Abstract
In nature, carotenoids are present as trans- and cis-isomers. Various physical and chemical factors like light, heat, acids, catalytic agents, and photosensitizers can contribute to the isomerization of carotenoids. Living organisms in the process of evolution have developed different mechanisms of adaptation to light stress, which can also involve isomeric forms of carotenoids. Particularly, light stress conditions can enhance isomerization processes. The purpose of this work is to review the recent studies on cis/trans isomerization of carotenoids as well as the role of carotenoid isomers for the light capture, energy transfer, photoprotection in light-harvesting complexes, and reaction centers of the photosynthetic apparatus of plants and other photosynthetic organisms. The review also presents recent studies of carotenoid isomers for the biomedical aspects, showing cis- and trans-isomers differ in bioavailability, antioxidant activity and biological activity, which can be used for therapeutic and prophylactic purposes.
Keywords: Cis-Carotenoid, Photosynthesis, Metabolic adaptation, Antioxidants, Carotenoid isomerization
Introduction
Carotenoids are one the most widespread classes of natural pigments (Britton et al. 2004). They are synthesized by higher plants, algae, phototrophic bacteria (including cyanobacteria) and some types of archaea, a number of chemotrophic bacteria, some filamentous fungi, and yeasts. Currently, about 1200 natural carotenoids are known (Yabuzaki 2017). Carotenoids are polyisoprenoids with an extended chain of conjugated double bonds, while the conjugation length is among the most important characteristics to determine different photophysical and photochemical properties of the molecule. Due to manyfold of possible structural modifications like cis/trans isomerism, cyclization, hydrogenation, and addition of different substituents, effective conjugation length must be introduced. The main absorption maxima of carotenoids depend on the effective conjugation length, and the range of 400–500 nm is typical for all-trans isomers with 10–11 (the most abundantly met) double bounds in the polyene chain, while cis-configurations lead to specific higher frequency bands. cis/trans Isomerization is achievable under the action of acids, heat, and light in the absence or presence of catalysts (including enzymes) and photosensitizers (Jensen et al. 1982; Milanowska and Gruszecki 2005; Liaaen-Jensen and Lutnœes 2008; Frank and Christensen 2008). Solution characteristics like polarizability, polarity (especially for carbonyl-contained carotenoids), and general environment organization (carotenoid-protein, carotenoid-carotenoid, etc.) are among the factors to change the electronic and vibrational properties of the molecule (Britton 2020). The energy levels of carotenoid singlet and triplet states are effectively used for energy transfer processes in photosynthesis (Liaaen-Jensen and Lutnœes 2008; Polívka and Sundström 2004), providing both light-harvesting (singlet–singlet energy transfer) and photoprotective (triplet–triplet energy transfer) functions. An additional mechanism for the photoprotective function is known for non-photochemical quenching (NPQ) of excited states of the chlorophyll molecule, in which excess energy of chlorophyll can be transferred to the carotenoid by singlet–singlet transfer (Staleva et al. 2015; Niedzwiedzki et al. 2016). Although all-trans isomers of carotenoids are generally more stable, the unique features of cis-configurations are also exploited in nature: 15-cis-β-carotene is found in the reaction centers (RCs) of green plants and phototrophic bacteria (Ohashi et al. 1996; Liaaen-Jensen and Lutnœes 2008; Sajilata et al. 2008). It can be assumed that the energy levels (e.g., the higher energy of S1 state) of cis-form are better suited for energy transfer mechanisms between carotenoids and chlorophylls while reported enhanced antioxidant properties of the cis-form can protect RCs from reactive oxygen species (ROS) more effectively (Koyama and Fujii 1999; Honda 2021).
In this review, carotenoids are examined in various aspects: general spectroscopic features, functioning in photosynthetic systems as well as their role in metabolic processes of living organisms. At the same time, the review is focused around a range of important questions where the structural form of the carotenoids (isomerization from one form to another under the influence of various physicochemical factors) affects the efficiency of energy transfer and quenching, interactions with other molecular systems, and solubility in various media. As a result, several studied forms of carotenoid isomers have different levels of antioxidant activity, bioavailability, and biological activity. Recently, more evidence has emerged on the effectiveness of cis-isomers of carotenoids for the treatment and prevention of a number of diseases such as cancer, inflammation, cardiovascular, and pathologies of organs that are exposed to light (skin, eyes).
The structure of natural carotenoids isomers
Strong coloration is the basic feature of carotenoids due to the extended system of conjugated double bonds. The linear hydrocarbon chain of carotenoids is subjected to various structural modifications. These include cis/trans isomerization, the level of hydrogenation, cyclization, and the addition of different substituents (often containing oxygen) (Fiedor and Burda 2014). Carotenoids are divided into two classes: carotenes, which contain only carbon and hydrogen atoms (like lycopene, β-carotene, α-carotene, etc.), and xanthophylls, with at least one oxygen atom in structure (lutein, zeaxanthin et al.) (Fig. 1).
Fig. 1.
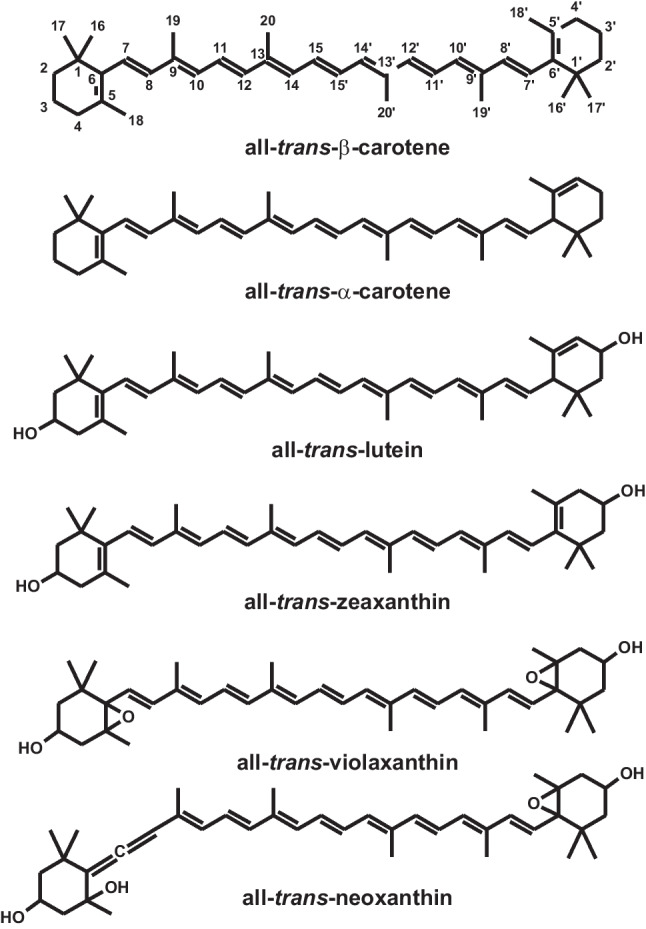
Structural formulas of some carotenoids
Comparison of the properties of cis- and all-trans-carotenoid isomers
Carotenoids can exist in different configurations due to the double bond isomerism (Fig. 2). The configurations around the double bond are called trans or cis (also referred to as E or Z), both having extended systems of conjugated double bonds. In general, cis-configurations are less thermodynamically stable than all-trans-isomers due to steric hindrance between neighboring cis-hydrogen and methyl groups in a non-linear structure. The structural transformations of the polyene chain for cis-isomers result in different electronic, vibration, and chemical properties of the molecule (Jomova and Valko 2013; Langi et al. 2018).
Fig. 2.
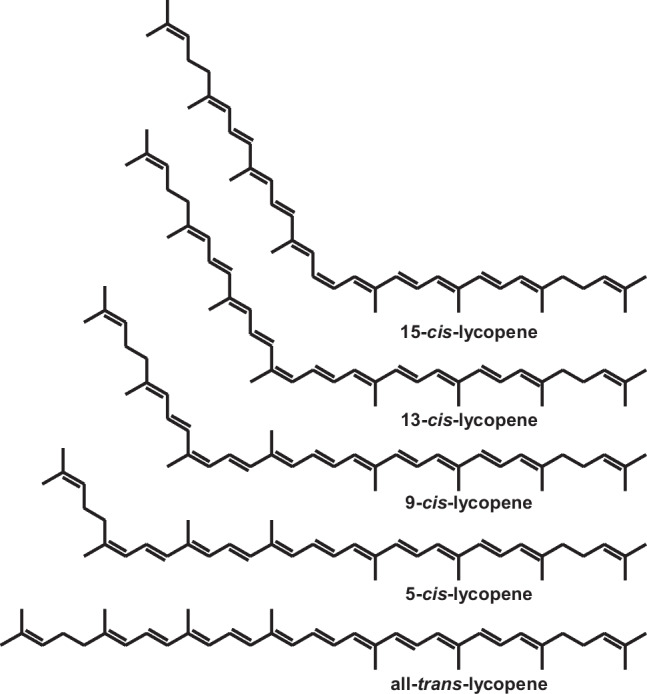
Geometric isomers of lycopene
Absorption spectroscopy
Carotenoids are effective UV, VIS light absorbers with a variety of colors depending on the molecular skeleton and its environment, which makes different absorption spectroscopy approaches (steady-state, variations of pump-probe time-resolved techniques like 2DES) among the most important for pigment detection, identification, excited state dynamics, and pigment interactions in complex natural and artificial systems (Britton et al. 2004; Ostroumov et al. 2013; Niedzwiedzki 2023). The absorption spectra of carotenoids have an electronic-vibrational structure in which three mutually overlapping bands of I, II, and III (Fig. 3) are usually well expressed due to the very high oscillator strength of S0 (11Ag−) → S2 (11Bu+) transition with extinction coefficients reaching up to 105 M−1 cm−1. The bands are also termed as 0–2, 0–1, and 0–0 (with an energy gap between peaks ~ 1350 cm−1), which correspond to the lowest vibronic bands of the electronic transition.
Fig. 3.
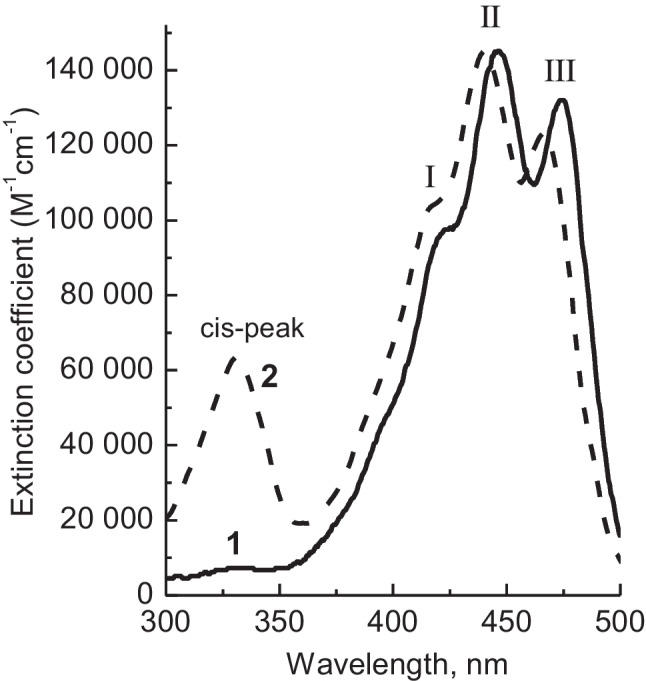
Absorption spectrum of (1) all-trans-lutein and (2) 13-cis-lutein in 85% CH3CN/15% CH3OH
The positions and resolution of I, II, and III in absorption spectra of carotenoids depend, at least, on the following factors (Table 1). First, the absorption bands shift to longer wavelengths with an increase of conjugation length (N the number of conjugated C = C bonds) of the polyene chain, reflecting energy gap S0 → S2 inversely proportional to N (Frank et al. 2002): difference ~ 2000 cm−1 (Table 1) between bands of β-carotene, lutein, astaxanthin (N = 9), and spirilloxanthin (N = 13). Second, cyclization can modify the effective conjugation length of the chain and lead to “out-of-plane” configurations between π-electrons of the ring and those of the chain, which can result in hypsochromic shifts and loss of I, II, and III bands resolution: The difference between dicyclic β-carotene (N = 9) linear counterpart lycopene can reach up to tens of nm. The absorption spectra virtually insensitive for the introduction of substituents have no significant effect on the conjugated chain (e.g., hydroxy groups of lutein and zeaxanthin with respect to β-carotene, Table 1). Third, slight hypsochromic shift (several nm), hypochromic effect, as well as redistribution of band intensities can be results of cis-isomerization of the polyene chain. Besides, the so-called cis-peak (the term introduced by Zechmeister and Polgar (1943)) is reliably seen for Z (cis) isomers, unlike the all-trans structures (Fig. 3), which is likely associated with S0 → S4 transition (Cerón-Carrasco et al. 2010). The transition moment of the band is perpendicular to the long axis of the molecule; therefore, hardly detected cis-band for all-trans isomers can be explained by slight geometry distortions of the polyene chain (Moore and Song 1974). The spectral position of the cis-peak is blue-shifted by ~ 140 nm relative to band III, while the intensity of the peak increases when the position of the cis-bond approaching the center of the polyene chain (Britton et al. 2004). Fourth, as it is seen from Table 1, positions of I, II, and III are solvent dependent; hence, the high polarizability of some solvents like CS2 can result in red shifts up to tens of nm (Ilagan et al. 2005). The polarity of solvent can have dramatic effect on the resolution of absorption bands (up to complete loss of three-peak character) when essential charge redistribution is possible due to polarity, e.g., carotenoids with conjugated carbonyl group in structure, like fucoxanthin, peridinin, spheroidenone, and β-apo-8′-carotenal (Frank et al. 2000; Zigmantas et al. 2004; Horiuchi et al. 2023). Low-temperature measurements can improve vibronic band resolution not only the main (S0 → S2) electronic transition but also the cis-band (Niedzwiedzki 2023). Fifth, large dipole moments of natural environment (causing conformational changes of carotenoids) and especially structures with excitonic interaction between carotenoids (oligomerization, H/J aggregation) could be a reason for substantial modifications of carotenoid absorption bands (Gottfried et al. 1991; Ruban et al. 2000; Spano 2009) including large bathochromic shifts (Ilagan et al. 2005; Christensson et al. 2013).
Table 1.
Spectral characteristics of the main carotenoids and their cis-isomers
| Carotenoid [m/z] | cis-/trans-isomer | Absorption maxima*, nm | |||
|---|---|---|---|---|---|
| cis-band | I | II | III | ||
| Lycopene, ψ,ψ-carotene [537] | all-transa,b,c,d,e | - | 443–458 | 470–487 | 501–522 |
| 5-cisd,e | 362 | 443–444 | 470 | 501–502 | |
| 7-cisd | 362 | 443 | 469 | 499 | |
| 9-cise | 361 | 438 | 464 | 495 | |
| 13-cise | 361 | 437 | 463 | 494 | |
| 15-cisd,e | 360 | 441–442 | 466–468 | 497–499 | |
| β-Carotene, β,β-carotene [537] | all-transa,b,c,f,g,h,i,j | - | 420–430 | 448–459 | 476–492 |
| 9-cisa,b,c,f,g,h,j | 330–344 | 410–428 | 445–452 | 469–476 | |
| 13-cisa,b,c,f,g,h,j | 325–344 | 418–424 | 439–448 | 467–476 | |
| 15-cisa,f | 328–340 | 418–425 | 441–447 | 470–474 | |
| α-Carotene, β,ε-carotene [537] | all-transa,b,f,h,i | - | 418–433 | 442–457 | 472–485 |
| 9- or 9'-cish | 344 | 421 | 446 | 470 | |
| 13- or 13'-cish | 332 | 416 | 440–446 | 464–470 | |
| Lutein, β,ε-carotene-3,3'-diol [569] | all-transa,b,f,g,h,i,j,q | - | 419–435 | 444–458 | 470–485 |
| 9-cisa,b,g,h,j | 330–334 | 415–421 | 439–446 | 467–470 | |
| 9'-cisa,b,g | 330–332 | 418–420 | 440–444 | 467–472 | |
| 13-cisa,b,f,g,h,q | 321–332 | 414–419 | 437–441 | 464–470 | |
| 13'-cisb,f | 322 | 410 | 437 | 464 | |
| 15-cisg,j | 330 | 414–416 | 439–440 | 465 | |
| Zeaxanthin, β,β-carotene-3,3'-diol [569] | all-transa,b,c,f,g,h,i,j,k,l | - | 420–440 | 448–463 | 475–493 |
| 9-cisb,g,j | 340 | 419–424 | 444–450 | 470–474 | |
| 13-cisb,k | 338 | 419 | 446–451 | 472–476 | |
| 15-cisb,j | 338 | 420–426 | 449–450 | 474–478 | |
| Neoxanthin, 5′,6′-epoxy-6,7-didehydro-5,6,5′,6′-tetrahydro-β,β-carotene-3,5,3′-triol [601] | all-transa,g,r | - | 418–426 | 441–452 | 469–482 |
| 9-cisg,j | 328 | 414–421 | 435–445 | 464–469 | |
| 9'-cisa,r | 330 | 413–422 | 436–447 | 464–477 | |
| Violaxanthin, 5,6:5′,6′-diepoxy-5,5′,6,6′-tetrahydro-β,β-carotene-3,3′-diol [601] | all-transa,c,f,g,h,i,g | - | 415–427 | 438–453 | 465–483 |
| 9-cisf,g | 305–329 | 399–412 | 421–435 | 447–462 | |
| Astaxanthin, 3,3′-dihydroxy-β,β-carotene-4,4′-dione [597] | all-transa,i,m,n,o | - | - | - | 466–485 |
| 9-cism,n,o | 365–367 | - | - | 464–474 | |
| 13-cism,n | 366–368 | - | - | 462–466 | |
| 15-ciso | 365–366 | - | - | 467–468 | |
| Spirilloxanthin, 1,1'-dimethoxy-3,4,3',4'-tetradehydro-l,2,1',2'-tetrahydro-ψ,ψ-carotene [597] | all-transa,p,q | - | 465–480 | 491–510 | 526–546 |
| 5-cisp | 386 | 463 | 492 | 525 | |
| 9-cisp | 370, 386 | 459 | 487 | 521 | |
| 13-cisp | 369, 386 | 459 | 487 | 520 | |
| 15-cisq | 388 | 459 | 492 | 523 | |
*The intervals for the absorption maxima of carotenoids are given, within which they vary in various solvents
References: aBritton et al. 2004; bKhachik et al. 1997b; cZaghdoudi et al. 2017; dHengartner et al. 1992; eHonda et al. 2015; fDiprat et al. 2017; gFernandes et al. 2020; hInbaraj et al. 2006; iMinyuk and Solovchenko 2018; jPatias et al. 2017; kAsker et al. 2018b; lSajilata et al. 2008; mAsker et al. 2018a; nDu et al. 2020; oHonda and Nishida 2023; pLindal and Liaaen-Jensen 1997; qNiedzwiedzki 2023; rStrand and Liaaen-Jensen 2000
Excited state energies and lifetimes of carotenoids have been studied intensively for the last decades to elucidate different mechanisms of energy transfer for photoprotective and light-harvesting functions. The low-lying singlet excited state S1 (21Ag−), first discovered back in the 1970s (Schulten and Karplus 1972), is “silent” for one-photon excitation, including isomers and substituted structures lacking C2h symmetry. This could probably be explained by the large displacement of potential energy surfaces of S0 and S1 (Fiedor et al. 2016), although high vibronic states of S1 could lead (Niedzwiedzki and Blankenship 2018) to slight shape modification of the S2 (0–0) band. The dark nature of the S1 state is also the reason for extremely low S1 → S0 fluorescence (quantum yield, QY ~ 10−4) for carotenoids with short conjugation lengths N < 8 (Gillbro and Cogdell 1989; DeCoster et al. 1992). In contrast to Kasha’s rule (Demchenko et al. 2017), S2 → S0 (with extremely low QY as well) emission detected for several carotenoids (e.g., lycopene, spheroidene, and spheroidenone) and dominates for N ≥ 9; dual fluorescence profiles with contribution both from S2 → S0 and S1 → S0 were also reported for N < 10 (Andersson et al. 1995; Frank et al. 2002). The QY of carotenoids can be essentially increased (almost ~ 2 orders of magnitude) for ionic liquids (Białek-Bylka et al. 2007). Both S1 and S2 states of carotenoids are considered to play a mediating role in different energy transfer mechanisms to chlorophylls (Chl) in light-harvesting complexes: (1) Upon S0 → S2 excitation (S2 pathway), the ultrafast (< 200 fs) energy transfer is possible to B and Qx bands of Chl-a and Chl-b, while S1 pathway assumes energy transfer (< 1 ps) to the lowest energy band of Chl-a (Qy) in LHCII complex; (2) S2, S1 pathways could be responsible for the energy transfer to Qx (< 180 fs) and Qy (< 25 ps) bands of BChl in LH2 complex (Polívka and Sundström 2004). One-photon S0 → S2 transition is commonly exploited to study excited states dynamics of different carotenoids in the frame of ultrafast time-resolved techniques like femtosecond pump-probe spectroscopy to trace: bleaching and recovery of S0 absorption, ultrafast conversion of S2 to hot state of S1, S1 → Sn transient absorption (presenting a fingerprint of the S1 state, red-shifted to S2 band) as well as other transitions like S* → Sn (spectrally between the ground state and S1 → Sn absorption, the presence of S* was reported to be a feature of triplet formation, Gradinaru et al. 2001), intramolecular charge–transfer states (ICT, the feature of polarity dependent carotenoids like peridinin), and energy transfer to highly fluorescence pigments. Besides, the transient counterpart of “cis-peak” to trace the ultrafast dynamics of carotenoid isomers was recently proposed for lutein and spirilloxanthin (Niedzwiedzki 2023). In many works, the lifetimes of S2 and S1 states were found to be in range of sub-picosecond (typical range of 50–300 fs) and picosecond (typically < 30 ps) timescales, respectively (Wasielewski and Kispert 1986; Shreve et al. 1991; Ilagan et al. 2005; Ostroumov et al. 2013), while ultrafast relaxation within S2 state is still scarcely studied with characteristic lifetimes less than 50 fs (Nakamura et al. 2004). The S1 lifetimes have a direct correlation with the S1 energy which decreases for the carotenoids with the increased effective conjugation N: sub-picosecond (0.8 ps) S1 lifetime was detected for diketospirilloxanthin, N ~ 14 (Niedzwiedzki et al. 2015), while for N ~ 8, S1 was found to be in the range of 160–180 ps (Zigmantas et al. 2001). The S2 lifetimes follow the energy gap S2-S1, which, in turn, decreases with an increase in solvent polarizability (Macpherson and Gillbro 1998). The early mentioned displacement of the S1 surface could be the reason for the non-linear dependence of the S2 lifetimes on the conjugation length reaching up to values of ~ 240 fs for N ~ 7: as opposite to the growing of S2-S1 gap with N, the lifetimes drop to ~ 70 fs for N ~ 13 (Kosumi et al. 2009). The relaxation dynamics of high-energy excited states (~ 30000 cm−1, pumped by UV light) of β-carotene and astaxanthin show a significantly broader S1 → Sn band, which assumes a specific relaxation pathway as opposite to S2 excitation (Kuznetsova et al. 2023). The lifetimes of ICT states were reported to be comparable with those of S1, while the involvement of ICT states (coupled S1/ ICT states) could essentially decrease the lifetime of excited states (Frank et al. 2000). It was also shown recently that different cis-isomers of β-apo-8′-carotenal have different S1 lifetime (in range of 6–10 ps), while 9-,13-, and 9,13-cis-isomers characterized by more pronounced stabilization of S1/ICT states which makes the isomers more effective for energy transfer functionality in photosynthetic apparatus (Horiuchi et al. 2023).
Vibrational spectroscopy of carotenoids
Raman spectroscopy (RS) has been widely used to study carotenoid content in vitro and in vivo for decades (Gill et al. 1970; Jehlička et al. 2014; Merlin 1985; Pfander 1992). The distinctive characteristic of the carotenoids (both carotenes and xanthophylls) is a large number of conjugated double bonds in a polyene chain which makes the pigments near the most efficient Raman scatterers among natural biomolecules in resonance (e.g., excitation wavelength at 532 nm or less) (Lu et al. 2018), pre-resonance, and off-resonance modes (e.g., red, NIR excitation wavelengths; Baranska et al. 2006; Subramanian et al. 2014; Yao et al. 2022). The main S0 → S2 (π-π*) electronic transition of carotenoids is commonly exploited for pre-resonance/resonance modes of the RS with accompanied enhancement factors reaching up to 104–106 (Horiue et al. 2020; Llansola-Portoles et al. 2022). In general, the resonance mode is preferable for selective molecule excitation in a heterogeneous medium, which is especially valuable to study the conformation of chromophores within proteins with no interference from other biological molecules. RS, as a vibrational and nondestructive approach that provides structural information of carotenoids, can predict at least the all-trans and some mono-cis-configurations and has been verified vs. high reproducibility techniques (i.e., TLC, HPLC) for different carotenoids like α/β-carotene, lutein, lycopene, zeaxanthin, crocetin, flavoxanthin, lycoxanthin, fucoxanthin, astaxanthin, and cis/trans-bixin (Ozaki et al. 1992; Jehlička et al. 2014; Schulz et al. 2005). Despite a very broad family of diverse chemical structures, the Raman spectra of carotenoids (Okamoto et al. 1994; Saito and Tasumi 1983) are dominated by several distinct bands in the fingerprint range stemming from different vibrations in polyene chain: conjugated C = C (ν1, 1500–1550 cm−1, very strong); C–C (ν2, 1130–1170 cm−1, strong) stretching vibrations of the polyene chain; and in-plane rocking vibration of methyl groups attached to the polyene chain and coupled with C–C (ν3, 1000–1020 cm−1, medium). Infrared spectroscopy (IR) has never been extensively applied to structure verification of carotenoids, mainly because these polyene chain vibrations are detected as weak or very weak (Schwieter et al. 1969). Additionally, water IR bands usually overlay the signals from other biological constituents for in vivo studies. The lower frequency RS bands (ν4, ~ 960 cm−1, medium-weak) are ascribed to the C-H vibrations outside of the chain, changes of the polyene chain angles (both valence and dihedral), deformations of the terminal rings, and the deformations of the whole carotenoid by twisting and waving. Non-planar carotenoid isomers involving rotation around C–C are not stable in solution. For planar structures, ν4 is weak (formally forbidden) (Rimai et al. 1973) in case of resonance excitation, while various skeleton distortions which include protein-pigments interactions usually enhance the band (Gruszecki et al. 2009): Slightly more intense ν4 band was detected of LH-bound carotenoid than in hexane for carotenoids from the spirilloxanthin series (Iwata et al. 1985). Moreover, the intensity of the ν4 band (5–10 times less intense for isolated carotenoids) was found to be the same order of magnitude as ν2 for spheroidenone bound to reaction centers of purple bacteria (Lutz et al. 1987). It was also noted that non-planar conformational changes reflected in the ν4 band are not accompanied with the spectral position of ν1 and ν3 (Robert 1999). Out-of-plane C-H vibrations of the C–CH = CH-C unit of the polyene chain are among the most prominent bands in the IR spectra of carotenoids and are typically located near ν4 (Lunde and Zechmeister 1955). The unique fingerprint of the band is cis/trans isomerization, which causes the single sharp peak (e.g., 966 cm−1 and 960 cm−1 for β-carotene and lycopene, respectively) transformation into a doublet (e.g., 952 cm−1 and 968 cm−1 for 15-cis-β-carotene), although for long polyene systems, the split can be obscured (Berezin and Nechaev 2005; Lunde and Zechmeister 1955; Schulz and Baranska 2007). The unmethylated mono-cis-isomers (7-cis, 11-cis, 15-cis) are supposed to be less stable than methylated ones (9-cis, 13-cis) because of steric interaction in the concave side of the cis-bend (Koyama and Fujii 1999) and can be (e.g., β-carotene, zeaxanthin) distinguished by the ~ 1380 cm−1 IR band which is assigned to deformation vibrations of methyl groups attached to a cis double bound (Lunde and Zechmeister 1955).
The exact spectral position and intensities of ν1, ν2, ν3, and ν4 depend on multiple physical and chemical conditions. Similar to absorption properties, an increase in conjugation length of the π-electron chain results in ν1 decrease (Rimai et al. 1973): The peaks at ~ 1535 cm−1 and ~ 1510 cm−1 correspond to carotenoids with 7 and 11 conjugated C = C bonds, respectively (Schulz and Baranska 2007). Temperature, environment polarizability, and cis/trans isomerization, among the factors, can influence the effective length of conjugated chain: A shift ~ 5 cm−1 of ν1 band is typically detected for temperature range between 293 and 77 K (Andreeva et al 2011); linear correlation was found between solvent polarizability and position of ν1 for different linear, linear with conjugated end-cycles and aryl-carotenoids (Mendes-Pinto et al. 2013); the average polarizability of binding sites of biological structures is dominant factor for tuning ν1 (along with S0 → S2 transition) of carotenoids and demonstrated for neurosporene, spheroidene, lycopene, and spirilloxanthin both for hexane solutions and binding to light-harvesting complexes (Llansola-Portoles et al. 2022); an increase in ν1 frequency is usually seen for all-trans, 7-cis, 9-cis, and 13-cis (the upshifts ~ 10–13 cm−1 for β-carotene) when a cis-bend introduced from the peripheral part toward the central part of the molecule; the effect is even larger for di-cis-isomers, reflecting further reduction of π-electrons delocalization (Koyama et al. 1988; Robert 1999). More detailed contributions of different isomers can be based on the 1100–1300 cm−1 range. The pronounced band around 1250 cm−1 appears for 15-cis-configurations: 1245 cm−1 and 1255 cm−1 for 15-cis and 13,15-di-cis for β-carotene, respectively (Koyama and Fujii 1999); 1251 cm−1 for 15-cis-isomer of astaxanthin, which comes from rocking vibrations of C15-H, C15’-H and stretching of C15 = C15’ (Yao et al. 2022). C–C stretching range is highly sensitive to the conformation of carotenoids. The most significant difference between all-trans and cis-isomers is the band detected in range of ~ 1130–1145 cm−1: typically weak for all-trans (shoulder to the main ν2 peak at ~ 1160 cm−1) and 15-cis-isomers; the band intensity is gradually increased for 7-cis- and 9-cis- and the strongest for 13-cis-isomer; the band is also clearly seen for di-cis-isomers like 9,13-di-cis- and 9,9’-di-cis-isomers (Koyama et al. 1982, 1988; Yao et al. 2022).
cis/trans Isomerization under the action of physicochemical factors
The cis/trans isomerization reaction is an equilibrium process. The process is influenced by two main factors: the thermodynamic stability of carotenoids and the activation energy of rotation of the C = C bond, which, in the case of carotenoids, is determined by the position of the bond relative to the center of the molecule and the presence of substituents (Guo et al. 2008). Heat, light, acids, microwave, ultrasound, organic solvents, metal ions, and adsorption to an active surface (such as alumina) promote the isomerization carotenoids (Yuan and Chen 1999; Milanowska and Gruszecki 2005; Zhao et al. 2005, 2006; De Bruijn et al. 2016; Brotosudarmo et al. 2020; Honda et al. 2020a; Fusi et al. 2023). Using quantum chemical calculations, it was shown that cis/trans isomerization of carotenoids can occur in both the ground singlet (S0) and triplet (T1) states (Guo et al. 2008). The characteristic lifetimes of triplet states of the carotenoid have been reported to be of microsecond time scales (Burke et al. 2000). In the S0 state, isomerization proceeds more slowly due to the high activation energy of the rotational barrier. In the T1 state, the activation energy decreases, resulting in an easier transition from one form to the other. Heating and irradiation help overcome the energy barrier and thus accelerate the isomerization process in both directions. The use of various catalysts (iodine, metal ions, etc.) accelerates the reaction, and various solvents, acids, or antioxidants affect the stability of carotenoids and, thus, also affect the ratio of isomeric forms in the equilibrium mixture. The stability of cis-isomers can be facilitated by interaction with fatty acids (De Bruijn et al. 2016), nonpolar solvents, and nanoparticles (Polyakov et al. 2023).
Isomerization usually results in a mixture of different isomers, with a predominance of the most thermodynamically stable all-trans and one of the cis-forms of carotenoids. Among all cis-isomers, the 9-cis-isomer is often thermodynamically stable, while the lowest activation energy barrier is characterized for 13-cis-form (Guo et al. 2008). These isomers are most often found when carotenoids are isolated from natural sources and are formed in the largest quantities in isomerization reactions. So, for example, in the photoisomerization of all-trans-β-carotene, the 9-cis-form dominates (Inbaraj et al. 2006), and the photoisomerization of all-trans-zeaxanthin – the 13-cis-form (Milanowska and Gruszecki 2005). It is generally accepted that the antioxidant effect of carotenoids occurs due to physical quenching. However, Fusi et al. (2023) showed that when interacting with 1O2, carotenoids also transform into a triplet state, which is quenched due to rotation of the C = C bond, leading to isomerization. This mechanism of ROS quenching via cis/trans isomerization may underlie the antioxidant effects of carotenoids. Since the energy barrier for reverse cis to trans isomerization is lower than for direct isomerization, this may explain why the cis-forms of carotenoids are often more effective antioxidants than the trans-forms.
Thermal isomerization is the simplest and most common method for obtaining cis-isomers. Isomerization usually occurs in organic solvents, and the yield of the resulting cis-isomers strongly depends on the nature of the solvent. Chloroform (CHCl3) and dibromomethane (CH2Br2) are more effective than acetone or hexane. But, these solvents do not comply with the food industry and medical applications. For these purposes, ethanol or vegetable oils are used as solvents (Honda 2022). To increase the yield of the resulting isomers, various catalysts are used, and antioxidants are added to reduce the degree of degradation (Kuki et al. 1991; von E Doering et al. 1995; Scheiber and Carle 2005; Sajilata et al. 2008; Yang et al. 2017b; Murakami et al. 2018; Honda et al. 2020a, b; Fusi et al. 2023).
The most effective cis/trans isomerization under the influence of light is observed while excitation of the main absorption maximum (band II) of carotenoid and proceeds through a triplet-excited state (Milanowska and Gruszecki 2005). The light-driven isomerization can be further improved by adding photosensitizers like iodine (Ladygin 2015; Yang et al. 2017b; Honda et al. 2020a). In biological objects, chlorophyll may act as a photosensitizer (Jensen et al. 1982). For processes of isomerization, two ways of T1 formation are possible: as a result of energy or electron transfer. Electron transfer is observed in the presence of electron donors or acceptors and leads to the formation of radical ions. For carotenoids, the reaction with the participation of radical cations is the most typical (Gao et al. 1996; Kispert et al. 2004; Polyakov and Leshina 2006; Honda et al. 2020a; Honda 2022). The same formation of radical cations occurs during electrochemical or simply chemical oxidation of carotenoids, which can also lead to cis/trans isomerization since the energy barrier for C = C bond rotation for radical cations is significantly lower than in a neutral molecule (Polyakov and Leshina 2006; Honda et al. 2020a; Polyakov et al. 2023).
Light is a stress factor and an inducer of carotenoid isomerization in living organisms. The halotolerant algae Dunaliella bardawil and Dunaliella salina are the richest known natural sources of β-carotene. A dry weight of the algae can contain up to 10% of β-carotene with an equal proportion of all-trans and 9-cis-isomers. The biosynthesis of β-carotene occurs in plastidic lipid globules, the formation of which is additionally induced by light stress. All-trans-/9-cis-β-carotene isomerase was found in these lipid globules, the biosynthesis of which is also induced in response to light stress (Davidi and Pick 2017). Thus, the biosynthesis of cis-carotenoids in response to light stress is an example of metabolic adaptation involving cis-carotenoids.
Biosynthesis of some cis-carotenoids and photoregulation of plant metabolism
The biosynthesis of carotenoids from geranylgeranyl diphosphate goes through a series of linear transformations from phytoene to lycopene (Ladygin 2015; Domonkos et al. 2013; Alagoz et al. 2018; Maoka 2020; Tóth et al. 2015). In plants, the biosynthesis of carotenoids goes through a number of additional stages in which various cis-forms of carotenoids are synthesized (Fig. 4). These cis-forms can accumulate in fruits or tissues in the absence of light for a long time. The first enzyme in carotenoid biosynthesis is phytoene synthase (PSY). The biosynthesis of 15-cis-phytoene by PSY is the rate-limiting step in the entire process of carotenoid biosynthesis. The PSY level is regulated by light and significantly decreases in the dark and can also decrease with the accumulation of excess intermediate cis-carotenoids in a feedback manner (Domonkos et al. 2013; Alagoz et al. 2018). Isomerization of carotenoids during biosynthesis can take place both with the participation of enzymes: ζ-carotene isomerase (Z-ISO) and carotenoid isomerase (CRTISO), and under the action of light, when enzymes are absent. Thus, photoregulation of biosynthesis and accumulation of cis-forms of carotenoids in plants occurs. In some cyanobacteria (for example, Synechocystis sp PCC 6803), the biosynthesis of carotenoids also occurs through the formation of intermediate cis-forms and is regulated by light, but with the participation of other enzymes (Masamoto et al. 2001; Tóth et al. 2015).
Fig. 4.
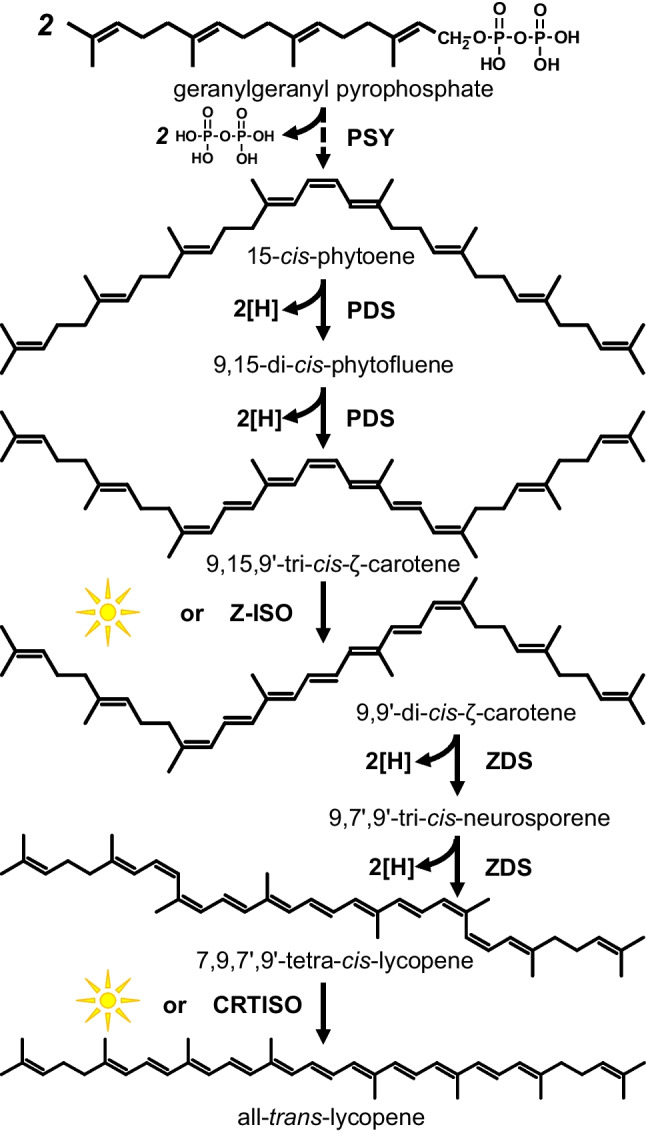
Biosynthesis of lycopene from gyranylgeranyl pyrophosphate in plants. Biosynthetic enzymes are phytoene synthase (PSY), ζ-carotene isomerase (Z-ISO), ζ-carotene desaturase (ZDS), phytoene desaturase (PDS), and carotenoid isomerase (CRTISO)
Carotenoid cleavage products (apocarotenoids) are precursors in the biosynthesis of signaling metabolites, such as phytohormones: abscisic acid and strigolactone. For example, the phytohormone cis-abscisic acid is synthesized from 9'-cis-neoxanthin (Parry et al. 1990; Takaichi and Mirauro 1998). Phytohormones, in turn, are involved in the regulation of leaf development, adaptation to stress, and retrograde signaling. Thus, not only the biosynthesis of carotenoids themselves is regulated but also the regulation of plant metabolism in general with the participation of carotenoid cis-forms. (Alagoz et al. 2018; Cazzonelli et al. 2020).
Involvement of carotenoids in the functioning of the photosynthetic apparatus
Photosynthesis is a biological process in which solar energy is converted into chemical bond energy and used to synthesize organic matter on Earth for over 2 billion years. The energy levels of the carotenoid singlet and triplet states are ideal for participating in the processes of solar energy transfer during photosynthesis.
The light reactions of photosynthesis in the photosynthetic apparatus (PSA) proceed in pigment-protein complexes, which are localized in the thylakoids of chloroplast membranes in eukaryotic organisms. In prokaryotic organisms, these complexes are localized in thylakoids located in the folds of the inner cytoplasmic membrane. In 2021, a new species of cyanobacteria, Anthocertibacter panamensis, was discovered that is capable of photosynthesis with a minimal set of genes and lacks thylakoids. These cyanobacteria release oxygen but grow more slowly than other cyanobacteria, which can be attributed to the absence of thylakoids (Rahmatpour et al. 2021). Thylakoids, which host numerous photosynthetic structures, are an important evolutionarily advanced morphological element. These pigment-protein complex structures include chlorophylls, carotenoids, and proteins. Pigment-protein complexes are organized into two photosystems: photosystem I (PSI) and photosystem II (PSII). Each photosystem includes an LHC and an RC. Xanthophylls are present in LHC, performing both light-harvesting and photoprotective roles there. Carotenes are present in the reaction centers and function as photoprotectors. The excess energy of the triplet state of the sensitizer (chlorophyll) or singlet oxygen 1O2 is transferred to the carotenoid molecule in the ground state, forming the triplet state carotenoid. The carotenoid then loses this energy as heat (Frank and Cogdell 1996; Polívka and Sundström 2004; Telfer et al. 2008; Saccon et al. 2020).
Thus, one of the main functions of carotenoids in PSA is to harvest light energy and transfer it to chlorophyll by singlet–singlet excitation transfer. Carotenoids are able to play the role of additional light-collecting pigments in that part of the solar spectrum (450–550 nm) where chlorophylls weakly absorb. Carotenoids, which have a high molar extinction coefficient, much higher than that of chlorophyll, can perfectly cope with the light-absorbing role. The functions of such additional pigments in plants are performed by lutein, violaxanthin, and neoxanthin in light-harvesting complexes in PSII (Polívka and Sundström 2004; Polívka and Frank 2010; Hashimoto et al. 2018).
Carotenoids can perform different functions within LHC depending on the conformation they adopt and the location and orientation relative to chlorophyll (Polívka and Frank 2010; Liguori et al. 2017). Under aerobic conditions, carotenoids can rapidly quench chlorophyll triplet states as well as scavenge 1O2 (Tamura and Ishikita 2020). There is another protective mechanism called non-photochemical quenching, when carotenoids quench excessive singlet excitation energy, turning it into heat (Telfer et al. 2008; Polívka and Frank 2010; Staleva et al. 2015; Niedzwiedzki et al. 2016).
Carotenoids are also required both for stabilization and proper folding of pigment-protein structures (Mohamed et al. 2005; Telfer et al. 2008; Zakar et al. 2016). A significant contribution to the formation of a carotenoid binding site in a protein is made by the interactions of carotenoids with chlorophylls, which are in close contact with each other (McDermott et al. 1995). Methods of molecular dynamics modeling and quantum chemical calculations have been used to study the interactions leading to the binding of carotenoids in LHCs. It has been found that carotenoid binding is predominantly stabilized by van der Waals interactions with chlorophylls rather than by hydrogen bonding to the protein. The protein binding pocket was shown to be relatively insensitive to the structure of the inserted carotenoid (Mascoli et al. 2021).
As part of the pigment-protein complexes, carotenoids are associated with chlorophylls and protein. The electronic properties of carotenoids are regulated by the binding site in the protein (Llansola-Portoles et al. 2017). Depending on the polarizability properties of the binding site, the properties of carotenoids change. For example, it was shown that in LHC PSII trimers of spinach, one lutein absorbs at 495 nm (lutein1), while the second (lutein2) absorbs at 510 nm. In monomers, these same two luteins absorb at 495 nm. Consequently, changes in the protein environment of pigments lead to conformational changes in carotenoids and their electronic characteristics, although they are bound to the protein by non-covalent bonds. This appears to be due to dispersion interactions between the environment (binding site) and the large dipole moment of carotenoids (Renge and Sild 2011). The interactions of carotenoids with chlorophylls, which are in close contact with each other, make a significant contribution to the polarizability of the binding site (McDermott et al. 1995).
Functions of carotenoids in LHCs
The transfer of excitation energy from carotenoid to chlorophyll in the LHC is very efficient and proceeds by singlet–singlet energy transfer (Fromme 2008; Green and Parson 2003; Koyama 1991). At present, there is no complete understanding of the mechanism of this transfer. This is primarily due to the fact that the photophysics and photochemistry of carotenoids are considered as if carotenoids were simple polyenes. But this is not the case since carotenoids have various substituents in the chain, methyl groups, OH groups, etc., which affect the linear polyene structure, disrupting its planarity, making it asymmetric, and therefore changing its conformation (Hashimoto et al. 2018; Ritz et al. 2000). Based on the excited states of linear polyenes, the lower singlet excited state S1 of carotenoids is believed to be symmetry forbidden. Recently, other views have appeared on the lower excited state of carotenoids and their possible participation in the transfer of excitation energy. It is assumed that there are additional energy states between S2 and S1, for example, S* with a lifetime of up to 12 ps. The distorted structures of the polyene chain are responsible for these intermediate states. Carotenoids with carbonyl groups can form intramolecular charge transfer states, and this allows the energy to be transferred from carotenoids to chlorophyll (Polívka and Sundström 2004). A state with a large dipole moment may arise, allowing the transfer of excitation energy from the carotenoid to chlorophyll due to a strong electronic dipole–dipole interaction (Kosumi et al. 2012). In general, under the influence of functional groups in the polyene chain, deformations occur in carotenoid molecules, and intermediate energy states appear, which are involved in the transfer of excitation energy to chlorophyll (Hashimoto et al. 2018).
The work of Hashimoto et al. (2018) is echoed by Artes Vivancos et al. (2020), who used femtosecond stimulated RS to investigate the function of spinach LHC PSII trimers. As a result, it was possible to characterize the excited states of carotenoids, the pathways for the transfer of excitation energy from carotenoids to chlorophylls, as well as the contribution of the deformation (the S1 energy level is higher for cis-conformations) of carotenoid molecules to this process. Spinach LHC PSII contains 14 chlorophylls and 4 carotenoids per monomer, specifically 2 luteins in the all-trans-form and neoxanthin in the cis-form and some other carotenoids in trace amounts. Luteins are found in two separate protein pockets. Lutein1 with an absorption λmax 495 nm is essential for the structural integrity of the complex and the transfer of excitation energy to chlorophyll. Lutein2 with an absorption λmax 510 nm is required for LHC trimerization and protection from harmful triplet states of chlorophyll. A comparison of the lifetimes of these three xanthophylls in the S1 state showed that lutein1 has the shortest lifetime, and it is this latter that transfers excitation to chlorophyll. The hot state S1 lutein1 has a lifetime of 500 fs and participates in the energy transfer. A comparison of the vibrational frequencies for these xanthophylls in tetrahydrofuran (THF) and in LHC showed that the S1 frequencies for neoxanthin in LHC and THF are close to 1793 cm−1 and 1790 cm−1, respectively. Whereas for lutein1 and lutein2, the frequencies are shifted down in LHC (1781 cm−1 and 1784 cm−1) compared to 1795 cm−1 in THF. This may be the result of a change in the properties of the lutein in the binding pocket in the LHC; therefore, there is a change in the conformation of the lutein and a deviation from the C2h symmetry, which can lead to a decrease in the frequency of S1. In general, the results of the work showed that the transfer of excitation energy from the S2 state of carotenoids to chlorophylls occurs in a sub-picosecond time interval and only the S1 state of lutein1 transfers energy to chlorophylls.
Photoprotective functions of carotenoid isomers
LHCs in plants and green algae adapt their structure and absorbing properties depending on light intensity, switching between light-harvesting and photoprotective states. Under conditions of excess light, when chlorophyll triplets 3Chl* accumulate and ROS, in particular, singlet oxygen, are formed, carotenoids in an altered configuration begin to function. In carotenoids, under the action of light in the presence of chlorophyll as a photosensitizer, cis/trans isomerization occurs with the formation of cis-forms. The neoxanthin present in the LHC operates in the 9'-cis-form under photoprotective conditions and performs several light-induced functions. One of them is associated with NPQ, and at the same time, conformational changes in 9'-cis-neoxanthin cause allosteric modifications in the LHC, and this leads to a dissipative state of the antenna complex (Zubik et al. 2011). Along with 9'-cis-neoxanthin, 9'-cis-violaxanthin can perform a photoprotective function as part of the LHC (Snyder et al. 2004). The next function is the quenching of excited states of chlorophyll and ROS scavenging; 9'-cis-neoxanthin also accelerates violaxanthin cycle reactions of xanthophylls by interacting with violaxanthin (Zubik et al. 2011; Giossi et al. 2020; Maslova et al. 2020). When light-induced functions are performed, the conformation of neoxanthin changes. In particular, neoxanthin has been shown to take on a twisted conformation (Ruban et al. 2007). In the RC of plants, algae, and other photosynthetic organisms, 15-cis-β-carotene functions in a photoprotective mode (Białek-Bylka et al. 1996, 1998; Jordan et al. 2001; Liaaen-Jensen and Lutnœes 2008). 9-cis-β-carotene was found in the cytochrome b6f complex of higher plants, green algae, and some cyanobacteria. It is assumed that it can perform a structural function and quench the triplet states of chlorophyll a, next to which it is located (Kurisu et al. 2003; Domonkos et al. 2013).
The functions of cis-carotenoids in membranes
The hydrophobic core of biomembranes, which consists of polyunsaturated fatty acids, is a potential target for ROS attack, directly leading to membrane degradation. Carotenoids, as highly lipophilic molecules, are usually located within cell membranes. Carotenes, such as β-carotene or lycopene, are found exclusively in the interior of the lipid bilayer. Xanthophylls in the all-trans-configuration (for example, lutein, zeaxanthin) are oriented perpendicular to the membrane surface, exposing their hydrophilic parts to the aquatic environment. It is also possible that all polar groups of the carotenoid molecule can remain in contact with the same polar side of the membrane. This orientation can be in xanthophylls in the cis-conformation and, in the case of lutein, in the all-trans-conformation. This is due to the fact that the system of conjugated double bonds of lutein does not extend to the ε-ring and it has a relative freedom of rotation around a single bond 6'-7'. A consequence of this freedom of rotation is the ability to change the orientation of the hydroxyl group located at the 3'-carbon atom, and due to this ability, lutein can assume different orientations with respect to the lipid bilayer (Gruszecki and Strzałka 2005; Widomska et al. 2023). The monomolecular layer method confirmed the ability of cis-xanthophylls (for example, 13-cis-zeaxanthin) to take a horizontal orientation at the polar-nonpolar boundary in two-component monomolecular layers. The existence of 13-cis- and all-trans-forms of zeaxanthin in two differently oriented positions seems to provide favorable conditions in terms of shielding short-wavelength radiation in biological membranes. It is also possible that horizontal orientation is more effective to protect the membrane from free radical attack from the outside of the membrane, while vertical is more effective against free radicals inside the membrane’s hydrophobic core (Milanowska et al. 2003).
The incorporation of carotenoids in various isomeric forms can also markedly affect the structural and dynamic properties of membranes such as rigidify, mechanical strength, thickness, fluidity, or permeability to small molecules, including oxygen (Gruszecki and Strzałka 2005). Stability and some other membrane-related processes, such as signal transduction, are also modified (Fiedor and Burda 2014); 13-cis-violaxanthin has been shown to be involved in the structural stabilization of the photosynthetic membrane (Grudziński et al. 2001).
Involvement of carotenoid isomers in metabolic adaptation
Carotenoids are very effective ROS quenchers (Zaghdoudi et al. 2017). The molecular mechanisms underlying these processes are still scarcely studied, especially in the context of the antioxidant and prooxidant activity of carotenoids. The carotenoid antioxidant potential is of particular importance for human health due to the fact that the imbalance between antioxidants and ROS leads to “oxidative stress,” the most important factor in the pathogenic processes of various chronic diseases. Evidence from epidemiological studies and clinical trials has shown that carotenoid supplementation in the human diet can significantly reduce the risk of ROS-mediated diseases such as cancer, cardiovascular disease, or photosensitivity disorders (Pagels et al. 2021; Raja et al. 2007; Srivastava et al. 2022). The human diet contains more than 50 carotenoids, of which only ~ 20 are absorbed and present in human blood and tissues. The most important of these include β-carotene, α-carotene, lycopene, lutein, zeaxanthin, β-cryptoxanthin, α-cryptoxanthin, γ-carotene, neurosporen, ζ-carotene, phytofluene, and phytoene (Fiedor and Burda 2014). At the same time, synthetic carotenoids are often less active than those isolated from natural sources because natural ones, in addition to the all-trans-form, usually contain small amounts of cis-carotenoids, which are considered more biologically active (Bechor et al. 2016; Khoo et al. 2011; Levin and Mokady 1994; Rotenstreich et al. 2013; Wang et al. 2022; Xu et al. 2018).
cis-Carotenoids as antioxidants
The interaction of carotenoids with ROS occurs mainly through physical quenching. The energy of singlet molecular oxygen is transferred to the carotenoid molecule to form oxygen in the ground state and a triplet-excited state of carotenoid, followed by energy dissipation (while returning to the ground state), before the cycle can be restarted (Stahl and Sies 2003; Maslova et al. 2020). ROS causes free radical oxidation of various biomolecules, leading to disruption of their functioning. Lipids are the most sensitive to such oxidation and are the main components of membranes. Carotenoids are known to protect lipids from peroxidation (Chisté et al. 2014; Lucas et al. 2022). Experimental data on who better performs the functions of antioxidants, all-trans-forms of carotenoids, or cis-forms are very contradictory (Hernandez-Marin et al. 2013). In some model experiments, 9-cis-β-carotene was shown to be better than all-trans-β-carotene or a mixture of both in preventing lipid peroxidation, which is associated with less stability of the cis-configuration and its higher potential energy (Levin and Mokady 1994). Other model experiments on peroxyl radical quenching (ROO•) have shown that cis-isomers of β-carotene are weaker antiradical agents than all-trans-β-carotene (Rodrigues et al. 2012). This is explained by the fact that the longer the polyene chain, the better the antiradical properties of carotenoids. For cis-forms of carotenoids, the polyene chain is distorted due to structural changes, which may affect its ability to quench radicals. Theoretical calculations using density functional theory showed that mono- and di-cis-β-carotene isomers are stronger antioxidants than all-trans-β-carotene, which may determine their functioning in cyanobacterial PSI (Cerezo et al. 2012). cis-Isomers of astaxanthin exhibit higher antioxidant activity than all-trans-astaxanthin (Liu and Osawa 2007; Yang et al. 2017b). Apparently, the antioxidant properties of cis- and all-trans-isomers of carotenoids differ and depend largely on the conditions under which experiments are carried out (Martínez-Hernández et al. 2019; Lucas et al. 2022; Wang et al. 2022).
cis-Carotenoids and vitamin A
Some carotenoids are provitamins of vitamin A, which include a group of retinol derivatives (dihydroretinol, retinal, retinoic acid, and their various cis/trans isomers). β-carotene entering the human intestine under the action of liver and intestinal enzymes is converted into various derivatives of retinal. All-trans-β-carotene and 13-cis-β-carotene mainly produce all-trans-retinal (about 70%) and 13-cis-retinal (about 20%), while 9-cis-β-carotene gives a mixture of 9-cis-retinal (30%), 13-cis-retinal (20%), and all-trans-retinal (50%). In fact, the conversion rate of cis-isomers is significantly lower than all-trans-β-carotene (Nagao and Olson 1994). Besides β-carotene, all-trans-α-carotene and β-cryptoxanthin also have provitamin A activity (Bohn et al. 2017; Diprat et al. 2017; Wang et al. 2022). 9-cis-Retinoic acid is formed only from 9-cis-β-carotene, whereas all-trans-β-carotene is transformed only into all-trans-retinoic acid (Wang et al. 1994). 9-cis-Retinoic acid acts as a hormone in signaling binding to nuclear receptors and controls the normal maintenance and reproduction of epithelial tissue. Retinoids are also involved in preventing carcinogenesis in various cancers (Bechor et al. 2016; Patrick 2000).
Bioavailability of cis-forms of carotenoids in comparison with all-trans-forms
There are a number of factors affecting the bioavailability, absorption, degradation, transport, and storage of carotenoids (Kopsell and Kopsell 2006; Liang et al. 2019). The release of carotenoids from the food matrix largely depends on their form: free form, microcrystals, or complex with proteins. Absorption of hydrophobic carotenoid molecules in the intestine involves the same steps as for dietary lipids or fat-soluble vitamins. Hydrophobic carotenes are distributed in the body mainly through the use of low-density lipoprotein (LDL), which contains the highest concentration of carotenoids in blood plasma. And less hydrophobic xanthophylls can also bind to other components of the blood plasma. The detectable concentration of carotenoids is found in the liver and adipose tissue, in the adrenal glands, testicles, skin, and retina (macula) (Krinsky et al. 1990; Bernstein et al. 2001; Dachtler et al. 2001; Fiedor and Burda 2014; Yang et al. 2017a). Also, small amounts of carotenoids accumulate in breast milk (including in the form of a significant proportion of cis-isomers), while the composition of carotenoids in milk differs from the composition of carotenoids in the mother’s blood. Milk preferentially accumulates lutein, β-cryptoxanthin, and β-carotene, while lycopene predominates in blood (Khachik et al. 1997b).
There is evidence that cis-isomers may be an important factor in the biological activity of carotenoids. cis-Isomers are more polar, less prone to crystallization, and have higher solubility than all-trans-isomers and are therefore more readily absorbed and transported into cellular compartments (Patrick 2000; Jomova and Valko 2013). Heat treatment of foods containing carotenoids increases their availability by destroying cell walls and loosening bonds and by converting carotenoids to the more readily available cis-form (Scheiber and Carle 2005). It was shown that the efficiency of micellarization of cis-isomers of β-carotene in the human intestine was superior to the efficiency of micellarization of all-trans-β-carotene. Whereas the transfer of cis-isomers through the brush surface of the enterocyte from mixed micelles proceeded with the same efficiency as the transfer of all-trans-β-carotene (Ferruzzi et al. 2006). In human blood serum, β-carotene is found mainly in the all-trans-form (95%), about 5% in the 13-cis-form, and in small amounts in the 9-cis-form. In tissues, cis-isomers accumulate in greater quantities than in the blood. In the liver, 9-cis-β-carotene reaches 25% of the total β-carotene, and 13-cis-β-carotene accumulates more in the adrenal glands (up to 18%), which, apparently, is associated with the functions of cis-carotenoids in these organs (Krinsky et al. 1990; Stahl et al. 1993).
Another picture is observed for the cis-isomers of lycopene. The content of cis-isomers of lycopene is approximately the same in blood plasma and in tissues and can reach up to 50% of the total amount of lycopene. At the same time, the level of cis-forms of lycopene in food is usually much lower (Krinsky et al. 1990; Stahl et al. 1993). The accumulation of cis-lycopene in large amounts in the serum and tissues of humans has prompted a debate among scientists as to whether cis-isomers can be preferentially absorbed by the body or whether all-trans-lycopene is converted to cis-isomers after consumption. The hypothesis that cis-isomers of lycopene are more bioavailable and more readily absorbed from food than all-trans-isomers has received the most support (Bernstein et al. 2001; Failla et al. 2008; Unlu et al. 2007; Zakynthinos and Varzakas 2016). The different bioavailability of lycopene isomers appears to be associated with different affinities for the scavenger receptor class B type I, which is a membrane transporter in the enterocyte and plays an important role in carotenoid absorption (Wang et al. 2023). An additional attraction in fortifying food with cis-isomers of lycopene is the fact that they can prevent breast and prostate cancer (Amorim et al. 2022; Natali et al. 2023).
In studies in vitro using the Caco-2 human intestinal cell model, it was shown that 13-cis-astaxanthin exhibited higher bioavailability and 9-cis-astaxanthin exhibited higher cellular transport efficiency than all-trans-astaxanthin (Yang et al. 2017a). It has also been shown that cis/trans isomerization can occur during digestion and cellular uptake processes (Yang et al. 2017a; Huang and Hui 2020). This apparently explains why the 9-cis- and 13-cis-isomers of astaxanthin accumulate in human blood and tissues, despite the fact that mainly all-trans-astaxanthin comes from food (Østerlie et al. 2000; Honda et al. 2021). Studies in rats have shown that a diet rich in cis-astaxanthin had higher total astaxanthin levels than a diet rich in trans-astaxanthin. At the same time, increased accumulation of cis-isomers was observed in the skin, eyes, lungs, and prostate (Honda et al. 2021).
cis-Carotenoids in various diseases
Retinitis pigmentosa is among the main causes of incurable blindness, affecting 1 in 3500 people. Retinitis pigmentosa is characterized by degeneration of the rod photoreceptor system, leading to night blindness at an early stage, then the disease progresses to tunnel vision, causing progressive loss of visual acuity. In many patients, retinitis pigmentosa is due to a defect in the enzymes of the retinoid cycle. The cycle is initiated by light-induced isomerization and cleavage of rhodopsin (consisting of opsin and 11-cis-retinal) into opsin and all-trans-retinal. The latter subsequently turns back into 11-cis-retinal in the retinal pigment epithelium. 9-cis-Retinal, which has a similar light absorption spectrum to 11-cis-retinal, can replace the latter when its availability is limited due to a defect in the retinoid cycle. 9-cis-β-Carotene is a known precursor of 9-cis-retinal (Nagao and Olson 1994), which can combine with opsin to form isorhodopsin. In addition, 9-cis-β-carotene (including its metabolites) can reduce inflammation and reveals antioxidant activity as well (Martínez-Hernández et al. 2019; Wang et al. 2022). Eating Dunaliella bardawil seaweed powder rich with 9-cis-β-carotene has shown positive results in the treatment of retinitis pigmentosa (Rotenstreich et al. 2013).
Age-related macular degeneration (a major cause of blindness in adults) is a degenerative condition of the macula characterized by the death or dysfunction of photoreceptors. The prevalence of the medical condition is expected to grow as the proportion of elderly persons in the population increases. Lutein and zeaxanthin are macular pigments involved in reducing the development of age-related macular degeneration (Carpentier et al. 2009). It has been shown that, in addition to lutein and zeaxanthin, macular pigments also contain their oxidation products (3'-epilutein, 3-hydroxy-ß,ε-carotene-3'-one, etc.), as well as their geometric isomers: 9-cis- and 13-cis-zeaxanthin and 9-cis-, 9'-cis-, 13-cis-, 13'-cis-lutein (Khachik et al. 1997a). The presence of oxidized carotenoids suggests that the pigments are susceptible to oxidation and thus act as antioxidants to protect the retina from photooxidation by short-wavelength visible light. The presence of cis-isomers in the retina, in addition to the fact that these isomers are also found in small amounts in human blood plasma and can thus enter the eye, is explained by the fact that carotenoids can isomerize under the action of light directly in the retina (Krinsky et al. al. 2003). Based on these data, it can be assumed that cis/trans isomerization may be one of the mechanisms for utilizing excessive light energy in the retina.
Atherosclerosis is a chronic inflammatory disease characterized by the interaction of oxidized lipoproteins with arterial wall cells, especially macrophages present there. Ingestion of modified lipoproteins by macrophages leads to the formation of foam cells derived from macrophages, which is an early sign of the development of atherosclerotic plaques. Reverse cholesterol transport is a process in which excess cholesterol is removed from the body: The cholesterol accumulated in peripheral cells is transported by high-density lipoprotein (HDL) to the liver for excretion. The efflux of cholesterol from macrophages to HDL is a key process in reverse cholesterol transport and therefore may inhibit the formation and progression of atherosclerosis. The export of cholesterol from macrophages to lipoproteins occurs either through passive diffusion or by active transport through the superfamily of ATP-binding cassette transporters. Retinoids regulate many biological activities, including inducing the expression of these carriers in macrophages and thus enhancing the efflux of cholesterol into HDL. Carotenoids are a source of retinoids and therefore may increase the efflux of cholesterol from macrophages. Cleavage of 9-cis-β-carotene produces 9-cis-retinoic acid (Wang et al. 1994), which induces the expression of transporters. It has been found that all-trans-β-carotene does not affect the induction of these carriers, but may influence cholesterol efflux through other mechanisms. The conversion of β-carotene to active metabolites in macrophages leads to the expression of cholesterol transporters, and this induction increases the efflux of cholesterol from macrophages to HDL. It has been shown that 9-cis-β-carotene accumulates in the macrophages of the vascular walls and can increase the outflow of cholesterol from the affected macrophages and, consequently, inhibit atherogenesis. (Bechor et al. 2016; Harari et al. 2013; Shaish et al. 2006; Zolberg Relevy et al. 2015). In addition, carotenoids, being strong antioxidants, can interfere with the oxidation of lipoproteins, which also has a positive effect in preventing the development of atherosclerosis and other cardiovascular diseases caused by lipid peroxidation (Giordano et al. 2012; Levy et al. 2000).
Prostate cancer. The chronic inflammation in the prostate can lead to benign prostatic hyperplasia and cancer. Hyperplasia can seriously impair the quality of life in one-third of people over 50 years of age and in about 90% of those over 80 years of age. Prostate cancer is one of the most common types of cancer among elderly men, with almost 1.5 million new cases each year. A large number of experimental and clinical studies on the prevention of prostatic hyperplasia have focused on lycopene present in various concentrations in tomato-derived products. Lycopene, found in tomatoes, has a wide spectrum of biological activity, which is also preserved in its metabolites. At the same time, it is mainly in an all-trans-isomer form with low bioavailability. The more bioavailable cis-lycopene, which is formed mainly when tomato products are heated, accumulates in certain human organs, including the prostate (Clinton et al. 1996). It has been shown that the nutrition of patients with products enriched with cis-lycopene has a positive effect on the dynamics of the development of age-related pathologies of the prostate and contributes to the prevention of these diseases (Natali et al. 2023). In addition to prostate cancer, the cis-isomers of lycopene, compared with the all-trans-isomer, are better at inhibiting the growth of human carcinoma cells (HepG2) and thus may enhance the treatment effect of liver cancer (Wang et al. 2023).
Astaxanthin as an anti-inflammatory, anti-cancer, and anti-aging agent. Astaxanthin is a xanthophyll found in marine microorganisms and animals. It is a natural and safe dye that is used in medicine because of its antioxidant, anti-inflammatory, anti-cancer, and anti-aging properties (Brotosudarmo et al. 2020). 9-cis-Astaxanthin has been shown to exhibit a greater anti-inflammatory effect than all-trans-astaxanthin due to inhibition of pro-inflammatory cytokine gene expression COX-2, TNF-α, and IL-8 (Yang et al. 2017a, 2019). Since inflammation is associated with oxidative stress, the antioxidant properties of astaxanthin additionally contribute to the improvement of any inflammation. Thus, it was shown in nematodes Caenorhabditis elegans that when fed with a high content of cis-isomers of astaxanthin, the average lifespan of nematodes increased (for 9-cis-astaxanthin, 2 times more than for all-trans-astaxanthin). At the same time, a decrease in the accumulation of ROS in nematode cells was observed, which correlated with increased life expectancy (Liu et al. 2018). In recent years, a number of studies have been conducted on the accumulation of cis-isomers of astaxanthin in the skin (Honda et al. 2021, 2022; Honda and Nishida 2023). It has been shown that even if all-trans-astaxanthin is consumed, significant amounts of the cis-isomers accumulate in the skin. cis-Isomers better protect the skin from exposure to UV light and aging and inhibit melanin formation and elastase production than all-trans-astaxanthin. The barrier function of cis-isomers appears to be due to the fact that they have additional absorption in the UV region (360–370 nm) (Honda et al. 2022; Honda and Nishida 2023). Similar results were shown for the cis-isomers of lycopene and β-carotene (Honda 2023); they also exhibit increased antioxidant, skin anti-aging, and skin-whitening activities compared to the trans isomers.
Conclusions
Numerous studies on carotenoids, conducted from the middle of the twentieth century to the present, have shown that carotenoids are present in nature in the form of all-trans- and cis-isomers. It has been established that cis-forms are involved in the biosynthesis of carotenoids and the regulation of metabolism. It has been shown that neutral or charged radicals participate as intermediates in the process of isomerization. All this demonstrates that carotenoids are rather labile compounds, both in terms of chemical activity and their spatial structure. As pigments with a high molar extinction coefficient, carotenoids form an integral part of the photosynthetic apparatus that carries out photosynthesis on Earth. Recent studies have established that while functioning as part of light-harvesting antenna complexes, carotenoids undergo conformational rearrangements, which make it possible to rearrange the spatial structure (change configuration) in such a way that efficient transfer of excitation energy from carotenoid to chlorophyll takes place. Carotenoids in cis-forms also participate in the photosynthetic apparatus, performing a photoprotective function both in antenna LHCs and in RC. Currently, the chemical properties of the cis- and all-trans-forms are being increasingly studied in the biomedical aspect. They have been shown to have different levels of bioavailability and antioxidant activity in different human organs. This opens up prospects for the use, in particular, of cis-forms for therapeutic and prophylactic purposes, taking into account the often higher bioavailability of cis-isomers.
Author contribution
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by T.A. Telegina, Yu. L. Vechtomova, and A.V. Aybush. The first draft of the manuscript was written by Telegina T.A., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Russian Science Foundation, grant number 21–74-20155.
Data availability
The review does not contain new data.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alagoz Y, Nayak P, Dhami N, Cazzonelli CI. cis-Carotene biosynthesis, evolution and regulation in plants: the emergence of novel signaling metabolites. Arch Biochem Biophys. 2018;654:172–184. doi: 10.1016/j.abb.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Amorim AGN, Vasconcelos AG, Souza J, Oliveira A, Gullón B, de Souza de Almeida Leite JR, Pintado M. Bio-availability, anticancer potential, and chemical data of lycopene: an overview and technological prospecting. Antioxidants. 2022;11(2):360. doi: 10.3390/antiox11020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson P, Bachilo S, Chen R, Gillbro T. Solvent and temperature effects on dual fluorescence in a series of carotenes. Energy Gap Dependence of the Internal Conversion Rate. J Phys Chem. 1995;99:16199–16209. doi: 10.1021/j100044a002. [DOI] [Google Scholar]
- Andreeva A, Apostolova I, Velitchkova M. Temperature dependence of resonance Raman spectra of carotenoids. Spectrochim Acta Part A. 2011;78:1261–1265. doi: 10.1016/j.saa.2010.12.071. [DOI] [PubMed] [Google Scholar]
- Artes Vivancos JM, van Stokkum IHM, Saccon F, Hontani Y, Kloz M, Ruban A, van Grondelle R, Kennis JTM. Unraveling the excited-state dynamics and light-harvesting functions of xanthophylls in light-harvesting complex II using femtosecond stimulated Raman spectroscopy. J Am Chem Soc. 2020;142:17346–17355. doi: 10.1021/jacs.0c04619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker D, Awad TS, Beppu T, Ueda K (2018a) Purification and identification of astaxanthin and its novel derivative produced by radio-tolerant Sphingomonas astaxanthinifaciens. In: Barreiro C, Barredo JL (eds) Microbial Carotenoids. Methods in Molecular Biology, V 1852 pp 171–192 HumanaPress, NewYork. 10.1007/978-1-4939-8742-9_10 [DOI] [PubMed]
- Asker D, Awad TS, Beppu T, Ueda K (2018b) Screening, isolation, and identification of zeaxanthin-producing bacteria. In: Barreiro C, Barredo JL (eds) Microbial Carotenoids. Methods in Molecular Biology, V 1852 pp 193–209 HumanaPress, NewYork. 10.1007/978-1-4939-8742-9_11 [DOI] [PubMed]
- Baranska M, Schütze W, Schulz H. Determination of lycopene and β-carotene content in tomato fruits and related products: comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal Chem. 2006;78:8456–8461. doi: 10.1021/ac061220j. [DOI] [PubMed] [Google Scholar]
- Bechor S, Zolberg Relevy N, Harari A, Almog T, Kamari Y, Ben-Amotz A, Harats D, Shaish A. 9-cis β-carotene increased cholesterol efflux to HDL in Macrophages. Nutrients. 2016;8:435. doi: 10.3390/nu8070435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin K, Nechaev V. Calculation of the IR spectrum and the molecularof β-carotene. J Appl Spectrosc. 2005;72:164–171. doi: 10.1007/s10812-005-0049-x. [DOI] [Google Scholar]
- Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Białek-Bylka GE, Hiyama T, Yumoto K, Koyama Y. 15-Cis-β-carotene found in the reaction center of spinach photosystem I. Photosynth Res. 1996;49:245–250. doi: 10.1007/BF00034785. [DOI] [PubMed] [Google Scholar]
- Białek-Bylka GE, Fujii R, Chen CH, Oh-oka H, Kamiesu A, Satoh K, Koike H, Koyama Y. 15-Cis-carotenoids found in the reaction center of a green sulfur bacterium Chlorobium tepidum and in the photosystem I reaction center of a cyanobacterium Synechococcus vulcanus. Photosynth Res. 1998;58:135–142. doi: 10.1023/A:1006112323144. [DOI] [Google Scholar]
- Białek-Bylka GE, Pawlak K, Jazurek B, Skrzypczak A, Koyama Y. Spectroscopic properties and temperature induced electronic configuration changes of all-trans and 15-cis β-carotenes in ionic liquids. Photosynthetica. 2007;45:161–166. doi: 10.1007/s11099-007-0027-z. [DOI] [Google Scholar]
- Bohn T, Desmarchelier C, Dragsted LO, Nielsen CS, Stahl W, Rühl R, Keijer J, Borel P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol Nutr Food Res. 2017;61:1600685. doi: 10.1002/mnfr.201600685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G. Carotenoid research: history and new perspectives for chemistry in biological systems. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158699. doi: 10.1016/j.bbalip.2020.158699. [DOI] [PubMed] [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids: handbook; Springer science & business media: Berlin/Heidelberg, Germany. p 647. 10.1007/978-3-0348-7836-4
- Brotosudarmo THP, Limantara L, Setiyono E, Heriyanto Structures of astaxanthin and their consequences for therapeutic application. Int J Food Sci. 2020;2020:2156582. doi: 10.1155/2020/2156582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke M, Land EJ, McGarvey DJ, Truscott TG (2000) Carotenoid triplet state lifetimes. 59:132–138. 10.1016/s1011-1344(00)00150-0 [DOI] [PubMed]
- Carpentier S, Knaus M, Suh M. Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr. 2009;49:313–326. doi: 10.1080/10408390802066979. [DOI] [PubMed] [Google Scholar]
- Cazzonelli CI, Hou X, Alagoz Y, Rivers J, Dhami N, Lee J, Marri S, Pogson BJ. A cis-carotene derived apocarotenoid regulates etioplast and chloroplast development. Elife. 2020;9:e45310. doi: 10.7554/eLife.45310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo J, Zúñiga J, Bastida A, Requena A, Cerón-Carrasco JP, Eriksson LA. Antioxidant properties of β-carotene isomers and their role in photosystems: insights from Ab initio simulations. J Phys Chem A. 2012;116:3498–3506. doi: 10.1021/jp301485k. [DOI] [PubMed] [Google Scholar]
- Cerón-Carrasco J, Requena A, Marian C. Theoretical study of the low-lying excited states of -carotene isomers by a multireference configuration interaction method. Chem Phys. 2010;373:98–103. doi: 10.1016/j.chemphys.2010.02.0. [DOI] [Google Scholar]
- Chisté RC, Freitas M, Mercadante AZ, Fernandes E. Carotenoids inhibit lipid peroxidation and hemoglobin oxidation, but not the depletion of glutathione induced by ROS in human erythrocytes. Life Sci. 2014;99:52–60. doi: 10.1016/j.lfs.2014.01.059. [DOI] [PubMed] [Google Scholar]
- Christensson N, Žídek K, Magdaong N, LaFountain A, Frank H, Zigmantas D. Origin of the bathochromic shift of astaxanthin in lobster protein: 2D electronic spectroscopy investigation of β-crustacyanin. J Phys Chem B. 2013;117:11209–11219. doi: 10.1021/jp401873k. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr cis-Trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- Dachtler M, Glaser T, Kohler K, Albert K. Combined HPLC-MS and HPLC-NMR on-line coupling for the separation and determination of lutein and zeaxanthin stereoisomers in spinach and in retina. Anal Chem. 2001;73:667–674. doi: 10.1021/ac000635g. [DOI] [PubMed] [Google Scholar]
- Davidi L, Pick U. Novel 9-cis/all-trans β-carotene isomerases from plastidic oil bodies in Dunaliella bardawil catalyze the conversion of all-trans to 9-cis β-carotene. Plant Cell Rep. 2017;36:807–814. doi: 10.1007/s00299-017-2110-7. [DOI] [PubMed] [Google Scholar]
- De Bruijn WJ, Weesepoel Y, Vincken JP, Gruppen H. Fatty acids attached to all-trans-astaxanthin alter its cis-trans equilibrium, and consequently its stability, upon light-accelerated autoxidation. Food Chem. 2016;194:1108–1115. doi: 10.1016/j.foodchem.2015.08.077. [DOI] [PubMed] [Google Scholar]
- DeCoster B, Christensen R, Gebhard R, Lugtenburg J, Farhoosh R, Frank H. Low-lying electronic states of carotenoids. Biochim Biophys Acta Bioenerg. 1992;1102:107–114. doi: 10.1016/0005-2728(92)90070-i. [DOI] [PubMed] [Google Scholar]
- Demchenko A, Tomin V, Chou P. Breaking the Kasha rule for more efficient photochemistry. Chem Rev. 2017;117:13353–13381. doi: 10.1021/acs.chemrev.7b00110. [DOI] [PubMed] [Google Scholar]
- Diprat AB, Menegol T, Boelter JF, Zmozinski A, Rodrigues Vale MG, Rodrigues E, Rech R. Chemical composition of microalgae Heterochlorella luteoviridis and Dunaliella tertiolecta with emphasis on carotenoids. J Sci Food Agric. 2017;97:3463–3468. doi: 10.1002/jsfa.8159. [DOI] [PubMed] [Google Scholar]
- Domonkos I, Kis M, Gombos Z, Ughy B. Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res. 2013;52:539–561. doi: 10.1016/j.plipres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Du X, Wang C, Wu L, Li Z, Sadiq FA, Jiang Z, Chen F, Ni H, Li Q. Two-dimensional liquid chromatography analysis of all-trans-, 9-cis-, and 13-cis-astaxanthin in raw extracts from Phaffia rhodozyma. J Sep Sci. 2020;43:3206–3215. doi: 10.1002/jssc.202000257. [DOI] [PubMed] [Google Scholar]
- Failla ML, Chitchumroonchokchai C, Ishida BK. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J Nutr. 2008;138:482–486. doi: 10.1093/jn/138.3.482. [DOI] [PubMed] [Google Scholar]
- Fernandes AS, Petry FC, Mercadante AZ, Jacob-Lopes E, Zepka LQ. HPLC-PDA-MS/MS as a strategy to characterize and quantify natural pigments from microalgae. Curr Res Food Sci. 2020;3:100–112. doi: 10.1016/j.crfs.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferruzzi MG, Lumpkin JL, Schwartz SJ, Failla M. Digestive stability, micellarization, and uptake of β-carotene isomers by Caco-2 human intestinal cells. J Agric Food Chem. 2006;54:2780–2785. doi: 10.1021/jf0530603. [DOI] [PubMed] [Google Scholar]
- Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedor L, Heriyanto FJ, Pilch M. Effects of molecular symmetry on the electronic transitions in carotenoids. J Phys Chem Lett. 2016;7:1821–1829. doi: 10.1021/acs.jpclett.6b00637. [DOI] [PubMed] [Google Scholar]
- Frank HA, Cogdell RJ. Carotenoids in photosynthesis. Photochem Photobiol. 1996;63:257–264. doi: 10.1111/j.1751-1097.1996.tb03022.x. [DOI] [PubMed] [Google Scholar]
- Frank H, Bautista J, Josue J, Pendon Z, Hiller R, Sharples F, Gosztola D, Wasielewski M. Effect of the solvent environment on the spectroscopic properties and dynamics of the lowest excited states of carotenoids. J Phys Chem B. 2000;104:4569–4577. doi: 10.1021/jp000079u. [DOI] [Google Scholar]
- Frank H, Josue J, Bautista J, van der Hoef I, Jansen F, Lugtenburg J, Wiederrecht G, Christensen R. Spectroscopic and photochemical properties of open-chain carotenoids. J Phys Chem B. 2002;106:2083–2092. doi: 10.1021/jp013321l. [DOI] [Google Scholar]
- Frank HA, Christensen RL (2008) Excited electronic states, photochemistry and photophysics of carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, vol 4. Birkhäuser Basel. 10.1007/978-3-7643-7499-0_9
- Fromme P (2008) Photosynthetic protein complexes; a structural approach. Weinheim, Germany: Wiley-Blackwell. 10.1002/9783527623464
- Fusi F, Romano G, Speranza G, Agati G. Photon- and singlet-oxygen-induced cis–trans isomerization of the water-soluble carotenoid crocin. Int J Mol Sci. 2023;24:10783. doi: 10.3390/ijms241310783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Wei CC, Jeevarajan AS, Kispert LD. Geometrical isomerization of carotenoids mediated by cation radical/dication formation. J Phys Chem. 1996;100:5362–5366. doi: 10.1021/jp9529230. [DOI] [Google Scholar]
- Gill D, Kilponen R, Rimai L. Resonance Raman scattering of laser radiation by vibrational modes of carotenoid pigment molecules in intact plant tissues. Nature. 1970;227:743–744. doi: 10.1038/227743a0. [DOI] [PubMed] [Google Scholar]
- Gillbro T, Cogdell R. Carotenoid fluorescence. Chem Phys Lett. 1989;158:312–316. doi: 10.1016/0009-2614(89)87342-7. [DOI] [Google Scholar]
- Giordano P, Scicchitano P, Locorotondo M, Mandurino C, Ricci G, Carbonara S, Gesualdo M, Zito A, Dachille A, Caputo P, Riccardi R, Frasso G, Lassandro G, Di Mauro A, Ciccone MM. Carotenoids and cardiovascular risk. Curr Pharm Des. 2012;18:5577–5589. doi: 10.2174/138161212803307527. [DOI] [PubMed] [Google Scholar]
- Giossi C, Cartaxana P, Cruz S. Photoprotective role of neoxanthin in plants and algae. Molecules. 2020;25:4617. doi: 10.3390/molecules25204617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried D, Steffen M, Boxer S. Large protein-induced dipoles for a symmetric carotenoid in a photosynthetic antenna complex. Science. 1991;251:662–665. doi: 10.1126/science.1992518. [DOI] [PubMed] [Google Scholar]
- Gradinaru C, Kennis J, Papagiannakis E, van Stokkum I, Cogdell R, Fleming G, Niederman R, van Grondelle R. An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc Nat Acad Sci USA. 2001;98:2364–2369. doi: 10.1073/pnas.051501298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BR, Parson WW. Light-harvesting antennas in photosynthesis. Dordrecht, The Netherland: Kluwer Academic Publishers; 2003. [Google Scholar]
- Grudziński W, Matuła M, Sielewiesiuk J, Kernen P, Krupa Z, Gruszecki WI. Effect of 13-cis violaxanthin on organization of light harvesting complex II in monomolecular layers. Biochim Biophys Acta. 2001;1503:291–302. doi: 10.1016/s0005-2728(00)00206-1. [DOI] [PubMed] [Google Scholar]
- Gruszecki WI, Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta. 2005;1740:108–115. doi: 10.1016/j.bbadis.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Gruszecki W, Gospodarek M, Grudzinski W, Mazur R, Gieczewska K, Garstka M. Light-induced change of configuration of the LHCII-bound xanthophyll (tentatively assigned to violaxanthin): a resonance Raman study. J Phys Chem B. 2009;113:2506. doi: 10.1021/jp8101755. [DOI] [PubMed] [Google Scholar]
- Guo WH, Tu CY, Hu CH. Cis-trans isomerizations of beta-carotene and lycopene: a theoretical study. J Phys Chem B. 2008;112:12158–12167. doi: 10.1021/jp8019705. [DOI] [PubMed] [Google Scholar]
- Harari A, Abecassis R, Relevi N, Levi Z, Ben-Amotz A, Kamari Y, Harats D, Shaish A. Prevention of atherosclerosis progression by 9-cis-β-carotene rich alga Dunaliella in apoE-deficient mice. Biomed Res Int. 2013;2013:169517. doi: 10.1155/2013/169517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Uragami C, Yukihira N, Gardiner AT, Cogdell RJ. Understanding/unravelling carotenoid excited singlet states. J R Soc Interface. 2018;15:20180026. doi: 10.1098/rsif.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner U, Bernhard K, Meyer K, Englert G, Glinz E. Synthesis, isolation, and NMR-spectroscopic characterization of fourteen (Z)-isomers of lycopene and of some acetylenic didehydro- and tetradehydrolycopenes. Helv Chim Acta. 1992;75:1848–1865. doi: 10.1002/hlca.19920750611. [DOI] [Google Scholar]
- Hernandez-Marin E, Galano A, Martínez A. Cis carotenoids: colorful molecules and free radical quenchers. J Phys Chem B. 2013;117:4050–4061. doi: 10.1021/jp401647n. [DOI] [PubMed] [Google Scholar]
- Honda M. Carotenoid isomers: a systematic review of the analysis, biological activity, physicochemical property, and methods for isomerization. Stud Nat Prod Chem. 2021;68:173–220. doi: 10.1016/B978-0-12-819485-0.00002-5. [DOI] [Google Scholar]
- Honda M. Application of E/Z-isomerization technology for enhancing processing efficiency, health-promoting effects, and usability of carotenoids: a review and future perspectives. J Oleo Sci. 2022;71:151–165. doi: 10.5650/jos.ess21338. [DOI] [PubMed] [Google Scholar]
- Honda M. Z-isomers of lycopene and β-carotene exhibit greater skin-quality improving action than their all-E-isomers. Food Chem. 2023;421:135954. doi: 10.1016/j.foodchem.2023.135954. [DOI] [PubMed] [Google Scholar]
- Honda M, Nishida Y. In vitro evaluation of skin-related physicochemical properties and biological activities of astaxanthin isomers. ACS Omega. 2023;8:19311–19319. doi: 10.1021/acsomega.2c08173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Takahashi N, Kuwa T, Takehara M, Inoue Y, Kumagai T. Spectral characterisation of Z-isomers of lycopene formed during heat treatment and solvent effects on the E/Z isomerisation process. Food Chem. 2015;171:323–329. doi: 10.1016/j.foodchem.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Honda M, Kageyama H, Hibino T, Ichihashi K, Takada W, Goto M. Isomerization of commercially important carotenoids (lycopene, β-carotene, astaxanthin) by natural catalysts: isothiocyanates and polysulfides. J Agric Food Chem. 2020;68:3228–3237. doi: 10.1021/acs.jafc.0c00316. [DOI] [PubMed] [Google Scholar]
- Honda M, Sowa T, Kawashima Y. Thermal- and photoinduced isomerization of all-E- and Z-isomer-rich xanthophylls: astaxanthin and its structurally-related xanthophylls, adonirubin, and adonixanthin. Eur J Lipid Sci Technol. 2020;122:1900462. doi: 10.1002/ejlt.201900462. [DOI] [Google Scholar]
- Honda M, Murakami K, Osawa Y, Kawashima Y, Hirasawa K, Kuroda I. Z-isomers of astaxanthin exhibit greater bioavailability and tissue accumulation efficiency than the all-E-isomer. J Agric Food Chem. 2021;69:3489–3495. doi: 10.1021/acs.jafc.1c00087. [DOI] [PubMed] [Google Scholar]
- Honda M, Kageyama H, Zhang Y, Hibino T, Goto M. Oral supplementation with Z-isomer-rich astaxanthin inhibits ultraviolet light-induced skin damage in Guinea pigs. Mar Drugs. 2022;20:414. doi: 10.3390/md20070414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Uragami C, Tao R, Kosumi D, Cogdell R, Hashimoto H. Excited-state dynamics of all the mono-cis and the major di-cis isomers of β-Apo-8′-carotenal as revealed by femtosecond time-resolved transient absorption spectroscopy. Molecules. 2023;28:4424. doi: 10.3390/molecules28114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiue H, Sasaki M, Yoshikawa Y, Toyofuku M, Shigeto S. Raman spectroscopic signatures of carotenoids and polyenes enable label-free visualization of microbial distributions within pink biofilms. Sci Rep. 2020;10:7704. doi: 10.1038/s41598-020-64737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Hui B. Rat intestinal homogenate and pancreatic juice can induce the Z-isomerization of all-E-lycopene in vitro. Sci Rep. 2020;10:9949. doi: 10.1038/s41598-020-67093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilagan R, Christensen R, Chapp T, Gibson G, Pascher T, Polívka T, Frank H. Femtosecond time-resolved absorption spectroscopy of astaxanthin in solution and in α-crustacyanin. J Phys Chem A. 2005;109:3120–3127. doi: 10.1021/jp0444161. [DOI] [PubMed] [Google Scholar]
- Inbaraj BS, Chien JT, Chen BH. Improved high performance liquid chromatographic method for determination of carotenoids in the microalga Chlorella pyrenoidosa. J Chromatogr A. 2006;1102:193–199. doi: 10.1016/j.chroma.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Iwata K, Hayashi H, Tasumi M. Resonance Raman studies of the conformations of all-trans carotenoids in light-harvesting systems of photosynthetic bacteria. Biochim Biophys Acta. 1985;810:269–273. doi: 10.1016/0005-2728(85)90141-0. [DOI] [Google Scholar]
- Jehlička J, Edwards HG, Osterrothová K, Novotná J, Nedbalová L, Kopecký J, Němec I, Oren A. Potential and limits of Raman spectroscopy for carotenoid detection in microorganisms: implications for astrobiology. Philos Trans A Math Phys Eng Sci. 2014;372:20140199. doi: 10.1098/rsta.2014.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen NH, Nielsen AB, Wilbrandt R. Chlorophyll a-sensitized trans-cis photoisomerization of all-trans-β-carotene. J Am Chem Soc. 1982;104:6117–6119. doi: 10.1021/ja00386a048. [DOI] [Google Scholar]
- Jomova K, Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur J Med Chem. 2013;70:102–110. doi: 10.1016/j.ejmech.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N. Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature. 2001;411:909–917. doi: 10.1038/35082000. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol vis Sci. 1997;38:1802–1811. [PubMed] [Google Scholar]
- Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem. 1997;69:1873–1881. doi: 10.1021/ac961085i. [DOI] [PubMed] [Google Scholar]
- Khoo H-E, Prasad KN, Kong K-W, Jiang Y, Ismail A. Carotenoids and their isomers: color pigments in fruits and vegetables. Molecules. 2011;16:1710–1738. doi: 10.3390/molecules16021710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert LD, Konovalova T, Gao Y. Carotenoid radical cations and dications: EPR, optical, and electrochemical studies. Arch Biochem Biophys. 2004;430:49–60. doi: 10.1016/j.abb.2004.03.036. [DOI] [PubMed] [Google Scholar]
- Kopsell DA, Kopsell DE. Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends Plant Sci. 2006;11:499–507. doi: 10.1016/j.tplants.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kosumi D, Fujiwara M, Fujii R, Cogdell R, Hashimoto H, Yoshizawa M. The dependence of the ultrafast relaxation kinetics of the S2 and S1 states in β-carotene homologs and lycopene on conjugation length studied by femtosecond time-resolved absorption and Kerr-gate fluorescence spectroscopies. J Chem Phys. 2009;130:214506. doi: 10.1063/1.3147008. [DOI] [PubMed] [Google Scholar]
- Kosumi D, Kita M, Fujii R, Sugisaki M, Oka N, Takaesu Y, Taira T, Iha M, Hashimoto H. Excitation energy-transfer dynamics of brown algal photosynthetic antennas. J Phys Chem Lett. 2012;3:2659–2664. doi: 10.1021/jz300612c. [DOI] [PubMed] [Google Scholar]
- Koyama Y. New trends in photobiology: structures and functions of carotenoids in photosynthetic systems. J Photochem Photobiol B. 1991;9:265–280. doi: 10.1016/1011-1344(91)80165-E. [DOI] [Google Scholar]
- Koyama Y, Kito M, Takii T, Saiki K, Tsukida K, Yamashita J. Configuration of the carotenoid in the reaction centers of photosynthetic bacteria. Comparison of the resonance Raman spectrum of the reaction center of Rhodopseudomonas sphaeroides G1C with those of cis-trans isomers of β-carotene. Biochim Biophys Acta Bioenerg. 1982;680:109–118. doi: 10.1016/0005-2728(82)90001-9. [DOI] [Google Scholar]
- Koyama Y, Takatsuka I, Nakata M, Tasumi M. Raman and infrared spectra of the all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of b-carotene: key bands distinguishing stretched or terminal-bent configurations form central-bent configurations. J Raman Spectrosc. 1988;19:37–49. doi: 10.1002/jrs.1250190107. [DOI] [Google Scholar]
- Koyama Y, Fujii R (1999) Cis-trans carotenoids in photosynthesis: configurations‚ excited-state properties and physiological functions. In: Frank HA, Young AJ, Britton G, Cogdell RJ (eds) Photochemistry of Carotenoids. Kluwer Acad Publ, pp 161–188. 10.1007/0-306-48209-6_9
- Krinsky NI, Russett MD, Handelman GJ, Snodderly DM. Structural and geometrical isomers of carotenoids in human plasma. J Nutr. 1990;120:1654–1662. doi: 10.1093/jn/120.12.1654. [DOI] [PubMed] [Google Scholar]
- Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- Kuki M, Koyama Y, Nagae H. Triplet-sesitized and thermal isomerization of all-trans, 7-cis, 13-cis, and 15-cis isomers of beta-carotene: configurational dependence of the quantum yield of isomerization via the T1 state. J Phys Chem. 1991;95:7171–7180. doi: 10.1021/j100172a016. [DOI] [Google Scholar]
- Kurisu G, Zhang H, Smith JL, Cramer WA. Structure of the cytochrome b6f complex of oxygenic photosynthesis: tuning the cavity. Science. 2003;302:1009–1014. doi: 10.1126/science.1090165. [DOI] [PubMed] [Google Scholar]
- Kuznetsova V, Fucimana M, Polívka T. Relaxation dynamics of high-energy excited states of carotenoids studied by UV excitation and pump–repump–probe transient absorption spectroscopy. Phys Chem Chem Phys. 2023;25:22336–22344. doi: 10.1039/D3CP02485G. [DOI] [PubMed] [Google Scholar]
- Ladygin VG (2015) Ways of biosynthesis, localization, metabolism and functions of carotenoids in chloroplasts of different types of algae. Issues of modern algology. p 87. http://algae.ru/633
- Langi P, Kiokias S, Varzakas T, Proestos C (2018) Carotenoids: from plants to food and feed industries. In: Barreiro C, Barredo JL (eds) Microbial Carotenoids. Methods in Molecular Biology, V 1852. Humana Press, New York. 10.1007/978-1-4939-8742-9_3 [DOI] [PubMed]
- Levin G, Mokady S. Antioxidant activity of 9-cis compared to all-trans beta-carotene in vitro. Free Radic Biol Med. 1994;17:77–82. doi: 10.1016/0891-5849(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Levy Y, Zaltsberg H, Ben-Amotz A, Kanter Y, Aviram M. Dietary supplementation of a natural isomer mixture of beta-carotene inhibits oxidation of LDL derived from patients with diabetes mellitus. Ann Nutr Metab. 2000;44:54–60. doi: 10.1159/000012821. [DOI] [PubMed] [Google Scholar]
- Liaaen-Jensen S, Lutnœes BF (2008) E/Z isomers and isomerization. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, vol 4. Birkhäuser Basel. 10.1007/978-3-7643-7499-0_3
- Liang X, Ma C, Yan X, Liu X, Liu F. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene. Trends Food Sci Technol. 2019;93:185–196. doi: 10.1016/j.tifs.2019.08.019. [DOI] [Google Scholar]
- Liguori N, Xu P, van Stokkum IHM, van Oort B, Lu Y, Karcher D, Bock R, Croce R. Different carotenoid conformations have distinct functions in light-harvesting regulation in plants. Nat Commun. 2017;8:1994. doi: 10.1038/s41467-017-02239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindal T-R, Liaaen-Jensen S. Bacterial carotenoids 56. On the Spirilloxanthin Stereoisomeric Set. Acta Chem Scand. 1997;51:1128–1131. doi: 10.3891/acta.chem.scand.51-1128. [DOI] [Google Scholar]
- Liu X, Osawa T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem Biophys Res Commun. 2007;357:187–193. doi: 10.1016/j.bbrc.2007.03.120. [DOI] [PubMed] [Google Scholar]
- Liu X, Chen X, Liu H, Cao Y. Antioxidation and anti-aging activities of astaxanthin geometrical isomers and molecular mechanism involved in Caenorhabditis elegans. J Funct Foods. 2018;44:127–136. doi: 10.1016/j.jff.2018.03.004. [DOI] [Google Scholar]
- Llansola-Portoles MJ, Pascal AA, Robert B. Electronic and vibrational properties of carotenoids: from in vitro to in vivo. J R Soc Interface. 2017;14:20170504. doi: 10.1098/rsif.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llansola-Portoles M, Pascal A, Robert B. Resonance Raman: a powerful tool to interrogate carotenoids in biological matrices. Methods Enzymol. 2022;674:113–135. doi: 10.1016/bs.mie.2022.03.068. [DOI] [PubMed] [Google Scholar]
- Lu L, Shi L, Secor J, Alfano R. Resonance Raman scattering of β-carotene solution excited by visible laser beams into second singlet state. J Photochem Photobiol B. 2018;179:18–22. doi: 10.1016/j.jphotobiol.2017.12.022. [DOI] [PubMed] [Google Scholar]
- Lucas M, Freitas M, Carvalho F, Fernandes E, Ribeiro D (2022) Antioxidant and pro-oxidant activities of carotenoids. In: Ekiert HM, Ramawat KG, Arora J (eds) Plant Antioxidants and Health. Reference Series in Phytochemistry. Springer, Cham. 10.1007/978-3-030-78160-6_4
- Lunde K, Zechmeister L. Infrared spectra and cis-trans configurations of some carotenoid pigments. J Am Chem Soc. 1955;77:1647–1653. doi: 10.1021/ja01611a071. [DOI] [Google Scholar]
- Lutz M, Szponarski W, Berger G, Robert B, Neumann J-M. The stereoisomerization of bacterial, reaction-center-bound carotenoids revisited: an electronic absorption, resonance Raman and NMR study. Biochem Biophys Acta. 1987;894:423–433. doi: 10.1016/0005-2728(87)90121-6. [DOI] [Google Scholar]
- Macpherson A, Gillbro T. Solvent dependence of the ultrafast S2–S1 internal conversion rate of beta-carotene. J Phys Chem A. 1998;102:5049–5058. doi: 10.1021/jp980979z. [DOI] [Google Scholar]
- Maoka T. Carotenoids as natural functional pigments. J Nat Med. 2020;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Hernández GB, Castillejo N, Artés-Hernández F. Effect of fresh–cut apples fortification with lycopene microspheres, revalorized from tomato by-products, during shelf life. Postharvest Biol Technol. 2019;156:110925. doi: 10.1016/j.postharvbio.2019.05.026. [DOI] [Google Scholar]
- Masamoto K, Wada H, Kaneko T, Takaichi S. Identification of a gene required for cis-to-trans carotene isomerization in carotenogenesis of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001;42:1398–1402. doi: 10.1093/pcp/pce167. [DOI] [PubMed] [Google Scholar]
- Mascoli V, Liguori N, Cupellini L, Elias E, Mennucci B, Croce R. Uncovering the interactions driving carotenoid binding in light-harvesting complexes. Chem Sci. 2021;12:5113–5122. doi: 10.1039/d1sc00071c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslova TG, Markovskaya EF, Slemnev NN (2020) Functions of carotenoids in leaves of higher plants (an overview). J Gen Biol 81:297–310. 10.31857/S0044459620040065
- McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ, Isaacs NW. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature. 1995;374:517–521. doi: 10.1038/374517a0. [DOI] [Google Scholar]
- Mendes-Pinto M, Sansiaume E, Hashimoto H, Pascal A, Gall A, Robert B. Electronic absorption and ground state structure of carotenoid molecules. J Phys Chem B. 2013;117:11015–11021. doi: 10.1021/jp309908r. [DOI] [PubMed] [Google Scholar]
- Merlin J. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl Chem. 1985;57:785–792. doi: 10.1351/pac198557050785. [DOI] [Google Scholar]
- Milanowska J, Gruszecki WI. Heat-induced and light-induced isomerization of the xanthophyll pigment zeaxanthin. J Photochem Photobiol B. 2005;80:178–186. doi: 10.1016/j.jphotobiol.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Milanowska J, Polit A, Wasylewski Z, Gruszecki WI. Interaction of isomeric forms of xanthophyll pigment zeaxanthin with dipalmitoylphosphatidylcholine studied in monomolecular layers. J Photochem Photobiol B. 2003;72:1–9. doi: 10.1016/j.jphotobiol.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Minyuk GS, Solovchenko AE (2018) Express analysis of microalgal secondary carotenoids by TLC and UV-vis spectroscopy. In: Barreiro C, Barredo JL (eds) Microbial Carotenoids. Methods in Molecular Biology, V 1852. Humana Press, New York. 10.1007/978-1-4939-8742-9_4 [DOI] [PubMed]
- Mohamed HE, van de Meene AM, Roberson RW, Vermaas WF. Myxoxanthophyll is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 2005;187:6883–6892. doi: 10.1128/JB.187.20.6883-6892.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Song P. Electronic spectra of carotenoids. β-Carotene. J Mol Spectrosc. 1974;52:209–215. doi: 10.1016/0022-2852(74)90111-8. [DOI] [Google Scholar]
- Murakami K, Honda M, Takemura R, Fukaya T, Wahyudiono KH, Goto M. Effect of thermal treatment and light irradiation on the stability of lycopene with high Z-isomers content. Food Chem. 2018;250:253–258. doi: 10.1016/j.foodchem.2018.01.062. [DOI] [PubMed] [Google Scholar]
- Nagao A, Olson JA. Enzymatic formation of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. FASEB J. 1994;8:968–973. doi: 10.1096/fasebj.8.12.8088462. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Fujii R, Nagae H, Koyama Y, Kanematsu Y. Vibrational relaxation in the 1Bu+ state of carotenoids as determined by Kerr-gate fluorescence spectroscopy. Chem Phys Lett. 2004;400:7–14. doi: 10.1016/j.cplett.2004.10.069. [DOI] [Google Scholar]
- Natali PG, Piantelli M, Minacori M, Eufemi M, Imberti L. Improving whole tomato transformation for prostate health: benign prostate hypertrophy as an exploratory model. Int J Mol Sci. 2023;24:5795. doi: 10.3390/ijms24065795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiedzki DM. Transient “cis-peak” is present in excited-state absorption of central-cis isomers of carotenoids. J Phys Chem Lett. 2023;14:1650–1655. doi: 10.1021/acs.jpclett.2c03855. [DOI] [PubMed] [Google Scholar]
- Niedzwiedzki D, Blankenship R. Excited-state properties of the central- cis isomer of the carotenoid peridinin. Arch Biochem Biophys. 2018;649:29–36. doi: 10.1016/j.abb.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Niedzwiedzki D, Dilbeck P, Tang Q, Mothersole D, Martin E, Bocian D, Holten D, Hunter C. Functional characteristics of spirilloxanthin and keto-bearing analogues in light-harvesting LH2 complexes from Rhodobacter sphaeroides with a genetically modified carotenoid synthesis pathway. Biochim Biophys Acta Bioenerg. 2015;1847:640–655. doi: 10.1016/j.bbabio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Niedzwiedzki DM, Tronina T, Liu H, Staleva H, Komenda J, Sobotka R, Blankenship RE, Polívka T. Carotenoid-induced non-photochemical quenching in the cyanobacterial chlorophyll synthase-HliC/D complex. Biochim Biophys Acta. 2016;1857:1430–1439. doi: 10.1016/j.bbabio.2016.04.280. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Ko-chi N, Kuki M, Shimamura T, Cogdell R, Koyama Y. The structures of S0 spheroidene in the light-harvesting (LH2) complex and S0 and T1 spheroidene in the reaction center of Rhodobacter sphaeroides 2.4.1 as revealed by Raman spectroscopy. Biospectroscopy. 1996;2:59–69. doi: 10.1002/(SICI)1520-6343(1996)2:1<59::AID-BSPY6>3.0.CO;2-N. [DOI] [Google Scholar]
- Okamoto H, Sekimoto Y, Tasumi M. Assignment and anharmonicity analysis of overtone and combination bands observed in the resonance Raman spectra of carotenoids. Spectrochim Acta A. 1994;50:1467–1473. doi: 10.1016/0584-8539(94)E0057-H. [DOI] [Google Scholar]
- Østerlie M, Bjerkeng B, Liaaen-Jensen S. Plasma appearance and distribution of astaxanthin E/Z and R/S isomers in plasma lipoproteins of men after single dose administration of astaxanthin. J Nutr Biochem. 2000;11:482–490. doi: 10.1016/s0955-2863(00)00104-2. [DOI] [PubMed] [Google Scholar]
- Ostroumov E, Mulvaney R, Cogdell R, Scholes G. Broadband 2D electronic spectroscopy reveals a carotenoid dark state in purple bacteria. Science. 2013;340:52–56. doi: 10.1126/science.1230106. [DOI] [PubMed] [Google Scholar]
- Ozaki Y, Cho R, Ikegaya K, Muraishi S, Kawauchi K. Potential of near-infrared Fourier transform Raman spectroscopy in food analysis. Appl Spectrosc. 1992;46:1503–1507. doi: 10.1366/000370292789619368. [DOI] [Google Scholar]
- Pagels F, Vasconcelos V, Guedes AC. Carotenoids from cyanobacteria: biotechnological potential and optimization strategies. Biomolecules. 2021;11:735. doi: 10.3390/biom11050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry AD, Babiano MJ, Horgan R. The role of cis-carotenoids in abscisic acid biosynthesis. Planta. 1990;182:118–128. doi: 10.1007/BF00239993. [DOI] [PubMed] [Google Scholar]
- Patias LD, Fernandes AS, Petry FC, Mercadante AZ, Jacob-Lopes E, Zepka LQ. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res Int. 2017;100:260–266. doi: 10.1016/j.foodres.2017.06.069. [DOI] [PubMed] [Google Scholar]
- Patrick L. Beta-carotene: the controversy continues. Altern Med Rev. 2000;5:530–545. [PubMed] [Google Scholar]
- Pfander H. Carotenoids: an overview. Methods Enzymol. 1992;213:3–13. doi: 10.1016/0076-6879(92)13105-7. [DOI] [Google Scholar]
- Polívka T, Frank HA. Molecular factors controlling photosynthetic light harvesting by carotenoids. Acc Chem Res. 2010;43:1125–1134. doi: 10.1021/ar100030m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polívka T, Sundström V. Ultrafast dynamics of carotenoid excited states-from solution to natural and artificial systems. Chem Rev. 2004;104:2021–2071. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- Polyakov NE, Leshina TV. Certain aspects of the reactivity of carotenoids. Redox processes and complexation. Russ Chem Rev. 2006;75:1049–1064. doi: 10.1070/RC2006v075n12ABEH003640. [DOI] [Google Scholar]
- Polyakov NE, Focsan AL, Gao Y, Kispert LD. The endless world of carotenoids—structural, chemical and biological aspects of some rare carotenoids. Int J Mol Sci. 2023;24:9885. doi: 10.3390/ijms24129885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmatpour N, Hauser DA, Nelson JM, Chen PY, Villarreal AJC, Ho MY, Li FW. A novel thylakoid-less isolate fills a billion-year gap in the evolution of Cyanobacteria. Curr Biol. 2021;31:2857–2867. doi: 10.1016/j.cub.2021.04.042. [DOI] [PubMed] [Google Scholar]
- Raja R, Hemaiswarya S, Balasubramanyam D, Rengasamy R. Protective effect of Dunaliella salina (Volvocales, Chlorophyta) against experimentally induced fibrosarcoma on Wistar rats. Microbiol Res. 2007;162:177–184. doi: 10.1016/j.micres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Renge I, Sild E. Absorption shifts in carotenoids—influence of index of refraction and submolecular electric fields. J Photochem Photobiol A. 2011;218:156–161. doi: 10.1016/j.jphotochem.2010.12.015. [DOI] [Google Scholar]
- Rimai L, Heyde ME, Gill D. Vibrational spectra of some carotenoids and related linear polyenes. Raman spectroscopic study. J Am Chem Soc. 1973;95:4493–4501. doi: 10.1021/ja00795a005. [DOI] [PubMed] [Google Scholar]
- Ritz T, Damjanović A, Schulten K, Zhang JP, Koyama Y. Efficient light harvesting through carotenoids. Photosynth Res. 2000;66:125–144. doi: 10.1023/A:1010750332320. [DOI] [PubMed] [Google Scholar]
- Robert B (1999) The electronic structure, stereochemistry and resonance Raman spectroscopy of carotenoids. In: Frank HA, Young AJ, Britton G, Cogdell RJ (eds) The Photochemistry of Carotenoids. Advances in Photosynthesis and Respiration, V 8. Springer, Dordrecht. 10.1007/0-306-48209-6_10
- Rodrigues E, Mariutti LR, Chisté RC, Mercadante AZ. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: application to carotenoids and structure-activity relationship. Food Chem. 2012;135:2103–2111. doi: 10.1016/j.foodchem.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Rotenstreich Y, Belkin M, Sadetzki S, Chetrit A, Ferman-Attar G, Sher I, Harari A, Shaish A, Harats D. Treatment with 9-cis β-carotene-rich powder in patients with retinitis pigmentosa: a randomized crossover trial. JAMA Ophthalmol. 2013;131:985–992. doi: 10.1001/jamaophthalmol.2013.147. [DOI] [PubMed] [Google Scholar]
- Ruban A, Pascal A, Robert B. Xanthophylls of the major photosynthetic light-harvesting complex of plants: identification, conformation and dynamics. FEBS Lett. 2000;477:181–185. doi: 10.1016/s0014-5793(00)01799-3. [DOI] [PubMed] [Google Scholar]
- Ruban AV, Berera R, Ilioaia C, van Stokkum IH, Kennis JT, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- Saccon F, Durchan M, Bína D, Duffy CDP, Ruban AV, Polívka T. A protein environment-modulated energy dissipation channel in LHCII antenna complex. iScience. 2020;23:101430. doi: 10.1016/j.isci.2020.101430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Tasumi M. Normal-coordinate analysis of retinal isomers and assignments of Raman and infrared bands. J Raman Spectrosc. 1983;14:236–245. doi: 10.1002/jrs.1250140405. [DOI] [Google Scholar]
- Sajilata MG, Singhal RS, Kamat MY. The carotenoid pigment zeaxanthin—a review. Compr Rev Food Sci Food Saf. 2008;7:29–49. doi: 10.1111/j.1541-4337.2007.00028.x. [DOI] [Google Scholar]
- Scheiber A, Carle R. Occurrence of carotenoid cis-isomers in foods: technological, analytical, and nutritional implications. Trends Food Sci Technol. 2005;16:416–422. doi: 10.1016/j.tifs.2005.03.018. [DOI] [Google Scholar]
- Schulten K, Karplus M. On the origin of a low-lying forbidden transition in polyenes and related molecules. Chem Phys Lett. 1972;14:305–309. doi: 10.1016/0009-2614(72)80120-9. [DOI] [Google Scholar]
- Schulz H, Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib Spectrosc. 2007;43:13–25. doi: 10.1016/j.vibspec.2006.06.001. [DOI] [Google Scholar]
- Schulz H, Baranska M, Baranski R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers. 2005;77:212–221. doi: 10.1002/bip.20215. [DOI] [PubMed] [Google Scholar]
- Schwieter U, Englert G, Rigassi N, Vetter W. Physical organic methods in carotenoid research. Pure Appl Chem. 1969;20:365–420. doi: 10.1351/pac196920040365. [DOI] [PubMed] [Google Scholar]
- Shaish A, Harari A, Hananshvili L, Cohen H, Bitzur R, Luvish T, Ulman E, Golan M, Ben-Amotz A, Gavish D, Rotstein Z, Harats D. 9-cis beta-carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis. 2006;189:215–221. doi: 10.1016/j.atherosclerosis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Shreve A, Trautman J, Owens T, Albrecht A. Determination of the S2 lifetime of beta-carotene. Chem Phys Lett. 1991;178:89–96. doi: 10.1016/0009-2614(91)85058-5. [DOI] [Google Scholar]
- Snyder AM, Clark BM, Robert B, Ruban AV, Bungard RA. Carotenoid specificity of light-harvesting complex II binding sites. Occurrence of 9-cis-violaxanthin in the neoxanthin-binding site in the parasitic angiosperm Cuscuta reflexa. J Biol Chem. 2004;279:5162–5168. doi: 10.1074/jbc.m309676200. [DOI] [PubMed] [Google Scholar]
- Spano F. Analysis of the UV/Vis and CD spectral line shapes of carotenoid assemblies: spectral signatures of ChiralH-aggregates. J Am Chem Soc. 2009;131:4267–4278. doi: 10.1021/ja806853v. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Kalwani M, Chakdar H, Pabbi S, Shukla P. Biosynthesis and biotechnological interventions for commercial production of microalgal pigments: a review. Bioresour Technol. 2022;352:127071. doi: 10.1016/j.biortech.2022.127071. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003;24:345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sundquist AR, Hanusch M, Schwarz W, Sies H. Separation of beta-carotene and lycopene geometrical isomers in biological samples. Clin Chem. 1993;39:810–814. doi: 10.1093/clinchem/39.5.810. [DOI] [PubMed] [Google Scholar]
- Staleva H, Komenda J, Shukla MK, Šlouf V, Kaňa R, Polívka T, Sobotka R. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat Chem Biol. 2015;11:287–291. doi: 10.1038/nchembio.1755. [DOI] [PubMed] [Google Scholar]
- Strand A, Liaaen-Jensen S. First preparation and characterization of the allenic carotenoids (all-E,6S)- and (9′Z,6S)-neoxanthin. J Chem Soc, Perkin Trans. 2000;1:595–598. doi: 10.1039/A907366C. [DOI] [Google Scholar]
- Subramanian B, Tchoukanova N, Djaoued Y, Pelletier C, Ferron M, Robichaud J. Investigations on the geometrical isomers of astaxanthin: Raman spectroscopy of conjugated polyene chain with electronic and mechanical confinement. J Raman Spectrosc. 2014;45:299–304. doi: 10.1002/jrs.4459. [DOI] [Google Scholar]
- Takaichi S, Mirauro M. Distribution and geometric isomerism of neoxanthin in oxygenic phototrophs: 9′-cis, a sole molecular form. Plant Cell Physiol. 1998;39:968–977. doi: 10.1093/oxfordjournals.pcp.a029461. [DOI] [Google Scholar]
- Tamura H, Ishikita H. Quenching of singlet oxygen by carotenoids via ultrafast superexchange dynamics. J Phys Chem A. 2020;124:5081–5088. doi: 10.1021/acs.jpca.0c02228. [DOI] [PubMed] [Google Scholar]
- Telfer A, Pascal A, Gall A (2008) Carotenoids in photosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, V 4. Birkhäuser Basel. 10.1007/978-3-7643-7499-0_14
- Tóth TN, Chukhutsina V, Domonkos I, Knoppová J, Komenda J, Kis M, Lénárt Z, Garab G, Kovács L, Gombos Z, van Amerongen H. Carotenoids are essential for the assembly of cyanobacterial photosynthetic complexes. Biochim Biophys Acta. 2015;1847:1153–1165. doi: 10.1016/j.bbabio.2015.05.020. [DOI] [PubMed] [Google Scholar]
- Unlu N, Bohn T, Francis D, Nagaraja H, Clinton S, Schwartz S. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br J Nutr. 2007;98:140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- von E Doering W, Sotiriu-Leventis C, Roth WR. Thermal Interconversions among 15-cis-, 13-cis-, and all-trans-β-carotene: kinetics, Arrhenius parameters, thermochemistry, and potential relevance to anticarcinogenicity of all-trans-β-carotene. J Am Chem Soc. 1995;117:2747–2757. doi: 10.1021/ja00115a010. [DOI] [Google Scholar]
- Wang XD, Krinsky NI, Benotti PN, Russell RM. Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro. Arch Biochem Biophys. 1994;313:150–155. doi: 10.1006/abbi.1994.1371. [DOI] [PubMed] [Google Scholar]
- Wang J, Hu X, Chen J, Wang T, Huang X, Chen G. The extraction of β-carotene from microalgae for testing their health benefits. Foods. 2022;11:502. doi: 10.3390/foods11040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lin Y, Liu Q, Zhou A, Bian H, Zhang W, Hui A, Wu Z. Antioxidant, anticancer activity and molecular docking study of lycopene with different ratios of Z-isomers. Curr Res Food Sci. 2023;6:100455. doi: 10.1016/j.crfs.2023.100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasielewski M, Kispert L. Direct measurement of the lowest excited singlet-state lifetime of all-trans-beta-carotene and related carotenoids. Chem Phys Lett. 1986;128:238–243. doi: 10.1016/0009-2614(86)80332-3. [DOI] [Google Scholar]
- Widomska J, Subczynski WK, Welc-Stanowska R, Luchowski R. An overview of lutein in the lipid membrane. Int J Mol Sci. 2023;24:12948. doi: 10.3390/ijms241612948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ibrahim IM, Wosu CI, Ben-Amotz A, Harvey PJ. Potential of new isolates of Dunaliella Salina for natural β-carotene production. Biology. 2018;7:14. doi: 10.3390/biology7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuzaki J (2017) Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database J Biol Databases Curation 2017:bax004. 10.1093/database/bax004 [DOI] [PMC free article] [PubMed]
- Yang C, Zhang H, Liu R, Zhu H, Zhang L, Tsao R. Bioaccessibility, cellular uptake, and transport of astaxanthin isomers and their antioxidative effects in human intestinal epithelial Caco-2 cells. J Agric Food Chem. 2017;65:10223–10232. doi: 10.1021/acs.jafc.7b04254. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang L, Zhang H, Sun Q, Liu R, Li J, Wu L, Tsao R. Rapid and efficient conversion of all-E-astaxanthin to 9Z- and 13Z-isomers and assessment of their stability and antioxidant activities. J Agric Food Chem. 2017;65:818–826. doi: 10.1021/acs.jafc.6b04962. [DOI] [PubMed] [Google Scholar]
- Yang C, Hassan YI, Liu R, Zhang H, Chen Y, Zhang L, Tsao R. Anti-inflammatory effects of different astaxanthin isomers and the roles of lipid transporters in the cellular transport of astaxanthin isomers in Caco-2 cell monolayers. J Agric Food Chem. 2019;67:6222–6231. doi: 10.1021/acs.jafc.9b02102. [DOI] [PubMed] [Google Scholar]
- Yao G, Muhammad M, Zhao J, Liu J, Huang Q. DFT-based Raman spectral study of astaxanthin geometrical isomers. Food Chem (oxf) 2022;4:100103. doi: 10.1016/j.fochms.2022.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JP, Chen F. Isomerization of trans-astaxanthin to cis-isomers in organic solvents. J Agric Food Chem. 1999;47:3656–3660. doi: 10.1021/jf981319u. [DOI] [PubMed] [Google Scholar]
- Zaghdoudi K, Ngomo O, Vanderesse R, Arnoux P, Myrzakhmetov B, Frochot C, Guiavarc’h Y. Extraction, identification and photo-physical characterization of persimmon (Diospyros kaki L.) Carotenoids. Foods. 2017;6:4. doi: 10.3390/foods6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakar T, Laczko-Dobos H, Toth TN, Gombos Z. Carotenoids assist in cyanobacterial photosystem II assembly and function. Front Plant Sci. 2016;7:295. doi: 10.3389/fpls.2016.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakynthinos G, Varzakas T (2016) Carotenoids: from plants to food industry. Curr Res Nutr Food Sci 4 (Special Issue Carotenoids). 10.12944/CRNFSJ.4.Special-Issue1.04
- Zechmeister L, Polgar A. cis-trans isomerization and spectral characteristics of carotenoids and some related compounds. J Am Chem Soc. 1943;65:1522–1528. doi: 10.1021/ja01248a025. [DOI] [Google Scholar]
- Zhao L, Chen F, Zhao G, Wang Z, Liao X, Hu X. Isomerization of trans- astaxanthin induced by copper(II) ion in ethanol. J Agric Food Chem. 2005;53:9620–9623. doi: 10.1021/jf0517750. [DOI] [PubMed] [Google Scholar]
- Zhao L, Zhao G, Chen F, Wang Z, Wu J, Hu X. Different effects of microwave and ultrasound on the stability of (all-E)-astaxanthin. J Agric Food Chem. 2006;54:8346–8351. doi: 10.1021/jf061876d. [DOI] [PubMed] [Google Scholar]
- Zigmantas D, Polivka T, Hiller R, Yartsev A, Sundstrom V. Spectroscopic and dynamic properties of the peridinin lowest singlet excited states. J Phys Chem A. 2001;105:10296–10306. doi: 10.1021/jp010022n. [DOI] [Google Scholar]
- Zigmantas D, Hiller R, Sharples F, Frank H, Sundström V, Polívka T. Effect of a conjugated carbonyl group on the photophysical properties of carotenoids. Phys Chem Chem Phys. 2004;6:3009–3016. doi: 10.1039/b315786e. [DOI] [Google Scholar]
- Zolberg Relevy N, Bechor S, Harari A, Ben-Amotz A, Kamari Y, Harats D, Shaish A. The Inhibition of macrophage foam cell formation by 9-cis β-carotene is driven by BCMO1 activity. PLoS ONE. 2015;10:e0115272. doi: 10.1371/journal.pone.0115272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubik M, Luchowski R, Grudzinski W, Gospodarek M, Gryczynski I, Gryczynski Z, Dobrucki JW, Gruszecki WI. Light-induced isomerization of the LHCII-bound xanthophyll neoxanthin: possible implications for photoprotection in plants. Biochim Biophys Acta. 2011;1807:1237–1243. doi: 10.1016/j.bbabio.2011.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The review does not contain new data.


