Abstract
Proton extrusion by roots of intact sunflower plants (Helianthus annuus L.) was studied in nutrient solutions or in agar media with a pH indicator. Proton extrusion was enhanced by either iron deficiency, addition of fusicoccin, or single salt solutions of ammonium or potassium salts. The three types of proton extrusion differ in both localization along the roots and capacity. From their sensitivity to ATPase inhibitors it seems justified to characterize them as proton pumps driven by plasma membrane APTases.
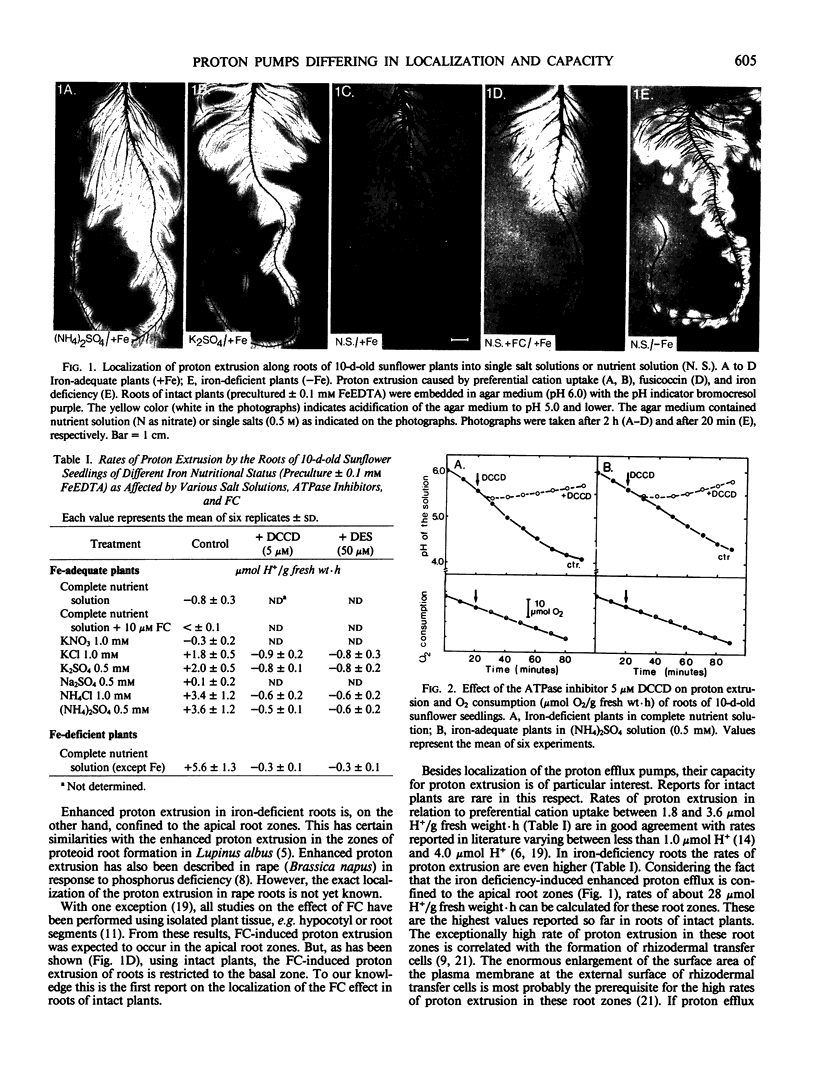
Enhanced proton extrusion induced by preferential cation uptake from (NH4)2SO4 or K2SO4 was uniformly distributed over the whole root system. In contrast, the enhancement effect of fusicoccin was confined to the basal root zones and that of iron deficiency to the apical root zones. Also the rates of proton extrusion per unit of root fresh weight differed remarkably and increased in the order: Fusicoccin ≪ K2SO4 < (NH4)2SO4 < iron deficiency.
Under iron deficiency the average values of proton extrusion for the whole root system are 5.6 micromoles H+ per gram fresh weight per hour; however, for the apical root zones values of about 28 micromoles H+ can be calculated. This high capacity is most probably related to the iron deficiency-induced formation of rhizodermal transfer cells in the apical root zones. It can be assumed that the various types of root-induced acidification of the rhizosphere are of considerable ecological importance for the plant-soil relationships in general and for mobilization of mineral nutrients from sparingly soluble sources in particular.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey T. J., Evans M. L. Geotropism in corn roots: evidence for its mediation by differential Acid efflux. Science. 1981 Apr 3;212(4490):70–71. doi: 10.1126/science.212.4490.70. [DOI] [PubMed] [Google Scholar]
- O'neill R. A., Scott T. K. Proton Flux and Elongation in Primary Roots of Barley (Hordeum vulgare L.). Plant Physiol. 1983 Sep;73(1):199–201. doi: 10.1104/pp.73.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Evidence for a Cl-Stimulated MgATPase Proton Pump in Oat Root Membranes. Plant Physiol. 1982 Apr;69(4):798–803. doi: 10.1104/pp.69.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel M. H., Dorn A., Jaffe L. F. Natural H Currents Traverse Growing Roots and Root Hairs of Barley (Hordeum vulgare L.). Plant Physiol. 1979 Sep;64(3):512–518. doi: 10.1104/pp.64.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]



