32- and 11.5-fold for the H217Q and C218A enzymes, respectively. Interestingly, the sequence alignment showed that M. jannaschii I-1-Pase has A199 in the position occupied by C218, and R198 replaces H217. This may contribute partially to the noninhibitory behavior of Li+ and high-concentration Mg2+. The function of the third metal binding site (5) formed by the E70 side chain oxygen (OE1) and three water molecules is not as well established as the first and second sites (40). Although ruled out as the catalytic metal binding site, metal at this site could also be involved in the inhibition of I-1-Pase at high concentrations of activating metal, based on the observation that the proposed metal bond water nucleophile is displaced by the third metal binding at this site (5). Given the difference in the archaeal I-1-Pase sequence and its altered kinetic behavior, a comparison of the structure of the archaeal I-1-Pase with that of the mammalian enzyme should eventually shed light on details of catalysis of both systems.
ACKNOWLEDGMENT
This work has been supported by grant DE-FG02-91ER20025 (to M.F.R.) from the Department of Energy Biosciences Division.
REFERENCES
- 1.Attwood P V, Ducep J-B, Chanal M-C. Purification and properties of myo-inositol-1-phosphatase from bovine brain. Biochem J. 1988;253:387–394. doi: 10.1042/bj2530387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge M J, Irvine R F. Inositol phosphates and cell signaling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 3.Bone R, Springer J P, Atack J R. Structure of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci USA. 1992;89:10031–10035. doi: 10.1073/pnas.89.21.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone R, Frank L, Springer J P, Pollack S J, Osborn S, Atack J R, Knowles M R, McAllister G, Ragan C I, Broughton H B, Baker R, Fletcher S R. Structure analysis of inositol monophosphatase complexes with substrates. Biochemistry. 1994;33:9460–9467. doi: 10.1021/bi00198a011. [DOI] [PubMed] [Google Scholar]
- 5.Bone R, Frank L, Springer J P, Pollack S J, Osborn S, Atack J R. Structural studies of metal binding by inositol monophosphatase: evidence for two metal ion catalysis. Biochemistry. 1994;33:9468–9476. doi: 10.1021/bi00198a012. [DOI] [PubMed] [Google Scholar]
- 6.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 7.Chen I-W, Charalampous C F. Biochemical studies on inositol. IX. d-Inositol-1-phosphate as intermediate in the biosynthesis of inositol from glucose-6-phosphate and characteristics of two reactions in this biosynthesis. J Biol Chem. 1966;241:2194–2199. [PubMed] [Google Scholar]
- 8.Chen I-W, Charalampous C F. Biochemical studies on inositol. VIII. Purification and properties of the enzyme system which converts glucose-6-phosphate to inositol. J Biol Chem. 1965;240:3507–3512. [PubMed] [Google Scholar]
- 9.Chen I-W, Charalampous C F. Biochemical studies on inositol. X. Partial purification of yeast inositol-1-phosphatase and its separation from glucose-6-phosphate cyclase. Arch Biochem Biophys. 1966;117:154–157. [Google Scholar]
- 10.Chen, L., E. Spiliotis, and M. F. Roberts. Biosynthesis of di-myo-inositol-1,1′-phosphate, a novel osmolyte in hyperthermophilic archaea. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 11.Ciulla R A, Burggraf S, Stetter K O, Roberts M F. Occurrence and role of di-myo-inositol-1,1′-phosphate in Methanococcus igneus. Appl Environ Microbiol. 1994;60:3660–3664. doi: 10.1128/aem.60.10.3660-3664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg F, Jr, Ranganathan P. Measurement of biosynthesis of myo-inositol from glucose-6-phosphate. Methods Enzymol. 1987;141:127–143. doi: 10.1016/0076-6879(87)41061-6. [DOI] [PubMed] [Google Scholar]
- 13.Frixos C, Chen I-W. Inositol-1-phosphate synthetase and inositol-1-phosphatase from yeast. Methods Enzymol. 1965;9:698–704. [Google Scholar]
- 14.Ganzhorn A J, Chanal M C. Kinetic studies with myo-inositol monophosphatase from bovine brain. Biochemistry. 1990;29:6065–6071. doi: 10.1021/bi00477a026. [DOI] [PubMed] [Google Scholar]
- 15.Gee N S, Ragan C I, Watling K J, Aspley S, Jackson R G, Reid G G, Gani D, Shute J K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988;249:883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillaspy G E, Keddie J S, Okda K, Gruissem W. Plant inositol monophosphatase is a lithium-sensitive enzyme encoded by a multigene family. Plant Cell. 1995;7:2175–2185. doi: 10.1105/tpc.7.12.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gore M G, Greasley P, McAllister G, Ragan C I. Mammalian inositol monophosphatase: the identification of residues important for the binding of Mg2+ and Li+ ions using fluorescence spectroscopy and site-directed mutagenesis. Biochem J. 1993;296:811–815. doi: 10.1042/bj2960811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumber S C, Loewus M W, Loewus F A. Further studies on myo-inositol-1-phosphatase from the pollen of lilium longiflorum thunb. Plant Physiol (Rockville) 1984;76:40–44. doi: 10.1104/pp.76.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallcher L M, Sherman W R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980;255:10896–10901. [PubMed] [Google Scholar]
- 20.Itaya K, Michio U. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966;14:361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- 21.Kwon O-S, Churchich J E. Binding of the activation ion Co(II) to myo-inositol monophosphatase monitored by fluorescence and phosphorescence spectroscopy. J Protein Chem. 1997;16:1–9. doi: 10.1023/a:1026353509519. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Leech A P, Baker G R, Shute J K, Cohen M A, Gani D. Chemical and kinetic mechanism of the inositol monophosphatase reaction and its inhibition by Li+ Eur J Biochem. 1993;212:693–704. doi: 10.1111/j.1432-1033.1993.tb17707.x. [DOI] [PubMed] [Google Scholar]
- 24.Loewus F A. Inositol biosynthesis. In: Moore D J, Boss W F, Loewus F A, editors. Inositol metabolism in plants. New York, N.Y: Wiley-Liss, Inc.; 1990. pp. 13–19. [Google Scholar]
- 25.Loewus M W, Loewus F A. myo-Inositol-1-phosphatase from the pollen of lilium longiflorum thunb. Plant Physiol. 1980;70:765–770. doi: 10.1104/pp.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry O H, Rosebrough N J, Randall R J. Protein measurements with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 27.Mach H, Middaugh C R, Lewis R V. Statistical determination of the average values of the extinction coefficient of tryptophan and tyrosine in native proteins. Anal Biochem. 1992;200:74–80. doi: 10.1016/0003-2697(92)90279-g. [DOI] [PubMed] [Google Scholar]
- 28.Majerus P W. Inositol phosphate biochemistry. Annu Rev Biochem. 1992;61:225–250. doi: 10.1146/annurev.bi.61.070192.001301. [DOI] [PubMed] [Google Scholar]
- 29.Matsuhisa A, Suzuki N, Noda T, Shiba K. Inositol monophosphatase activity from the Escherichia suhB gene product. J Bacteriol. 1995;177:200–205. doi: 10.1128/jb.177.1.200-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAllister G, Whiting P, Hammond E A, Knowles M R, Atack J R, Bailey F J, Maigetter R, Ragan C I. cDNA cloning of human and rat brain myo-inositol monophosphatase: expression and characterization of the human recombinant enzyme. Biochem J. 1992;284:749–754. doi: 10.1042/bj2840749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meek J L, Rice T J, Anton E. Rapid purification of inositol monophosphate phosphatase from beef brain. Biochem Biophys Res Commun. 1988;156:143–148. doi: 10.1016/s0006-291x(88)80816-7. [DOI] [PubMed] [Google Scholar]
- 32.Murray M, Greenberg M L. Regulation of inositol monophosphatase in Saccharomyces cerevisiae. Mol Biol. 1997;25:541–546. doi: 10.1046/j.1365-2958.1997.4881840.x. [DOI] [PubMed] [Google Scholar]
- 33.Parthasarathy L, Vadnal R E, Ramesh T G, Shyamaladevi C S, Parthasarathy R. myo-Inositol monophosphatase from rat testes: purification and properties. Arch Biochem Biophys. 1993;304:94–101. doi: 10.1006/abbi.1993.1326. [DOI] [PubMed] [Google Scholar]
- 34.Pollack S J, Atack J R, Knowles M R, McAllister G, Ragan C I, Baker R, Fletcher S R, Iversen L L, Broughton H B. Mechanism of inositol monophosphatase, the putative target of lithium therapy. Proc Natl Acad Sci USA. 1994;91:5766–5770. doi: 10.1073/pnas.91.13.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollack S J, Knowles M R, Atack J R, Broughton H B, Ragan C I, Osborne S, McAllister G. Probing the role of metal ions in the mechanism of inositol monophosphatase by site-directed mutagenesis. Eur J Biochem. 1993;217:281–287. doi: 10.1111/j.1432-1033.1993.tb18244.x. [DOI] [PubMed] [Google Scholar]
- 36.Ramakrishnan V, Verhagen M F J M, Adams M W W. Characterization of di-myo-inositol-1,1′-phosphate in the hyperthermophilic bacterium Thermotoga maritima. Appl Environ Microbiol. 1997;63:347–350. doi: 10.1128/aem.63.1.347-350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rana R S, Hokin L E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990;70:115–161. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- 38.Roth S C, Harkness D R, Isaacks R E. Studies on avian erythrocyte metabolism: purification of myo-inositol-1-phosphatase from chick erythrocytes. Arch Biochem Biophys. 1981;210:465–473. doi: 10.1016/0003-9861(81)90210-1. [DOI] [PubMed] [Google Scholar]
- 39.Saiki R H, Gelfand D H, Stoffel S, Scharf S J, Higuchi R, Horn G T, Mullis K B, Erlich H A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 40.Saudek V, Vincendon P, Do Q T, Atkinson R A, Sklenar V, Pelton P D, Piriou F, Ganzhorn A J. 7Li nuclear magnetic resonance study of lithium binding to myo-inositol monophosphatase. Eur J Biochem. 1996;240:288–291. doi: 10.1111/j.1432-1033.1996.0288h.x. [DOI] [PubMed] [Google Scholar]
- 41.Scholz S, Sonnenbichler J, Schafer W, Hensel R. Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett. 1992;306:239–242. doi: 10.1016/0014-5793(92)81008-a. [DOI] [PubMed] [Google Scholar]
- 42.Takimoto K, Okada M, Matsuda Y, Nakagawa H. Purification and properties of myo-inositol-1-phosphatase from rat brain. J Biochem. 1985;98:363–370. doi: 10.1093/oxfordjournals.jbchem.a135290. [DOI] [PubMed] [Google Scholar]
- 43.Van Leeuwen S H, van der Marel G A, Hensel R, van Boom J H. Synthesis of l,l di-myo-inositol-1,1′-phosphate: a novel inositol phosphate from Pyrococcus woesei. Recl Trav Chim Pays-Bas Belg. 1994;113:335–336. [Google Scholar]