Abstract
Objective
Currently, pre-treatment prediction of patients with pancreatic neuroendocrine tumors with liver metastases (PNELM) receiving surufatinib treatment was unsatisfying. Our objective was to examine the association between radiological characteristics and efficacy/prognosis.
Methods
We enrolled patients with liver metastases in the phase III, SANET-p trial (NCT02589821) and obtained contrast-enhanced computed tomography (CECT) images. Qualitative and quantitative parameters including hepatic tumor margins, lesion volumes, enhancement pattern, localization types, and enhancement ratios were evaluated. The progression-free survival (PFS) and hazard ratio (HR) were calculated using Cox’s proportional hazard model. Efficacy was analyzed by logistic-regression models.
Results
Among 152 patients who had baseline CECT assessments and were included in this analysis, the surufatinib group showed statistically superior efficacy in terms of median PFS compared to placebo across various qualitative and quantitative parameters. In the multivariable analysis of patients receiving surufatinib (N=100), those with higher arterial phase standardized enhancement ratio-peri-lesion (ASER-peri) exhibited longer PFS [HR=0.039; 95% confidence interval (95% CI): 0.003−0.483; P=0.012]. Furthermore, patients with a high enhancement pattern experienced an improvement in the objective response ratio [31.3% vs. 14.7%, odds ratio (OR)=3.488; 95% CI: 1.024−11.875; P=0.046], and well-defined tumor margins were associated with a higher disease control rate (DCR) (89.3% vs. 68.2%, OR=4.535; 95% CI: 1.285−16.011; P=0.019) compared to poorly-defined margins.
Conclusions
These pre-treatment radiological features, namely high ASER-peri, high enhancement pattern, and well-defined tumor margins, have the potential to serve as predictive markers of efficacy in patients with PNELM receiving surufatinib.
Keywords: Neuroendocrine tumors, liver metastases, computed tomography, surufatinib
Introduction
The pancreas is one of the most common primary sites among gastroenteropancreatic neuroendocrine tumors (NET) (1). Among all metastatic sites in pancreatic NETs, pancreatic neuroendocrine tumors with liver metastases (PNELM) are most commonly seen, with a prevalence of 82% in registries and 64% in the SEER database in United States (1). Liver metastases (LM) are associated with poor prognosis and a 5-year survival of 13%−54% compared to 75%−99% without LM (2,3). The European Neuroendocrine Tumor Society consensus guidelines suggest that over 80% of PNELM can only be treated systemically (4-7). PNELM are usually accompanied by abundant blood supply, which is characteristically hypervascularized by a dense and specialized capillary network and high levels of vascular endothelial growth factor (8). These characteristics provide opportunities for the therapeutic use of anti-angiogenesis agents (9).
Surufatinib is a novel oral tyrosine kinase inhibitor that simultaneously targets angiogenesis [vascular endothelial growth factor receptor and tumor-immune evasion (colony-stimulating factor-1 receptor)]. In the randomized placebo-controlled phase III trial, SANET-p, surufatinib demonstrated a prolonged median progression-free survival (PFS) than placebo [10.9 vs. 3.7 months; hazard ratio (HR)=0.49, 95% confidence interval (95% CI): 0.32−0.76; P=0.001], indicating its clinical benefits for pancreatic NET (pNET) (10). Notably, PNELM accounted for 94.2% of the SANET-p population, hinting at the potential strong impact of surufatinib on a subgroup of patients with LM.
Contrast-enhanced computed tomography (CECT) is widely used for the diagnosis and evaluation of treatment responses in cancer patients (11-13). Pre-treatment radiological evaluation may have considerable clinical implications for identifying optimal subgroups. Radiology-based prognosis stratification has demonstrated the association between blood-supply-related imaging features and treatment efficacy (12). By using more quantitative and precise approaches, pre-treatment imaging has become a convenient method for predicting the prognosis and efficacy (14). High-vascularized lesions, for instance, the PNELM, theoretically had the future. They would most benefit treatment with anti-angiogenic therapy (15). Surufatinib, an efficacious angiogenic therapy, may also benefit from this approach.
This study aimed to investigate the association between qualitative and quantitative radiological parameters and the prognosis and efficacy of PNELM treated with surufatinib, hoping to optimize an effective therapy strategy for subgroups of patients receiving surufatinib treatment.
Materials and methods
Patient selection and eligibility
The analysis was conducted in all patients with PNELM from SANET-p, a multicenter, randomized controlled phase III trial. SANET-p was conducted in accordance with good clinical practice principles and relevant local regulations. Pathology was reviewed and diagnosis was confirmed by two independent pathologists according to the 2019 5th edition of the World Health Organization (WHO) Classification of Neuroendocrine Tumors. Clinical information was retrieved using the hospital information system. The basic eligibility criteria mainly complied with the trial. Key inclusion criteria in this analysis included: 1) above 18 years old; 2) pathological diagnosis of unresectable or metastatic, well differentiated pancreatic NETs (pathological grade 1 or 2 according to the 2010 WHO classification); 3) Eastern Cooperative Oncology Group performance status <2; 4) CECT completed within 4 weeks before the first dose of surufatinib; 5) estimated survival of more than 3 months; and 6) at least one dose of surufatinib or placebo. Key exclusion criteria mainly included: 1) no confirmed liver lesions or no measurable lesions (diameter <1 cm) (n=12); 2) no CECT assessment at baseline (n=3); or 3) images could not be analyzed because of artifacts. All subjects gave written informed consent and the study protocol was approved by the institutional review committee of Peking University Cancer Hospital according to the Helsinki Declaration.
CT technique and assessment criteria
All patients received pre-treatment CECT of the abdomen (liver dual phase) and pelvis, with the scanning coverage ranging from the dome of the right diaphragm to the symphysis pubis or lower according to the standard scanning protocol (the tube voltage was 120 kVp and current was 200 mA). The intravenous administration of 100−150 mL (150−300 mg/mL) of nonionic contrast material at a rate of 2−3 mL/s was required. Images were acquired first for the arterial phase and subsequently for the portal venous phase. The slice thickness was 5 mm with no slice gap, and followed by contiguous reconstruction increments of 5 mm. The image acquisition guideline (Supplementary Table S1) was followed by all centers involved in the SANET-p trial. All pre-treatment and subsequent CECT data were retrieved in a workstation (picture archiving and communication system, PACS) for further assessment. Evaluation of CECT images was performed by two qualified radiologists, and when inconsistencies occurred, the assessment was arbitrated by an independent third member. All the imaging reviewers were blinded to the clinical information.
Table S1. CT scanning protocol in the study.
| Variables | SCAN1 | SCAN2 | SCAN3 |
| CT, computed tomography; FOV, field of view. | |||
| Scan phase/delay | Pre-contrast | Ensure high-quality imaging of the liver | |
| Arterial phase | Portal phase | ||
| Scan location/coverage | Dome of the right diaphragm through the symphysis pubis or lower to ensure complete coverage of the pelvis | Lung apices through entire liver | Dome of the right diaphragm through the symphysis pubis or lower to ensure complete coverage of the pelvis |
| Patient orientation | Supine | Supine | Supine |
| Breathing instruction | Abdomen and pelvis performed in one breath-hold | Chest and entire liver performed in one breath-hold | Abdomen and pelvis in one breath-hold |
| Scan FOV | Large | Large | Large |
| Display FOV | Unique to patient size | Unique to patient size | Unique to patient size |
| Slice thickness | 5 mm | 5 mm | 5 mm |
| Reconstruction interval | 5 mm | 5 mm | 5 mm |
| Gap (slice spacing) | None (i.e. contiguous) | None (i.e. contiguous) | None (i.e. contiguous) |
| Contrast media instructions | |||
| i.v. contrast | Required 100−150 mL non-ionic only | Required 100−150 mL non-ionic only | Required 100−150 mL non-ionic only |
| i.v. contrast concentration | 150−300 mg/mL | 150−300 mg/mL | 150−300 mg/mL |
| Injection rate | Single injection: 2−3 cc/sec (power injector preferred) | Single injection: 2−3 cc/sec (power injector preferred) | Single injection: 2−3 cc/sec (power injector preferred) |
| Oral contrast | Required (use standard protocol) | Required (use standard protocol) | Required (use standard protocol) |
Target liver metastatic lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were selected from baseline CECT images, avoiding blood vessels and normal liver parenchyma. The region of interest (ROI) was delineated manually in the arterial phase. The ROI were traced along the outer margin of the metastatic tumor of each slice to contain the whole lesion. Subsequently, the CT values of each region were measured in HU on the workstation (HUtumorART). ROI was then measured at the periphery of the lesion and at the maximum slice to record the parameter-peri (parameters are described below). The parameter-whole was measured as the averaged values of the peripheral area and central area (except for obvious necrotic areas in cores). HUliverART and aorta (HUaortaART) were measured. Finally, HUtumor, HUliver, and HUaorta were measured in non-enhanced and HUtumorPORT, HUliverPORT were obtained in portal venous phase slice target lesions.
Imaging parameters
The radiologic features involved qualitative and quantitative parameters. The qualitative parameters included the total hepatic lesion volume (<25% vs. ≥25%) (16), hepatic tumor localization type classified by Frilling et al. [single metastasis (type I), isolated metastatic bulk accompanied by smaller deposits (type II), disseminated metastatic spread (type III)] (3), tumor margins (poorly-defined or well-defined), enhancement pattern [high enhancement and other (including low enhancement and heterogeneous enhancement) patterns], and necrosis proportions of lesions (≤25% vs. >25%) (17).
Quantitative parameters were mainly set as ratios between the tumor tissues and reference vessels, to avoid the inevitable variability between examinations and patients (18). Quantitative parameters included:
(a) Maximum diameter (MD) was defined as the longest diameter of the maximum lesion, measured by the longest transverse diameter axially in slices.
(b) Relative enhancement ratio (RER) was defined as the tumor density compared to the adjacent parenchyma in arterial (HUtumorART/HUliver, ARER) or portal venous phases (HUtumorPORT/HUliver, PRER). The RER-peri and RER-whole were calculated depending on the specific position of the lesions (19,20).
(c) Standardized enhancement ratio (SER) was defined as the tumor density compared with aortic enhancement (HUtumorART/HUaorta, ASER) of the arterial phase and portal phase, and portal enhancement (HUtumorPORT/HUaorta, PSER). The SER-peri and RER-whole were calculated depending on the specific position of the lesions (20,21).
Efficacy assessment
Patients were re-assessed by investigators using consistent imaging evaluating methods per RECIST version 1.1 every 8 weeks (±3 d) for the first 12 months and then every 12 weeks (±3 d) until progression disease (PD). SANET-p was performed in parallel by the investigators and blinded independent image review committee for efficacy analysis in SANET-p (10). Efficacy variables in the analysis included: the primary end point was defined as the time from randomization to documented PD or death, and the median PFS was the time taken for half of the patients to reach the primary end point. The objective response (OR) was defined as a complete response (CR) or partial response (PR). The disease control rate (DCR) was defined as the proportion of patients with CR, PR, or stable disease (SD).
Statistical analysis
Continuous variables were tested by the t-test (normally distributed variables), and categorical variables were compared using Chi-square test or Fisher’s exact test, where appropriate. We generated PFS curves using the Kaplan-Meier method and log-rank test. Forest plots depicting the PFS of subgroups were presented. The quantitative radiological parameters were stratified below and above the 50th percentile as low or high levels (e.g. ASER-peri low or high). To assess the association between PFS and the prognostic value of different radiological parameters, multivariate analysis following univariate analysis was conducted using Cox’s proportional hazards model. Logistical regressions with univariate/multivariate analysis were performed to evaluate the efficacy. Statistical significance was set at P<0.05. All analyses were performed with SAS Enterprise® (Version 8.2; Cary, NC, USA).
Results
Overview of whole cohort
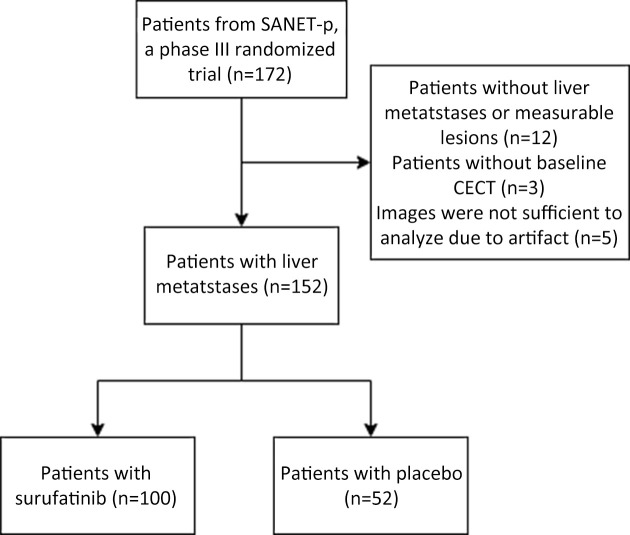
Among 172 patients in the SANET-p trial, patients with no confirmed liver lesions or no measurable lesions (n=12); no CECT assessment at baseline (n=3); and inadequate images for analysis (n=5) were excluded according to the eligibility criteria. We included 152 patients [surufatinib (n=100) and placebo (n=52)] diagnosed with PNELM, who had baseline and at least one post baseline CECT evaluation from the SANET-p study. The flowchart illustrating the exclusion process is shown in Supplementary Figure S1. In the surufatinib group, the overall median (range) age was 50.0 (25.0−75.0) years and 87% patients were G2. Baseline characteristics were balanced between the surufatinib and placebo groups (Table 1). The radiological deciphering of enhancement and margin-related features are shown in Supplementary Figure S2 and Figure 1.
Figure S1.
Flowchart of the study. CECT, contrast-enhanced computed tomography.
Table 1. Baseline clinical information of surufatinib vs. placebo in SANET-p of whole cohort.
| Variables | n (%) | ||
| Surufatinib group (N=100) | Placebo group (N=52) | Total | |
| IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group. | |||
| Age (year) | |||
| Median (IQR) | 50.0 (43.5−57.0) | 48.0 (41.0−60.0) | 49.5 (43.0−57.0) |
| Range | 25.0−75.0 | 20.0−77.0 | 20.0−77.0 |
| Sex | |||
| Female | 48 (48) | 28 (54) | 76 (50) |
| Male | 52 (52) | 24 (46) | 76 (50) |
| ECOG performance status | |||
| 0 | 65 (65) | 39 (75) | 104 (68) |
| 1 | 35 (35) | 13 (25) | 48 (32) |
| WHO pathological grade | |||
| G1 | 13 (13) | 5 (10) | 18 (12) |
| G2 | 87 (87) | 47 (90) | 134 (88) |
| Proliferation marker protein Ki-67 index | |||
| <5% | 36 (36) | 15 (29) | 51 (34) |
| 5%−10% | 50 (50) | 30 (58) | 80 (53) |
| >10% | 14 (14) | 7 (13) | 21 (14) |
| Functional status | |||
| Functioning | 9 (9) | 3 (6) | 12 (8) |
| Non-functioning | 91 (91) | 48 (92) | 139 (91) |
| Unknown | 0 (0) | 1 (2) | 1 (1) |
| Extra-hepatic metastatic sites | |||
| Lymph nodes | 35 (35) | 18 (35) | 53 (35) |
| Lung | 7 (7) | 2 (4) | 9 (6) |
| Bone | 12 (12) | 1 (2) | 13 (9) |
| Other | 21 (21) | 5 (10) | 26 (17) |
| No. of organs involved | |||
| ≤2 | 46 (46) | 30 (58) | 76 (50) |
| >2 | 54 (54) | 22 (42) | 76 (50) |
| Previous-line systemic anti-tumor drug | |||
| Any previous systemic anti-tumor treatment | 65 (65) | 36 (69) | 101 (66) |
| Previous somatostatin analogue treatment | 41 (41) | 28 (54) | 69 (45) |
| Previous systemic chemotherapy | 29 (29) | 10 (19) | 39 (26) |
| Previous everolimus treatment | 11 (11) | 3 (6) | 14 (9) |
| Previous anti-angiogenic treatment | 5 (5) | 6 (12) | 11 (7) |
| Sunitinib | 4 (4) | 6 (12) | 10 (7) |
| Endostatin | 1 (1) | 1 (2) | 2 (1) |
| Famitinib | 1 (1) | 0 (0) | 1 (1) |
| Apatinib | 0 (0) | 1 (2) | 1 (1) |
| Surgery history of primary tumor | |||
| Yes | 40 (40) | 27 (52) | 67 (45) |
| No | 60 (60) | 25 (48) | 85 (55) |
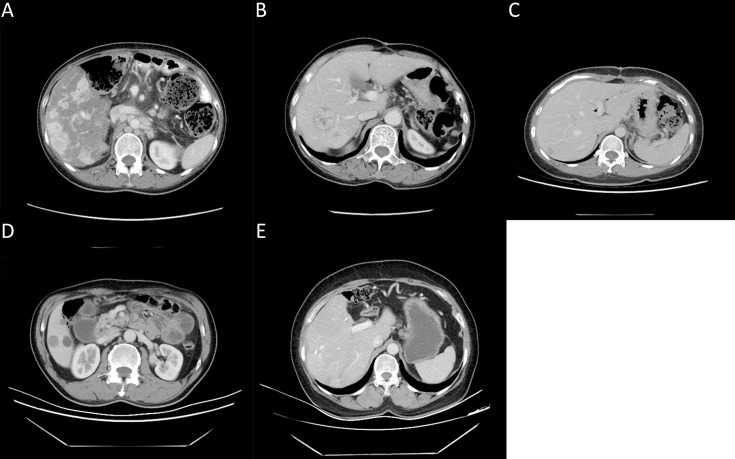
Figure S2.
Examples of some radiological features seen on pre-treatment CECT of qualitative enhancement measurements. (A) Low enhancement; (B) High enhancement; (C) Heterogeneous enhancement; (D) Well-defined margins; (E) Poorly-defined margins. CECT, contrast-enhanced computed tomography
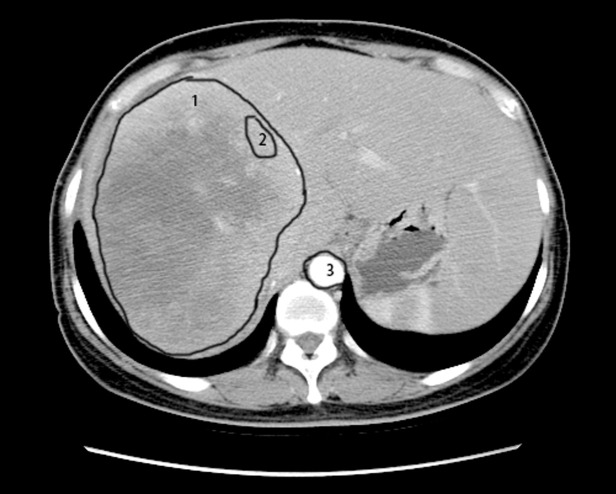
Figure 1.
Delineation of target lesions and ROIs. ROI (number 1) was depicted along the contours of the tumor and the average of CT values was calculated as the whole-lesion values. In addition, the ROI (number 2) was generated on the periphery of the tumor; while the ROI (number 3) inside the aorta was depicted to calculate the peri-lesion values. ROI, region of interest; CT, computed tomography.
The summary of imaging parameters is listed in Supplementary Table S2. In the surufatinib group, 56 (56.0%) and 44 (44.0%) patients had well- or poorly-defined tumor margins, respectively. Thirty-two patients (32.0%) had high enhancement pattern, mean [standard deviation (SD)] of ASER-peri and ASER-whole was 0.46 (0.15), and 0.38 (0.14); mean (SD) of PSER-peri and PSER-whole was 0.80 (0.14), and 0.69 (0.15); and mean (SD) of ARER-peri and ARER-whole was 1.51 (0.97) and 1.27 (0.90); mean (SD) of PRER-peri and ARER-whole was 1.11 (0.34) and 0.95 (0.31), respectively. No major differences in baseline imaging features were observed between the surufatinib and placebo groups.
Table S2. Baseline qualitative and quantitative radiological information of surufatinib vs. placebo.
| Level | Overall | Surufatinib | Placebo | P* |
| ARER/PRER, arterial phase/portal venous phase relative enhancement ratio; ASER/PSER, arterial phase/portal venous phase standard enhancement ratio; AEI, arterial enhancement index; PEI, portal-venous enhancement index. *, Continuous variables were tested by the t-test (normally distributed variables), and categorical variables were compared using Chi-square test or Fisher’s exact test, where appropriate. | ||||
| Qualitative parameters [n (%)] | ||||
| Tumor margin | 0.264 | |||
| Well-defined | 90 (59.21) | 56 (56.00) | 34 (65.38) | |
| Poorly-defined | 62 (40.79) | 44 (44.00) | 18 (34.62) | |
| Necrosis proportion | 0.144 | |||
| 0−25% | 102 (67.11) | 67 (67.00) | 35 (67.31) | |
| >25% | 50 (32.89) | 33 (33.00) | 17 (32.69) | |
| High enhancement pattern | 0.370 | |||
| High | 53 (34.87) | 32 (32.00) | 21 (40.38) | |
| Not high | 99 (65.13) | 68 (68.00) | 31 (59.62) | |
| Hepatic localization type | 0.727 (exact) | |||
| Type I | 7 (4.64) | 4 (4.04) | 3 (5.77) | |
| Type II | 90 (59.60) | 61 (61.62) | 29 (55.77) | |
| Type III | 54 (35.76) | 34 (34.34) | 20 (38.46) | |
| Tumor volume | 0.442 | |||
| ≤25% | 105 (69.08) | 67 (67.00) | 38 (73.08) | |
| >25% | 47 (30.92) | 33 (33.00) | 14 (26.92) | |
| Quantitative parameters [Mean (SD)] | ||||
| Maximum diameters | 41.7 (28.84) | 44.4 (30.20) | 36.7 (25.57) | 0.121 |
| ARER-whole | 1.31 (0.87) | 1.27 (0.90) | 1.39 (0.82) | 0.467 |
| ARER-peri | 1.54 (0.94) | 1.51 (0.97) | 1.60 (0.87) | 0.619 |
| PRER-whole | 0.96 (0.29) | 0.95 (0.31) | 0.98 (0.25) | 0.592 |
| PRER-peri | 1.12 (0.32) | 1.11 (0.34) | 1.13 (0.28) | 0.658 |
| ASER-whole | 0.38 (0.13) | 0.38 (0.14) | 0.37 (0.11) | 0.536 |
| ASER-peri | 0.44 (0.15) | 0.46 (0.15) | 0.42 (0.13) | 0.271 |
| PSER-whole | 0.69 (0.14) | 0.69 (0.15) | 0.69 (0.14) | 0.909 |
| PSER-peri | 0.80 (0.14) | 0.80 (0.14) | 0.80 (0.15) | 0.999 |
| AEI | 5.44 (9.43) | 5.91 (10.81) | 4.58 (6.18) | 0.421 |
| PEI | 1.67 (0.97) | 1.75 (1.11) | 1.52 (0.65) | 0.156 |
Efficacy analysis of whole cohort
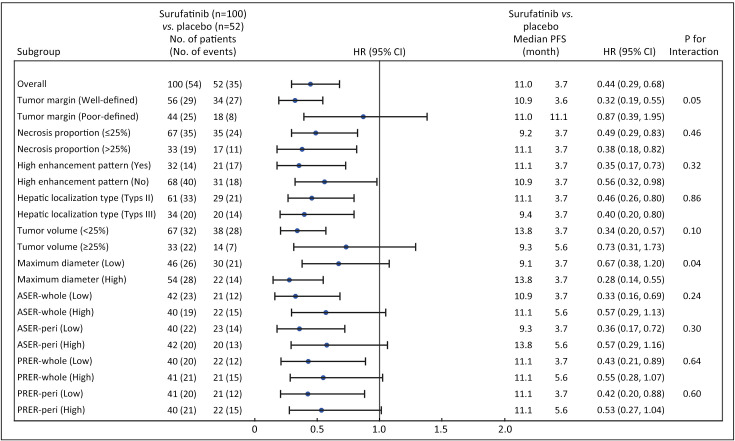
The PFS was 11.0 months in the surufatinib group compared to 3.7 months in the placebo group, with an HR of 0.44 (95% CI, 0.29−0.68), indicating a 56% reduction in the risk of disease progression with surufatinib (Figure 2). Subgroup analyses of PFS consistently demonstrated benefit across most imaging parameters in the PNELM population, with no suggested influence of baseline covariates on PFS (Supplementary Table S3).
Figure 2.
Forest plot of subgroup analysis in imaging characteristics and PFS assessed by the investigators. ASER, arterial standardized enhancement ratio; PRER, portal venous relative enhancement ratio; HR, hazard ratio; 95% CI, 95% confidence interval; PFS, progression-free survival.
Table S3. Univariate analysis of PFS by investigator considering potential influence of baseline covariates.
| Variables | Subsets (n/n) | HR | 95% CI | P |
| PFS, progression-free survival; NET, neuroendocrine tumor; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; 95% CI, 95% confidence interval. The second level is the reference level for interpretation of HRs for categorical characteristics. If <10% of patients are assigned to a particular stratum, those particular covariates are not included in the analysis. | ||||
| Treatment (Surufatinib/Placebo) | 100/52 | 0.444 | 0.29, 0.68 | <0.001 |
| NET pathological grade (Grade 1/Grade 2) | 18/134 | 0.882 | 0.44, 1.76 | 0.722 |
| ECOG performance status (0/1) | 104/48 | 0.724 | 0.46, 1.14 | 0.162 |
| Age (year) (<65/≥65) | 135/17 | 0.814 | 0.43, 1.53 | 0.523 |
| Gender (Male/Female) | 76/76 | 1.402 | 0.92, 2.14 | 0.119 |
| Functioning tumor (Yes/No) | 12/139 | − | − | − |
| No. of organs involved by tumor (≤2/≥3) | 76/76 | 0.783 | 0.51, 1.20 | 0.257 |
| Prior systemic chemotherapy (Yes/No) | 39/113 | 0.930 | 0.57, 1.52 | 0.772 |
| Previous systemic anti-tumor drug for advanced disease (Yes/No) | 101/51 | 1.252 | 0.80, 1.95 | 0.321 |
| Prior anti-angiogenesis treatment (Yes/No) | 11/141 | − | − | − |
Notably, a significant reduction in PFS events was observed in subgroups defined by blood-supply-associated imaging parameters (HR<0.4), including high enhancement pattern, well-defined tumor margins, type III localization metastases, high MDs, necrosis proportions >25%, and hepatic tumor volume <25%.
In terms of response, the surufatinib group demonstrated a best overall response (BOR) of PR in 20 (20.0%) patients, SD in 60 (60.0%) patients, PD in 7 (7.0%) patients, and not evaluable (NE) in 13 (13.0%) patients. In comparison, the placebo group had a BOR of PR in 1 (1.9%) patient, SD in 30 (57.7%) patients, PD in 14 (26.9%) patients, and NE in 7 (13.5%) patients. The objective response ratio (ORR) was 20.0% in the surufatinib group and 1.9% in the placebo group. The ORR results were consistent with those observed in the SANET-p study (Supplementary Table S4).
Table S4. Objective responses in patients of surufatinib cohort.
| Variables | n/N | P | n/N | P | |||
| ORR | Non-ORR | DCR | Non-DCR | ||||
| Parameters with P<0.1 were entered into the multivariate analysis. ASER, arterial standardized enhancement ratio; ORR, objective response ratio; DCR, disease control rate. | |||||||
| Tumor margin | 0.159 | − | |||||
| Well-defined | 14/56 | 42/56 | − | − | |||
| Poorly-defined | 6/44 | 38/44 | − | − | |||
| Necrosis proportion | 0.832 | 0.440 (Exact) | |||||
| 0−25% | 13/67 | 54/67 | 52/67 | 15/67 | |||
| >25% | 7/33 | 26/33 | 28/33 | 5/33 | |||
| High enhancement pattern | 0.054 | 0.830 | |||||
| No | 10/68 | 58/68 | 54/68 | 14/68 | |||
| Yes | 10/32 | 22/32 | 26/32 | 6/32 | |||
| Hepatic metastases localization type | 0.048 (Exact) | 0.459 (Exact) | |||||
| Type I | 2/4 | 2/4 | 4/4 | 0/4 | |||
| Type II | 8/61 | 53/61 | 47/61 | 14/61 | |||
| Type III | 10/34 | 24/34 | 29/34 | 5/34 | |||
| Tumor volume | 0.457 | 0.832 | |||||
| <25% | 12/67 | 55/67 | 54/67 | 13/67 | |||
| ≥25% | 8/33 | 25/33 | 26/33 | 7/33 | |||
| ASER-peri | 0.594 | 0.414 | |||||
| Low | 8/41 | 33/41 | 31/41 | 10/41 | |||
| High | 10/41 | 31/41 | 34/41 | 7/41 | |||
| Maximum diameter | 0.617 | 0.617 | |||||
| Low | 9/50 | 41/50 | 39/50 | 11/50 | |||
| High | 11/50 | 39/50 | 41/50 | 9/50 | |||
Efficacy analysis of surufatinib group
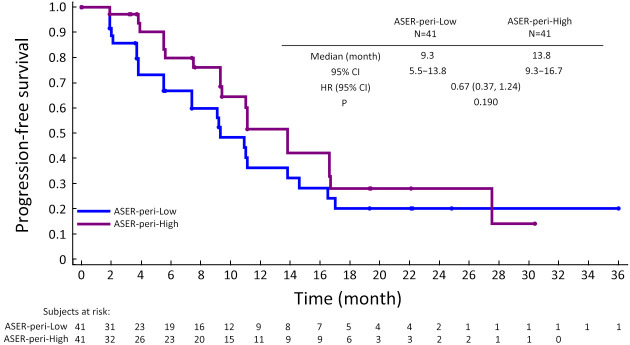
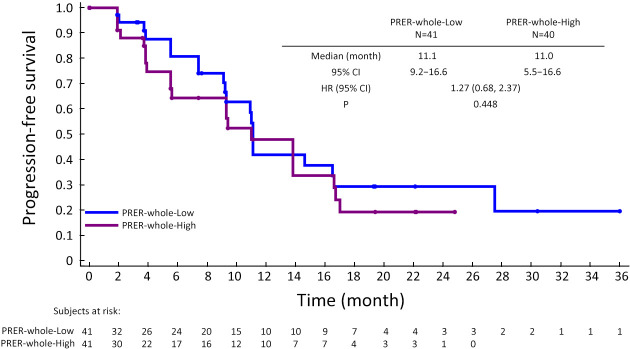
Further exploration of qualitative and quantitative enhancement parameters and efficacy in the surufatinib group revealed potential predictive factors for longer mPFS using univariate Cox regression analysis: high ASER-peri (HR=0.094, 95% CI: 0.009−0.931, P=0.043), low PRER-whole (HR=2.080, 95% CI: 0.881−4.908, P=0.095), and low PRER-peri (HR=2.051, 95% CI: 0.959−4.388, P=0.064). Proceeding with the multivariate analysis, ASER-peri (HR=0.039, 95% CI: 0.003−0.483, P=0.012) and PRER-whole (HR=2.872, 95% CI: 1.299−6.348, P=0.009) were significantly associated with PFS (Table 2). PFS curves stratified by ASER-peri (low/high) and PRER-whole (low/high) are shown in Supplementary Figure S3 and Supplementary Figure S4. These figures demonstrate that ASER-peri-high is associated with longer mPFS compared to the other groups.
Table 2. Cox proportional hazards regression models of variables associated with PFS outcomes in surufatinib.
| Co-variate | Univariate analysis† | Multivariate analysis | ||||||
| HR (95% CI) | P | N | HR (95% CI) | P | N | |||
| †, Patients with evaluable assessment, N=81 (Other patients had uncalculating lesions); Parameters with P<0.1 from univariate analysis entered into the multivariate analysis. Stepwise selection (entry and removal significance level =0.1) was performed to find the best performing model. PFS, progression-free survival; ARER, arterial relative enhancement ratio; PRER, portal venous relative enhancement ratio; ASER, arterial standardized enhancement ratio; PSER, portal venous standard enhancement ratio; HR, hazard ratio; 95% CI, 95% confidence interval. PRER-peri did not enter the final model during the construction of models by stepwise selection (P<0.1). | ||||||||
| Tumor margin [Poorly-defined (n=44) vs. Well-defined (n=56)] | 0.958 (0.560, 1.650) | 0.878 | 100 | |||||
| Necrosis proportion [>25% (n=33) vs. ≤25% (n=67)] | 0.690 (0.393, 1.213) | 0.198 | 100 | |||||
| High enhancement pattern [Others (n=68) vs. High (n=32)] | 0.940 (0.506, 1.749) | 0.846 | 100 | |||||
| Tumor volume [≥25% (n=33) vs. <25% (n=67)] | 1.269 (0.736, 2.187) | 0.392 | 100 | |||||
| Hepatic localization type | 99 | |||||||
| Type II (n=61) vs. Type I (n=4) | 4.707 (0.640, 34.623) | 0.128 | ||||||
| Type III (n=34) vs. Type I (n=4) | 3.861 (0.516, 28.869) | 0.188 | ||||||
| Maximum diameters | 0.992 (0.983, 1.002) | 0.101 | 100 | |||||
| ARER-whole | 1.182 (0.875, 1.596) | 0.276 | 82 | |||||
| ARER-peri | 1.153 (0.860, 1.546) | 0.341 | 82 | |||||
| PRER-whole | 2.080 (0.881, 4.908) | 0.095 | 81 | 2.872 (1.299, 6.348) |
0.009 | 81 | ||
| PRER-peri | 2.051 (0.959, 4.388) | 0.064 | 81 | |||||
| ASER-whole | 0.124 (0.010, 1.514) | 0.102 | 82 | |||||
| ASER-peri | 0.094 (0.009, 0.931) | 0.043 | 82 | 0.039 (0.003, 0.483) |
0.012 | 81 | ||
| PSER-whole | 1.544 (0.260, 9.182) | 0.633 | 81 | |||||
| PSER-peri | 1.811 (0.297, 11.031) | 0.520 | 81 | |||||
Figure S3.
Kaplan-Meier plot showing PFS according to ASER-peri extent (high vs. low). PFS, progression-free survival; ASER, arterial standardized enhancement ratio; HR, hazard ratio; 95% CI, 95% confidence interval.
Figure S4.
Kaplan-Meier plot showing PFS according to PRER-whole extent (high vs. low). PFS, progression-free survival; PRER, portal venous relative enhancement ratio; HR, hazard ratio; 95% CI, 95% confidence interval.
When examining the association between radiological factors and response in the surufatinib group using logistic analysis, we found that a high enhancement pattern was associated with a better ORR [high enhancement pattern vs. other enhancement pattern, 31.3% vs. 14.7%, OR=3.488 (95% CI: 1.024−11.875), P=0.046]. Well-defined tumor margins also showed a tendency towards better response in the surufatinib group [25.0% vs. 13.6%, OR=3.142 (95% CI: 0.808−12.224), P=0.099] (Table 3). Furthermore, well-defined tumor margins were associated with a better DCR [89.3% vs. 68.2%, OR=4.535 (95% CI: 1.285−16.011), P=0.019] in the multivariate logistic regression analysis (Supplementary Table S5). No differences in ORR/DCR were observed in comparisons with different localization types, tumor volumes, necrosis proportions, and ASER-peri values.
Table 3. Logistic regression of variables associated with ORR in surufatinib groups.
| Co-variate | Univariate analysis | Multivariate analysis | |||||||||
| OR | 95% CI | P | Type 3 tests |
N | OR | 95% CI | P | Type 3 tests |
N | ||
| ORR, objective response ratio; ASER, arterial standardized enhancement ratio; OR, odds ratio; 95% CI, 95% confidence interval. The parameters with P values <0.1 were entered into the multivariate analysis. | |||||||||||
| Tumor margin [Well-defined (n=56) vs.
poorly-defined (n=44)] |
2.110 | 0.737, 6.061 | 0.164 | 0.164 | 100 | 3.142 | 0.808, 12.224 | 0.099 | 0.099 | 82 | |
| Necrosis proportion [>25% (n=33) vs.
≤25% (n=67)] |
1.118 | 0.399, 3.136 | 0.832 | − | 100 | − | − | − | − | − | |
| Tumor volume [≥25% (n=33) vs.
<25% (n=67)] |
1.467 | 0.533, 4.035 | 0.458 | − | 100 | − | − | − | − | − | |
| High enhancement pattern [High (n=32) vs. others (n=68)] |
2.636 | 0.965, 7.199 | 0.059 | 0.059 | 100 | 3.488 | 1.024, 11.875 | 0.046 | 0.046 | 82 | |
| Hepatic localization type | 99 | 82 | |||||||||
| Type I (n=4) vs. Type III (n=34) | 2.400 | 0.296, 19.485 | 0.413 | 0.068 | 0.517 | 0.042, 6.413 | 0.607 | 0.080 | |||
| Type II (n=61) vs. Type III (n=34) | 0.362 | 0.127, 1.032 | 0.057 | − | 0.222 | 0.058, 0.849 | 0.028 | − | |||
| ASER-peri | 0.752 | 0.263, 2.150 | 0.594 | 0.594 | 82 | 0.834 | 0.255, 2.727 | 0.764 | 0.764 | 82 | |
Table S5. Logistic regression models of variables associated with DCR of surufatinib cohort.
| Level | Univariate analysis | Multivariate analysis | |||||||
| OR | 95% CI | P | Type 3 tests |
OR | 95% CI | P | Type 3 tests |
||
| DCR, disease control rate; ASER-peri, arterial standard enhancement rate-peri; OR, odds ratio; 95% CI, 95% confidence interval. No patients with surufatinib with type I hepatic metastases localization types had objective response, thus DCR cannot be estimated. | |||||||||
| Tumor margin (Poorly-defined vs. well-defined) | 0.257 | 0.089, 0.741 | 0.012 | 0.012 | 4.535 | 1.285, 16.011 | 0.019 | 0.019 | |
| Necrosis proportion (>25% vs. ≤25%) | 1.615 | 0.532, 4.909 | 0.398 | − | − | − | − | ||
| Tumor volume (≥25% vs. <25%) | 0.894 | 0.319, 2.507 | 0.832 | − | − | − | − | ||
| High enhancement pattern (High vs. others) | 1.123 | 0.387, 3.258 | 0.830 | 0.830 | 0.887 | 0.226, 3.487 | 0.864 | 0.864 | |
| Hepatic localization type | |||||||||
| Type I vs. Type III | − | − | 0.981 | 0.633 | − | − | 0.982 | 0.315 | |
| Type II vs. Type III | 0.579 | 0.189, 1.776 | 0.339 | 0.358 | 0.095, 1.347 | 0.129 | |||
| ASER-peri | 0.568 | 0.013, 24.404 | 0.769 | − | − | − | − | ||
Discussion
Although surufatinib provided significant clinical benefit regarding the PFS of PNELM patients, more precise methods are warranted to distinguish non-beneficiaries. We utilized blood-supply-associated imaging parameters as markers for surufatinib to evaluate its potential in PNELM. The results suggested that high ASER-peri, high enhancement patterns, and well-defined tumor margins could be predictors for better PFS and optimal ORR/DCR in surufatinib-treated patients.
We hypothesized that high blood-supply pre-treatment CECT parameters might correlate with the response to surufatinib, because vascularization positively correlated to efficacy, lesions with high blood supply may provide greater access to anti-angiogenesis agents (surufatinib) as well as markers of tumors with well-developed tumor vessels (9). Some identified radiological predictors, including the pre-treatment enhancement ratio (ER), have already been associated with treatment effectiveness (20,22). Such quantitative indexes have advantages in standardization for precise clinical practice and future research. Studies have mainly focused on enhancement patterns, which had positive relationships with promising objective responses in patients treated with anti-angiogenesis agents (23). Volume of lesion enhancement and tumor micro-vessel density were also significant prognostic factors (21,22,24,25). The baseline CT arterial enhancement pattern/fraction also improved the PFS and response in patients with transcatheter arterial embolization, chemotherapy, radioembolization, or surgery (13,15,19,26-29).
We demonstrated that blood supply-associated radiological features (high ASER-peri and low PRER-whole values) revealed PFS benefit in the surufatinib group, which is applicable for future treatment judgement. Given the necessity to exclude the interference of different scan conditions, we employed standard enhancement ratios. Among these parameters, the peri-lesion enhancement reflecting the blood supply may have similar practical value to whole-lesions. The measurement of central-lesion parameters, however, was inferior to that of peri/whole-lesions (30,31), which may be due to the core of the lesions being filled with necrosis components and introducing some miscalculation. The ASER-peri, thereafter, is affirmed as the ideal precise measurement because it was positively correlated to the blood supply.
Patients with well-defined margins and high enhancement patterns were more likely to have higher ORR (32). We observed the in-concordance of efficacy by imaging markers between ORR and PFS, which might be caused by the imperfect association between the PFS and ORR benefit generated from the slow progression nature of pNET (long duration SD was calculated as PFS benefit, but was not recorded as remission).
Our study had some noteworthy strengths. First, all data were collected from a multicenter double-blinding phase III clinical trial, excluding commonly-seen heterogeneity in retrospective cohorts. Second, we analyzed the data with large samples to increase the reliability. We revealed that ordinary pre-treatment CECT assessments can be extraordinary prognostic parameters for PNELM patients. Furthermore, the major populations were G2 patients, eliminating common pathological confounding factors (13,33,34).
Our study had some limitations. The analysis of CT scans was performed in different centers, which introduced variability in the measurement of parameters. Additionally, subjectivity was present in manual procedures such as defining the ROI on CT images. In the future, artificial intelligence might offer solutions to address these challenges and ensure more consistent and objective measurements (15).
Conclusions
Our study emphasized the association between pre-treatment high blood-supply radiological parameters of tumors and the efficacy of surufatinib in patients with PNELM. Specifically, high ASER-peri emerged as a significant factor that benefited the PFS. Moreover, a high enhancement pattern and well-defined tumor margins were associated with a better ORR and DCR in these challenging cases of refractory PNELM. These pre-treatment CECT characteristics have the potential to enhance patient selection and contribute to improving management strategies for PNELM.
Acknowledgements
None.
Contributor Information
Ming Lu, Email: qiminglu@126.com.
Jianming Xu, Email: Jmxu2003@163.com.
References
- 1.Dasari A, Shen C, Halperin D, et al Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavel M, O’Toole D, Costa F, et al ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103:172–85. doi: 10.1159/000443167. [DOI] [PubMed] [Google Scholar]
- 3.Frilling A, Modlin IM, Kidd M, et al Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8–21. doi: 10.1016/S1470-2045(13)70362-0. [DOI] [PubMed] [Google Scholar]
- 4.Frilling A, Li J, Malamutmann E, et al Treatment of liver metastases from neuroendocrine tumors in relation to the extent of hepatic disease. Br J Surg. 2009;96:175–84. doi: 10.1002/bjs.6468. [DOI] [PubMed] [Google Scholar]
- 5.Cloyd JM, Wiseman JT, Pawlik TM Surgical management of pancreatic neuroendocrine liver metastases. J Gastrointest Oncol. 2020;11:590–600. doi: 10.21037/jgo.2019.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demirkan BH, Eriksson B Systemic treatment of neuroendocrine tumors with hepatic metastases. Turk J Gastroenterol. 2012;23:427–37. doi: 10.4318/tjg.2012.0552. [DOI] [PubMed] [Google Scholar]
- 7.Rinke A, Müller HH, Schade-Brittinger C, et al Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 8.Couvelard A, O’Toole D, Turley H, et al Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumors: negative correlation of microvascular density and VEGF expression with tumor progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Shen L, Bai C, et al Surufatinib in advanced pancreatic neuroendocrine tumors (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1489–99. doi: 10.1016/S1470-2045(20)30493-9. [DOI] [PubMed] [Google Scholar]
- 11.Heneweer C, Holland JP, Divilov V, et al Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J Nucl Med. 2011;52:625–33. doi: 10.2967/jnumed.110.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KW, Lee JM, Klotz E, et al Quantitative CT color mapping of the arterial enhancement fraction of the liver to detect hepatocellular carcinoma. Radiology. 2009;250:425–34. doi: 10.1148/radiol.2501072196. [DOI] [PubMed] [Google Scholar]
- 13.Cappelli C, Boggi U, Mazzeo S, et al Contrast enhancement pattern on multidetector CT predicts malignancy in pancreatic endocrine tumors. Eur Radiol. 2015;25:751–9. doi: 10.1007/s00330-014-3485-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Wang W, Jin K, et al Special issue “the advance of solid tumor research in China”: Prediction of sunitinib efficacy using computed tomography in patients with pancreatic neuroendocrine tumors. Int J Cancer. 2023;152:90–9. doi: 10.1002/ijc.34294. [DOI] [PubMed] [Google Scholar]
- 15.Okabe H, Hashimoto D, Chikamoto A, et al Shape and enhancement characteristics of pancreatic neuroendocrine tumor on preoperative contrast-enhanced computed tomography may be prognostic indicators. Ann Surg Oncol. 2017;24:1399–405. doi: 10.1245/s10434-016-5630-4. [DOI] [PubMed] [Google Scholar]
- 16.Panzuto F, Pusceddu S, Faggiano A, et al Prognostic impact of tumor burden in stage IV neuroendocrine neoplasia: A comparison between pancreatic and gastrointestinal localizations. Pancreatology. 2019;19:1067–73. doi: 10.1016/j.pan.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Beleù A, Rizzo G, De Robertis R, et al Liver tumor burden in pancreatic neuroendocrine tumors: CT features and texture analysis in the prediction of tumor grade and 18F-FDG uptake. Cancers (Basel) 2020;12:1486. doi: 10.3390/cancers12061486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulpinar B, Peker E, Soydal C, et al Can we differentiate histologic subtypes of neuroendocrine tumor liver metastases at a single phase contrast-enhanced CT-correlation with Ga-68 DOTATATE PET/CT findings. Br J Radiol. 2020;93:20190735. doi: 10.1259/bjr.20190735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Onofrio M, Ciaravino V, Cardobi N, et al CT enhancement and 3D texture analysis of pancreatic neuroendocrine neoplasms. Sci Rep. 2019;9:2176. doi: 10.1038/s41598-018-38459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagayama Y, Inoue T, Kato Y, et al Relative enhancement ratio of portal venous phase to unenhanced CT in the diagnosis of lipid-poor adrenal adenomas. Radiology. 2021;301:360–8. doi: 10.1148/radiol.2021210231. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Chen W, Cui W, et al Quantitative pretreatment CT parameters as predictors of tumor response of neuroendocrine tumor liver metastasis to transcatheter arterial bland embolization. Neuroendocrinology. 2020;110:697–704. doi: 10.1159/000504257. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura Y, Kobayashi M, Shindoh J, et al Pretreatment heterogeneous enhancement pattern of hepatocellular carcinoma may be a useful new predictor of early response to lenvatinib and overall prognosis. Liver Cancer. 2020;9:275–92. doi: 10.1159/000505190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebauer L, Moltz JH, Mühlberg A, et al Quantitative imaging biomarkers of the whole liver tumor burden improve survival prediction in metastatic pancreatic cancer. Cancers (Basel) 2021;13:5732. doi: 10.3390/cancers13225732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Yan H, Xu L, et al Correlation between radiologic features on contrast-enhanced CT and pathological tumor grades in pancreatic neuroendocrine neoplasms. J Biomed Res. 2020;35:179–88. doi: 10.7555/JBR.34.20200039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colagrande S, Calistri L, Campani C, et al CT volume of enhancement of disease (VED) can predict the early response to treatment and overall survival in patients with advanced HCC treated with sorafenib. Eur Radiol. 2021;31:1608–19. doi: 10.1007/s00330-020-07171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrache F, Vullierme MP, Roy C, et al Arterial phase enhancement and body mass index are predictors of response to chemoembolisation for liver metastases of endocrine tumors. Br J Cancer. 2007;96:49–55. doi: 10.1038/sj.bjc.6603526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo I, Lee JM, Kim KW, et al Liver metastases on quantitative color mapping of the arterial enhancement fraction from multiphasic CT scans: Evaluation of the hemodynamic features and correlation with the chemotherapy response. Eur J Radiol. 2011;80:e278–83. doi: 10.1016/j.ejrad.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Jarraya H, Borde P, Mirabel X, et al Lobulated enhancement evaluation in the follow-up of liver metastases treated by stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:292–8. doi: 10.1016/j.ijrobp.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Ye Y, Hu Y, et al Extent of enhancement on multiphase contrast-enhanced CT images is a potential prognostic factor of stage I-III colon cancer. Eur Radiol. 2019;29:1114–23. doi: 10.1007/s00330-018-5689-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhu HB, Xu D, Zhang XY, et al Prediction of therapeutic effect to treatment in patients with colorectal liver metastases using functional magnetic resonance imaging and RECIST criteria: A pilot study in comparison between bevacizumab-containing chemotherapy and standard chemotherapy. Ann Surg Oncol. 2022;29:3938–49. doi: 10.1245/s10434-021-11101-y. [DOI] [PubMed] [Google Scholar]
- 31.Sun YQ, Xiao Q, Hu FX, et al Diffusion kurtosis imaging in the characterisation of rectal cancer: utilizing the most repeatable region-of-interest strategy for diffusion parameters on a 3T scanner. Eur Radiol. 2018;28:5211–20. doi: 10.1007/s00330-018-5495-y. [DOI] [PubMed] [Google Scholar]
- 32.Froelich MF, Heinemann V, Sommer WH, et al. CT attenuation of liver metastases before targeted therapy is a prognostic factor of overall survival in colorectal cancer patients. Results from the randomised, open-label FIRE-3/AIO KRK0306 trial. Eur Radiol 2018;28:5284-92.
- 33.Kim DW, Kim HJ, Kim KW, et al Neuroendocrine neoplasms of the pancreas at dynamic enhanced CT: comparison between grade 3 neuroendocrine carcinoma and grade 1/2 neuroendocrine tumour. Eur Radiol. 2015;25:1375–83. doi: 10.1007/s00330-014-3532-z. [DOI] [PubMed] [Google Scholar]
- 34.Belousova E, Karmazanovsky G, Kriger A, et al Contrast-enhanced MDCT in patients with pancreatic neuroendocrine tumors: correlation with histological findings and diagnostic performance in differentiation between tumour grades. Clin Radiol. 2017;72:150–8. doi: 10.1016/j.crad.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]