Abstract
Unhealthy vascular aging promotes atherogenesis, which may lead to significant internal carotid artery stenosis (CAS) in 5 to 7.5% of older adults. The pathogenic factors that promote accelerated vascular aging and CAS also affect the downstream portion of the cerebral microcirculation in these patients. Primary treatments of significant CAS are eversion endarterectomy or endarterectomy with patch plasty. Factors that determine adequate hemodynamic compensation and thereby the clinical consequences of CAS as well as medical and surgical complications of carotid reconstruction surgery likely involve the anatomy of the circle of Willis (CoW), the magnitude of compensatory inter-hemispheric blood flow, and the effectiveness of cerebral microcirculatory blood flow autoregulation. This study aimed to test two hypotheses based on this theory. First, we hypothesized that patients with symptomatic and asymptomatic CAS would exhibit differences in autoregulatory function and inter-hemispheric blood flow. Second, we predicted that anatomically compromised CoW would associate with impaired inter-hemispheric blood flow compensation. We enrolled older adults with symptomatic or asymptomatic internal CAS (>70% NASCET criteria; n = 46) and assessed CoW integrity by CT angiography. We evaluated transient hyperemic responses in the middle cerebral arteries (MCA) after common carotid artery compression (CCC; 10 s) by transcranial Doppler sonography (TCD). We compared parameters reflecting autoregulatory function (e.g., transient hyperemic response ratio [THRR], return to baseline time [RTB], changes of vascular resistance) and inter-hemispheric blood flow (residual blood flow velocity). Our findings revealed that CAS was associated with impaired cerebral vascular reactivity. However, we did not observe significant differences in autoregulatory function or inter-hemispheric blood flow between patients with symptomatic and asymptomatic CAS. Moreover, anatomically compromised CoW did not significantly affect these parameters. Notably, we observed an inverse correlation between RTB and THRR, and 49% of CAS patients exhibited a delayed THRR, which associated with decreased inter-hemispheric blood flow. Future studies should investigate how TCD-based evaluation of autoregulatory function and inter-hemispheric blood flow can be used to optimize surgical techniques and patient selection for internal carotid artery revascularization.
Keywords: Cerebrovascular reactivity, Transcranial Doppler sonography, Carotid artery stenosis, Transient hyperemic response, Cerebral hemodynamics
Introduction
Internal carotid artery stenosis (CAS) is a steno-occlusive arterial disease, usually caused by atherosclerosis associated with unhealthy vascular aging [1] The prevalence of CAS is approximately 5 to 7.5% in older adults [2–4] There is significant variability in the severity of clinical manifestations of CAS. The atherosclerotic plaque causing the narrowing of the internal carotid artery can be stable and asymptomatic, or it can be a source of thromboembolization to the brain, leading to ischemic events (“symptomatic CAS,” approximately one-third of all CAS cases [5]). Symptomatic CAS is characterized by either symptoms associated with temporary brain ischemia (transient ischemic attack [TIA]) or ischemic stroke. Atherosclerotic CAS is a leading risk factor for ischemic stroke, which is a major cause of mortality and long-term disability in developed countries [6–8]. To prevent stroke, eligible patients with significant CAS are treated with either eversion endarterectomy or endarterectomy with patch plasty [1, 9].
The mechanisms by which CAS promotes cerebral ischemic events are thought to involve hemodynamic mechanisms in addition to thromboembolism [10–16]. It has also been proposed that dysregulation of cerebral blood flow regulation in CAS patients may also contribute to perioperative complications during carotid endarterectomy or carotid artery reconstruction surgery [5, 17, 18]. Factors that determine adequate hemodynamic compensation and thereby the clinical consequences of CAS as well as medical and surgical complications of carotid reconstruction surgery likely involve the anatomy of the circle of Willis (CoW) [13, 19–31], the magnitude of compensatory inter-hemispheric blood flow, and the effectiveness of cerebral microcirculatory blood flow autoregulation [10–16, 19, 20, 32–38].
The pathogenic factors that accelerate vascular aging, exacerbate atherogenesis in the large arteries, and promote the genesis of CAS also affect the function and phenotype of resistance arteries in the brain [39–47]. It has been proposed that the resulting functional impairment of microvessels may compromise cerebral blood flow regulation [10–16, 19, 20, 32–38] and exacerbate the deleterious effects of thromboembolism, increasing the risk of infarction in the border zone areas of the brain [48–55]. Autoregulation of cerebral blood flow is a homeostatic mechanism that is critical for the maintenance of oxygen and nutrient supply to the brain when perfusion pressure decreases [16, 39, 56–59]. Importantly, CAS has been linked to autoregulatory dysfunction in older patients [11, 14–16, 37]. Yet, the differential effects of symptomatic and asymptomatic CAS on autoregulation of cerebral blood flow remain elusive.
A well-developed collateral circulation is critical for the maintenance of cerebral perfusion through inter-hemispheric blood flow compensation in patients with CAS [10]. Impairment of inter-hemispheric blood flow due to anatomical or functional alterations of the collateral circulation increases the risk of stroke and exacerbates perioperative complications in these patients [18, 27, 60]. Among the factors contributing to impaired inter-hemispheric blood flow compensation in CAS, decreased cerebral primary anastomosis capacity based on abnormal anatomical variations of the CoW [21–31] communicating connections plays a central role [61, 62]. The prevalence of CoW variations is unexpectedly high [63]. It is presently unknown how absence of collateral flow via the CoW and impaired vasomotor reactivity are related.
The present study was conducted to test two related hypotheses: (1) that patients with symptomatic and asymptomatic CAS exhibit differences in autoregulatory function and inter-hemispheric blood flow, and (2) that anatomically compromised CoW results in impaired inter-hemispheric blood flow compensation. To test these predictions, in older adults with symptomatic or asymptomatic internal CAS (>70%), CoW integrity was assessed by CT angiography and cerebral vasoreactivity and autoregulatory function was assessed by a transcranial Doppler sonography (TCD)-based method.
TCD is one of the most widely used, reliable method for assessing cerebral vasoreactivity and autoregulatory function. Using TCD changes in cerebral blood flow velocity (BFV) in proximal intracranial arteries can be measured with excellent temporal sensitivity [64–66]. Several TCD methods have been developed to assess cerebrovascular function (e.g., hyperventilation test, apnea test and acetazolamide test, thigh cuff test). In the present study, we used the common carotid artery compression (CCC) test, which is a validated method to estimate cerebral vasoreactivity and autoregulatory function [67–71]. Importantly, the CCC test is the only functional TCD test that mimics the hemodynamic conditions associated with clamping during carotid reconstruction surgery [72]. During CCC, the perfusion pressure in the ipsilateral middle cerebral artery (MCA) is significantly reduced, which is associated with an immediate decrease in BFV. When compression is released, a rapid transient increase in BFV is measured. This phenomenon is known as the transient hyperemic response (THR) from which cerebral vasoreactivity can be estimated [73–75]. Due to the significant changes in blood pressure [76, 77] and BFV occurring in a short period of time, the CCC test is often interpreted primarily as a dynamic autoregulation test. Yet, in addition to the pressure-sensitive autoregulatory myogenic function of the cerebral resistance arteries and arterioles, the characteristics of the transient hyperemic response are likely influenced by local vasoregulatory mechanisms activated by the sudden decline in oxygen delivery, shear stress-induced, endothelium-mediated regulation of vascular tone and arterial wall stiffness as well. Thus, analysis of transient hyperemic responses in CAS patients may provide useful information on multiple aspects of macro- and microvascular health. We performed simultaneous bilateral measurement of BFV in the right and left MCAs during unilateral CCC to characterize compensatory inter-hemispheric blood flow as well.
Materials and methods
This study was conducted in accordance with the ethical principles outlined by the Regional and Institutional Committee of Science and Research Ethics of Semmelweis University (SE-RKEB, protocol number: 256/2018) and was registered at ClinicalTrials.gov (Identifier: NCT03840265). We consecutively enrolled patients who were admitted to the Semmelweis University Department of Vascular Surgery for the evaluation of internal carotid artery (ICA) stenosis between January 2019 and September 2021. Control participants with an absence of CAS diagnosis and other major diseases were recruited by flyers distributed at the clinic and by word of mouth.
Written informed consent was obtained from all participants. The severity of ICA stenosis and circle of Willis (CoW) morphology were assessed by CT angiography in CAS patients, and patients with significant ICA stenosis (>70%) were included in the study. Severity of the ICA stenosis was given based on the North American Symptomatic Carotid Endarterectomy Trial (NASCET [78]) criteria [79]. Transcranial Doppler (TCD) tests were performed only if an appropriate temporal insonation bone window was present, and exclusion criteria included features such as atrial fibrillation, extensive emboligenic plaques in the common carotid artery, and carotid sinus hyperesthesia that may compromise the performance or evaluation of the functional TCD tests. Symptomatic CAS was defined when an ipsilateral non-lacunar ischemic event was present (TIA or minor stroke in the previous 6 months), while patients with asymptomatic CAS had none of the aforementioned factors present. We collected patients’ demographic and anthropometric data (sex, age, BMI), medical history (hypertension, diabetes mellitus, smoking status, ischemic heart disease, chronic lung disease, contralateral stenosis), and results of standard laboratory tests (including thrombocyte level, serum cholesterol, low-density lipoprotein cholesterol [LDL], high-density lipoprotein cholesterol [HDL], triglyceride level, creatinine, estimated glomerular filtration rate [eGFR]). Previous medical history was based on patient interviews and prior medical documentation.
Study protocol: transcranial Doppler sonography
The Doppler Laboratory of the Department of Neurology at Semmelweis University performed TCD examinations on patients. A bilateral, fixed TCD transducer (2 MHz, DWL Multi-Dop T2, Sipplingen, Germany) was used to record blood flow velocity in the MCAs while the patient was in a supine position and at rest through the transtemporal insonation window at a depth of 50–55 mm [80, 81]. The transducers were fixed in a head frame and adjusted for the maximum signal strength. The analog output of the TCD equipment was the envelope that was derived from the maximum of the flow velocity power spectrum following fast-Fourier transformation. Continuous, non-invasive, beat-to-beat arterial blood pressure monitoring was performed using radial artery applanation tonometry (Colin-BP508, Hayashi Komaki Aichi, Japan), which was calibrated with a standard upper arm arterial blood pressure cuff on the contralateral side. Heart rate was measured with a 3-lead surface ECG, and respiratory rate was monitored using exhaled air CO2 (EtCO2) with a capnograph connected to a nasal cannula (Colin-BP508, Hayashi Komaki Aichi, Japan).
A baseline (BL) registration was recorded after a minimum rest period of 15 min. Radial arterial blood pressure was calibrated before each test, and patients were monitored clinically during the tests constantly. If there were any complaints, the tests were suspended. Of the 82 patients who met the inclusion criteria, those who were unable to have TCD measurements due to a lack of an insonation window were excluded. TCD signal quality criteria were also used for patient selection. Only signals of appropriate quality that allowed for long-term monitoring were accepted, while artifact-distorted registrations with poor quality were deemed unsuitable for the study. To ensure reproducibility and achieve the best signal-to-noise ratio, the tests were performed three times. The technically and qualitatively most optimal registration was evaluated for each patient. The compression with inadequate efficiency and improper patient cooperation can lead to distortion during the CCC test. In this study, to avoid these biases, the compression was considered effective when the blood flow velocity (BFV) reduction was at least 40% [75]. A 10-s compression was used based on the results of previous studies, as this duration can achieve the maximum THR, and a longer duration of compression does not increase the THR [75]. After excluding patients with sub-optimal measurements, measurements of 5 control participants and 46 CAS patients were found eligible for further analysis (Fig. 1).
Fig. 1.
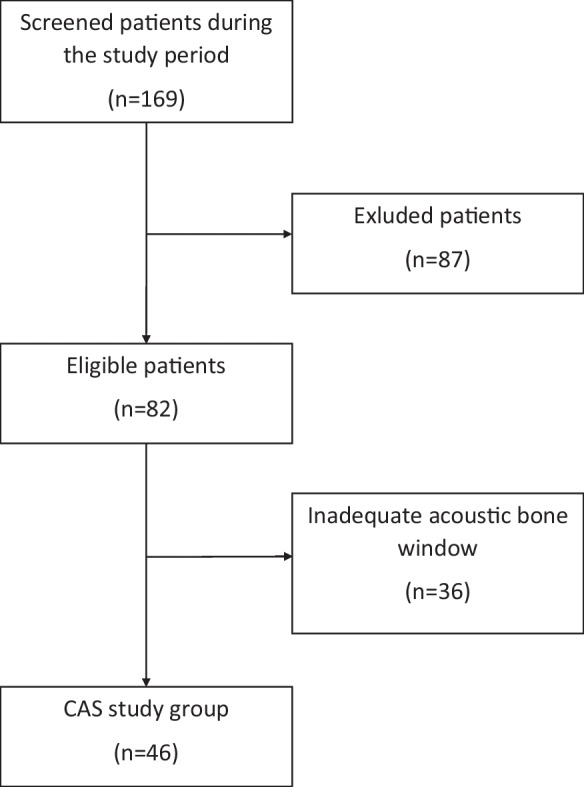
Study group flow chart
Data processing
The analog signals were digitized simultaneously on 5 channels (TCD1, TCD2, ECG, capnograph, tonometry) at a sampling frequency of 500 Hz. Raw data for each patient were saved in European Data Format (EDF) files, which were imported into LabChart software (AdInstruments, LabChart ver. 8, Colorado Springs, CO, USA) for digital filtering and segmentation. The resulting pre-processed data segments were stored in separate text files, which were then processed by an in-house Python code. The code linearly interpolated the five channels into 0.5-s intervals and exported the interpolated data to Microsoft Excel. The amplitude and time variables of the cerebrovascular reactivity were subsequently calculated.
Calculation of BFV variables
The response to CCC stimulus can be described as occurring in two distinct phases, each characterized by changes in opposite directions (Figs. 2 and 3). Initially, the dilation of downstream resistance vessels induced by the pressure drop leads to an increase in blood flow (and thereby flow velocity) in the MCA after the compression is released. This increase often surpasses the baseline value observed under normal conditions which is commonly referred to as the THR. This hyperperfusion state triggers an opposite process, namely, pressure-induced (myogenic) vasoconstriction in the resistance arteries and arterioles. The return of cerebral blood flow velocity in the MCA to resting levels following this process is likely due to a combination of active myogenic [43, 82] and shear stress-induced [57, 83] vasoconstriction.
Fig. 2.
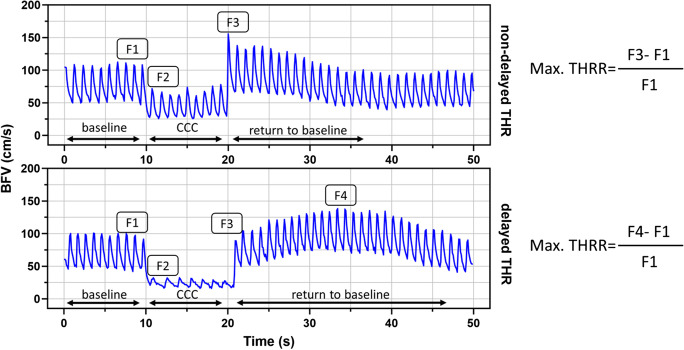
Representative registration of patients with carotid artery stenosis (CAS), illustrating non-delayed transient hyperemic response (THR) in the upper panel and delayed THR in the lower panel. Equations for calculating the maximal transient hyperemic response ratio (THRR) are provided for each group. BFV denotes blood flow velocity, CCC represents the common carotid artery compression test, RTB indicates return to baseline, F1 represents baseline BFV, F2 represents initial BFV during CCC, F3 corresponds to the early peak BFV, and F4 denotes the maximal BFV if the maximum blood flow velocity did not occur immediately after the end of CCC, but after a few cardiac cycles
Fig. 3.
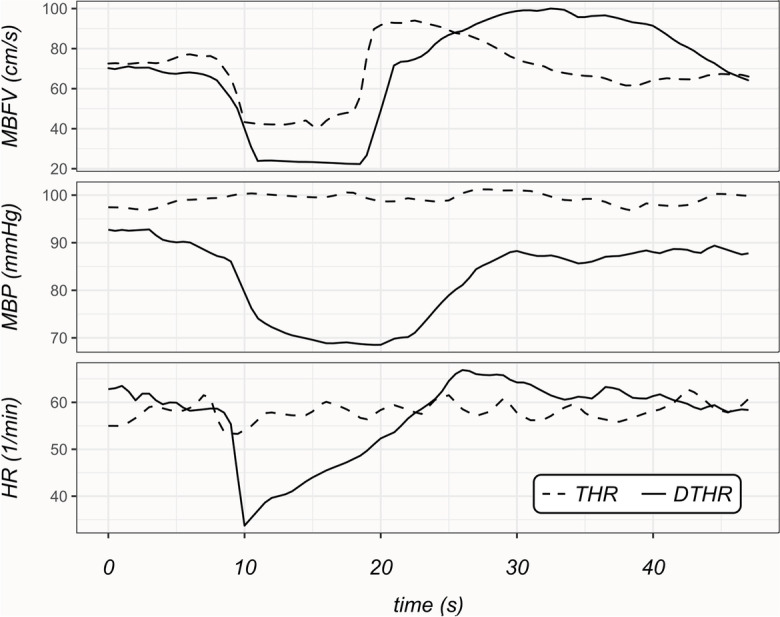
Representative recordings of patients with carotid artery stenosis (CAS) categorized into two groups based on the dynamics of transient hyperemic response: normal (THR) and delayed-type transient hyperemic response (DTHR). The recordings include mean blood flow velocity (MBFV), mean blood pressure (MBP), and heart rate (HR) measurements
The BFV variables were calculated based on the distinctive changes observed in the MCAs during the CCC test. The analysis identified four distinct phases and peaks (F1–F4) (see Fig. 2). The baseline value (F1) was recorded immediately prior to the CCC test. F2 was defined as the BFV plateau that occurred immediately after the start of the CCC. F3 referred to the early peak of BFV increases that occurred immediately after the end of the CCC. This reaction type was considered a normal transient hyperemic response. In some patients, the maximum BFV response was not observed immediately after the end of CCC, but rather after a few cardiac cycles (F4). This type of BFV response was defined as a delayed transient hyperemic response.
The transient hyperemic response ratio (THRR) was calculated as the increase in BFV measured immediately after CCC, expressed relative to baseline ((F3 − F1)/F1). THRR was determined using previously published methods [75, 84, 85] based on mean BFV. Delayed THRR was calculated as the maximal BFV increase after the release of CCC, expressed relative to baseline ((F4 − F1)/F1). Patients could be categorized into intact vasoreactivity (THRR > 10%) and impaired vasoreactivity (THRR < 10%) groups, based on the literature suggesting that a minimum BFV increase of 10% indicates intact cerebral vasoreactivity [75].
To estimate compressible perfusion, the percentage by which BFV decreases during CCC compared to baseline was calculated.
Cumulative blood flow and repayment during post-CCC hyperemia were estimated by calculating the area under the curve (AUC) from the mean BFV curve, from the end of CCC to the return to baseline. Excess cumulative blood flow was estimated as the integrated BFV during THR minus the integrated pre-CCC BFV for a period corresponding to the duration of the hyperemic response.
Cerebrovascular resistance (CVR) was defined as the quotient of mean arterial blood pressure and BFV [86]. CVR was determined at F1, F3, and F4 points. CVR-THRR and CVR-delayed THRR values were defined similarly to THRR, where the change in resistance from baseline (CVRF1) to the minimum CVR value after compression (F3 or F4) was calculated as CVRTHRR or CVRdelayed_THRR (CVRTHRR = (CVRF3 − CVRF1)/CVRF1 and CVRdelayed_THRR = (CVRF4 − CVRF1)/CVRF1). CRTHRR was calculated for all patients, while CRdelayed_THR values were calculated for patients with delayed THR reaction.
Return to baseline time (RTB) was defined as the time required for BFV to return to baseline value after manual compression release (Fig. 2). The decrease in arterial blood pressure during compression was considered if a change larger than 2 standard deviations in arterial blood pressure was present compared to baseline measurements. Heart rate reduction was considered based on a similar calculation. Time to peak (TTP) was defined as the time (in seconds) required for maximum BFV increase (F3 or F4) to occur from the end of CCC.
Assessment of CoW morphology
Two expert neuroradiologists evaluated CoW morphology using CT angiography. Segments were classified as normal (diameter > 0.8 mm), hypoplastic (diameter ˂ 0.8 mm), or aplastic (non-visualized) based on previous studies [61, 87]. A compromised CoW was defined as at least one of the posterior or anterior CoW segments (anterior communicating artery, anterior cerebral artery A1 segment, posterior communicating artery) being aplastic or hypoplastic. All segments were present in the intact CoW group.
Statistical analysis
The statistical analysis and visualization of data was performed with GraphPad Prism 9.2.0 program. The significance level was defined as p < 0.05 for all tests. Categorical variables of groups were compared with χ2 test or Fisher’s exact test. Normal distribution of data was determined using the Kolmogorov-Smirnov test. Outlier values were excluded using the ROUT test (Q = 1%), and the number of excluded outliers are reported where applicable. For multigroup comparisons of normally distributed data, the one-way ANOVA test with post hoc Tukey’s test or two-way ANOVA with post hoc Bonferroni test was conducted. For comparisons of non-normally distributed data, Mann-Whitney U test and Kruskal-Wallis test with Dunn’s post hoc test were used. The relationship between non-normally distributed data was investigated using the Spearman correlation test.
Results
Our study included a total of 46 patients, comprising 14 women (mean age: 69 ± 7.4 years) and 32 men (mean age: 68 ± 6.8 years), as shown in Fig. 1. Almost one-third of the patients reported being smokers, and one-third had diabetes mellitus. All patients had treated hypertension. Of the total patients, 15 (32%) had symptomatic ICA stenosis, while 31 (67%) had asymptomatic ICA stenosis. None of the patients experienced any discomfort or adverse reaction during the CCC tests.
Comparison of patients with asymptomatic vs. symptomatic CAS
Table 1 presents the baseline characteristics of patients with asymptomatic and symptomatic CAS. Notably, compromised CoW was found to be significantly more prevalent in the symptomatic group, affecting 40% of patients as compared to only 10% of asymptomatic patients. Additionally, the symptomatic CAS group showed a lower incidence of delayed THR, with only 27% of patients exhibiting this symptom, as opposed to 58% in the asymptomatic CAS group (p = 0.046).
Table 1.
Demographic and clinical characteristics of patients with carotid artery stenosis (CAS) grouped by symptomatic status
| Symptomatic (n = 15) | Asymptomatic (n = 31) | p value | Statistical test | |
|---|---|---|---|---|
| Age | 67 ± 1.4 | 68 ± 1.3 | 0.68 | t-Test |
| Sex (female/male) [n, (%)] | 4/11 (27%/73%) | 10/21 (32%/68%) | 0.7 | χ2 test |
| BMI (median [IQR]) | 25.6 [23.1 to 27.1] | 28.7 [27.1 to 30.8] | 0.16 | Mann-Whitney U test |
| Hypertension [n, (%)] | 13 (87%) | 30 (94%) | 0.19 | χ2 test |
| Diabetes [n, (%)] | 6 (40%) | 12 (39%) | 0.93 | χ2 test |
| Smoking [n, (%)] | 8 (53%) | 8 (39%) | 0.067 | χ2 test |
| Degree of stenosis (mean %) | 80% | 80% | 0.26 | Mann-Whitney U test |
| Contralateral stenosis [n, (%)] | 2 (13%) | 7 (23%) | 0.46 | χ2 test |
| Compromised circle of Willis [n, (%)] | 6 (40%) | 9 (10%) | 0.015* | χ2 test |
| Delayed THR peak present [n, %] | 4 (27%) | 18 (58%) | 0.0625 | Fisher’s exact test |
| Impaired vasoreactivity [n, %] | 6 (40%) | 11 (35%) | 0.76 | χ2 test |
Data are presented as median [interquartile range] for continuous variables and as number of patients and percentage for categorical variables. BMI, body mass index; THR, transient hyperemic response; IQR, interquartile range; SD, standard deviation. The table includes the following parameters: age, sex distribution, BMI, presence of hypertension, diabetes, and smoking, degree of stenosis, contralateral stenosis, compromised circle of Willis, and delayed THR peak present. The symptomatic status of the patients (i.e., symptomatic or asymptomatic) is used as the grouping variable, * = significant.
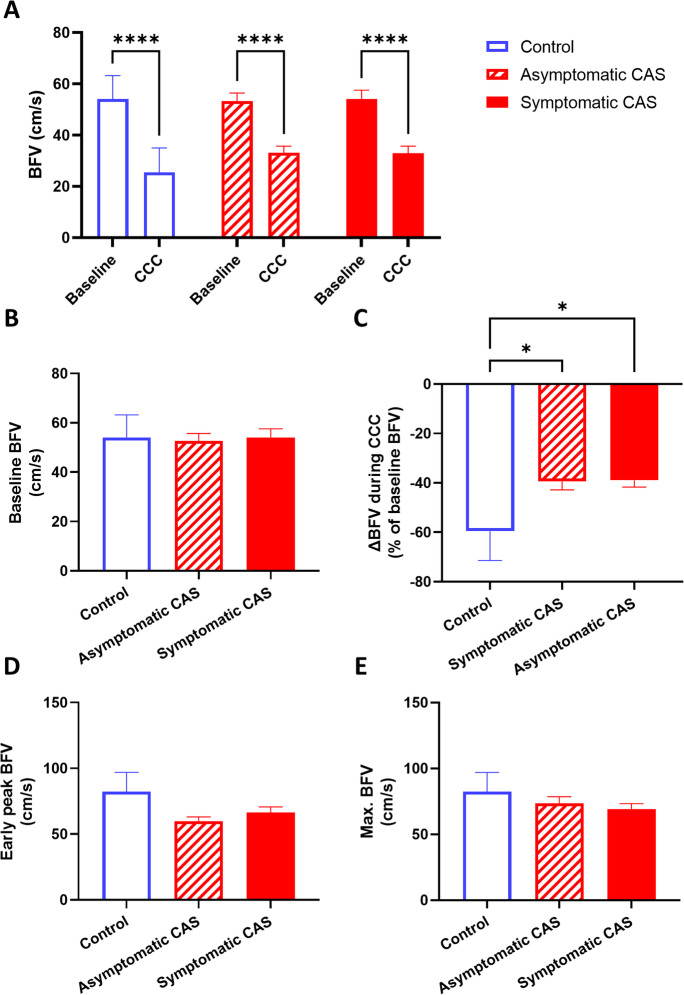
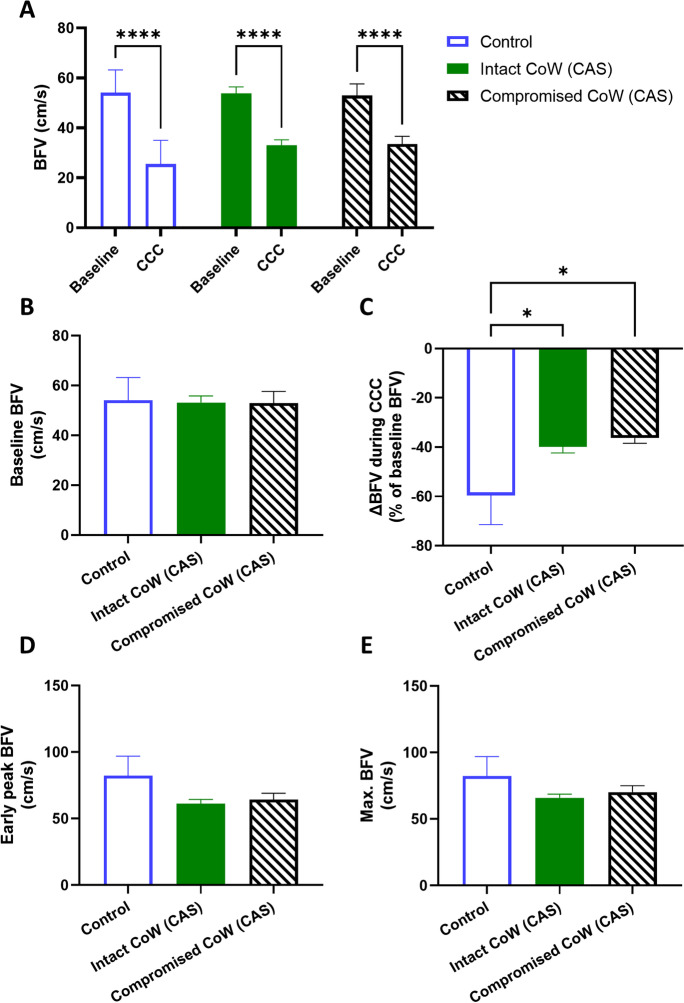
Blood flow velocity (BFV) was compared in the middle cerebral artery (MCA) in three groups—asymptomatic CAS patients, symptomatic CAS patients, and controls—before, during, and after CCC was performed, as shown in Fig. 4. Two-way ANOVA testing revealed a significant effect of CCC, causing a significant change in BFV (F[1, 47] = 200.2, p < 0.01), and post hoc testing confirmed the significant decrease of BFV in all three groups (Fig. 4A). However, baseline BFV did not differ significantly between the groups (Fig. 4A–B). While there was a significant effect of patient group in the change of BFV caused by CCC (F[2, 46] = 3.843, p = 0.03), post hoc testing only found differences between the control and CAS groups (control vs. asymptomatic CAS: p = 0.02; control vs. symptomatic CAS: p = 0.04), but not between the two CAS groups. No differences were found in the early peak (F3) or maximal (F3 or F4) BFV in the three groups.
Fig. 4.
Middle cerebral artery (MCA) blood flow velocity (BFV) measured by transcranial Doppler sonography in control, asymptomatic CAS, and symptomatic CAS groups under resting and common carotid artery compression (CCC) testing conditions. A Comparison of baseline BFV and BFV during CCC. Two-way ANOVA with post hoc Bonferroni test was performed, and the results of pairwise comparisons are displayed. B Comparison of baseline BFV among the groups. C Comparison of the maximal change in BFV during CCC. D Comparison of the early peak BFV after CCC release. E Comparison of the maximal BFV during the hyperemic response. For B–E, one-way ANOVA was conducted on normally distributed data, followed by post hoc Tukey’s test. The results of pairwise comparisons are presented. The mean values with standard error of the mean (SEM) are depicted. Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
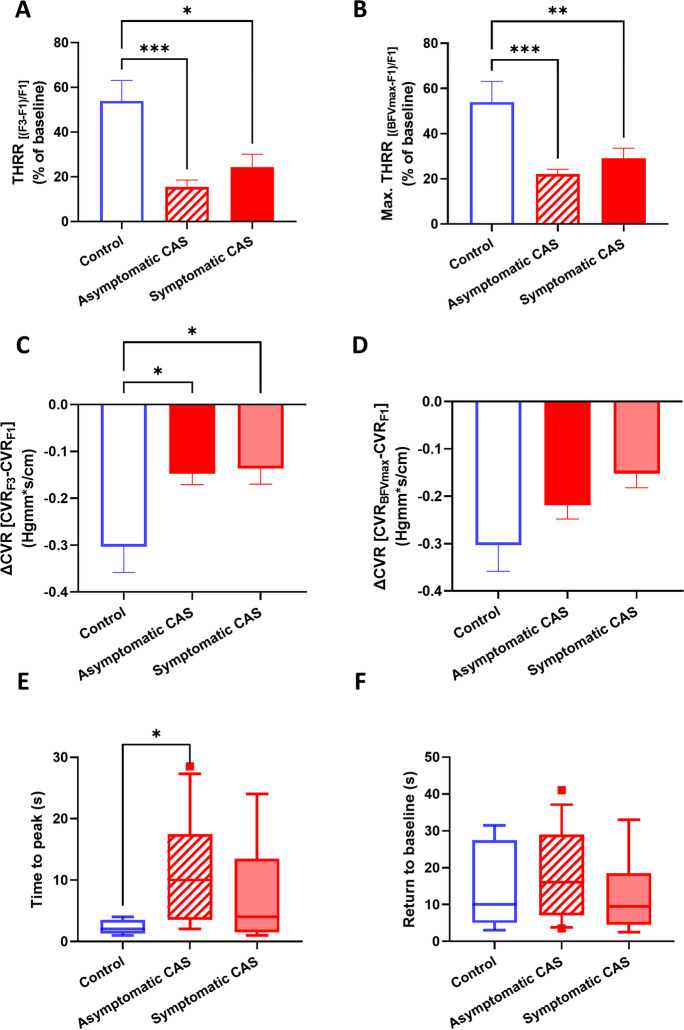
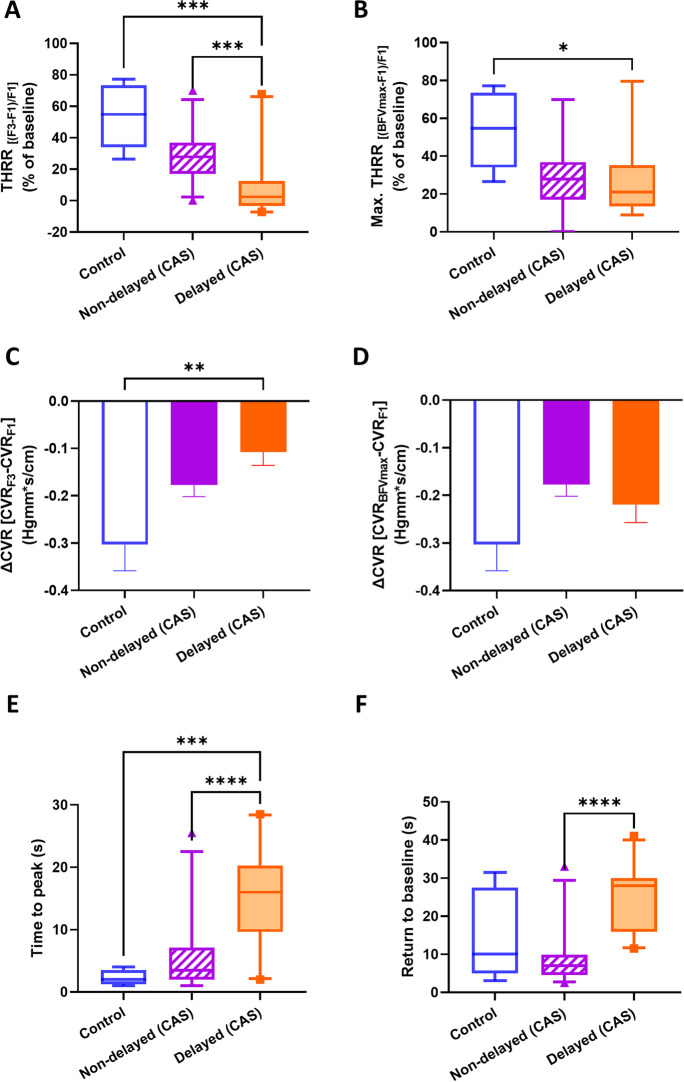
Transient hyperemic responses evoked by CCC were compared in the same three groups. Significant differences in THRR were found between the groups (F[2, 46] = 9.16, p < 0.01), and post hoc testing revealed differences between controls and both asymptomatic CAS (p < 0.01) and symptomatic CAS (p < 0.01), but not between the two CAS groups (Fig. 5A). When maximal THRR was evaluated, ROUT test identified five outliers in the asymptomatic CAS group, who were excluded. Similarly to THRR, one-way ANOVA found a significant effect of patient group on maximal THRR (F[2, 40] = 10.57, p = 0.03), and pairwise comparisons revealed differences between the control and CAS patient groups (asymptomatic CAS: p < 0.01; symptomatic CAS: p < 0.01, Fig. 5B).
Fig. 5.
Parameters describing the transient hyperemic response (THR) elicited by the common carotid artery compression (CCC) test in control subjects, asymptomatic CAS patients, and symptomatic CAS patients. A Transient hyperemic response ratio (THRR) calculated from the first peak of recorded blood flow velocity (BFV) following CCC release. B Transient hyperemic response ratio (THRR) calculated from the maximal BFV recorded after CCC release. C Change in cerebrovascular resistance index (CVR) at the first peak of BFV after CCC release. D Change in cerebrovascular resistance index (CVR) at the maximal BFV after CCC release. E Time elapsed to reach the maximal BFV after CCC release. F Time for BFV to return to baseline after CCC release. For A–D, one-way ANOVA was conducted on normally distributed data, followed by post hoc Tukey’s test for pairwise comparisons. The mean values with standard error of the mean (SEM) are depicted. For E–F, the Kruskal-Wallis test was performed due to non-normally distributed data, and post hoc Dunn’s test was used for pairwise comparisons. The median values with interquartile range (boxes) and 5–95 percentiles (whiskers) are displayed. Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
When the change in cerebrovascular resistance (ΔCVR) was compared, significant group effects were found (F[2, 46] = 3.396, p = 0.04), and post hoc test highlighted differences between the control and CAS groups only (asymptomatic CAS: p = 0.04, symptomatic CAS: p = 0.04, Fig. 5C). No significant differences were found when the same analysis was performed on ΔCVR calculated from CVR at BFVmax (Fig. 5D). Significant differences were found in the timing required to reach BFVmax between the three groups (p < 0.01), as revealed by Kruskal-Wallis test, and post hoc testing found significant differences between the controls and asymptomatic CAS patients (p = 0.01, Fig. 5E). However, return to baseline (RTB) did not show significant differences when compared among the three groups (Fig. 5F).
The CCC-evoked reactive hyperemia was measured as the area under the curve (AUC) above the baseline, and no differences were found when reactive hyperemia of the three groups was compared (Fig. 6A). However, when the correlation between the reactive hyperemia AUC and BFV during CCC that evoked the reactive hyperemia was analyzed, significant correlation was found only in the symptomatic CAS group (Fig. 6B).
Fig. 6.
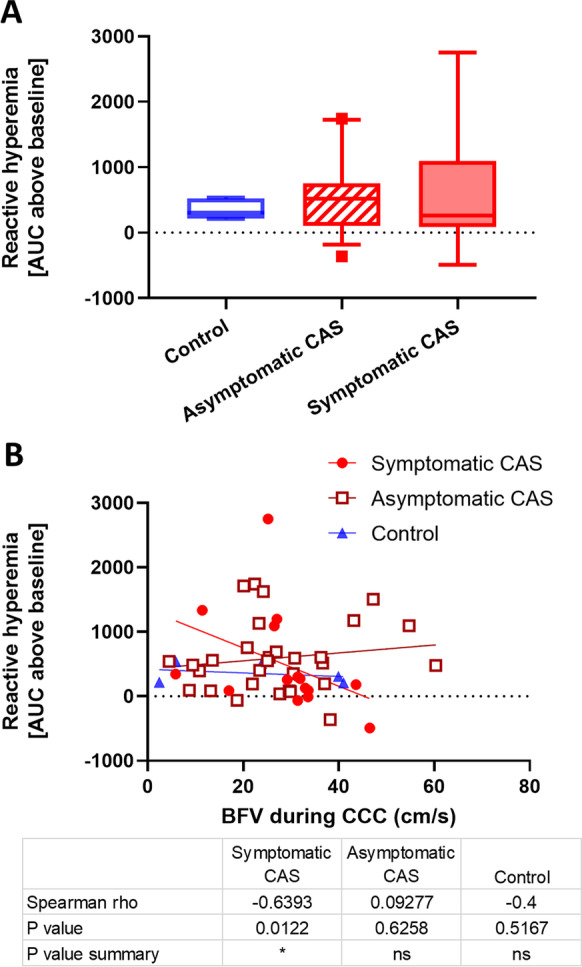
Reactive hyperemia responses in control subjects, asymptomatic CAS patients, and symptomatic CAS patients induced by common carotid artery compression (CCC) testing. A Area under the curve (AUC) of hyperemic responses following CCC. The Kruskal-Wallis test was performed due to non-normally distributed data, and post hoc Dunn’s test was applied. No significant differences were found in the post hoc testing. The median values with interquartile range (boxes) and 5–95 percentiles (whiskers) are shown. B Correlation analysis of the evoked reactive hyperemia responses plotted against the blood flow velocity (BFV) measured during CCC. Linear regression lines are fitted to display linear trendlines, and the Spearman’s rho and p values are provided in the accompanying table
Comparison of CAS patients with non-delayed vs. delayed transient hyperemic responses
The present study also aimed to compare patients with CAS who had non-delayed vs. delayed transient hyperemic responses following CCC. Table 2 presents a comparison of the characteristics between CAS patients with delayed and non-delayed THRs. Of the total 46 CAS patients included in the study, 22 (48%) exhibited delayed THR while 24 (52%) showed non-delayed THR. The delayed THR group was found to have a significantly higher mean age compared to the non-delayed THR group.
Table 2.
Comparing characteristics of non-delayed THR and delayed THR groups
| Data | Non-delayed THR group | Delayed THR group | Test | p value |
|---|---|---|---|---|
| Demographic data | ||||
| Number of patients (N/%) | 24/52 | 22/48 | NA | |
| Age, years median (IQR) | 67 (62.3–72.8) | 70 (65.5–72.3) | Mann-Whitney U | 0.46 |
| Sex, male (N/%) | 15/65 | 14/52 | Chi-square | 0.36 |
| Medical history | ||||
| Hypertension (N/%) | 24/100 | 21/100 | NA | |
| Symptomatic (N/%) | 11/45 | 4/18 | Fisher’s exact | 0.06 |
| Contralateral sign. stenosis or occlusion (N/%) | 3/13 | 7/30 | Fisher’s exact | 0.14 |
| Diabetes (N/%) | 9/39 | 7/30 | Chi-square | 0.53 |
| Smoking (N/%) | 7/31 | 7/30 | Fisher’s exact | 0.92 |
| Ischemic heart disease (N/%) | 6/26 | 9/39 | Chi-square | 0.77 |
| Chronic lung disease (N/%) | 2/8 | 2/8 | Fisher’s exact | 0.69 |
| Multimodal TCD metrics | ||||
| THRR (median, IQR) | 0.28 (0.2–0.3) | 0.01 (−0.03–0.1) | Mann-Whitney U | ˂0.001 |
| RTB (median, IQR) | 6.9 (4.4–10.0) | 27.8 (15.9–29.4) | Mann-Whitney U | ˂0.001 |
| Baseline CVR (median, IQR) | −0.17 (−0.4–0.09) | −0.09 (−0.3–0.1) | Mann-Whitney U | 0.04 |
| HR decrease (N/%) | 18/75 | 19/86 | Fisher’s exact | 0.27 |
| ABP decrease (N/%) | 12/52 | 19/82 | Fisher’s exact | 0.02 |
| Impaired vasoreactivity (N/%) | 3/13 | 8/36 | Fisher’s exact | ˂0.001 |
Data are presented as median [interquartile range] for continuous variables and as number of patients and percentage for categorical variables. THR, transient hyperemic response; IQR, interquartile range; The table includes the following parameters: age, sex distribution, presence of hypertension, diabetes, and smoking, symptomatic senosis, contralateral stenosis or occlusion, ischemic heart disease, chronic lung disease, THRR, transient hyperemic response ratio; RTB, return to baseline; baseline CVR, cerebrovascular resistance; HR, heart rate; ABP, arterial blood pressure. The non-delayed THR and delayed THR status of the patients is used as the grouping variable. Significant difference was found by THRR, RTB, baseline CVR, HR decrease, ABP decrease and impaired vasoreactivity
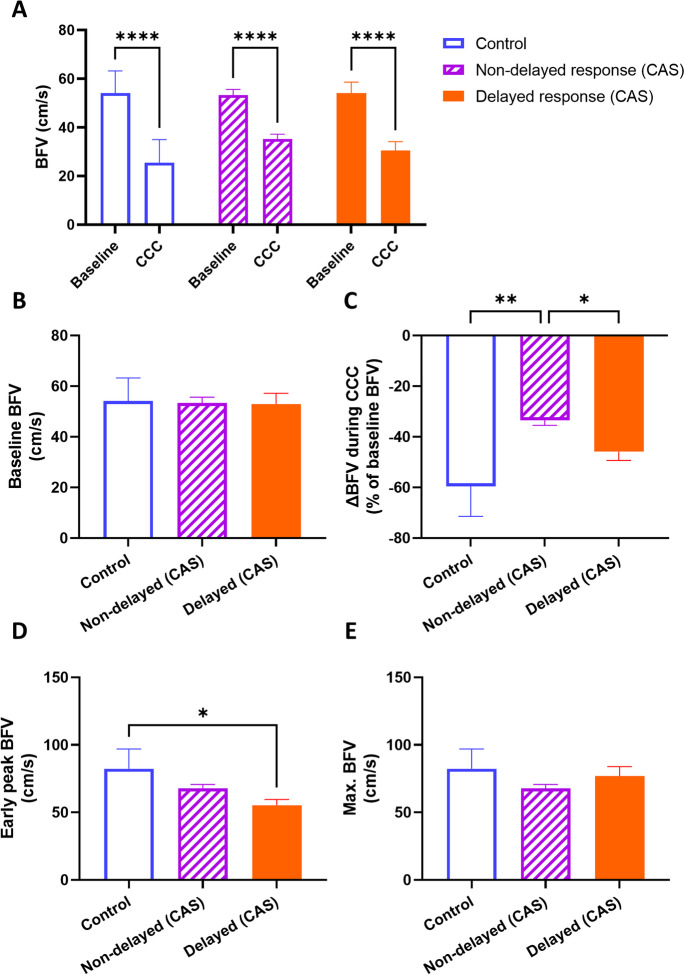
We conducted a comparison of BFV and CCC-evoked transient hyperemic response parameters in CAS patients with non-delayed THR, delayed THR, and controls. Baseline BFV did not differ significantly between the groups (Fig. 7A–B). Two-way ANOVA revealed a significant effect of CCC intervention on BFV (F[1, 47] = 222.1, p < 0.01), with a significant decrease in all three groups (p < 0.01, Fig. 7A). The change of BFV caused by CCC was significantly affected by the patient group (F[2, 46] = 8.532, p < 0.01), and post hoc testing found significant differences between the control vs. non-delayed THR (p < 0.01) and non-delayed THR CAS vs. delayed THR CAS (p = 0.02) comparisons (Fig. 7C). The early peak (F3) BFV was also influenced by the patient group (F[2, 46] = 5.125, p < 0.01), with a pairwise comparison-detected significant difference between the control and delayed THR CAS group (p = 0.02, Fig. 7D). However, no differences were found in the maximal (F3 or F4) BFV in the observed three groups (Fig. 7E).
Fig. 7.
Middle cerebral artery (MCA) blood flow velocity (BFV) measured by transcranial Doppler sonography in different carotid artery stenosis (CAS) patient groups classified by the presence of delayed or non-delayed transient hyperemic response during resting and common carotid artery compression (CCC) testing conditions. A Comparison of baseline BFV and BFV during CCC. Two-way ANOVA with post hoc Bonferroni test was conducted, and the results of pairwise comparisons are displayed. B Comparison of baseline BFV among the patient groups. C Comparison of the maximal change in BFV during CCC. D Comparison of the early peak BFV after CCC release. E Comparison of the maximal BFV during the hyperemic response. For B–E, one-way ANOVA was performed on normally distributed data, followed by post hoc Tukey’s test for pairwise comparisons. The mean values with standard error of the mean (SEM) are depicted. Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
We also evaluated CCC-evoked transient hyperemic response parameters, including THRR (calculated at F3 waveform), maximal THRR (calculated from BFVmax), ΔCVR (calculated from CVR at F3 and BFVmax), time to peak BFV, and time for BFV to return to baseline (RTB). THRR was significantly different between groups (p < 0.01), and post hoc testing found differences in the controls vs. delayed THR CAS (p < 0.01) and non-delayed THR CAS vs. delayed THR CAS groups (p < 0.01, Fig. 8A). Kruskal-Wallis comparison found differences between groups in maximal THRR, and pairwise comparisons found differences between the control and delayed THR CAS group (p = 0.03, Fig. 8B). One-way ANOVA revealed a significant effect of patient group on ΔCVR (F[2, 46] = 5.338, p < 0.01), with post hoc tests highlighting differences between the control and delayed THR CAS groups (p < 0.01, Fig. 8C). However, when the same analysis was performed on ΔCVR, calculated from CVR at BFVmax, no significant differences were found (Fig. 8D). The time to peak BFV was found to be different (p < 0.01), and the post hoc test detected significant differences between the controls and the delayed THR CAS (p < 0.01) and also between the delayed THR CAS and non-delayed THR CAS (Fig. 8E). Kruskal-Wallis test also found the time for BFV to return to baseline (RTB) significantly different (p < 0.01), and pairwise comparisons highlighted differences between the control and the delayed THR CAS group (Fig. 8F). Additionally, we observed a significant, negative correlation between THRR (calculated with F3) and RTB values (Spearman rho = −0.44, p < 0.001, Fig. 9A) (Table 3), while no significant correlation was found for THRR calculated with BFVmax (Fig. 9B).
Fig. 8.
Parameters characterizing the transient hyperemic response (THR) induced by the common carotid artery compression (CCC) test in control subjects and carotid artery stenosis (CAS) patients with delayed and non-delayed transient hyperemic response. A Transient hyperemic response ratio (THRR) calculated from the first peak of blood flow velocity (BFV) recorded after CCC release. B Transient hyperemic response ratio (THRR) calculated from the maximal BFV recorded after CCC release. C Change in cerebrovascular resistance index (CVR) at the first peak of BFV after CCC release. D Change in cerebrovascular resistance index (CVR) at the maximal BFV after CCC release. E Time elapsed to reach the maximal BFV after the release of CCC. F Time taken for BFV to return to baseline after CCC release. The Kruskal-Wallis test was used to analyze non-normally distributed data for A–B, E–F, and post hoc Dunn’s test was applied for pairwise comparisons. The results are presented as median values with interquartile range (boxes) and 5–95 percentiles (whiskers). One-way ANOVA with post hoc Tukey’s test was conducted on normally distributed data for C–D, and the pairwise comparisons are shown as mean ± SEM. Statistical significance is indicated as follows: * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001
Fig. 9.
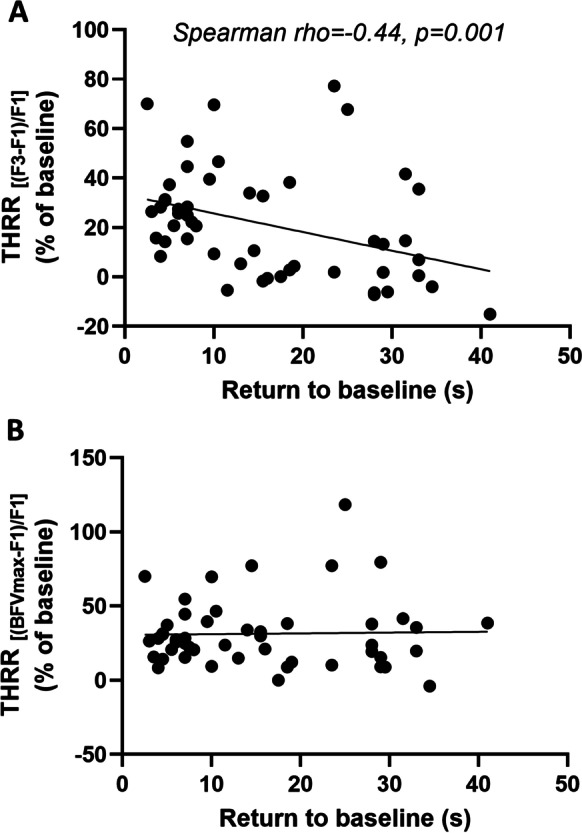
Correlation analysis of the transient hyperemic response ratios (THRR) elicited by the common carotid artery compression (CCC) test plotted against the time taken for the middle cerebral artery (MCA) blood flow velocity (BFV) to return to its baseline value. A THRR calculated from the first peak of BFV (F3) after CCC release. B THRR calculated from the maximal peak of BFV (F3 or F4) after CCC release. Spearman rho = 0.16, p = 0.46. Linear regression lines were fitted to display linear trends, and Spearman’s rho and p values are reported to indicate the strength and significance of the correlations
Table 3.
Return to baseline time analysis
| Multimodal TCD characteristics | Statistical tests for the analysis of RTB | Spearman rho | p value |
|---|---|---|---|
| THRR | Spearman correlation | −0.44 | ˂0.001 |
| CVR-THRR | Spearman correlation | 0.21 | 0.05 |
| HR decrease | Mann-Whitney U | N/A | 0.56 |
| ABP decrease | Mann-Whitney U | N/A | 0.31 |
| Impaired vasoreactivity | Mann-Whitney U | N/A | 0.007 |
RTB, return to baseline time; THRR, transient hyperemic response ratio; CVR-THRR, cerebrovascular resistance-transient hyperemic response ratio; HR, heart rate; ABP, arterial blood pressure. Significant correlation was found by THRR and CVR-THRR, significant difference was found by impaired vasoreactivity
Comparison of CAS patients with intact CoW vs. CAS patients with compromised CoW
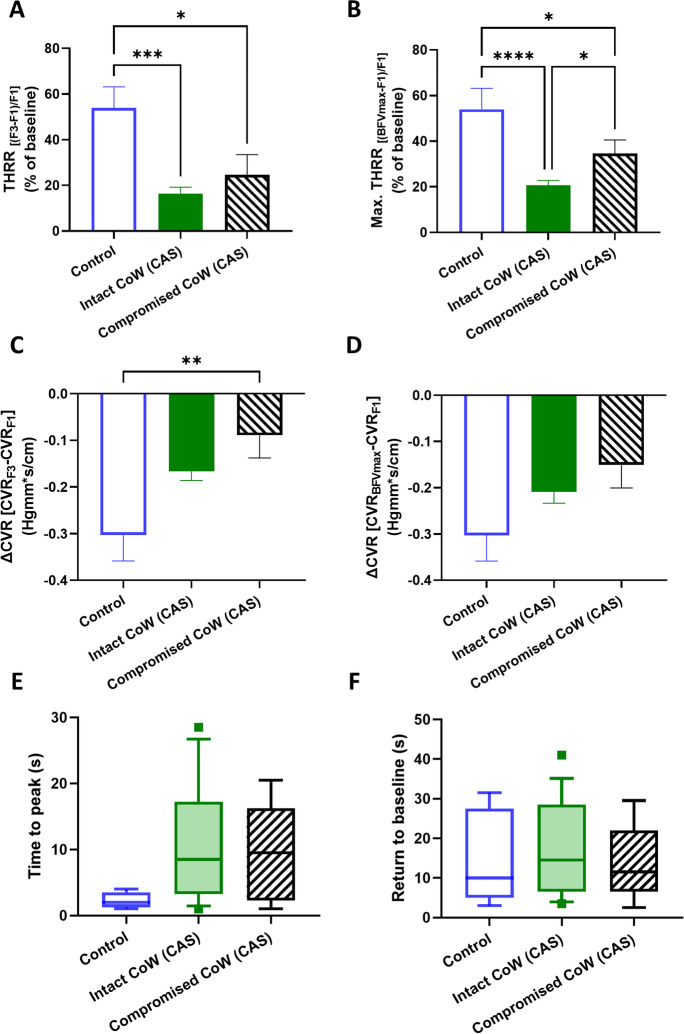
The study evaluated the CoW anatomy in patients with CAS and identified that 9 out of 45 patients (20%) had a compromised CoW. The subsequent analysis compared various blood flow velocity and THR parameters during CCC testing in subgroups of CAS patients with intact CoW, compromised CoW, and controls (Figs. 10 and 11).
Fig. 10.
Middle cerebral artery (MCA) blood flow velocity (BFV) measured by transcranial Doppler sonography in carotid artery stenosis (CAS) patient groups categorized by the presence of an intact or compromised circle of Willis (CoW) under resting and common carotid artery compression (CCC) testing conditions. A Comparison of baseline BFV and BFV during CCC. Two-way ANOVA with post hoc Bonferroni test was performed, and the results of pairwise comparisons are presented. B Comparison of baseline BFV among the patient groups. C Comparison of the maximal change in BFV during CCC. D Comparison of the early peak BFV after CCC release. E Comparison of the maximal BFV during the hyperemic response. For B–E, one-way ANOVA was conducted on normally distributed data, followed by post hoc Tukey’s test for pairwise comparisons. The mean values with standard error of the mean (SEM) are depicted. Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Fig. 11.
Parameters characterizing the transient hyperemic response (THR) induced by the common carotid artery compression (CCC) test in control subjects and carotid artery stenosis (CAS) patients categorized by the presence of an intact or compromised circle of Willis (CoW). A Transient hyperemic response ratio (THRR) calculated from the first peak of blood flow velocity (BFV) recorded after CCC release. B Transient hyperemic response ratio (THRR) calculated from the maximal BFV recorded after CCC release. C Change in cerebrovascular resistance index (CVR) at the first peak of BFV after CCC release. D Change in cerebrovascular resistance index (CVR) at the maximal BFV after CCC release. E Time elapsed to reach the maximal BFV after CCC release. F Time taken for BFV to return to baseline after CCC release. For A–D, one-way ANOVA was performed on normally distributed data, followed by post hoc Tukey’s test for pairwise comparisons. The mean values with standard error of the mean (SEM) are depicted. For E–F, the Kruskal-Wallis test was conducted on non-normally distributed data, followed by post hoc Dunn’s test for pairwise comparisons. The results are presented as median values with interquartile range (boxes) and 5–95 percentiles (whiskers). Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Blood flow velocity parameters during CCC testing showed that CCC intervention significantly decreased BFV in all three groups (p < 0.01), without significant differences in baseline BFV (Fig. 10A–B). However, the BFV drop caused by CCC was influenced by the patient group (F[2, 46] = 4.134, p = 0.02), with significant differences between controls vs. intact CoW CAS (p = 0.03) and controls vs. compromised CoW CAS (p = 0.03) (Fig. 10C). No significant differences were found in early peak (F3) and maximal (F3 or F4) BFV among the groups (Fig. 10D–E).
Transient hyperemic responses evoked by CCC were significantly different between the control group and the CAS groups with intact (p < 0.01) or compromised (p = 0.02) CoW (Fig. 11A). The comparison of maximal transient hyperemic response calculated from BFVmax showed significant group effects (F[2, 42] = 14.11, p < 0.01), with significant differences found between controls vs. intact CoW CAS (p < 0.01), controls vs. compromised CoW CAS (p = 0.04), and intact CoW CAS vs. compromised CoW CAS (p = 0.03) (Fig. 11B).
The change in cerebrovascular resistance (ΔCVR) calculated from CVR at F3 was significantly different between patient groups (F[2, 46] = 4.856, p = 0.01), with significant differences between the control and compromised CoW CAS groups (p < 0.01) (Fig. 11C). However, no significant differences were observed in ΔCVR calculated from CVR at BFVmax (Fig. 11D) or in time to peak BFV or RTB among the three groups (Fig. 11E–F).
Overall, these findings suggest that compromised CoW in CAS patients may lead to altered cerebral hemodynamics during CCC testing, with reduced blood flow velocity and transient hyperemic response, and increased cerebrovascular resistance compared to controls and CAS patients with intact CoW.
Discussion
The primary objective of this study was to investigate the impact of CAS on cerebral hemodynamics and autoregulation in both symptomatic and asymptomatic patients. Our results demonstrated that CAS was associated with impaired cerebral vascular reactivity, corroborating previous findings [88–90]. Notably, a larger proportion of symptomatic CAS patients exhibited incomplete CoW anatomy, which potentially contributed to their more severe clinical presentation. Contrary to our initial hypothesis, no significant differences were observed in autoregulatory function or inter-hemispheric blood flow between symptomatic and asymptomatic CAS patients. These findings suggest that impaired autoregulation of cerebral blood flow and inefficient inter-hemispheric blood flow may not be major contributing factors to the symptomatic manifestations of CAS in older adults.
In cases of ICA stenosis, cerebral infarction can occur due to both hemodynamic hypoperfusion and embolization. Various clinical and imaging features have been identified as risk factors for late cerebral infarction in asymptomatic ICA stenosis patients [91]. These features include stenosis progression, silent infarction on CT, contralateral transient ischemic attack (TIA) or stroke, intra-plaque hemorrhage on MRI, impaired cerebrovascular reactivity, and spontaneous embolization on TCD [92–101]. These findings underscore the importance of accurately categorizing atherosclerotic ICA stenosis to enhance outcome prediction using novel or repurposed examination methods.
Current international guidelines for the treatment of atherosclerotic CAS consider the degree of stenosis, previous ischemic events (cerebral infarction or TIA), and the overall condition of the patient [91]. Conservative therapy is recommended for CAS that is not hemodynamically significant (less than 70%), while invasive procedures are advised for symptomatic CAS with hemodynamic significance (greater than 70%) [79, 102]. Carotid endarterectomy (CEA) has been shown to effectively prevent secondary events in cases of symptomatic significant (>70%) ICA stenosis [91]. In asymptomatic patients with significant ICA stenosis, CEA can reduce the long-term risk of ischemic events by 50%. However, the perioperative risk of ischemic events is reported to be twice as high in asymptomatic patients (6%) compared to symptomatic patients (3%) [103].
In our study, we did not find significant differences in postoperative complications and surgical outcomes between the groups. The occurrence of adverse effects and postoperative complications was minimal, suggesting that the surgical outcomes were predominantly determined by the quality of the surgery itself. Therefore, the preoperative hemodynamic measurements we recorded had limited predictive value for surgical outcomes in this particular cohort. These findings are consistent with previous studies, emphasizing the critical role of surgical skill and experience in achieving successful outcomes for patients undergoing CAS surgery. Nonetheless, preoperative hemodynamics may still have relevance in guiding the choice of surgical approach.
Several TCD methods are available for evaluating cerebrovascular reactivity, including the hyperventilation test, apnea test, acetazolamide test, and thigh cuff test [66, 67, 104–106]. However, the CCC test stands out as the only functional TCD test that mimics preoperatively the hemodynamic environment similar to clamping during carotid endarterectomy (CEA). Thus, this test has the potential to impact the choice of carotid reconstruction technique. According to previous research, the CCC test has a preoperative predictive value of 66.7% for a positive outcome, 100% for a negative outcome, 100% sensitivity, and 97.8% specificity in predicting the need for a shunt during CEA [107]. In another study with a larger sample size and a broader population of patients with CAS, the CCC test was reported to have a 60% likelihood of indicating the need for intraoperative shunt use [72, 107]. To strengthen this indication, the CCC test can be combined with other functional TCD tests [72, 107].
In this study, we conducted a comprehensive analysis of the amplitude and time variables of blood flow velocity response in the middle cerebral artery (MCA) following the release of carotid compression challenge (CCC). Our observations revealed a unique pattern, characterized by the presence of two peaks in blood flow velocity response after CCC release, in a subset of patients with carotid artery stenosis (CAS). This pattern, referred to as delayed transient hyperemic response (DTHR), was observed in nearly half (48%) of CAS patients, indicating a delayed peak in blood flow during the hyperemic phase. We believe that this phenomenon may be influenced by multifaceted mechanisms involving alterations in local autoregulatory mechanisms, such as myogenic constriction and flow-induced vasoregulation. Thus, we further investigated whether the presence of this late blood flow velocity peak was associated with specific patient characteristics or correlated with static and dynamic blood flow velocity changes.
While we did not observe differences in maximal blood flow evoked by CCC, our findings demonstrated that patients with delayed THRR had lower maximal THRR values. Delayed THRR is characterized by a prolonged reduction in blood flow velocity, indicated by a longer return to baseline (RTB), which may be suggestive of impaired cerebral vascular reactivity. Our results indicate that a significant immediate increase in MCA blood flow velocity after carotid compression cessation, reflected by a high transient hyperemic response ratio, is often accompanied by a rapid induction of vasoconstriction in response to increased flow, as quantified by the rate of blood flow velocity reduction time (RTB). We propose that RTB can serve as a reliable marker of vasoreactivity. Notably, our correlation analysis revealed that prolongation of RTB was associated with decreases in vasoreactivity, as evidenced by delayed or absent transient hyperemia. A smaller increase in blood flow velocity suggests impaired cerebral vasoreactivity, while a prolonged RTB may indicate compensation for the less efficient restoration of blood flow following CCC. In our study, impaired vasoreactivity was associated with longer RTBs. These findings highlight the potential role of RTB as an indicator of impaired vasoreactivity and suggest its utility in assessing cerebrovascular function.
More than half of the patients included in our study experienced a decrease in both heart rate and arterial blood pressure during the CCC maneuver. This observation suggests that a reduction in systemic arterial blood pressure during CCC may contribute to the lower THRR observed after release of the compression cuff. We hypothesize that the normalization of systemic arterial pressure can lead to an additional increase in blood flow, contributing to the delayed transient hyperemia phenomenon. Considering the changes in arterial blood pressure during CCC, we also calculated cerebrovascular resistance (CVR) values. Typically, CVR values at the end of the CCC test, reflecting maximal vasodilation, are low, indicating a well-functioning autoregulation. However, our study’s CVR data revealed reduced autoregulatory capacity in the group exhibiting delayed THR reactions. Autonomic dysregulation may contribute to the observed alterations in heart rate and arterial blood pressure during the CCC test. Previous research has demonstrated a higher prevalence of autonomic dysfunction in patients with carotid atherosclerosis, which has been associated with increased mortality in individuals with cerebrovascular disease [108, 109]. This phenomenon is believed to occur due to decreased carotid sinus distensibility, resulting in reduced baroreceptor sensitivity and abnormal cardiovascular regulatory responses [110]. Clinical trials have confirmed that patients with carotid atherosclerosis exhibit more severe autonomic dysfunction compared to elderly control groups and other individuals with cerebrovascular disease [108]. These findings highlight the importance of monitoring arterial blood pressure concurrently with the assessment of cerebral vascular reactivity.
Furthermore, we found a significant correlation between patient age and decreased amplitude, delayed THR, and prolonged RTB following CCC. Additionally, there was a correlation between age and eGFR values, as well as a higher occurrence of significant drops in blood pressure during CCC in older patients. These results support the detrimental effects of aging on both cerebral and systemic microcirculation and cardiovascular homeostasis, which likely contribute to impaired cerebral vascular reactivity and dysregulation of cerebral blood flow. The cellular and molecular mechanisms underlying cerebrovascular aging and accelerated cerebrovascular aging associated with atherosclerotic diseases are multifaceted, involving dysregulation of calcium signaling pathways and myogenic machinery in smooth muscle cells, endothelial dysfunction, alterations in the extracellular matrix, heightened inflammatory status of the vascular wall, and structural remodeling and rarefaction of cerebral microvessels [39–41, 43, 82, 111–115].
The CoW serves as a crucial network connecting the internal carotid arteries to the vertebral arteries, supplying collateral blood flow to the brain. In patients with CAS, where blood flow through the affected carotid artery is compromised, an intact CoW can play a vital role in maintaining sufficient blood supply to the brain by providing an alternative pathway for blood to reach the affected hemisphere. This collateral circulation helps prevent cognitive dysfunction caused by ischemia and reduces the risk and severity of ischemic strokes. Remarkably, our study found that a larger proportion of symptomatic CAS patients exhibited compromised CoW anatomy compared to asymptomatic CAS patients. This aligns with previous research demonstrating a higher prevalence of absent or hypoplastic segments in the CoW among symptomatic CAS patients [87, 116–119]. In healthy individuals, the prevalence of a complete CoW ranges from 27 to 90%, while in individuals with cerebrovascular disease, it ranges from 18 to 55%. The prevalence of absent or hypoplastic CoW segments can be influenced by various factors, including ethnic and population differences [61, 63, 120].
Variations in the CoW can impact both the temporal and amplitude characteristics of transient hyperemic response and residual cerebral blood flow. Surprisingly, our study did not find significant differences in the amplitude variables of THR or residual CBF during the CCC test between patients with an intact CoW and those with a compromised CoW. However, we did observe variation in delayed THR values between the two patient groups. Our findings demonstrated that patients with CAS and an intact CoW had a lower maximal THRR compared to those with a compromised CoW. These results suggest that the intact CoW may compensate for reduced cerebral blood supply in the affected hemisphere by enhancing inter-hemispheric blood flow. Although the exact mechanisms underlying this compensation are not yet fully understood, it is possible that the intact CoW facilitates increased blood flow through the contralateral hemisphere, leading to improved cerebral perfusion. Another plausible explanation is that compromised CoW could be indicative of more extensive vascular disease, affecting cerebral autoregulation and resulting in a higher maximal THRR. Further research is necessary to explore these hypotheses and elucidate the potential clinical implications of our findings. The unexpected results in our study may be attributed to the heterogeneity within the compromised CoW group. While studies on the anastomosis capacity of the circle of Willis often emphasize the significance of the anterior communicating artery, others emphasize the role of the posterior CoW [121, 122]. In our study, we did not analyze the anterior and posterior CoW systems separately, and the presence of contralateral steno-occlusive CAS might also act as a modifying factor. It is possible that our patient group consisted of subgroups with varying degrees of CoW compromise, which could have influenced the study outcomes. Further research with larger sample sizes and more homogeneous participant groups is necessary to validate and expand upon these findings. Accurately characterizing global cerebral hemodynamics also requires assessing the capacity of the secondary cerebral collateral system, including leptomeningeal and extra-intracranial arterial anastomoses [123].
While our study offers valuable insights, it is crucial to acknowledge its limitations. One limitation is the relatively small and selective sample of patients included. However, it is worth noting that the number of patients recruited is comparable to previous studies conducted with TCD [104, 105, 124, 125]. Although efforts were made to include both symptomatic and asymptomatic patients, there was an imbalance in the representation of these groups, with a predominance of male patients. Moreover, certain subgroups of ICA stenosis were excluded from the study due to the absence of insonation windows and exclusion criteria, potentially introducing bias into the results. Additionally, the adequacy of the temporal insonation window can be influenced by age-related changes in bone structure, as reported in the literature [126]. In our study, the higher proportion of inadequate insonation windows compared to previous reports may be attributed to the older age of our patient cohort. It is also possible that the rigorous study protocol outlined in the methods section contributed to these differences. The described study procedure is operator-dependent, as both TCD and the CCC test require a skilled operator for accurate performance and interpretation. Additionally, it is important to note that TCD measures blood flow velocity in large arteries, which may not fully reflect regional differences in blood flow within smaller vessels. The velocity of blood flow and its change in the proximal cerebral vessels measured by TCD without knowing the arterial pressure that maintains perfusion does not in itself reflect the change in blood flow in the relevant brain tissue. Furthermore, patient safety is a concern during the CCC test; however, it is noteworthy that none of the patients in our study reported any complaints during the examinations. Moreover, it is essential to acknowledge that the various calculated indices introduced in this study, characterizing cerebrovascular responses, are not yet widely employed in clinical practice. Nevertheless, we hope that these indices will be adopted by other researchers, enabling them to compare their findings with those presented in our study.
In conclusion, the evaluation of cerebrovascular reactivity presented in this study offers a new perspective on characterizing patients with significant CAS. By analyzing the duration and type of THR during the CCC test, we provide a more complex understanding of cerebral vasoreactivity. This study is the first to comprehensively analyze time variables, CoW anatomy, and changes in blood pressure and heart rate in characterizing the CCC test in older adults with symptomatic and asymptomatic CAS. Further follow-up studies on a larger cohort of older CAS patients are needed to assess additional relevant clinical outcome measures. The mechanisms responsible for the transient hyperemic response after the CCC test are complex and involve several physiological processes, including myogenic autoregulation, metabolic vasodilation, sympathetic nervous system activation, and endothelium-mediated vasomotor responses. Aging and several risk factors that promote accelerated cardiovascular aging (e.g., hypertension, diabetes mellitus, smoking, hyperlipidemia) can impact the function and phenotype of vascular smooth muscle cells, pericytes, and endothelial cells and the regulation of the tone of resistance arteries, arterioles, and capillaries by mediators released from astrocytes, neurons and perivascular microglia, and immunocytes. Further mechanistic studies are needed to determine how alterations in these synergistic vasoregulatory mechanisms induced by cardiovascular risk factors affect the components of the THR and inter-hemispheric blood flow in older adults and patients with accelerated biological age [127].
Funding
Open access funding provided by Semmelweis University. The study was supported by the National Office for Research, Development and Innovation (NKFIH, K 129277). Project no. TKP2021-NKTA-47 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme. Funding for the project through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) was provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund. This work was also supported by funding from the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/EUniWell/EAC-A02-2019/EAC-A02-2019-1). This study was also supported by Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund (Grant No: TKP2021-EGA/TKP2021-NVA/TKP2021-NKTA) and the NKFI-1 K OTKA 132596 K_19, TKP2021-EGA-37 of MIT of Hungary-NRDI TKP2021-EGA funding and HAS/MTA Post-Covid 2021-34. The funding sources had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declarations
Competing interests
Dr. Tamas Csipo serves as Associate Editor for GeroScience. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and as Consulting Editor for The American Journal of Physiology-Heart and Circulatory Physiology.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bonati LH, Jansen O, de Borst GJ, Brown MM. Management of atherosclerotic extracranial carotid artery stenosis. Lancet Neurol. 2022;21:273–283. doi: 10.1016/S1474-4422(21)00359-8. [DOI] [PubMed] [Google Scholar]
- 2.de Weerd M, Greving JP, de Jong AW, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke. 2009;40:1105–1113. doi: 10.1161/STROKEAHA.108.532218. [DOI] [PubMed] [Google Scholar]
- 3.de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, Rosvall M, Sitzer M, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–1297. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, Rosvall M, Sitzer M, de Borst GJ, Buskens E, Bots ML. Prediction of asymptomatic carotid artery stenosis in the general population: identification of high-risk groups. Stroke. 2014;45:2366–2371. doi: 10.1161/STROKEAHA.114.005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmid S, Tsantilas P, Knappich C, Kallmayer M, Breitkreuz T, Zimmermann A, Eckstein HH, Kuehnl A. Age but not sex is associated with higher risk of in-hospital stroke or death after carotid artery stenting in symptomatic and asymptomatic carotid stenosis. J Vasc Surg. 2019;69:1090–1101 e3. doi: 10.1016/j.jvs.2018.03.439. [DOI] [PubMed] [Google Scholar]
- 6.Collaborators GS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Bai X, Wang T, Dmytriw AA, Patel AB, Jiao L. Carotid stenting versus endarterectomy for asymptomatic carotid artery stenosis: a systematic review and meta-analysis. Stroke. 2022;53:3047–3054. doi: 10.1161/STROKEAHA.122.038994. [DOI] [PubMed] [Google Scholar]
- 10.Zirak P, Delgado-Mederos R, Dinia L, Marti-Fabregas J, Durduran T. Microvascular versus macrovascular cerebral vasomotor reactivity in patients with severe internal carotid artery stenosis or occlusion. Acad Radiol. 2014;21:168–174. doi: 10.1016/j.acra.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Bokkers RP, van Osch MJ, van der Worp HB, de Borst GJ, Mali WP, Hendrikse J. Symptomatic carotid artery stenosis: impairment of cerebral autoregulation measured at the brain tissue level with arterial spin-labeling MR imaging. Radiology. 2010;256:201–208. doi: 10.1148/radiol.10091262. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt M, Pfadenhauer K, Zentner J, Grimmer S, Wachenfeld-Wahl C, Heidenreich P, Loeprecht H, Wolfle KD. Impaired cerebral autoregulation in asymptomatic patients with carotid artery stenosis: comparison of acetazolamide-SPECT and transcranial CO(2)-dopplersonography. Zentralbl Chir. 2004;129:178–182. doi: 10.1055/s-2004-822798. [DOI] [PubMed] [Google Scholar]
- 13.Koppl T, Schneider M, Pohl U, Wohlmuth B. The influence of an unilateral carotid artery stenosis on brain oxygenation. Med Eng Phys. 2014;36:905–914. doi: 10.1016/j.medengphy.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Reinhard M, Hetzel A, Lauk M, Lücking CH. Dynamic cerebral autoregulation testing as a diagnostic tool in patients with carotid artery stenosis. Neurol Res. 2001;23:55–63. doi: 10.1179/016164101101198299. [DOI] [PubMed] [Google Scholar]
- 15.Semenyutin VB, Asaturyan GA, Nikiforova AA, Aliev VA, Panuntsev GK, Iblyaminov VB, Savello AV, Patzak A. Predictive value of dynamic cerebral autoregulation assessment in surgical management of patients with high-grade carotid artery stenosis. Front Physiol. 2017;8:872. doi: 10.3389/fphys.2017.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White RP, Markus HS. Impaired dynamic cerebral autoregulation in carotid artery stenosis. Stroke. 1997;28:1340–1344. doi: 10.1161/01.STR.28.7.1340. [DOI] [PubMed] [Google Scholar]
- 17.Weise J, Kuschke S, Bahr M. Gender-specific risk of perioperative complications in carotid endarterectomy patients with contralateral carotid artery stenosis or occlusion. J Neurol. 2004;251:838–844. doi: 10.1007/s00415-004-0438-8. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JE, Gomori JM, Itshayek E, Pikis S, Keigler G, Eichel R, Leker RR. Ischemic complications after tailored carotid artery stenting in different subpopulations with high-grade stenosis: feared but rare. J Clin Neurosci. 2015;22:189–194. doi: 10.1016/j.jocn.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Chuang YM, Huang KL, Chang YJ, Chang CH, Chang TY, Wu TC, Lin CP, Wong HF, Liu SJ, Lee TH. Associations between circle of Willis morphology and white matter lesion load in subjects with carotid artery stenosis. Eur Neurol. 2011;66:136–144. doi: 10.1159/000329274. [DOI] [PubMed] [Google Scholar]
- 20.Marshall RS, Pavol MA, Cheung YK, Strom I, Slane K, Asllani I, Lazar RM. Dissociation among hemodynamic measures in asymptomatic high grade carotid artery stenosis. J Neurol Sci. 2016;367:143–147. doi: 10.1016/j.jns.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando T, Sekine T, Murai Y, Orita E, Takagi R, Amano Y, Iwata K, Nakaza M, Ogawa M, Obara M, Kumita SI. Multiparametric flow analysis using four-dimensional flow magnetic resonance imaging can detect cerebral hemodynamic impairment in patients with internal carotid artery stenosis. Neuroradiology. 2020;62:1421–1431. doi: 10.1007/s00234-020-02464-2. [DOI] [PubMed] [Google Scholar]
- 22.Chuang YM, Lin CP, Wong HF, Chang YJ, Chang CH, Chang TY, Wu TC, Wu HC, Lee TH. Plasticity of circle of Willis: a longitudinal observation of flow patterns in the circle of Willis one week after stenting for severe internal carotid artery stenosis. Cerebrovasc Dis. 2009;27:572–578. doi: 10.1159/000214221. [DOI] [PubMed] [Google Scholar]
- 23.Dankbaar JW, Kerckhoffs KGP, Horsch AD. van der Schaaf IC, Kappelle LJ, Velthuis BK and investigators D. Internal carotid artery stenosis and collateral recruitment in stroke patients. Clin Neuroradiol. 2018;28:339–344. doi: 10.1007/s00062-017-0568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunieda T, Miyake K, Sakamoto H, Iwasaki Y, Iida S, Morise S, Fujita K, Nakamura M, Kaneko S, Kusaka H. Leptomeningeal collaterals strongly correlate with reduced cerebrovascular reactivity measured by acetazolamide-challenged single-photon emission computed tomography using a stereotactic extraction estimation analysis in patients with unilateral internal carotid artery stenosis. Intern Med. 2017;56:2857–2863. doi: 10.2169/internalmedicine.8397-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long Q, Luppi L, Konig CS, Rinaldo V, Das SK. Study of the collateral capacity of the circle of Willis of patients with severe carotid artery stenosis by 3D computational modeling. J Biomech. 2008;41:2735–2742. doi: 10.1016/j.jbiomech.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Waaijer A, van Leeuwen MS, van der Worp HB, Verhagen HJ, Mali WP, Velthuis BK. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidetector row CT angiography. Cerebrovasc Dis. 2007;23:267–274. doi: 10.1159/000098326. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Ji J, Qiu J, Wang Y. Incompleteness of circle of Willis and silent brain infarction in patients with internal carotid artery stenosis. J Clin Neurosci. 2022;98:73–77. doi: 10.1016/j.jocn.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Wei W, Yi X, Ruan J, Duan X, Luo H, Lv Z. Influence of collateral circulation on cerebral blood flow and frontal lobe cognitive function in patients with severe internal carotid artery stenosis. BMC Neurol. 2019;19:151. doi: 10.1186/s12883-019-1380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye H, Wu X, Yan J, Wang J, Qiu J, Wang Y. Completeness of circle of Willis and white matter hyperintensities in patients with severe internal carotid artery stenosis. Neurol Sci. 2019;40:509–514. doi: 10.1007/s10072-018-3683-9. [DOI] [PubMed] [Google Scholar]
- 30.Zarrinkoob L, Wahlin A, Ambarki K, Birgander R, Eklund A, Malm J. Blood flow lateralization and collateral compensatory mechanisms in patients with carotid artery stenosis. Stroke. 2019;50:1081–1088. doi: 10.1161/STROKEAHA.119.024757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu G, Yuan Q, Yang J, Yeo JH. The role of the circle of Willis in internal carotid artery stenosis and anatomical variations: a computational study based on a patient-specific three-dimensional model. Biomed Eng Online. 2015;14:107. doi: 10.1186/s12938-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokkers RP, Wessels FJ, van der Worp HB, Zwanenburg JJ, Mali WP, Hendrikse J. Vasodilatory capacity of the cerebral vasculature in patients with carotid artery stenosis. AJNR Am J Neuroradiol. 2011;32:1030–1033. doi: 10.3174/ajnr.A2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottler J, Kaczmarz S, Kallmayer M, Wustrow I, Eckstein HH, Zimmer C, Sorg C, Preibisch C, Hyder F. Flow-metabolism uncoupling in patients with asymptomatic unilateral carotid artery stenosis assessed by multi-modal magnetic resonance imaging. J Cereb Blood Flow Metab. 2019;39:2132–2143. doi: 10.1177/0271678X18783369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igarashi T, Sakatani K, Fujiwara N, Murata Y, Suma T, Shibuya T, Hirayama T, Katayama Y. Monitoring of hemodynamic change in patients with carotid artery stenosis during the tilt test using wearable near-infrared spectroscopy. Adv Exp Med Biol. 2013;789:463–467. doi: 10.1007/978-1-4614-7411-1_62. [DOI] [PubMed] [Google Scholar]
- 35.Mendelow AD, Graham DI, Tuor UI, Fitch W. The hemodynamic effects of internal carotid artery stenosis and occlusion. J Neurosurg. 1987;66:755–763. doi: 10.3171/jns.1987.66.5.0755. [DOI] [PubMed] [Google Scholar]
- 36.Milanlioglu A, Yaman A, Kolukisa M, Asil T. Evaluation of cerebral hemodynamic status in patients with unilateral symptomatic carotid artery stenosis during motor tasks, through use of transcranial Doppler sonography. Arq Neuropsiquiatr. 2022;80:339–343. doi: 10.1590/0004-282x-anp-2020-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minhas PS, Smielewski P, Kirkpatrick PJ, Pickard JD, Czosnyka M. Pressure autoregulation and positron emission tomography-derived cerebral blood flow acetazolamide reactivity in patients with carotid artery stenosis. Neurosurgery. 2004;55:63–67. doi: 10.1227/01.NEU.0000126876.10254.05. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph JL, Sorond FA, Pochay VE, Haime M, Treanor P, Crittenden MD, Babikian VL. Cerebral hemodynamics during coronary artery bypass graft surgery: the effect of carotid stenosis. Ultrasound Med Biol. 2009;35:1235–1241. doi: 10.1016/j.ultrasmedbio.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ungvari Z, Toth P, Tarantini S, Prodan CI, Sorond F, Merkely B, Csiszar A. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balasubramanian P, Kiss T, Tarantini S, Nyul-Toth A, Ahire C, Yabluchanskiy A, Csipo T, Lipecz A, Tabak A, Institoris A, Csiszar A, Ungvari Z. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–H761. doi: 10.1152/ajpheart.00736.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018; in press [DOI] [PMC free article] [PubMed]
- 46.Tucsek Z, Toth P, Sosnowsk D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood brain barrier disruption, neuroinflammation and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Sette M, Eliasziw M, Streifler JY, Hachinski VC, Fox AJ, Barnett HJ. Internal borderzone infarction: a marker for severe stenosis in patients with symptomatic internal carotid artery disease. For the North American Symptomatic Carotid Endarterectomy (NASCET) Group. Stroke. 2000;31:631–636. doi: 10.1161/01.STR.31.3.631. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Hong H, Liu D, Xu G, Wang Y, Zeng J, Zhang R, Liu X. Lesion patterns and mechanism of cerebral infarction caused by severe atherosclerotic intracranial internal carotid artery stenosis. J Neurol Sci. 2011;307:79–85. doi: 10.1016/j.jns.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Hong CT, Chan HF, Lee SP, Jang JS. Cerebral infarct in patients with bilateral high-grade internal carotid artery stenosis: analysis by diffusion-weighted imaging. Eur Neurol. 2011;65:88–93. doi: 10.1159/000323514. [DOI] [PubMed] [Google Scholar]
- 51.Hupperts RM, Warlow CP, Slattery J, Rothwell PM. Severe stenosis of the internal carotid artery is not associated with borderzone infarcts in patients randomised in the European Carotid Surgery Trial. J Neurol. 1997;244:45–50. doi: 10.1007/s004150050049. [DOI] [PubMed] [Google Scholar]
- 52.Kashiwazaki D, Akioka N, Kuwayama N, Noguchi K, Tanaka K, Kuroda S. Pathophysiology of acute cerebrovascular syndrome in patients with carotid artery stenosis: a magnetic resonance imaging/single-photon emission computed tomography study. Neurosurgery. 2015;76:427–433. doi: 10.1227/NEU.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 53.Lythgoe D, Simmons A, Pereira A, Cullinane M, Williams S, Markus HS. Magnetic resonance markers of ischaemia: their correlation with vasodilatory reserve in patients with carotid artery stenosis and occlusion. J Neurol Neurosurg Psychiatry. 2001;71:58–62. doi: 10.1136/jnnp.71.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schroder J, Heinze M, Gunther M, Cheng B, Nickel A, Schroder T, Fischer F, Kessner SS, Magnus T, Fiehler J, Larena-Avellaneda A, Gerloff C, Thomalla G. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. Neuroimage Clin. 2019;22:101779. doi: 10.1016/j.nicl.2019.101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiart M, Berthezene Y, Adeleine P, Feugier P, Trouillas P, Froment JC, Nighoghossian N. Vasodilatory response of border zones to acetazolamide before and after endarterectomy: an echo planar imaging-dynamic susceptibility contrast-enhanced MRI study in patients with high-grade unilateral internal carotid artery stenosis. Stroke. 2000;31:1561–1565. doi: 10.1161/01.STR.31.7.1561. [DOI] [PubMed] [Google Scholar]
- 56.Harder DR, Narayanan J, Gebremedhin D. Pressure-induced myogenic tone and role of 20-HETE in mediating autoregulation of cerebral blood flow. Am J Physiol Heart Circ Physiol. 2011;300:H1557–H1565. doi: 10.1152/ajpheart.01097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49:375–389. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Zhu YS, Hill C, Armstrong K, Tarumi T, Hodics T, Hynan LS, Zhang R. Cerebral autoregulation of blood velocity and volumetric flow during steady-state changes in arterial pressure. Hypertension. 2013;62:973–979. doi: 10.1161/HYPERTENSIONAHA.113.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension. 1994;23:565–568. doi: 10.1161/01.HYP.23.5.565. [DOI] [PubMed] [Google Scholar]
- 60.Sato K, Suzuki S, Yamada M, Oka H, Kurata A, Okamoto H, Fujii K, Kumabe T. Selecting an appropriate surgical treatment instead of carotid artery stenting alone according to the patient’s risk factors contributes to reduced perioperative complications in patients with internal carotid stenosis: a single institutional retrospective analysis. Neurol Med Chir (Tokyo) 2015;55:124–132. doi: 10.2176/nmc.oa.2014-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varga A, Di Leo G, Banga PV, Csobay-Novák C, Kolossváry M, Maurovich-Horvat P, Hüttl K. Multidetector CT angiography of the circle of Willis: association of its variants with carotid artery disease and brain ischemia. Eur Radiol. 2019;29:46–56. doi: 10.1007/s00330-018-5577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spacek M, Tesar D, Veselka J. The paramount role of the anterior communicating artery in the collateral cerebral circulation. Int J Angiol. 2015;24:236–240. doi: 10.1055/s-0034-1370889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones JD, Castanho P, Bazira P, Sanders K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: a literature review and meta-analysis. Clin Anat. 2021;34:978–990. doi: 10.1002/ca.23662. [DOI] [PubMed] [Google Scholar]
- 64.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods. 2011;196:221–237. doi: 10.1016/j.jneumeth.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 65.Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol. 2012;32:411–420. doi: 10.1055/s-0032-1331812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norcliffe-Kaufmann L, Galindo-Mendez B, Garcia-Guarniz AL, Villarreal-Vitorica E, Novak V. Transcranial Doppler in autonomic testing: standards and clinical applications. Clin Auton Res. 2018;28:187–202. doi: 10.1007/s10286-017-0454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol. 2013;591:5809–5821. doi: 10.1113/jphysiol.2013.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorteberg A, Sorteberg W, Bakke SJ, Lindegaard KF, Boysen M, Nornes H. Varying impact of common carotid artery digital compression and internal carotid artery balloon test occlusion on cerebral hemodynamics. Head Neck. 1998;20:687–694. doi: 10.1002/(SICI)1097-0347(199812)20:8<687::AID-HED5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 69.Smielewski P, Czosnyka M, Iyer V, Piechnik S, Whitehouse H, Pickard J. Computerised transient hyperaemic response test—a method for the assessment of cerebral autoregulation. Ultrasound Med Biol. 1995;21:599–611. doi: 10.1016/0301-5629(94)00154-6. [DOI] [PubMed] [Google Scholar]
- 70.Smielewski P, Czosnyka M, Kirkpatrick P, McEroy H, Rutkowska H, Pickard JD. Assessment of cerebral autoregulation using carotid artery compression. Stroke. 1996;27:2197–2203. doi: 10.1161/01.STR.27.12.2197. [DOI] [PubMed] [Google Scholar]
- 71.Smielewski P, Czosnyka M, Kirkpatrick P, Pickard JD. Evaluation of the transient hyperemic response test in head-injured patients. J Neurosurg. 1997;86:773–778. doi: 10.3171/jns.1997.86.5.0773. [DOI] [PubMed] [Google Scholar]
- 72.Visser GH, Wieneke GH, van Huffelen AC, Eikelboom BC. The use of preoperative transcranial Doppler variables to predict which patients do not need a shunt during carotid endarterectomy. Eur J Vasc Endovasc Surg. 2000;19:226–232. doi: 10.1053/ejvs.1999.1009. [DOI] [PubMed] [Google Scholar]
- 73.Hetzel A, von Reutern G, Wernz MG, Droste DW, Schumacher M. The carotid compression test for therapeutic occlusion of the internal carotid artery. Comparison of angiography with transcranial Doppler sonography. Cerebrovasc Dis. 2000;10:194–199. doi: 10.1159/000016056. [DOI] [PubMed] [Google Scholar]
- 74.Giller CA. A bedside test for cerebral autoregulation using transcranial Doppler ultrasound. Acta Neurochir (Wien). 1991;108:7–14. doi: 10.1007/BF01407660. [DOI] [PubMed] [Google Scholar]
- 75.Cavill G, Simpson EJ, Mahajan RP. Factors affecting assessment of cerebral autoregulation using the transient hyperaemic response test. Br J Anaesth. 1998;81:317–321. doi: 10.1093/bja/81.3.317. [DOI] [PubMed] [Google Scholar]
- 76.Calviello LA, Zeiler FA, Donnelly J, Uryga A, de Riva N, Smielewski P, Czosnyka M. Estimation of pulsatile cerebral arterial blood volume based on transcranial Doppler signals. Med Eng Phys. 2019;74:23–32. doi: 10.1016/j.medengphy.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Trofimov AO, Kalent’ev GV, Agarkova DI. Cerebrovascular resistance in patients with severe combined traumatic brain injury. Zh Vopr Neirokhir Im N N Burdenko. 2015;79:28–33. doi: 10.17116/neiro201579528-33. [DOI] [PubMed] [Google Scholar]
- 78.Alexandrov AV, Bladin CF, Maggisano R, Norris JW. Measuring carotid stenosis. Time for a reappraisal. Stroke. 1993;24:1292–1296. doi: 10.1161/01.STR.24.9.1292. [DOI] [PubMed] [Google Scholar]
- 79.North American Symptomatic Carotid Endarterectomy Trial Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.STR.22.6.711. [DOI] [PubMed] [Google Scholar]
- 80.Debreczeni R, Amrein I, Kamondi A, Szirmai I. Hypocapnia induced by involuntary hyperventilation during mental arithmetic reduces cerebral blood flow velocity. Tohoku J Exp Med. 2009;217:147–154. doi: 10.1620/tjem.217.147. [DOI] [PubMed] [Google Scholar]
- 81.Szirmai I, Amrein I, Pálvölgyi L, Debreczeni R, Kamondi A. Correlation between blood flow velocity in the middle cerebral artery and EEG during cognitive effort. Brain Res Cogn Brain Res. 2005;24:33–40. doi: 10.1016/j.cogbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Springo Z, Toth P, Tarantini S, Ashpole NM, Tucsek Z, Sonntag WE, Csiszar A, Koller A, Ungvari ZI. Aging impairs myogenic adaptation to pulsatile pressure in mouse cerebral arteries. J Cereb Blood Flow Metab. 2015;35:527–530. doi: 10.1038/jcbfm.2014.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011;31:2096–2105. doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahajan RP, Cavill G, Simpson EJ. Reliability of the transient hyperemic response test in detecting changes in cerebral autoregulation induced by the graded variations in end-tidal carbon dioxide. Anesth Analg. 1998;87:843–849. doi: 10.1213/00000539-199810000-00018. [DOI] [PubMed] [Google Scholar]
- 85.Girling KJ, Cavill G, Mahajan RP. The effects of nitrous oxide and oxygen on transient hyperemic response in human volunteers. Anesth Analg. 1999;89:175–180. doi: 10.1213/00000539-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 86.Moir ME, Klassen SA, Al-Khazraji BK, Woehrle E, Smith SO, Matushewski BJ, Kozić D, Dujić Ž, Barak OF. Shoemaker JK. Impaired dynamic cerebral autoregulation in trained breath-hold divers. J Appl Physiol. 2019;126:1694–1700. doi: 10.1152/japplphysiol.00210.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Banga PV, Varga A, Csobay-Novák C, Kolossváry M, Szántó E, Oderich GS, Entz L, Sótonyi P. Incomplete circle of Willis is associated with a higher incidence of neurologic events during carotid eversion endarterectomy without shunting. J Vasc Surg. 2018;68:1764–1771. doi: 10.1016/j.jvs.2018.03.429. [DOI] [PubMed] [Google Scholar]
- 88.Silvestrini M, Troisi E, Matteis M, Cupini LM and Caltagirone C. Transcranial Doppler assessment of cerebrovascular reactivity in symptomatic and asymptomatic severe carotid stenosis. Stroke. 1996;27:1970-3. [DOI] [PubMed]
- 89.Reinhard M, Schwarzer G, Briel M, Altamura C, Palazzo P, King A, Bornstein NM, Petersen N, Motschall E, Hetzel A, Marshall RS, Klijn CJ, Silvestrini M, Markus HS, Vernieri F. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology. 2014;83:1424–1431. doi: 10.1212/WNL.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prokin AL, Slankamenac P, Kovačević P, Kaloci SR, Živanović Ž. Cerebral vasomotor reactivity and apnea test in symptomatic and asymptomatic high-grade carotid stenosis. Srp Arh Celok Lek. 2015;143:520–524. doi: 10.2298/SARH1510520L. [DOI] [PubMed] [Google Scholar]
- 91.Naylor AR, JB. de Borst, GJ. Debus, S. de Haro, J. Halliday, A. Hamilton, G. Kakisis, J. Kakkos, S. Lepidi, S. Markus, HS. McCabe, DJ. Roy, J. Sillesen, H. van den Berg, JC. Vermassen, F. Esvs Guidelines Committee None, P K, N C, RJ H, I K, JS L, M VdC, F V and Esvs Guideline Reviewers None Archie JB, S. Chaudhuri, A. Koelemay, M. Lindahl, AK. Padberg, F. Venermo, M. Editor’s choice - management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery (ESVS). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2018;55:3-81. [DOI] [PubMed]
- 92.Hirt LS. Progression rate and ipsilateral neurological events in asymptomatic carotid stenosis. Stroke. 2014;45:702–706. doi: 10.1161/STROKEAHA.111.613711. [DOI] [PubMed] [Google Scholar]
- 93.Gupta A, Kesavabhotla K, Baradaran H, Kamel H, Pandya A, Giambrone AE, Wright D, Pain KJ, Mtui EE, Suri JS, Sanelli PC, Mushlin AI. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke. 2015;46:91–97. doi: 10.1161/STROKEAHA.114.006091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kakkos SK, Sabetai M, Tegos T, Stevens J, Thomas D, Griffin M, Geroulakos G, Nicolaides AN, Group ACSaRoSAS Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg. 2009;49:902–909. doi: 10.1016/j.jvs.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 95.Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS, Investigators A. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology. 2011;77:751–758. doi: 10.1212/WNL.0b013e31822b00a6. [DOI] [PubMed] [Google Scholar]
- 96.Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, Tegos T, Geroulakos G, Labropoulos N, Doré CJ, Morris TP, Naylor R, Abbott AL, Group ACSaRoSAS Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486–1496.e1-5. doi: 10.1016/j.jvs.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 97.Nicolaides AN, Kakkos S, Griffin M, Geroulakos G, Ioannidou E. Severity of asymptomatic carotid stenosis and risk of ipsilateral hemispheric ischaemic events: results from the ACSRS study. Nicolaides et al.: EJVES 2005; 30: 275-284. Eur J Vasc Endovasc Surg. 2006;31:336. doi: 10.1016/j.ejvs.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, Bornstein NM, Schaafsma A. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9:663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.King A, Serena J, Bornstein NM, Markus HS, Investigators A. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke. 2011;42:1550–1555. doi: 10.1161/STROKEAHA.110.609057. [DOI] [PubMed] [Google Scholar]
- 100.Kakkos SK, Griffin MB, Nicolaides AN, Kyriacou E, Sabetai MM, Tegos T, Makris GC, Thomas DJ, Geroulakos G, Group ACSaRoSAS The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg. 2013;57:609–618.e1. doi: 10.1016/j.jvs.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 101.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–3077. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 102.AF AR, Avgerinos ED, Chang RW, Darling RC, III, Duncan AA, Forbes TL, Malas MB, Murad MH, Perler BA, Powell RJ, Rockman CB. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J Vasc Surg. 2022;75:4S–22S. doi: 10.1016/j.jvs.2021.04.073. [DOI] [PubMed] [Google Scholar]
- 103.Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. [DOI] [PMC free article] [PubMed]
- 104.Rosenkranz K, Hierholzer J, Langer R, Hepp W, Palenker J, Felix R. Acetazolamide stimulation test in patients with unilateral internal carotid artery obstructions using transcranial Doppler and 99mTc-HM-PAO-Spect. Neurol Res. 1992;14:135–138. doi: 10.1080/01616412.1992.11740033. [DOI] [PubMed] [Google Scholar]
- 105.Markus HS, Harrison MJ. Estimation of cerebrovascular reactivity using transcranial Doppler, including the use of breath-holding as the vasodilatory stimulus. Stroke. 1992;23:668–673. doi: 10.1161/01.STR.23.5.668. [DOI] [PubMed] [Google Scholar]
- 106.Mahony PJ, Panerai RB, Deverson ST, Hayes PD, Evans DH. Assessment of the thigh cuff technique for measurement of dynamic cerebral autoregulation. Stroke. 2000;31:476–480. doi: 10.1161/01.STR.31.2.476. [DOI] [PubMed] [Google Scholar]
- 107.Naraynsingh V, Harnarayan P, Maharaj R, Dan D, Hariharan S. Preoperative digital carotid compression as a predictor of the need for shunting during carotid endarterectomy. Open Cardiovasc Med J. 2013;7:110–112. doi: 10.2174/1874192401307010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiong L, Leung HW, Chen XY, Han JH, Leung WH, Soo OY, Lau YL, Wong KS. Autonomic dysfunction in ischemic stroke with carotid stenosis. Acta Neurol Scand. 2012;126:122–128. doi: 10.1111/j.1600-0404.2011.01617.x. [DOI] [PubMed] [Google Scholar]
- 109.Mense L, Reimann M, Rüdiger H, Gahn G, Reichmann H, Hentschel H, Ziemssen T. Autonomic function and cerebral autoregulation in patients undergoing carotid endarterectomy. Circ J. 2010;74:2139–2145. doi: 10.1253/circj.CJ-10-0365. [DOI] [PubMed] [Google Scholar]
- 110.Nasr N, Pavy-Le Traon A, Larrue V. Baroreflex sensitivity is impaired in bilateral carotid atherosclerosis. Stroke. 2005;36:1891–1895. doi: 10.1161/01.STR.0000177890.30065.cb. [DOI] [PubMed] [Google Scholar]
- 111.Owens CD, Mukli P, Csipo T, Lipecz A, Silva-Palacios F, Dasari TW, et al. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am J Physiol Heart Circ Physiol. 2022;322(6):H924–35. [DOI] [PMC free article] [PubMed]
- 112.Istvan L, Czako C, Elo A, Mihaly Z, Sotonyi P, Varga A, et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: from diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience. 2021;43(4):1703–23. [DOI] [PMC free article] [PubMed]
- 113.Gardner AW, Montgomery PS, Wang M, Shen B, Casanegra AI, Silva-Palacios F, et al. Cognitive decrement in older adults with symptomatic peripheral artery disease. Geroscience. 2021;43(5):2455–65. [DOI] [PMC free article] [PubMed]
- 114.Toth P, Csiszar A, Tucsek Z, Sosnowska D, Gautam T, Koller A, Schwartzman ML, Sonntag WE, Ungvari Z. Role of 20-HETE, TRPC channels, and BKCa in dysregulation of pressure-induced Ca2+ signaling and myogenic constriction of cerebral arteries in aged hypertensive mice. Am J Physiol Heart Circ Physiol. 2013;305:H1698–H1708. doi: 10.1152/ajpheart.00377.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Göksu E, Koç P, Küçükseymen E, Ünal A, Genç F, Gencer ES, Yaman A. The association of the circle of Willis anomaly and risk of stroke in patients with carotid artery disease. Arq Neuropsiquiatr. 2017;75:429–432. doi: 10.1590/0004-282x20170054. [DOI] [PubMed] [Google Scholar]
- 117.Manojlovic V, Popovic V, Nikoloc D, Milosevic D, Pasternak J, Budakov N. Completeness of circle of Willis in asymptomatic and symptomatic extracranial carotid disease. Med Pregl. 2016;69:351–355. doi: 10.2298/MPNS1612351M. [DOI] [PubMed] [Google Scholar]
- 118.Park BJ, Kim KM, Lee WJ, Chun IK, Kim I, Lee SJ, Kim S. Clinical significance of the circle of Willis in patients with symptomatic internal carotid artery occlusion. World Neurosurg. 2018;115:e585–e591. doi: 10.1016/j.wneu.2018.04.104. [DOI] [PubMed] [Google Scholar]
- 119.Hoksbergen AW, Legemate DA, Csiba L, Csáti G, Síró P, Fülesdi B. Absent collateral function of the circle of Willis as risk factor for ischemic stroke. Cerebrovasc Dis. 2003;16:191–198. doi: 10.1159/000071115. [DOI] [PubMed] [Google Scholar]
- 120.Klimek-Piotrowska W, Rybicka M, Wojnarska A, Wójtowicz A, Koziej M, Hołda MK. A multitude of variations in the configuration of the circle of Willis: an autopsy study. Anat Sci Int. 2016;91:325–333. doi: 10.1007/s12565-015-0301-2. [DOI] [PubMed] [Google Scholar]
- 121.Chuang YM, Liu CY, Pan PJ, Lin CP. Posterior communicating artery hypoplasia as a risk factor for acute ischemic stroke in the absence of carotid artery occlusion. J Clin Neurosci. 2008;15:1376–1381. doi: 10.1016/j.jocn.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 122.Schomer DF, Marks MP, Steinberg GK, Johnstone IM, Boothroyd DB, Ross MR, Pelc NJ, Enzmann DR. The anatomy of the posterior communicating artery as a risk factor for ischemic cerebral infarction. N Engl J Med. 1994;330:1565–1570. doi: 10.1056/NEJM199406023302204. [DOI] [PubMed] [Google Scholar]
- 123.Saqqur M, Khan K, Derksen C, Alexandrov A, Shuaib A. Transcranial Doppler and transcranial color duplex in defining collateral cerebral blood flow. J Neuroimaging. 2018;28:455–476. doi: 10.1111/jon.12535. [DOI] [PubMed] [Google Scholar]
- 124.Müller M, Voges M, Piepgras U, Schimrigk K. Assessment of cerebral vasomotor reactivity by transcranial Doppler ultrasound and breath-holding. A comparison with acetazolamide as vasodilatory stimulus. Stroke. 1995;26:96–100. doi: 10.1161/01.STR.26.1.96. [DOI] [PubMed] [Google Scholar]
- 125.Al-Jehani H, Angle M, Marcoux J, Teitelbaum J. Early abnormal transient hyperemic response test can predict delayed ischemic neurologic deficit in subarachnoid hemorrhage. Crit Ultrasound J. 2018;10:1. doi: 10.1186/s13089-017-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leoni RF, Oliveira IA, Pontes-Neto OM, Santos AC, Leite JP. Cerebral blood flow and vasoreactivity in aging: an arterial spin labeling study. Braz J Med Biol Res. 2017;50:e5670. doi: 10.1590/1414-431x20175670. [DOI] [PMC free article] [PubMed] [Google Scholar]