Abstract
Nuclear medicine plays an irreplaceable role in the diagnosis and treatment of tumors. Radiopharmaceuticals are important components of nuclear medicine. Among the radiopharmaceuticals approved by the Food and Drug Administration (FDA), radio-tracers targeting prostate-specific membrane antigen (PSMA) and somatostatin receptor (SSTR) have held essential positions in the diagnosis and treatment of prostate cancers and neuroendocrine neoplasms, respectively. In recent years, FDA-approved serials of immune-therapy and targeted therapy drugs targeting programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), and nectin cell adhesion molecule 4 (Nectin 4). How to screen patients suitable for these treatments and monitor the therapy? Nuclear medicine with specific radiopharmaceuticals can visualize the expression level of those targets in systemic lesions and evaluate the efficacy of treatment. In addition to radiopharmaceuticals, imaging equipment is also a key step for nuclear medicine. Advanced equipment including total-body positron emission tomography/computed tomography (PET/CT) and positron emission tomography/magnetic resonance imaging (PET/MRI) has been developed, which contribute to the diagnosis and treatment of tumors, as well as the development of new radiopharmaceuticals. Here, we conclude most recently advances of radiopharmaceuticals in nuclear medicine, and they substantially increase the “arsenal” of clinicians for tumor therapy.
Keywords: Nuclear medicine, radiopharmaceuticals, neoplasms
Introduction
Nuclear medicine plays an irreplaceable role in the diagnosis and treatment of tumors. With the emergence of immunotherapy and targeted therapy, nuclear medicine plays a greater role in the diagnosis and treatment of tumors. Knowing the expression of biomarkers throughout the body is crucial for clinical treatment. However, conventional clinical methods such as immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) to analyze the expression level of biomarkers in tumor tissues have poor reproducibility because they are unable to obtain the expression of biomarkers throughout the body. Meanwhile, they are invasive. Radiopharmaceuticals represented by 18F-fluorodeoxyglucose (18F-FDG), as the important components of nuclear medicine, have played a center role in many kinds of diseases, especially in tumors. However, 18F-FDG reflects the glucose metabolism of the lesions and is not a tumor-specific imaging agent. Researchers have been committed to finding ideal targets and developing new radiopharmaceuticals (Figure 1). And radiopharmaceuticals targeting special tumor biomarkers have been gradually developed. Compared to 18F-FDG, they are specific and can visualize the biomarker expression by imaging, which is helpful to diagnose specifically, detect lesions (including primary lesions and metastases) systemically, screen suitable patients for these treatments precisely, non-invasively as well. Many of them have been approved by the Food and Drug Administration (FDA) or have entered the clinical research stage. Radiopharmaceuticals targeting prostate-specific membrane antigen (PSMA) or somatostatin receptor (SSTR) have played an important role in the diagnosis and treatment of prostate cancer and neuroendocrine tumors.
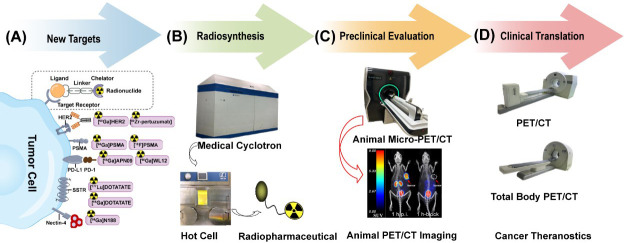
Figure 1.
Radiopharmaceuticals: process from bench to bedside. (A) Radiopharmaceuticals are consisted of four parts including radionuclide, chelator, linker and ligand which could specially bind to cell surface receptors or targets such as PSMA, HER2, PD-1/PD-L1, SSTR and Nectin-4; (B) Preparation of radiopharmaceuticals includes production of nuclides, radiolabeling, and quality control. Animal PET/CT imaging demonstrated high accumulation in tumor region (1); (C) A series of preclinical evaluations are performed to screen out the optimal probes; (D) Optimal probe for clinical translation. Nuclear medicine equipment such as total-body PET/CT is applied to perform whole-body dynamic imaging with quite low doses to achieve visual diagnosis and treatment. PSMA, prostate-specific membrane antigen; HER2, human epidermal growth factor receptor 2; PD-1, programmed death 1; PD-L1, programmed death ligand 1; SSTR, somatostatin receptor; Nectin 4, nectin cell adhesion molecule 4; PET/CT, positron emission tomography/computed tomography.
In addition to radiopharmaceuticals, the nuclear medicine equipment also plays a positive role in the diagnosis and treatment of tumors. The novel nuclear medicine equipment includes total-body positron emission tomography/computed tomography (PET/CT) and positron emission tomography/magnetic resonance imaging (PET/MRI). Compared to conventional PET/CT, total-body PET/CT can reduce patient and staff doses (2), shorten acquisition time (3), optimize image quality, reduce radiopharmaceutical costs, and increase patient throughput (4). In addition, it can observe the distribution and metabolism of radiopharmaceuticals in the body in real-time and dynamically, which is very helpful for pharmacokinetic research. The soft tissue resolution of PET/MRI is better than conventional PET/CT, so it can better display the morphology of lesions and their relationship with adjacent tissues.
As easily can be found in the new advances, the rush hour of nuclear medicine is coming.
Concept of nuclear medicine and radiopharmaceuticals
In 1898, Pierre Curie and Marie Curie discovered the radioactive element radium (mainly 226Ra) and polonium, pioneered the theory of radioactivity, and invented the radioisotope 226Ra separation technique, which releases α rays that kill cells and bacteria. In 1905, Marie Curie performed the first radioisotope insertion therapy using a radium needle. Since then, humans began to study the application of radioactive elements in medical treatment. Nuclear medicine is a discipline using nuclear technology, mainly using radionuclides or nuclear radiation, to diagnose, treat and research diseases. It includes not only imaging diagnosis, functional determination, in vitro analysis and nuclide therapy, but also various tracer experiments in the field of basic medical research.
Radiopharmaceuticals are a class of special drugs containing radionuclides for medical diagnosis and treatment. It can be simple radionuclide inorganic compounds, such as 99mTcO4−, 201TlCl, Na131I, etc, while most clinical radiopharmaceuticals are composed of radionuclides and non-radioactive labeled substances. Non-radioactive labeled parts include chemical compounds, biochemical agents (peptides, hormones, etc.), biological products (monoclonal antibodies, etc.), blood components (red blood cells, white blood cells) and antibiotics.
Generally, radiopharmaceuticals mainly consist of four parts: radionuclides, chelators, linkers, and ligands which can specifically bind to cell surface receptors or targets, such as PSMA, SSTR, human epidermal growth factor receptor 2 (HER2), programmed death 1 (PD-1)/programmed death ligand 1(PD-L1), and nectin cell adhesion molecule 4 (Nectin-4). The characteristics are as follows: 1) Radioactivity. Radiopharmaceuticals mainly use the particles or rays released by their radionuclides to achieve the purpose of diagnosis and treatment; 2) Instability. Radionuclides in radiopharmaceuticals spontaneously decay into another nuclide or nuclear state, and not only does the amount of radioactivity but also their intrinsic mass change over time. Therefore, the concept of “recording time” should be emphasized during the whole process, from production, preparation, and quality control to clinical use; 3) Radiation self-decomposition. The physical, chemical and biological effects of the particles or rays emitted by the decay of radionuclides directly affect the radiopharmaceutical itself, causing the change of the structure of the compound or the loss of biological activity, which can lead to the change of the biological behavior of the radiopharmaceutical in vivo; and 4) Low dose. The dose of radiopharmaceuticals introduced into the body is mostly at the level of milligrams and micrograms, and thus usually using radioactive activity as a unit of measurement.
Functional imaging, molecular imaging and imaging probe
Functional imaging
Functional imaging is a way to demonstrate the functional changes of tissues and organs by imaging, primarily including MRI (diffusion imaging, perfusion imaging, brain functional localization imaging), CT (perfusion imaging), PET/CT (perfusion imaging, mainly used 18F, 11C, 13N, 15O and other labeled compounds for brain and myocardial blood perfusion imaging), single photon emission computed tomography (SPECT) (mainly includes regional cerebral blood flow and myocardial perfusion imaging). But it is insufficient for prediction of tumor markers. Thus, it remains unclear how to evaluate the efficacy of targeted therapies and optimize the therapeutic strategy. Therefore, novel imaging modality and biomarkers are being developed to better evaluate targeted therapy’s effects.
Molecular imaging
Molecular imaging is a non-invasive medical imaging method that enables visualization, characterization, and measurement of biological processes at the molecular and cellular levels in humans or other organisms. Compared to conventional imaging modalities, which mainly image the structural differences of tissues or organs, molecular imaging can demonstrate the physiological and biochemical activities of normal and diseased tissue cells in active state. The essence of molecular imaging is a technology based on functional imaging, which is used for diagnosis of disease-related molecular changes, drug development and treatment monitoring. Molecular imaging accelerates tumor detection, surgical guidance, targeted drug delivery, image-guided therapy, and efficacy evaluation.
Molecular imaging technologies mainly comprise nuclear medicine molecular imaging technology, magnetic resonance molecular imaging technology, optical imaging technology and ultrasonic imaging technology. However, because CT and MRI contrast agents are mostly non-specific, and nuclear medicine imaging molecules are based on molecular tracers, they have unique advantages and convenient conditions in molecular imaging. PET imaging can visualize the expression of therapeutic targets of whole body with specific targeted molecular probes, such as various 68Ga and 18F-labeled PSMA ligands. The presence and degree of target protein expression were related to treatment response. Therefore, PET imaging probes have been introduced as predictive biomarkers (5). For example, measuring SSTR in patients with neuroendocrine tumors with 68Ga-DOTATATE/TOC/NOC can predict peptide receptor radionuclide therapy response. PSMA-targeted PET imaging can not only help to differentiate the primary and metastatic prostate cancer, but also improve the detection rate of lymph nodes and bone metastases, especially at low prostate-specific antigen levels.
Nuclear medicine imaging probe
Tumor-specific imaging probes have been used to evaluate the efficacy of targeted cancer therapies. Tumor-specific imaging probes specifically bind carrier molecules labeled with positron emitters (for PET imaging), single photon emitters (for SPECT imaging), or fluorophores (for fluorescence imaging) to a specific tumor target. Carrier molecules can be monoclonal antibodies, monoclonal antibody fragments, adhesion molecules, small peptides, or small molecules that specifically target certain cell surface markers that are overexpressed in tumors. In addition, carrier molecules can also be small molecules that detect the acidic microenvironment of tumors. Therefore, the development and maturity of nuclear medicine imaging largely depend on the search for ideal targets and the design of ideal molecules as radiolabeled probes. In general, the ideal radiopharmaceuticals should have the following properties: high target-to-nontarget ratio after the drug enters the body, fast clearance in the blood, appropriate ray type, half-life and energy, suitable for SPECT and PET imaging, long retention time of the probes in the diseased tissue, convenient drug source, low price, and non-toxic. Moreover, in the synthesis of radiopharmaceuticals, the probes should be avoided to be destroyed and decomposed in the body (6).
Radiopharmaceuticals promote precise diagnosis and treatment of tumors
The discovery of various biomarkers has enabled the application of radiopharmaceuticals in clinical diagnosis and therapy to flourish. Encouraged by this, more and more radiopharmaceuticals have been approved by the FDA. With the popularity of SPECT and PET, the diagnostic research and development of radiopharmaceuticals represented by 99mTc, 18F and 68Ga has developed rapidly in recent years, and has been developed for the diagnosis of various cancers, cardiovascular and cerebrovascular diseases, kidney function and nervous system diseases. Representative radiopharmaceuticals including 68Ga-PSMA-11 and 68Ga-DOTATATE are approved for the diagnosis of prostate cancers and neuroendocrine neoplasms (NENs), respectively. In recent years, with the great success of the FDA-approved 177Lu-DOTATATE for the treatment of NENs and 177Lu-PSMA-617 for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in clinical applications, targeted radionuclide therapy has received extensive attention. In addition, radiopharmaceuticals targeting some therapeutic targets can visualize the expression level of corresponding targets in systemic lesions (including primary lesions and metastases) and can evaluate treatment efficacy.
In this section, we will describe the clinical translational research on PSMA, SSTR2, PD1/PD-L1 and HER2, which have been highly studied in recent years. In addition, we will also introduce the clinical translation of Nectin-4, a target that has been less studied in the field of nuclear medicine, in order to provide some thoughts on the research of new targets in the field of radiopharmaceuticals.
Radiopharmaceuticals targeting PSMA
PSMA is a type II transmembrane glycoprotein that exists on the cell surface. The expression level of PSMA is significantly upregulated in the membranes of almost all prostate cancer cells. 68Ga-PSMA PET/CT can identify and locate tumors at the molecular level due to the specific binding of PSMA ligands, and detect tumors at early stages by imaging tiny lesions of 4 mm (7). FDA-approved radiopharmaceuticals targeting PSMA include 68Ga-PSMA-11, 18F-DCFPyL, and 177Lu-PSMA-617 (Table 1). The VISION trial of the phase III study of 177Lu-PSMA-617 in patients with mCRPC (8) facilitated the approval of 177Lu-PSMA-617. PSMA PET is helpful for primary tumor staging, biochemical recurrence detection, restaging, efficacy evaluation, and treatment management (7).
Table 1. FDA-approved radiopharmaceuticals targeting PSMA/SSTR for imaging/therapy.
| Radiopharmaceutical name | Brand name | Effect | Tumor type | Target molecule | Approved date | Indications and usage |
| PSMA, prostate specific membrane antigen; SSTR, somatostatin receptor; PCa, prostate cancer; NET, neuroendocrine tumor; mCRPC, metastatic castration-resistant prostate cancer; PET, positron emission tomography. | ||||||
| Fluorine-18 flotufolast | POSLUMA | Imaging | PCa | PSMA | 2023.05.25 | To identify suspected metastasis or recurrence of prostate cancer |
| Gallium-68 Gozetotide | Locametz Illuccix | Imaging | PCa | PSMA | 2022.03.23 | To identify suspected metastasis or recurrence of prostate cancer |
| Fluorine-18 piflufolastat | Pylarify | Imaging | PCa | PSMA | 2021.05.27 | To identify suspected metastasis or recurrence of prostate cancer |
| Gallium-68 PSMA-11 | − | Imaging | PCa | PSMA | 2020.12.01 | To identify suspected metastasis or recurrence of prostate cancer |
| Lutetium-177 PSMA-617 | Pluvicto | Therapy | PCa | PSMA | 2022.03.23 | Treatment of PSMA-positive mCRPC adult patients who have been treated with androgen receptor pathway inhibition and taxane-based chemotherapy |
| Copper-64 dotatate | Detectnet | Imaging | NETs | SSTR | 2020.09.03 | Localization of SSTR-positive NETs in adult patients using PET |
| Gallium-68 dotatoc | − | Imaging | NETs | SSTR | 2019.08.22 | Localization of SSTR-positive NETs in adult and pediatric patients using PET |
| Gallium-68 dotatate | NETSPOT | Imaging | NETs | SSTR | 2016.06.01 | Localization of SSTR-positive NETs in adult and pediatric patients using PET |
| Lutetium-177 dotatate | LUTATHERA | Therapy | NETs | SSTR | 2018.01.26 | Treatment of SSTR-positive NETs of gastroenteropancreatic origin, including foregut, midgut, and hindgut NETs |
PSMA-targeted imaging
Several studies indicated that PSMA PET imaging agents are diagnostically superior to other imaging examinations. Liu et al. (9) found that 68Ga-PSMA-617 PET demonstrates a high detection rate of 75% (30/40) for primary prostate cancer, and even higher sensitivity and specificity. A meta-analysis (10) including 5 studies and 497 patients with prostate cancer showed that the sensitivity and specificity of PSMA PET/MRI to diagnose the primary lesion of prostate cancer were 91% and 64%, respectively, which is better than that of PSMA PET and MRI alone.
PSMA imaging is also valuable in pre-biopsy localization. PSMA PET scan before biopsy helps to avoid false negative pathological diagnoses when determining the puncture position, especially for small lesions (11).
The staging ability of PSMA PET/CT or PET/MRI is superior to the conventional imaging. The National Comprehensive Cancer Network (NCCN) guidelines (https://jnccn.org/view/journals/jnccn/21/10/article-p1067.xml) indicate that 18F-pilufolastat PSMA or 68Ga-PSMA-11 PET/CT or PET/MRI can be considered as an alternative to standard imaging of bone and soft tissues for initial staging, the detection of biochemically recurrent disease, and as workup for progression. A prospective, randomized and multi-center study (12) using PSMA PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy found that the accuracy, sensitivity, and specificity of 68Ga-PSMA-11 PET/CT in assessing pelvic lymph node metastasis were higher than those of the conventional imaging examinations.
PSMA PET shows great clinical application prospects in detecting lesions in biochemical recurrence (BCR) patients, and can still achieve high sensitivity even if prostate-specific antigen (PSA) is very low (<0.2 ng/mL). It is difficult for conventional imaging examinations to diagnose BCR. When PSA is less than 2.0 ng/mL, the sensitivity of the whole-body bone scan is low, with only 9.4%. The detection ability of CT for BCR is only 11%−14%, while PSMA PET/CT or PSMA PET/MRI has high diagnostic accuracy due to their high specificity (13). A meta-analysis (14) that included 16 studies and 1,309 BCR prostate cancer patients showed that the sensitivity and specificity of 68Ga-PSMA PET imaging in diagnosing BCR prostate cancer metastasis were 80% and 97%, respectively. The sensitivity and specificity of pelvic lymph nodes and distant metastasis are both 86%, and it still expresses good diagnostic performance when the PSA level is low (<1.0 ng/mL).
With the continuous development of clinical research, scholars have discovered that some non-prostate tumors also take up 68Ga/18F-PSMA, including liver cancer, lung cancer, renal cell carcinoma, thyroid cancer, breast cancer, central nervous system tumors, etc. The potential reason may be that PSMA is highly expressed in new blood vessels of these kind of tumors. Schmidt et al. (15) showed that detecting PSMA expression in lung cancer can be used to accurately screen beneficiaries of targeted PSMA therapy. In addition, a study (16) found that 68Ga/18F-PSMA can detect lesions with no 18F-FDG uptake, suggesting that 68Ga/18F-PSMA PET/CT can be an effective supplement to 18F-FDG PET/CT. However, it is worth noting that most studies on the application of 68Ga/18F-PSMA PET/CT or PET/MRI in the diagnosis and treatment of non-prostate cancer tumors have small sample sizes or even only case reports, so large sample studies are needed to confirm it in the future.
PSMA-targeted radioligand therapy
The NCCN guidelines (https://jnccn.org/view/journals/jnccn/21/10/article-p1067.xml) indicate that 177Lu-PSMA-617 is a treatment option for patients with ≥1 PSMA-positive lesion and/or metastatic disease that is predominately PSMA-positive and with no dominant PSMA-negative metastatic lesions who have been treated previously with androgen receptor-directed therapy and a taxane-based chemotherapy. Some studies (17-23) demonstrated that 177Lu-PSMA-617 has a positive effect on overall survival (OS), progression-free survival (PFS), PSA response and improvement of quality of life in mCRPC patients.
177Lu-PSMA-I&T is a great potentially promising radiopharmaceutical. A study (24) conducted in an East Asian population to evaluate the efficacy and safety of 177Lu-PSMA-I&T in mCRPC patients found that radioligand therapy using 177Lu-PSMA-I&T achieved good response in mCRPC patients, including 75% of patients achieved partial response (PR) or stable disease (SD), significantly improved quality of life, and were well tolerated. In addition, PSMA-I&T is easy to produce, which promotes its clinical application.
225Ac-targeted PSMA therapy is a new option for patients with advanced prostate cancer who are ineffective with other therapies, including 177Lu-PSMA therapy. Some studies (25-27) have evaluated the efficacy of 225Ac-PSMA-617 in mCRPC patients (including patients resistant to 177Lu-PSMA-617), and preliminary results show its therapeutic potential. Among 26 mCRPC patients (who had received abiraterone or enzalutamide, docetaxel chemotherapy and 177Lu-PSMA), 65% of the patients had a decrease in PSA>50%, and the median OS was 7.7 months after receiving 225Ac-PSMA-617 treatment (27).
PSMA-targeted radionuclide therapy with different radiopharmaceuticals has different biodistribution characteristics and toxicity. The combination of low-dose multiple radiopharmaceuticals can improve the efficacy. A study (28) suggested that a single course of tandem therapy with low-activity 225Ac-PSMA-617/full-activity 177Lu-PSMA-617 may safely enhance response to PSMA-targeted radioligand therapy in patients with late-stage/end-stage mCRPC, and it can minimize xerostomia severity. Another study (27) showed that 15 mCRPC patients received 225Ac-PSMA-617 after progressing on 177Lu-PSMA treatment, and 5 cases experienced a PSA decrease of >50%, but the overall treatment tolerance was poor.
Radiopharmaceuticals targeting SSTR
NENs are a type of heterogeneous malignancies that originate from the neuroendocrine system. In terms of pathology, according to the degree of differentiation, NENs is divided into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). Well-differentiated NENs can express high levels of SSTR. Somatostatin analogues (SSA) can specifically bind to SSTR. SSA drugs include SSTR agonists and SSTR antagonists. The radiopharmaceuticals targeting SSTR currently approved by the FDA include 68Ga-DOTATATE, 68Ga-DOTATOC, 64Cu-DOTATATE, and 177Lu-DOTATATE (Table 1). They are all SSTR agonists.
SSTR-targeted imaging
SSTR PET imaging is of great value in the diagnosis, staging, recurrence monitoring, guiding treatment, and monitoring prognosis of NENs (29). The NCCN guidelines (https://jnccn.org/view/journals/jnccn/19/7/article-p839.xml) recommend SSTR-PET/CT or SSTR-PET/MRI as an imaging way for NENs. Haidar et al. (30) performed 68Ga-DOTA-NOC PET/CT examination on 104 patients of NETs diagnosed by pathology and follow-up, and found that the diagnostic sensitivity, specificity, negative predictive value, and positive prediction value were greater or equal to 90.0%, and the accuracy was 0.931. 68Ga-DOTA-SSTR PET/CT is superior to conventional imaging and SPECT imaging in initial tumor staging (31,32). In addition, SSTR PET imaging is also valuable in guiding treatment (33-35). Patients with positive 68Ga-DOTA-SSTR PET/CT imaging may benefit from peptide radioreceptor therapy (PRRT) and SSTR analog treatment (35).
However, there are some disadvantages about radionuclide-labeled SSTR agonists. The tumor detection rates, especially the detection rate of liver metastases, are still limited (36). The clinical value of SSTR agonists is mainly limited by the following factors: 1) low detection sensitivity for small tumors; and 2) relatively high background activity in normal organs (especially liver, kidneys, and spleen). This limits the detection in small metastases with length less than 1 cm or poorly differentiated (G3 grade) NENs (37). In recent years, in vivo and in vitro research results have shown that NENs has higher uptake rate of SSTR antagonists than SSTR agonists, and the retention time of the former in tumor cells is longer than that of the latter (36,38-40). Therefore, the use of radionuclide-labeled SSTR antagonists emerges as a new approach to molecular imaging and treatment of NEN. SSTR antagonists currently successfully developed include 68Ga-NODAGA-JR11, 68Ga-DOTA-JR11, 68Ga-NODAGA-LM3 and 68Ga-DOTA-LM3.
In addition to NENs, SSTR imaging has also shown value in other tumors such as meningiomas (41-45) and multiple myeloma (46). A systematic review (47) found that 68Ga-DOTA-SSTR PET studies may revolutionize the routine neuro-oncology practice, especially in meningiomas, by improving diagnostic accuracy, delineating radiotherapy targets, and evaluating patient eligibility for radionuclide therapies.
SSTR-targeted radioligand therapy
The NCCN guidelines (48) and the European Neuroendocrine Tumor Society (ENETS) consensus (49,50) recommend that PRRT is the first choice of treatment for patients with unresectable or metastatic NENs. In a NETTER-1 trial (51), the researchers found that the median OS with 177Lu-DOATATE plus long-acting octreotide is 11.7 months longer than with high-dose long-acting octreotide alone in patients with midgut NETs. Despite final OS not reaching statistical significance, the results might be considered clinically relevant. The current clinical protocol for PRRT is to use 177Lu or 90Y-labeled SSTR agonists (such as 90Y-DOTATOC and 177Lu-DOTATATE), which can exert a tumor inhibitory effect, alleviate patients’ clinical symptoms, and prolong PFS (52). The combined application of 90Y and 177Lu nuclides can exert their complementary effects and improve the therapeutic effect on tumors of different sizes. NENs patients who used the combination of 90Y-DOTATOC and 177Lu-DOTATATE had a higher objective response rate, and the incidence of adverse reactions of both regimens was low (53).
PRRT with 177Lu-DOTATATE is generally administered via intravenous injection for systemic radionuclide therapy (SRT). In SRT, a considerable amount of the radioactive dose is dissipated within the systemic circulation due to intravenous administration of radionuclides for therapy and therefore only part of the radiopharmaceuticals reaches the target sites. Some dosimetry studies (52,54) have also demonstrated the toxicity to bone marrows and kidneys from 177Lu-DOTATATE SRT treatment due to systemic and off-target exposure. Intra-arterial radionuclide therapy (IART) allows direct delivery of 177Lu-DOTATATE to the tumor sites within the liver via intra-arterial cannulation of the hepatic artery. Some studies (55-57) about NENs demonstrated that IART led to higher tumor concentration and lower overall systemic toxicity (57) compared with SRT. In addition, in the treatment of meningiomas, some studies (58,59) have shown that IART can also increase the radioactive doses of tumor sites. As a result, IART seems to deliver high radioactive doses to tumor sites, therefore reducing overall systemic toxicity in comparison to SRT. However, large samples and prospective studies are still needed to verify that.
Radiopharmaceuticals targeting PD-1/PD-L1
In recent years, immune checkpoint inhibitor therapy has become an attractive research direction in tumor therapy. Among many new therapeutic targets, immune checkpoint inhibitors targeting PD-1 and PD-L1 have been widely used in clinical practice. However, not all tumor patients have good response to PD-1/PD-L1 targeted therapy because of the variable PD-1 or PD-L1 expression in tumors (60). Radiopharmaceutical imaging targeting PD-1/PD-L1 can screen potential beneficiaries in real-time, non-invasively, and dynamically during immunotherapy and can predict treatment effects, thereby guiding PD-1/PD-L1 targeted therapy and prognosis assessment.
Several radiopharmaceuticals targeting PD-1 or PD-L1 are currently investigated in clinical trials. The PD-1 imaging agents include 89Zr-pembrolizumab, 64Cu-pembrolizumab, and 89Zr-nivolumab. A study (61) indicated that 89Zr-nivolumab showed a significant tumor uptake rate in patients with PD-1-positive advanced non-small cell lung cancer. Tumor uptake of PD-L1 radiopharmaceuticals (89Zr-atezolizumab and 68Ga-BMS-986192) is superior to predictive biomarkers based on immunohistochemistry or RNA sequencing in assessing clinical response to immune checkpoint inhibitor therapy (62,63).
In addition, some novel radiopharmaceuticals targeting PD-1/PD-L1 are also being studied. 68Ga-NOTA-WL12 is a peptide-based PET imaging agent. The first-in-human findings (64) showed the safety and feasibility of 68Ga-NOTA-WL12 for noninvasive, in vivo detection of PD-L1 expression levels in non-small cell lung cancer patients, which indicates the potential benefits for clinical PD-L1 therapy. Additionally, 68Ga-THP-APN09 is a novel 68Ga-labeled nanobody. A study (65) found that this tracer can detect PD-L1 expression levels in tumors, which may make it possible to predict response to PD-1 immunotherapy combined with chemotherapy.
Radiopharmaceuticals targeting HER2
HER2 is overexpressed mainly in breast cancer but also expressed in gastrointestinal, ovarian, and bladder cancer. Currently, various types of radionuclide-labeled probes including monoclonal antibodies and their fragments, nanobodies, affibodies and peptides are used in HER2 imaging.
Radiolabeled trastuzumab and pertuzumab can highly accumulate in HER2+ tumor tissue, which is helpful for the detection and treatment of these tumors. In the first human study of 89Zr-pertuzumab, Ulaner et al. (66) indicated that the imaging of the HER2 tracer is significant for assessing HER2 heterogeneity and for targeting patients with metastases unsuitable for biopsy, because they found 20% of patients with HER2− primary tumors will develop HER2+ metastases finally. Tamura et al. (67) used 64Cu-DOTA-trastuzumab to image six HER2+ breast cancer patients and they found that the radiation exposure of 64Cu-DOTA-trastuzumab was 2.5 times lower than 89Zr-trastuzumab. In addition, 64Cu-DOTA-trastuzumab could be used to image other HER2+ malignancies, such as advanced HER2+ gastric cancer (67).
A novel affibody 68Ga-NOTA-MAL-MZHER2 (68Ga-HER2) was developed by the team of the Department of Nuclear Medicine of Peking University Cancer Hospital and the team of Min Yang of the Jiangsu Institute of Atomic Medicine. They found that it is a feasible method to noninvasively detect the HER2 status in advanced gastric cancer patients and enable early detection with a low dose. Ongoing anti-HER2 therapy did not influence 68Ga-HER2 affibody imaging, which allowed repeated evaluations to monitor the HER2 status after anti-HER2 therapy. This method provides an in vivo understanding of advanced gastric cancer biology, and it can help oncologists improve individualized therapy plans (68).
Radiopharmaceuticals targeting Nectin-4
Nectin-4 has been reported to be overexpressed in 69% of tumor specimens, including breast cancer, lung cancer, pancreatic cancer, esophageal cancer, ovarian cancer, etc. For urothelial carcinoma, more than 80% of tumor specimens express Nectin-4, and 60% show moderate-to-high staining (69).
Because the doses of antibody-drug conjugates (ADC) that can reach the targeted lesions are mainly related to the Nectin-4 expression level, it is necessary to monitor Nectin-4 expression before and during the ADC treatment (70).
Recently, the Peking University team developed a new radioactive PET tracer 68Ga-N188 and conducted the first human study of PET/CT imaging. The tracer has fast blood clearance rate, high tumor cell target affinity and specificity, and can provide high-contrast PET images to quantitatively evaluate the Nectin-4 expression levels of tissue and tumors. 68Ga-N188 PET imaging can be used to assess Nectin-4 expression in urothelial carcinoma, helping to select patients who are most likely to benefit from enfortumab vedotin therapy. The research results of this article provide strong support for the application of 68Ga-N188 PET that can be used as an auxiliary diagnostic tool for optimizing treatments targeting Nectin-4 (70).
Triple-negative breast cancer is a subtype of breast cancer that does not express all of progesterone receptor, estrogen receptor and HER-2, and it is related to an unfavorable prognosis. A study (71) confirmed the high expression of Nectin-4 in triple-negative breast cancer by SPECT/CT and explored the feasibility of photothermal therapy with Nectin-4 as the target.
Nuclear medicine equipment advances diagnosis and treatment of tumor
In this section, we will introduce the novel nuclear medicine equipment, namely total-body PET/CT and PET/MRI. They have various advantages compared to conventional PET/CT and play a significant role in the diagnosis and treatment of tumors.
Total-body PET/CT
The uExplorer, a long axial field-of-view (LAFOV) PET/CT instrument, has been used in clinical, and it has an axial field of view of 1.94 m (2). This allows the entire adult human body to be scanned in a single-bed position. LAFOV PET/CT scanners offer greater flexibility to optimize image acquisition in terms of image quality, radiation exposure of the patients, and acquisition time (72). These changes can reduce patient and staff doses (2), shorten acquisition time (3), optimize image quality, reduce radiopharmaceutical costs, and increase patient throughput (4). The reduction in the doses of radiopharmaceuticals can reduce the radiation exposure of the patients, which is especially meaningful for children. The short acquisition time is meaningful for claustrophobic patients, severely ill patients, and children, and it avoids the risks associated with the use of sedative medications such as chloral hydrate.
Using two or more molecular probes for imaging is one of the ways to improve the diagnostic performance of PET/CT. Affected by the limitations of conventional PET/CT, it is difficult to implement dual tracer imaging in clinical work. Total-body PET/CT has high sensitivity, so dual-tracer imaging is feasible when using total-body PET/CT. Liu et al. (73) investigated the feasibility of a one-stop FDG-FAPI dual-tracer imaging protocol with dual-low activity for oncological imaging. They reported that the one-stop dual-tracer dual-low-activity PET imaging protocol combines the advantages of 18F-FDG and 68Ga-DOTA-FAPI-04 with shorter duration and lesser radiation and is thus clinically applicable (73).
Additionally, total-body PET/CT is helpful in the development of new radiopharmaceuticals. It can observe the distribution and metabolism of radiopharmaceuticals in the body in real-time and dynamically, which is helpful for pharmacokinetic research. In addition, the sensitivity of total-body PET is high, so it can be detected even if the low dose of radiopharmaceuticals, allowing research on lower drug doses to ensure safety. A study (74) used an ultra-low dose (0.37 MBq/kg) of 18F-FDG to perform total-body dynamic imaging, and found the pharmacokinetic results were consistent with conventional doses (3.7 MBq/kg). In addition, using 1/10 of the dose, the radiation dose received by the patient was greatly reduced compared to the conventional dose group (median: 0.419 vs. 4.886 mSv; P<0.001) (74). What’s more, due to the high sensitivity of total-body PET/CT, it can detect signals generated by the decay of radionuclides with long half-lives. Imaging can be possible over many half-lives without impairment in imaging quality (75).
PET/MRI
PET/MRI, as a new and promising multi-modal molecular imaging technology, began to be used in clinical since 2010. PET/MRI has great application in tumors, the cardiovascular system and the nervous system. This article mainly introduces its application in tumors.
PET/MRI can better display the morphology of lesions and their relationship with adjacent tissues, especially small lesions that cannot be displayed on PET/CT, owing to the good soft tissue resolution. In 2023, an international consensus recommendation for PET/MRI in oncology (76) summarized the application of PET/MR in many kinds of tumors. It demonstrates the significant value of PET/MRI in diagnosis, staging, restaging, biochemical recurrence, prediction and evaluation of therapy effect, prognosis, and evaluation of tumor aggressiveness. PET/MRI is superior to PET/CT in some aspects such as neuro-oncology and pleural invasion detection.
In addition, the combination of whole-body PET/CT imaging and local PET/MRI imaging can make up for the shortcomings of long PET/MRI examination time to a certain extent, and at the same time can give full play to the respective advantages of PET/CT and PET/MRI.
Conclusions
Nowadays nuclear medicine has shown significant value in the diagnosis and treatment of tumors, and radiopharmaceuticals are the cornerstone of the development of it. Both diagnostic and therapeutic radiopharmaceuticals are being developed. Therapeutic radiopharmaceuticals provide a new treatment method for tumor patients and have shown value in clinical practice. Radiopharmaceuticals targeting different targets promote precise diagnosis and treatment of tumors. New nuclear medicine equipment has also furtherly promoted the diagnosis and treatment of tumors and the research and development of new radiopharmaceuticals. It is believed that inspired by the novel radiopharmaceuticals, the rush hour of nuclear medicine is coming.
Acknowledgements
None.
Contributor Information
Hua Zhu, Email: zhuhuaBCH@pku.edu.cn.
Nan Li, Email: rainbow6283@sina.com.
References
- 1.Ren Y, Liu C, Liu T, et al Preclinical evaluation and first in human study of Al18F radiolabeled ODAP-urea-based PSMA targeting ligand for PET imaging of prostate cancer. Front Oncol. 2022;12:1030187. doi: 10.3389/fonc.2022.1030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawi RD, Shi H, Hu P, et al First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60:299–303. doi: 10.2967/jnumed.119.226498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberts I, Hünermund JN, Prenosil G, et al Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur J Nucl Med Mol Imaging. 2021;48:2395–404. doi: 10.1007/s00259-021-05282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slart RHJA, Tsoumpas C, Glaudemans AWJM, et al Long axial field of view PET scanners: a road map to implementation and new possibilities. Eur J Nucl Med Mol Imaging. 2021;48:4236–45. doi: 10.1007/s00259-021-05461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He K, Zeng S, Qian L Recent progress in the molecular imaging of therapeutic monoclonal antibodies. J Pharm Anal. 2020;10:397–413. doi: 10.1016/j.jpha.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau J, Jacobson O, Niu G, et al Bench to bedside: albumin binders for improved cancer radioligand therapies. Bioconjug Chem. 2019;30:487–502. doi: 10.1021/acs.bioconjchem.8b00919. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Shao S, Wu P, et al Diagnostic performance of 68Ga-PSMA PET/CT in the detection of prostate cancer prior to initial biopsy: comparison with cancer-predicting nomograms. Eur J Nucl Med Mol Imaging. 2019;46:908–20. doi: 10.1007/s00259-018-4255-1. [DOI] [PubMed] [Google Scholar]
- 8.Sartor O, de Bono J, Chi KN, et al Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Liu T, Zhang N, et al 68Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2018;45:1852–61. doi: 10.1007/s00259-018-4037-9. [DOI] [PubMed] [Google Scholar]
- 10.Kawada T, Yanagisawa T, Rajwa P, et al Diagnostic performance of prostate-specific membrane antigen positron emission tomography-targeted biopsy for detection of clinically significant prostate cancer: A systematic review and meta-analysis. Eur Urol Oncol. 2022;5:390–400. doi: 10.1016/j.euo.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Qiu DX, Li J, Zhang JW, et al Dual-tracer PET/CT-targeted, mpMRI-targeted, systematic biopsy, and combined biopsy for the diagnosis of prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2022;49:2821–32. doi: 10.1007/s00259-021-05636-1. [DOI] [PubMed] [Google Scholar]
- 12.Hofman MS, Lawrentschuk N, Francis RJ, et al Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. doi: 10.1016/s0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 13.Kane CJ, Amling CL, Johnstone PA, et al Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–11. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 14.Perera M, Papa N, Roberts M, et al Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77:403–17. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt LH, Heitkötter B, Schulze AB, et al Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PloS One. 2017;12:e0186280. doi: 10.1371/journal.pone.0186280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jochumsen MR, Gormsen LC, Nielsen GL 68Ga-PSMA avid primary adenocarcinoma of the lung with complementary low 18F-FDG uptake. Clin Nucl Med. 2018;43:117–9. doi: 10.1097/rlu.0000000000001935. [DOI] [PubMed] [Google Scholar]
- 17.Calopedos RJS, Chalasani V, Asher R, et al Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:352–60. doi: 10.1038/pcan.2017.23. [DOI] [PubMed] [Google Scholar]
- 18.Violet J, Sandhu S, Iravani A, et al Long-term follow-up and outcomes of retreatment in an expanded 50-patient single-center phase II prospective trial of 177Lu-PSMA-617 theranostics in metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:857–65. doi: 10.2967/jnumed.119.236414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofman MS, Emmett L, Sandhu S, et al [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/s0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadzadehfar H, Wegen S, Yordanova A, et al Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 21.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 22.Hofman MS, Violet J, Hicks RJ, et al [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. doi: 10.1016/s1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 23.Yadav MP, Ballal S, Bal C, et al Efficacy and safety of 177Lu-PSMA-617 radioligand therapy in metastatic castration-resistant prostate cancer patients. Clin Nucl Med. 2020;45:19–31. doi: 10.1097/rlu.0000000000002833. [DOI] [PubMed] [Google Scholar]
- 24.Bu T, Zhang L, Yu F, et al 177Lu-PSMA-I&T radioligand therapy for treating metastatic castration-resistant prostate cancer: a single-centre study in East Asians. Front Oncol. 2022;12:835956. doi: 10.3389/fonc.2022.835956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kratochwil C, Bruchertseifer F, Giesel FL, et al 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–4. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]
- 26.Sathekge MM, Bruchertseifer F, Lawal IO, et al Treatment of brain metastases of castration-resistant prostate cancer with 225Ac-PSMA-617. Eur J Nucl Med Mol Imaging. 2019;46:1756–7. doi: 10.1007/s00259-019-04354-z. [DOI] [PubMed] [Google Scholar]
- 27.Feuerecker B, Tauber R, Knorr K, et al Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79:343–50. doi: 10.1016/j.eururo.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Khreish F, Ebert N, Ries M, et al 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8. doi: 10.1007/s00259-019-04612-0. [DOI] [PubMed] [Google Scholar]
- 29.Hope TA, Bergsland EK, Bozkurt MF, et al Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74. doi: 10.2967/jnumed.117.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haidar M, Shamseddine A, Panagiotidis E, et al The role of 68Ga-DOTA-NOC PET/CT in evaluating neuroendocrine tumors: real-world experience from two large neuroendocrine tumor centers. Nucl Med Commun. 2017;38:170–7. doi: 10.1097/mnm.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 31.Frilling A, Sotiropoulos GC, Radtke A, et al The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850–6. doi: 10.1097/SLA.0b013e3181fd37e8. [DOI] [PubMed] [Google Scholar]
- 32.Ezziddin S, Attassi M, Yong-Hing CJ, et al Predictors of long-term outcome in patients with well-differentiated gastroenteropancreatic neuroendocrine tumors after peptide receptor radionuclide therapy with 177Lu-octreotate. J Nucl Med. 2014;55:183–90. doi: 10.2967/jnumed.113.125336. [DOI] [PubMed] [Google Scholar]
- 33.Calais J, Czernin J, Eiber M, et al Most of the intended management changes after 68Ga-DOTATATE PET/CT are implemented. J Nucl Med. 2017;58:1793–6. doi: 10.2967/jnumed.117.192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrio M, Czernin J, Fanti S, et al The impact of somatostatin receptor-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. 2017;58:756–61. doi: 10.2967/jnumed.116.185587. [DOI] [PubMed] [Google Scholar]
- 35.Haug AR, Auernhammer CJ, Wängler B, et al 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J Nucl Med. 2010;51:1349–56. doi: 10.2967/jnumed.110.075002. [DOI] [PubMed] [Google Scholar]
- 36.Nicolas GP, Schreiter N, Kaul F, et al Sensitivity comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase II imaging study. J Nucl Med. 2018;59:915–21. doi: 10.2967/jnumed.117.199760. [DOI] [PubMed] [Google Scholar]
- 37.Zhu W, Cheng Y, Wang X, et al Head-to-head comparison of 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT in patients with metastatic, well-differentiated neuroendocrine tumors: a prospective study. J Nucl Med. 2020;61:897–903. doi: 10.2967/jnumed.119.235093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolas GP, Beykan S, Bouterfa H, et al Safety, biodistribution, and radiation dosimetry of 68Ga-OPS202 in patients with gastroenteropancreatic neuroendocrine tumors: a prospective phase I imaging study. J Nucl Med. 2018;59:909–14. doi: 10.2967/jnumed.117.199737. [DOI] [PubMed] [Google Scholar]
- 39.Ginj M, Zhang H, Waser B, et al Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Nati Acad Sci USA. 2006;103:16436–41. doi: 10.1073/pnas.0607761103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs S, Pandit-Taskar N, Reidy D, et al Biodistribution and radiation dose estimates for 68Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2019;46:677–85. doi: 10.1007/s00259-018-4193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelak MJ, Flechl B, Mumot M, et al The value of SSTR2 receptor-targeted PET/CT in proton irradiation of grade I mningioma. Cancers (Basel) 2021;13:4707. doi: 10.3390/cancers13184707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashir A, Vestergaard MB, Binderup T, et al Pharmacokinetic analysis of [68Ga]Ga-DOTA-TOC PET in meningiomas for assessment of in vivo somatostatin receptor subtype 2. Eur J Nucl Med Mol Imaging. 2020;47:2577–88. doi: 10.1007/s00259-020-04759-1. [DOI] [PubMed] [Google Scholar]
- 43.Unterrainer M, Ilhan H, Vettermann F, et al Whole-body staging of metastatic atypical meningioma using 68Ga-DOTATATE PET/CT. Clin Nucl Med. 2019;44:227–8. doi: 10.1097/rlu.0000000000002422. [DOI] [PubMed] [Google Scholar]
- 44.Unterrainer M, Ruf V, Ilhan H, et al 68Ga-DOTATOC PET/CT differentiates meningioma from dural metastases. Clin Nucl Med. 2019;44:412–3. doi: 10.1097/rlu.0000000000002513. [DOI] [PubMed] [Google Scholar]
- 45.Dressen MS, Muthukrishnan A, Tragon TR, et al Complementary molecular and metabolic characterization of meningiomas with DOTATATE and FDG-PET: advancing treatment planning and prognostication. Clin Nucl Med. 2019;44:e26–7. doi: 10.1097/rlu.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 46.Sonmezoglu K, Vatankulu B, Elverdi T, et al The role of 68Ga-DOTA-TATE PET/CT scanning in the evaluation of patients with multiple myeloma: preliminary results. Nucl Med Commun. 2017;38:76–83. doi: 10.1097/mnm.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 47.Palmisciano P, Watanabe G, Conching A, et al The role of [68Ga]Ga-DOTA-SSTR PET radiotracers in brain tumors: a systematic review of the literature and ongoing clinical trials. Cancers (Basel) 2022;14:2925. doi: 10.3390/cancers14122925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2. 2018. J Natl Compr Canc Netw 2018;16:693-702.
- 49.Kwekkeboom DJ, Krenning EP, Lebtahi R, et al ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220–6. doi: 10.1159/000225951. [DOI] [PubMed] [Google Scholar]
- 50.Perren A, Couvelard A, Scoazec JY, et al ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology. 2017;105:196–200. doi: 10.1159/000457956. [DOI] [PubMed] [Google Scholar]
- 51.Strosberg JR, Caplin ME, Kunz PL, et al 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:1752–63. doi: 10.1016/s1470-2045(21)00572-6. [DOI] [PubMed] [Google Scholar]
- 52.Strosberg J, El-Haddad G, Wolin E, et al Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunikowska J, Zemczak A, Kołodziej M, et al Tandem peptide receptor radionuclide therapy using 90Y/177Lu-DOTATATE for neuroendocrine tumors efficacy and side-effects — polish multicenter experience. Eur J Nucl Med Mol Imaging. 2020;47:922–33. doi: 10.1007/s00259-020-04690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forrer F, Krenning EP, Kooij PP, et al Bone marrow dosimetry in peptide receptor radionuclide therapy with [177Lu-DOTA(0), Tyr(3)]octreotate. Eur J Nucl Med Mol Imaging. 2009;36:1138–46. doi: 10.1007/s00259-009-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nautiyal A, Jha AK, Konuparamban A, et al A dosimetric comparison of systemic peptide receptor radionuclide therapy and intra-arterial peptide receptor radionuclide therapy in patients with liver dominant gastroenteropancreatic neuroendocrine tumours. Nucl Med Commun. 2023;44:585–95. doi: 10.1097/mnm.0000000000001696. [DOI] [PubMed] [Google Scholar]
- 56.Kratochwil C, Giesel FL, López-Benítez R, et al Intraindividual comparison of selective arterial versus venous 68Ga-DOTATOC PET/CT in patients with gastroenteropancreatic neuroendocrine tumors. Clin Cancer Res. 2010;16:2899–905. doi: 10.1158/1078-0432.Ccr-10-0004. [DOI] [PubMed] [Google Scholar]
- 57.Pool SE, Kam BL, Koning GA, et al [111In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29:179–87. doi: 10.1089/cbr.2013.1552. [DOI] [PubMed] [Google Scholar]
- 58.Verburg FA, Wiessmann M, Neuloh G, et al Intraindividual comparison of selective intraarterial versus systemic intravenous 68Ga-DOTATATE PET/CT in patients with inoperable meningioma. Nuklearmedizin. 2019;58:23–7. doi: 10.1055/a-0802-5039. [DOI] [PubMed] [Google Scholar]
- 59.Vonken EPA, Bruijnen RCG, Snijders TJ, et al Intraarterial administration boosts 177Lu-HA-DOTATATE accumulation in salvage meningioma patients. J Nucl Med. 2022;63:406–9. doi: 10.2967/jnumed.121.262491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibney GT, Weiner LM, Atkins MB Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016;17:e542–51. doi: 10.1016/s1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.England CG, Jiang D, Ehlerding EB, et al 89Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur J Nucl Med Mol Imaging. 2018;45:110–20. doi: 10.1007/s00259-017-3803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulgaonkar A, Elias R, Woolford L, et al ImmunoPET imaging with 89Zr-labeled atezolizumab enables in vivo evaluation of PD-L1 in tumorgraft models of renal cell carcinoma. Clin Cancer Res. 2022;28:4907–16. doi: 10.1158/1078-0432.Ccr-22-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robu S, Richter A, Gosmann D, et al Synthesis and preclinical evaluation of a 68Ga-Labeled adnectin, 68Ga-BMS-986192, as a PET agent for imaging PD-L1 expression. J Nucl Med. 2021;62:1228–34. doi: 10.2967/jnumed.120.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou X, Jiang J, Yang X, et al First-in-humans evaluation of a PD-L1-binding peptide PET radiotracer in non-small cell lung cancer patients. J Nucl Med. 2022;63:536–42. doi: 10.2967/jnumed.121.262045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma X, Zhou X, Hu B, et al Preclinical evaluation and pilot clinical study of [68Ga]Ga-THP-APN09, a novel PD-L1 targeted nanobody radiotracer for rapid one-step radiolabeling and PET imaging. Eur J Nucl Med Mol Imaging. 2023;50:3838–50. doi: 10.1007/s00259-023-06373-3. [DOI] [PubMed] [Google Scholar]
- 66.Ulaner GA, Lyashchenko SK, Riedl C, et al First-in-human human epidermal growth factor receptor 2-targeted imaging using 89Zr-pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med. 2018;59:900–6. doi: 10.2967/jnumed.117.202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamura K, Kurihara H, Yonemori K, et al 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54:1869–75. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 68.Zhou N, Liu C, Guo X, et al Impact of 68Ga-NOTA-MAL-MZHER2 PET imaging in advanced gastric cancer patients and therapeutic response monitoring. Eur J Nucl Med Mol Imaging. 2021;48:161–75. doi: 10.1007/s00259-020-04898-5. [DOI] [PubMed] [Google Scholar]
- 69.Challita-Eid PM, Satpayev D, Yang P, et al Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–13. doi: 10.1158/0008-5472.Can-15-1313. [DOI] [PubMed] [Google Scholar]
- 70.Duan X, Xia L, Zhang Z, et al First-in-human study of the radioligand 68Ga-N188 targeting nectin-4 for PET/CT imaging of advanced urothelial carcinoma. Clin Cancer Res. 2023;29:3395–407. doi: 10.1158/1078-0432.Ccr-23-0609. [DOI] [PubMed] [Google Scholar]
- 71.Shao F, Pan Z, Long Y, et al Nectin-4-targeted immunoSPECT/CT imaging and photothermal therapy of triple-negative breast cancer. J Nanobiotechnology. 2022;20:243. doi: 10.1186/s12951-022-01444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cherry SR, Jones T, Karp JS, et al Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59:3–12. doi: 10.2967/jnumed.116.184028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu G, Mao W, Yu H, et al One-stop [18F]FDG and [68Ga]Ga-DOTA-FAPI-04 total-body PET/CT examination with dual-low activity: a feasibility study. Eur J Nucl Med Mol Imaging. 2023;50:2271–81. doi: 10.1007/s00259-023-06207-2. [DOI] [PubMed] [Google Scholar]
- 74.Liu G, Hu P, Yu H, et al Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of 18F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48:2373–83. doi: 10.1007/s00259-020-05173-3. [DOI] [PubMed] [Google Scholar]
- 75.Alberts I, Prenosil G, Mingels C, et al Feasibility of late acquisition [68Ga]Ga-PSMA-11 PET/CT using a long axial field-of-view PET/CT scanner for the diagnosis of recurrent prostate cancer-first clinical experiences. Eur J Nucl Med Mol Imaging. 2021;48:4456–62. doi: 10.1007/s00259-021-05438-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veit-Haibach P, Ahlström H, Boellaard R, et al International EANM-SNMMI-ISMRM consensus recommendation for PET/MRI in oncology. Eur J Nucl Med Mol Imaging. 2023;50:3513–37. doi: 10.1007/s00259-023-06406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]