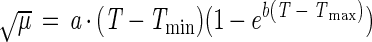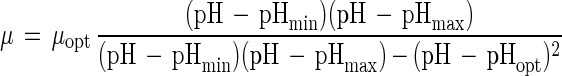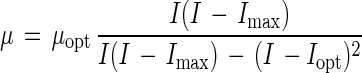Abstract
We investigated the effect of the ecological factors pH, temperature, ionic strength, and lactate, acetate, and ethanol levels on Candida milleri and two strains of Lactobacillus sanfranciscensis, organisms representative of the microflora of sourdough. A mathematical model describing the single and combined effects of these factors on the growth of these organisms was established in accordance with the following criteria: quality of fit, biological significance of the parameters, and applicability of the in vitro data to in situ processes. The growth rates of L. sanfranciscensis LTH1729 and LTH2581 were virtually identical under all conditions tested. These organisms tolerated >160 mmol of undissociated acetic acid per liter. Growth occurred in the pH range of 3.9 to 6.7 and was completely inhibited by 4% NaCl. C. milleri had a lower optimum temperature for growth (27°C) than the lactobacilli. The growth of the yeast was not affected by pH in the range of 3.5 to 7, and up to 8% NaCl was tolerated. Complete inhibition of growth occurred at 150 mmol of undissociated acetic acid per liter, but acetate at concentrations of up to 250 mmol/liter exerted virtually no effect. The model provides insight into factors contributing to the stability of the sourdough microflora and can facilitate the design of novel sourdough processes.
Sourdough fermentation is a process to obtain bread from wheat or rye flours by the combined metabolic activity of lactic acid bacteria and yeasts. Due to the superior sensory quality and the prolonged shelf life of the resulting baked goods, sourdough processes have retained their importance in modern baking technology (11, 20, 34). These processes are employed in the production of more than 20% of the bread produced in Central Europe, France, and Italy and are essential for a wide variety of specialty products.
Sourdough fermentations are characterized by a stable association of yeasts and lactobacilli. In sourdoughs with a tradition of continuous propagation, Lactobacillus sanfranciscensis (37) (“Lactobacillus brevis subsp. lindneri” [30]), Candida milleri, and Saccharomyces exiguus (anamorphic form: Torulopsis holmii) are the predominant microorganisms (1, 26, 35). The process conditions of traditional fermentations ensure a high level of metabolic activity for these organisms and permit the production of breads with sensory qualities superior to those prepared from pure cultures of lactobacilli or yeasts (33). The raw materials of bread are essentially flavorless. Therefore, the formation of flavor compounds relies on endogenous cereal enzymes, microbial metabolism, and the baking process. The significant contribution of lactic acid bacteria as well as yeasts to the formation of aroma volatiles or precursors available for thermal transformation to aroma compounds is well established (6, 13, 28).
The adaptation of artisanal processes to new products and technologies requires profound knowledge of the factors determining microbial metabolism and the stability of the microflora. In order to achieve a balanced metabolic activity of lactobacilli and yeasts in sourdough, interactions between these organisms have to be taken into account. Recent research has focused mainly on the metabolism of carbohydrates and amino acids (3, 10, 31). Little information is available on the response of the sourdough microflora to the ecological factors temperature, pH, ionic strength, and accumulation of metabolic end products. A useful tool to assess the effects of environmental factors on the growth of microorganisms is the development of mathematical models that meet the criteria proposed by Rosso et al. (24): simplicity of the model, biological significance of the parameters, applicability, and quality of fit. Models based on in vitro experiments permit the evaluation of the single effect of an environmental factor independent of the choice of raw materials, whereas during in situ fermentations, usually only the sum of several factors can be evaluated. Wijtzes et al. (38), Rosso et al. (24), and Cuppers et al. (5) have validated models describing the combined effects of temperature and pH, as well as temperature and NaCl concentration, on microbial growth. It was the aim of our study to expand the scope of the proposed models to more than two factors, taking into account the combined effects of pH, temperature, salt concentration, and accumulation of metabolic end products, and to use the model to identify the most important factors contributing to the stable association of lactobacilli and yeasts.
MATERIALS AND METHODS
Organisms and media.
The strains used in this study are L. sanfranciscensis LTH2581 and LTH1729 and C. milleri LTH H198. These organisms were isolated by Böcker et al. (2) from a commercial mother sponge. L. sanfranciscensis LTH2581 and LTH1729 were differentiated by their carbohydrate fermentation pattern, plasmid content, and colony morphology on mMRS agar (2). The strains have been maintained in this sourdough starter for at least 20 years, accounting for more than 90% of the microflora of that product. The lactobacilli were grown in modified MRS medium (mMRS) containing the following per liter: 10 g of tryptone, 5 g of meat extract, 5 g of yeast extract, 2.5 g of maltose, 1.25 g of fructose, 8 g of KH2PO4, 3 g of diammonium citrate, 3 g of NH4Cl, 0.5 g of cysteine HCl, 1 g of Tween 80, 6 g of dl-lactic acid (90%), 4.9 g of sodium acetate · 3H2O, 0.2 mg of MgSO4 · 7H2O, 0.05 g of MnSO4 · H2O, 0.5 μg each of cobalamin, folic acid, niacin, pantothenic acid, pyridoxal, and thiamin. The pH was 5.44 after autoclaving. Sugars were autoclaved separately, and vitamins were sterilized by filtration. For cultivation of yeast, maltose and fructose were replaced by 2.5 g of glucose. To determine the effect of pH, NaCl, ethanol, lactate, and acetate, the medium composition was changed as follows.
(i) pH.
The pH of mMRS media was adjusted to values ranging from 3.5 to 7 with 4 N NaOH or HCl.
(ii) NaCl.
mMRS media containing 3.6, 4.8, 7.2, 9.6, 10.8, or 16.2% NaCl (wt/vol) were diluted with mMRS medium (0% NaCl) to obtain the desired NaCl concentrations in the ranges of 0 to 4% (for lactobacilli) and 0 to 8% (for yeasts).
(iii) Ethanol.
mMRS media were adjusted to a concentration of 6, 8, or 18% ethanol (vol/vol) and mixed with mMRS medium to obtain ethanol concentrations ranging from 0 to 8%. Media containing ethanol were prepared immediately prior to inoculation to minimize evaporation losses.
(iv) Lactate and acetate.
mMRS media containing 360, 240, or 0 mmol of acetate or lactate per liter were mixed with mMRS without acetate or lactate to obtain the desired concentration of acetate and lactate in the range of 0 to 240 mmol/liter. The pH of these media was 4.45.
(v) Combination of the effects of pH, acetate, NaCl, and lactate.
The pH of mMRS media (0 mmol of acetate and lactate per liter) containing 12 g of K2HPO4 · 3H2O was adjusted to 6.5, 6.0, 5.5, 5.0, 4.5, 4.0, and 3.5 with 0, 22, 49, 78, 91, 140, and 230 mmol of lactic acid/liter, respectively. For each pH level, media were prepared containing 0, 4, and 8% NaCl (wt/vol). Salt containing mMRS was diluted with mMRS (0% NaCl) to give NaCl concentrations of 0, 1.8, 2.7, and 4% for lactobacilli and 0, 3.6, 5.3, and 8% for yeasts. The acetate concentration was adjusted to 0, 30, 60, 90, 120, or 150 mmol of acetate/liter. Blanks were prepared for each combination of parameters (144 for both lactobacilli and yeasts), and the pH was measured.
Determination of the growth rates.
Standardized inocula were prepared by growing overnight cultures in mMRS broth to exponential growth phase (optical density at 595 nm [OD595], 0.2 to 0.4). The growth media were inoculated to an OD595 of ca. 0.001 and incubated at 30°C in microtiter plates. The total volume was 150 μl, and the media were overlaid with 100 μl of paraffin to achieve anoxic growth conditions and to avoid evaporation losses of water, ethanol, and acetic acid. The growth of the organisms was monitored by measuring the OD of the growth media with a Bioscreen C microbiological analyzer (Labsystems, Frankfurt, Germany) for automated measuring or a microtiter plate reader, model 450 (Bio-Rad, Munich, Germany). The effects of temperature and pH were studied by using 15-ml reagent tubes incubated in a water bath at the desired temperature ± 0.5°C. For OD measurements, the tubes were vortexed and 150-μl samples were transferred to microtiter plates. The ODs of the cultures were correlated to cell counts by using the same shaking regimen as that applied in the measurements. The OD measurements on microtiter plates were correlated to the cell counts of C. milleri LTH H198 and L. sanfranciscensis LTH2581 and LTH1729 with correlation coefficients, r2, of 0.972, 0.977, and 0.950, respectively. The threshold sensitivity of the OD measurements was about 106 cells/ml for both yeast and lactobacilli. Growth below this threshold level was not recorded or accounted for as lag-phase growth. Thus, OD measurements are not the most suitable method to assess the growth of food pathogens or spoilage organisms but are adequate for modeling the growth of food-fermenting organisms, where the emphasis is exclusively on growth conditions that allow growth to a high population density. From each growth curve, the maximum growth rate, μmax, the lag phase, λ, and the asymptote, A, were obtained by fitting the OD readings to the logistic growth curve (41). SigmaPlot 1.02 software was used for all curve fit routines.
Model development.
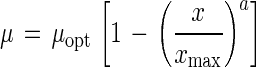
The model equations used to describe the effects of temperature, pH, NaCl, ethanol, lactate, and acetate on the growth of C. milleri and L. sanfranciscensis are shown in Table 1. All functions were of the form μ(x) = μopt γ(x). The parameter μopt equals μmax at the growth conditions with the factor x at its optimum value. The function γ(x) describes the response of the growth rate to changes in the factor x, with values ranging from 0 (no growth) to 1 (optimum growth). The models are valid only in the range of xmin ≤ x ≤ xmax. This scheme allows the description of the combined effects of the factors x1, x2, …, xn by models of the type μ(x1, x2, …, xn) = μopt · γ(x1) · γ(x2) · … · γ(xn).
TABLE 1.
Model development to describe the effect of temperature, pH, ionic strength, ethanol, acetate, and lactic and acetic acids on microbial growth
| Factor described | Equation(s) used | Model no. (reference) | |
|---|---|---|---|
| Temperature | μ = a · xb · e−c · x | 1a (this study) | |
| x = Tmax − T | |||
|
1b (40) | ||
| pH |
|
2 (24) | |
| Ionic strength |
|
3 (this study) | |
| Ionic strength and ethanol, acetic acid, acetate, and lactic acid concna |
|
4 (21) | |
In the equation, x is ionic strength or the concentration of ethanol (in percent) or acetic acid, acetate, or lactic acid (in moles per liter).
RESULTS
Effect of temperature on growth.
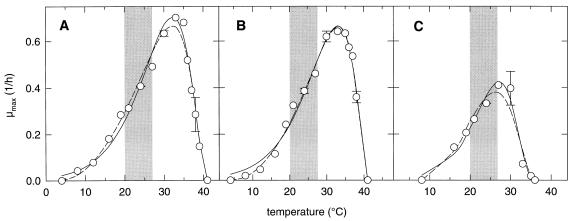
The dough temperature is affected by the temperatures of the raw materials, water and flour, and the incubation temperature. In practice, these are not strictly controlled but are subject to changes on a daily and seasonal basis. The effect of temperature on the growth rates of L. sanfranciscensis LTH2581 and LTH1729 and C. milleri LTH H198 is shown in Fig. 1, and the parameter estimates for models 1a and 1b fitted to the experimental data are compiled in Table 2. From parameters a, b, and c of model 1a, the optimum temperature for growth (Topt), μopt, and a′ were calculated as (Tmax − b/c), μ(Topt), and a/μopt, respectively (see Appendix), where Tmax is as defined below. L. sanfranciscensis LTH1729 and LTH2581 have Topt values of 33 and 32°C, respectively, whereas C. milleri grows fastest at 27°C. With model 1a, the quality of fit was not improved if Tmax was incorporated as a regression parameter; Tmax was therefore defined as the lowest temperature above Topt at which no visible growth occurred after 7 days of incubation. Tmax was 41°C for both strains of L. sanfranciscensis and 36°C for C. milleri. These values do not differ appreciably from those obtained with model 1b. Model 1a gave the better curve fit results for all strains except LTH1729. Compared with model 1b, model 1a gave a poor estimate around the minimum growth-permitting temperature (Tmin), whereas the prediction of growth rates around Topt is more accurate. The growth rates of L. sanfranciscensis LTH1729 and LTH2581 are almost identical over the entire temperature range, with a higher μopt and a slightly lower Topt determined for strain LTH2581. In comparison to the lactobacilli, C. milleri has a lower Topt and Tmax. Sourdough fermentations are commonly performed at temperatures between 20 and 28°C. Remarkably, L. sanfranciscensis and C. milleri exhibit the same response to changes at temperatures of <26°C.
FIG. 1.
Effect of temperature on the μmax values for L. sanfranciscensis LTH2581 (A) and LTH1729 (B) and C. milleri LTH H198 (C). The solid and dashed lines represent growth rates predicted with models 1a and 1b, respectively. Error bars indicate the standard deviations from the means of two independent experiments. The shaded area represents the range commonly encountered during sourdough fermentations.
TABLE 2.
Parameter values and correlation coefficients for effect of temperature on growth
| Strain | Model 1a
|
Model 1b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter estimates
|
r2 | Parameter estimates
|
r2 | |||||||
| μopt | a′ | b | c | Tmax | Tmin | a | b | |||
| LTH2581 | 0.71 | 0.097 ± 0.01 | 2.0 ± 0.1 | 0.22 ± 0.02 | 0.966 | 41.0 ± 0.1 | 3.0 ± 0.6 | 0.22 ± 0.02 | 0.032 ± 0.001 | 0.963 |
| LTH H198 | 0.42 | 0.029 ± 0.007 | 3.0 ± 0.4 | 0.34 ± 0.04 | 0.967 | 35.9 ± 0.3 | 8.0 ± 1 | 0.12 ± 0.04 | 0.05 ± 0.01 | 0.949 |
| LTH1729 | 0.67 | 0.19 ± 0.02 | 1.5 ± 0.1 | 0.19 ± 0.01 | 0.987 | 41.0 ± 0.07 | 4.1 ± 0.5 | 0.29 ± 0.02 | 0.031 ± 0.001 | 0.991 |
Effect of pH on growth.
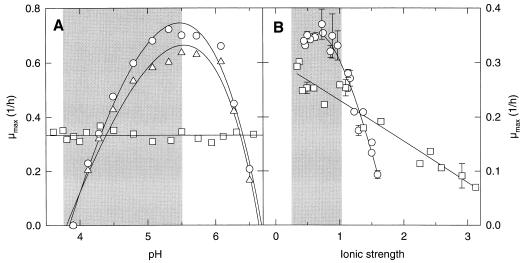
The initial pH of the sourdough fermentation ranges from 4.5 to 5.5, depending on the size of the inoculum. Sourdoughs are acidified to a pH of 3.5 to 3.7. The effect of pH on the growth rates of L. sanfranciscensis LTH2581 and LTH1729 and C. milleri LTH H198 is shown in Fig. 2A; the parameter estimates for model 2 are given in Table 3. Growth of C. milleri is not affected by the pH in the range tested, i.e., 3.5 to 7. The model describing growth therefore simplifies to μ = μopt. Remarkably, the same values of pHmin, pHmax, and pHopt (the minimum, maximum, and optimum pH values for growth, respectively) and thus the same γ(pH) were obtained for the two strains of L. sanfranciscensis.
FIG. 2.
Effect of pH on μmax values for L. sanfranciscensis LTH2581 (○) and LTH1729 (▵) and C. milleri LTH H198 (□) (A) and effect of NaCl addition expressed as ionic strength on the growth of L. sanfranciscensis LTH2581 and C. milleri LTH H198 (B). The lines represent the predicted growth rates. Error bars indicate the standard deviations from the means of three independent experiments. The shaded areas represent the ranges commonly encountered during sourdough fermentations.
TABLE 3.
Parameters and correlation coefficients for growth effects of single factors
| Factor and model | Strain | Parameter estimatesa
|
r2 | |||
|---|---|---|---|---|---|---|
| μopt | pHmin | pHopt | pHmax | |||
| pH | ||||||
| 2 | LTH2581 | 0.72 ± 0.02 | 3.94 ± 0.05 | 5.47 ± 0.05 | 6.67 ± 0.05 | 0.979 |
| 2 | LTH H198 | 0.33 ± 0.02 | n.v. | n.v. | n.v. | 0 |
| 2 | LTH1729 | 0.66 ± 0.02 | 3.90 ± 0.06 | 5.53 ± 0.06 | 6.64 ± 0.05 | 0.966 |
| Ionic strength | ||||||
| 3 | LTH2581 | 0.356 ± 0.006 | 0.960 | |||
| 4 | LTH H198 | 0.30 ± 0.03 | 0.935 | |||
| Ethanol concn | ||||||
| 4 | LTH2581 | 0.36 ± 0.01 | 0.942 | |||
| 4 | LTH H198 | 0.29 ± 0.3 | 0.843 | |||
| Acetate concn | ||||||
| 4 | LTH2581 | 0.24 ± 0.01 | 0.813 | |||
| 4 | LTH H198 | 0.29 ± 0.01 | 0.960 | |||
| Lactate concn | ||||||
| 4 | LTH2581 | 0.22 ± 0.01 | 0.839 | |||
| 4 | LTH H198 | 0.32 ± 0.01 | 0.682 | |||
| Imax | Iopt |
| 1.80 ± 0.04 | 0.64 ± 0.03 |
| xmax | a |
| 3.9 ± 0.4 | 1.0 ± 0.3 |
| 8.12 ± 0.05 | 9.5 ± 1.5 |
| 8.2 ± 0.9 | 1.0 ± 0.3 |
| 0.28 ± 0.02 | 3.2 ± 0.8 |
| 0.166 ± 0.006 | 1.9 ± 0.3 |
| 0.28 ± 0.02 | 2.2 ± 0.5 |
| 0.36 ± 0.06 | 2.9 ± 0.9 |
Parameter estimates are shown in boldface if the values of xmax calculated were outside the factor range applied in the assays. n.v., not valid.
Effect of ionic strength on growth.
The ionic strength of sourdough is affected by the ash content of the flour, the dough yield (grams of dough/100 g of flour), and the formula. The effect of ionic strength on growth was studied by adjusting ionic strength by the addition of NaCl. The results are shown in Fig. 2B; the models used and the parameter estimates are shown in Table 3. Model 3 was used to describe the effect of ionic strength on the growth of L. sanfranciscensis. It is based on model 2 with Imin = 0, where Imin is the minimum ionic strength for growth. As a stimulatory effect of the ionic strength on the growth of C. milleri was not observed (i.e., Iopt [optimum ionic strength for growth] and Imin are outside the range of investigation), the use of model 4 was considered to be more appropriate. The yeast grows in up to 8% NaCl (I = 3.2), whereas the growth of lactobacilli is inhibited by 4% NaCl (I = 1.9). An increasing ionic strength will therefore inhibit the growth of lactobacilli to a much greater degree than it inhibits the growth of yeasts.
Effects of ethanol, lactate, and acetate.
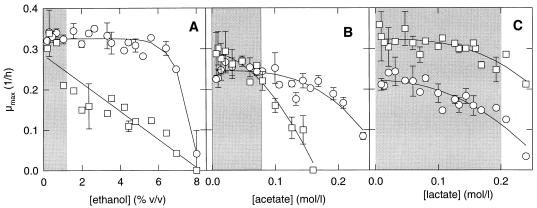
Lactate, acetate, ethanol, and CO2 are the major metabolic end products of cofermentations with L. sanfranciscensis and C. milleri. Their accumulation in the dough may lead to an inhibition of growth of the microflora. The production of lactate and acetate is influenced by appropriate technological measures, e.g., buffering capacity of the dough, aeration, and the addition of citrate or fructose (12). The effects of ethanol, acetate, and lactate on microbial growth are shown in Fig. 3; the regression parameters are presented in Table 3. Differences in the μopt obtained with model 4 for the effects of lactate and acetate reflect experimental error. The values of maximum lactate concentration for lactic acid bacteria and yeasts as well as those of maximum acetate concentration for lactobacilli are not supported by experimental data. The maximum ethanol concentration is approximately the same for both organisms. For the yeasts a linear decrease of the growth rate is evident with increasing ethanol concentration, whereas the growth of L. sanfranciscensis LTH2581 is affected by ethanol only at concentrations of >5%. A more pronounced inhibitory effect of acetate on the growth of yeasts than on the growth of lactobacilli is evident. The growth of C. milleri was completely inhibited by 166 mmol of acetate per liter, corresponding to 110 mmol of undissociated acid per liter, while L. sanfranciscensis LTH2581 tolerated more than 240 mmol of acetate per liter. The experimental design does not allow differentiation between the inhibitory effects of acetate and undissociated acid. As opposed to acetate, lactate inhibited the growth of lactobacilli and yeasts to the same degree. As pointed out above for acetate, the inhibitory effects of lactate and lactic acid could not be distinguished.
FIG. 3.
Effect of ethanol (A), acetate (B), and lactate (C) on μmax of L. sanfranciscensis LTH2581 (○) and C. milleri LTH H198 (□). The lines represent the growth rates predicted with model 4. Error bars indicate the standard deviations from the means of three independent experiments. The shaded areas represent the ranges commonly encountered during sourdough fermentations.
Combined effects of pH, NaCl, lactic acid, acetic acid, and acetate on growth.
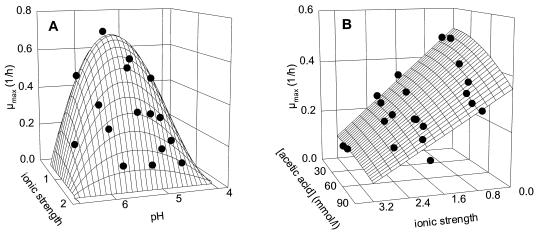
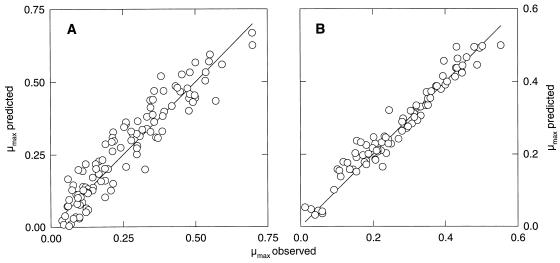
It was the intent of the study to verify that a model of the type μ(x1, x2, … , xn) = μopt · γ(x1) · γ(x2) · … · γ(xn) gives an accurate prediction of the combined effects of the factors of importance in sourdough on the growth of the fermentation flora. Therefore, the combined effects of pH, NaCl, lactate, and acetate were evaluated. The models describing the single effects of these factors on growth were used to predict by extrapolation the combined effects of two factors on appropriate data subsets. The predictions of the combined effects of pH and ionic strength on L. sanfranciscensis LTH2581 and acetic acid and ionic strength on C. milleri LTH H198 are compared to the experimental data in Fig. 4A and 4B, respectively. The value of r2 was 0.829 for LTH2581 and 0.880 for LTH H198. The experimental design allows distinction between the inhibitory effects of acetic acid and acetate as well as between those of lactic acid and lactate, because several concentrations of undissociated acid-sodium salt were tested at different pH values. Thus, the data were fitted to an expanded model taking into account the combined effect of these factors. The models used and parameters obtained are compiled in Table 4; plots of the observed growth rates against the predicted growth rates are shown in Fig. 5. The values determined for pHopt, pHmax, and Iopt (LTH2581) and Imax (maximum ionic strength for growth; LTH2581 and LTH H198) correspond to those determined for the single effects of the factors on growth (Table 3). The pHmin determined for L. sanfranciscensis LTH2581 was slightly higher (4.16 versus 3.94) than that determined for the single effect of pH on growth. This deviation can be explained by the experimental design, as pH values between 3.9 and 4.1 were not evaluated. The growth of C. milleri is completely inhibited by 150 mmol of acetic acid per liter, while the inhibitory concentration of acetate was about 10 times higher. It is of special interest that growth inhibition of L. sanfranciscensis was correlated to acetate concentration, with little or no effect of the undissociated acid.
FIG. 4.
Three-dimensional plot of observed growth rates (•) versus surfaces of predicted growth rates. Observed growth rates are taken from data subsets for the evaluation of combined effects; calculated growth rates were extrapolated with the models describing single effects. (A) L. sanfranciscensis LTH2581; (B) C. milleri LTH H198.
TABLE 4.
Parameters and correlation coefficients for the effects of combined factors on growtha
| Strain | Model | μopt | γ(pH)
|
||
|---|---|---|---|---|---|
| pHmin | pHopt | pHmax | |||
| L. sanfranciscensis LTH2581 | μopt · γ(pH) · γ(I) · γ(Ac−) | 0.68 ± 0.02 | 4.16 ± 0.06 | 5.43 ± 0.03 | 6.68 ± 0.08 |
| C. milleri LTH H198 | μopt · γ(I) · γ(HAc) · γ(Ac−) · γ(HLac) | 0.52 ± 0.01 | |||
| γ(I)
|
γ(Ac−)
|
γ(HAc)
|
γ(HLac)
|
r2 (DOF) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Imax | Iopt | a | Ac−max | a | HAcmax | a | HLacmax | a | |
| 1.97 ± 0.09 | 0.45 ± 0.03 | 1.0 ± 0.5 | 0.8 ± 0.3 | 0.887 (104) | |||||
| 3.2 ± 0.2 | 1.4 ± 0.2 | 1.5 ± 0.2 | 0.71 ± 0.2 | 0.15 ± 0.03 | 1.3 ± 0.2 | 0.18 ± 0.02 | 4 ± 1 | 0.951 (80) | |
Ac−, acetate; HAc, acetic acid; HLac, lactic acid; DOF, degrees of freedom. Parameter estimates are shown in boldface if the xmax values calculated were outside the factor range applied in the assays.
FIG. 5.
Plot of observed growth rates versus growth rates predicted with the model for the combined effects. (A) L. sanfranciscensis LTH2581; (B) C. milleri LTH H198.
DISCUSSION
The experimental design used in this study has several limitations with respect to conditions during sourdough fermentations: (i) in practice, fermentations are not performed under static conditions for environmental factors; (ii) the differences in nutrient supply between mMRS medium and wheat or rye doughs may alter the response of the microorganisms to these factors; and (iii) the microflora of the dough may be affected by antagonistic or synergistic interactions such as competition for nutrients or by release of nutrients by extracellular enzymes during growth in mixed culture. Therefore, the model predictions require careful validation by comparison with data from sourdough fermentations.
The parameter estimates for the effect of temperature on the growth of L. sanfranciscensis and C. milleri determined in our work are in good agreement with literature data. Böcker et al. (1) and Spicher (29) reported that strains of L. sanfranciscensis have a Topt in the range between 30 and 37°C. C. milleri and S. exiguus do not grow at temperatures above 35°C (17, 39). The observation that L. sanfranciscensis LTH2581 and LTH1729 and C. milleri LTH H198 exhibit the same response to temperatures below 26°C provides an explanation for the stable association of these organisms in a sourdough for more than 20 years (2). Our data are furthermore in agreement with the “baker’s rule” that low temperatures during sourdough fermentations (20 to 26°C) are better for yeast growth than higher temperatures (30).
Sourdoughs usually are prepared without the addition of salt; nevertheless, several processes that make use of the incorporation of 2 to 5% NaCl in the sourdough have been developed (30). Our data are consistent with the observations of Röcken et al. (22) and Gianotti et al. (9) that increasing dough yields leads to faster acidification of doughs. The addition of salt may alter the composition of the microflora, because C. milleri is much less sensitive to salt than the strains of L. sanfranciscensis. Yeast growth in dough may even be stimulated by the addition of NaCl. In agreement with this assumption, it was observed that the addition of NaCl to wheat doughs inhibited the growth of lactic acid bacteria while it exerted a stimulating effect on yeast (9). We expressed the salt concentration as ionic strength rather than water activity. This was considered justified on the basis of the results of Dossmann (7), who observed a more pronounced growth inhibition of Lactobacillus sakei if NaCl replaced glycerol for adjusting the water activity to values ranging from 0.92 to 0.99.
The response of L. sanfranciscensis to changes in pH described in this study is in accordance with the original species description of Kline and Sugihara (15), who reported a pHopt of about 5 and a pHmin between 3.6 and 4.0. We observed that the growth of lactobacilli is favored over yeast growth at pH values of >4.5, corresponding to the first stage of dough fermentation. L. sanfranciscensis does not grow below pH 3.8, indicating that the pH is a decisive growth-limiting factor for this organism in sourdough. This conclusion is confirmed by the observation that factors increasing the buffering capacity of the dough do not alter the final pH of the fermentation but result in a higher content of lactic acid (23, 25). The parameter estimates describing the pH effect on the growth of L. sanfranciscensis LTH1729 and LTH2581 are strikingly similar, suggesting that the stable coexistence of these organisms in the same substrate is explained in part by their identical growth rates. The finding that the pH has little effect on the growth of C. milleri is in accordance with our earlier observations of a cereal-based growth medium (8).
During sourdough fermentation, acidification is achieved by the production of lactate and acetate to levels of 100 to 200 mmol/liter and 40 to 60 mmol/liter, respectively. The lactate content is determined by the buffering capacity of the sourdough, and the acetate content depends on the availability of substrates used as electron acceptors by the lactobacilli (12). Heterofermentative lactobacilli including L. sanfranciscensis tolerated up to 250 mmol of acetate and lactate per liter in rye and wheat doughs (18, 23). Because lactate and acetate production by lactobacilli has various effects on microbial growth, i.e., increase of ionic strength, decrease of pH, and additional effects of undissociated organic acids, it is necessary to distinguish between these effects. Remarkably, the growth of L. sanfranciscensis was not inhibited by undissociated acetic acid.
The growth of C. milleri was strongly inhibited by acetic acid, and was inhibited to a much lesser extent by lactic acid. The inhibitory concentrations of these organic acids are in good agreement with our earlier studies of a cereal-based medium, rye bran extract (8). Increased acetate contents of sourdough resulting from technological measures such as those proposed by Röcken et al. (22) and Martinez Anaya et al. (18) are therefore likely to result in the inhibition of yeast growth. The acetate tolerances of other sourdough yeasts such as T. holmii were reported to be in the same range as that of C. milleri LTH H198 (36).
Certain strains of heterofermentative lactobacilli are known to grow at ethanol concentrations as high as 18% (4). Because the ethanol concentration in doughs does not exceed 1%, it is unlikely to exert an inhibitory effect on lactobacilli but may contribute to the inhibition of yeast growth. Unlike the metabolic end products lactate, acetate, and ethanol, CO2 does not accumulate in the dough during fermentation but rather is dissolved in equilibrium to a 100% CO2 athmosphere. As the liquid media used in our work were overlaid with paraffin, the conditions matched those of sourdough fermentation, and a possible effect of CO2 on the growth of C. milleri and L. sanfranciscensis was not further considered. L. sanfranciscensis is known to grow optimally at a CO2 atmosphere of 25 to >90% (15).
The model used in this study to describe the combined effects of several factors on growth is based on the hypothesis that the cardinal parameters of growth for a given factor are independent of the values of any other factor and of the medium composition. Therefore, all parameters with the exception of μopt, and thus γ(x), should be independent of the medium composition. This hypothesis is supported by the satisfactory quality of fit obtained by extrapolation of single effects to the combined effect of different factors. Wijtzes et al. (38) demonstrated that the cardinal parameters of temperature and pH for Lactobacillus curvatus are independent of each other. A possible influence of salt concentration on the Topt values for five fungi was suggested by Cuppers et al. (5). These authors nevertheless preferred a model that neglected this effect of NaCl on Topt. However, there are limitations to the use of γ functions to describe the combined effects of environmental factors on microbial growth. For example, the addition of cationic compatible solutes, e.g., betaine and choline, decreased the inhibitory effect of NaCl on the growth of Lactobacillus plantarum (14); thus, γ(NaCl) and not μopt has been modified by the medium composition.
The importance of antagonistic and synergistic interactions between yeasts and lactobacilli based on the metabolism of carbohydrates and amino acids was emphasized by Gobbetti and Corsetti (10). However, sourdough yeasts do not affect the cell yield of L. sanfranciscensis in sourdough (8, 27, 32). This is consistent with the conclusion that under practical conditions the pH is the limiting factor for growth of lactobacilli. The maltose, amino acid, and peptide concentrations are not depleted during sourdough fermentations in wheat or rye doughs (16, 19, 31). Maltose is the preferred carbon source for L. sanfranciscensis but is not utilized by either C. milleri or S. exiguus (12). The cell yield of C. milleri and S. exiguus is greatly reduced in the presence of lactobacilli both in wheat and in rye doughs (8, 27). The accumulation of metabolic end products of the heterolactic fermentation inhibits the growth of these yeasts in sourdough. The glucose concentration in rye flours and whole-wheat flours remains high enough to support yeast growth throughout the fermentation (19, 23). Fermentations that employ white wheat flours as the raw materials are characterized by low concentrations of glucose, and small amounts of lactic acid are produced because of the low buffering capacity. In these doughs, depletion of glucose and fructose may occur and limit the growth of yeasts (19, 27).
Generally, the predictions made with the model are in good agreement with the literature data on dough available, indicating that the most important factors contributing to the microbial stability of sourdough fermentations have been taken into account. A more detailed verification of the model in situ is in progress. The model allows the assessment of factors contributing to the stable association of lactobacilli and yeasts in traditional sourdough fermentations, and it can therefore provide important information for the design of novel sourdough processes.
ACKNOWLEDGMENT
This work was supported by a grant of the European Union (FAIR project no. CT96-1126).
Appendix
With μ(x) = axbe−cx, differentiation yields 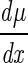 = axbe−cx(b/x − c). With e−cx =/ 0 for any x, the function has a maximum at x = b/c, corresponding to (Tmax − Topt). Substitution of μ(b/c) = μopt and a′μopt = a into model 1a gives μ(x) = μopt a′xbe−cx.
= axbe−cx(b/x − c). With e−cx =/ 0 for any x, the function has a maximum at x = b/c, corresponding to (Tmax − Topt). Substitution of μ(b/c) = μopt and a′μopt = a into model 1a gives μ(x) = μopt a′xbe−cx.
REFERENCES
- 1.Böcker G, Stolz P, Hammes W P. Neue Erkenntnisse zum Ökosystem Sauerteig und zur Physiologie der sauerteigtypischen Stämme Lactobacillus sanfrancisco und Lactobacillus pontis. Getreide Mehl Brot. 1995;49:370–374. [Google Scholar]
- 2.Böcker G, Vogel R F, Hammes W P. Lactobacillus sanfrancisco als stabiles Element in einem Reinzucht-Sauerteig-Präparat. Getreide Mehl Brot. 1990;44:269–274. [Google Scholar]
- 3.Collar C, Mascaros A R, Benedito de Barber C. Amino acid metabolism by yeasts and lactic acid bacteria during bread dough fermentation. J Food Sci. 1992;57:1423–1427. [Google Scholar]
- 4.Couto J A, Rozes N, Hogg T. Ethanol-induced changes in the fatty acid composition of Lactobacillus hilgardii, its effects on plasma membrane fluidity and relationship with ethanol tolerance. J Appl Bacteriol. 1996;81:126–132. [Google Scholar]
- 5.Cuppers H G, Oomes S, Brul S. A model for the combined effects of temperature and salt concentration on growth rate of food spoilage molds. Appl Environ Microbiol. 1997;63:3764–3769. doi: 10.1128/aem.63.10.3764-3769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damiani P, Gobbetti M, Cossignani L, Corsetti A, Simonetti M S, Rossi J. The sourdough microflora. Characterization of hetero- and homofermentative lactic acid bacteria, yeasts and their interactions on the basis of the volatile compounds produced. Lebensm-Wiss Technol. 1996;29:63–70. [Google Scholar]
- 7.Dossmann M U. Einfluß von ökologischen Parametern auf die physiologischen Leistungen von Laktobazillen in Rohwurst. Dissertation. Stuttgart, Germany: Universität Hohenheim; 1995. [Google Scholar]
- 8.Gänzle M G, Häusle S, Hammes W P. Wechselwirkungen zwischen Laktobazillen und Hefen des Sauerteiges. Getreide Mehl Brot. 1997;51:209–215. [Google Scholar]
- 9.Gianotti A, Vannini L, Gobbetti M, Corsetti A, Gardini F, Guerzoni M E. Modelling of the activity of selected starters during sourdough fermentation. Food Microbiol. 1997;14:327–337. [Google Scholar]
- 10.Gobbetti M, Corsetti A. Lactobacillus sanfrancisco, a key sourdough lactic acid bacterium: a review. Food Microbiol. 1997;14:175–187. [Google Scholar]
- 11.Hammes W P, Gänzle M G. Sourdough breads and related products. In: Wood B J B, editor. Microbiology of fermented foods. London, United Kingdom: Chapman and Hall; 1997. pp. 199–216. [Google Scholar]
- 12.Hammes W P, Stolz P, Gänzle M. Metabolism of lactobacilli in traditional sourdoughs. Adv Food Sci. 1996;18:176–184. [Google Scholar]
- 13.Hansen B, Hansen A. Volatile compounds in wheat sourdoughs produced by lactic acid bacteria and sourdough yeasts. Z Lebensm-Unters-Forsch. 1994;198:202–209. [Google Scholar]
- 14.Kets E P W, Nierop Groot M, Galinski E A, de Bont J A M. Choline and acetylcholine: novel cationic osmolytes in Lactobacillus plantarum. Appl Microbiol Biotechnol. 1997;48:94–98. [Google Scholar]
- 15.Kline L, Sugihara T F. Microorganisms of the San Francisco sour dough bread process. II. Isolation and characterization of undescribed bacterial species responsible for the souring activity. Appl Microbiol. 1971;21:459–465. doi: 10.1128/am.21.3.459-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kratochvil J, Holas J. Untersuchungen über proteolytische Vorgänge im Roggensauerteig. Getreide Mehl Brot. 1984;38:330–332. [Google Scholar]
- 17.Kreger van Rij N J W. The yeasts, a taxonomic study. 3rd ed. Amsterdam, The Netherlands: Elsevier Science Publisher; 1984. [Google Scholar]
- 18.Martinez Anaya M A, Llin M L, Macias M P, Collar C. Regulation of acetic acid production by homo- and heterofermentative lactobacilli in whole wheat sourdoughs. Z Lebensm-Unters-Forsch. 1994;199:186–190. doi: 10.1007/BF01193440. [DOI] [PubMed] [Google Scholar]
- 19.Martinez Anaya M A, Rouzaud O. Influence of wheat flour and Lactobacillus strains on the dynamics of by-products from amylolytic activities. Food Sci Technol Int. 1997;3:123–136. [Google Scholar]
- 20.Ottogalli G, Galli A, Foschino R. Italian bakery products obtained with sourdough: characterization of the typical microflora. Adv Food Sci. 1996;18:131–144. [Google Scholar]
- 21.Passos F V, Fleming H P, Ollis D F, Hassan H P, Felder R M. Modeling the specific growth rate of Lactobacillus plantarum in cucumber extract. Appl Microbiol Biotechnol. 1993;40:143–150. [Google Scholar]
- 22.Röcken W, Rick M, Reinkemeier M. Controlled production of acetic acid in wheat sour doughs. Z Lebensm-Unters-Forsch. 1992;195:259–263. [Google Scholar]
- 23.Röcken W, Voysey P A. Sour-dough fermentation in bread making. J Appl Bacteriol. 1992;79:38S–48S. [Google Scholar]
- 24.Rosso L, Lobry J R, Bajard S, Flandrois J P. Convenient model to describe the combined effects of temperature and pH on microbial growth. Appl Environ Microbiol. 1995;61:610–616. doi: 10.1128/aem.61.2.610-616.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salovaara H, Valjakka T. The effect of fermentation temperature, flour type, and starter on the properties of sour wheat bread. Int J Food Sci Technol. 1987;22:591–597. [Google Scholar]
- 26.Salovaara H, Savolainen J. Yeast type isolated from Finnish sour rye dough starters. Acta Aliment Pol. 1984;10:241–246. [Google Scholar]
- 27.Saunders R M, Ng H, Kline L. The sugars of flour and their involvement in the San Francisco sour dough French bread process. Cereal Chem. 1972;49:86–91. [Google Scholar]
- 28.Schieberle P. Intense aroma compounds—useful tools to monitor the influence of processing and storage on bread aroma. Adv Food Sci. 1996;18:237–244. [Google Scholar]
- 29.Spicher G. Einige neue Aspekte der Biologie der Sauerteiggärung. Getreide Mehl Brot. 1982;36:12–16. [Google Scholar]
- 30.Spicher G, Stephan H. Handbuch Sauerteig: Biologie, Biochemie, Technologie. 4th ed. Hamburg, Germany: B. Behr’s Verlag; 1993. [Google Scholar]
- 31.Spicher G, Nierle W. Proteolytic activity of sourdough bacteria. Appl Microbiol Biotechnol. 1988;28:487–492. [Google Scholar]
- 32.Spicher G, Rabe E, Sommer R, Stephan H. Die Mikroflora des Sauerteiges. XV. Mitteilung: über das Verhalten heterofermentativer Sauerteigbakterien und Hefen bei gemeinsamer Kultur. Z Lebensm-Unters-Forsch. 1982;174:222–227. [Google Scholar]
- 33.Spicher G, Schröder R, Stephan H. Die Mikroflora des Sauerteiges. X. Mitteilung: die backtechnische Wirkung der in „Reinzuchtsauern“ auftretenden Milchsäurebakterien (Genus Lactobacillus Beijerinck) Z Lebensm-Unters-Forsch. 1980;171:119–124. [Google Scholar]
- 34.Stolz P, Böcker G. Technology, properties and applications of sourdough products. Adv Food Sci. 1996;18:234–236. [Google Scholar]
- 35.Sugihara T F, Kline L, McCready L B. Nature of the San Francisco sour dough French bread process. Baker’s Dig. 1970;44:50–52. [Google Scholar]
- 36.Suhiko M-L, Mäkinen V. Tolerance of acetate, propionate and sorbate by Saccharomyces cerevisiae and Torulopsis holmii. Food Microbiol. 1984;1:105–110. [Google Scholar]
- 37.Trüper H G, De’ Clari L. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) “in apposition.”. Int J Syst Bacteriol. 1997;47:908–909. [Google Scholar]
- 38.Wijtzes T, de Wit J C, Huis in ’t Veld J H J, van ’t Riet K, Zwietering M H. Modelling bacterial growth of Lactobacillus curvatus as a function of acidity and temperature. Appl Environ Microbiol. 1995;61:2533–2539. doi: 10.1128/aem.61.7.2533-2539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarrow D. Candida milleri sp. nov. Int J Syst Bacteriol. 1978;28:608–610. [Google Scholar]
- 40.Zwietering M H, de Wit J C, Cuppers H G A M, van ’t Riet K. Modeling of bacterial growth with shifts in temperature. Appl Environ Microbiol. 1994;60:204–213. doi: 10.1128/aem.60.1.204-213.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwietering M H, Jongenburger I, Rombouts F M, van ’t Riet K. Modeling of the bacterial growth curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]