Abstract
Background/Aims
Nonalcoholic fatty liver disease (NAFLD) has been observed in patients after partial pancreatectomy. Previous studies have been performed on oncologic patients who underwent partial pancreatectomy and received adjuvant chemotherapy. By studying a cohort of patients with intraductal papillary mucinous neoplasms (IPMNs) who did not receive chemotherapy, the authors investigate the isolated effect of partial pancreatectomy on the development of fatty liver.
Methods
A retrospective search for patients with pancreatic IPMNs who underwent partial pancreatectomy at an academic center from 2006 to 2014 identified 63 patients, including 42 who had pancreaticoduodenectomy (PD) and 21 who had distal pancreatectomy (DP). Fourteen patients with preoperative hepatic steatosis, diabetes, obesity, on steroid therapy, history of malignancy, or incomplete data were excluded. No patient received chemotherapy. Liver fat signal fraction (LFSF) was computed by the Dixon method using pre- and postoperative in- and out-of-phase MRI.
Results
Of the 49 patients included in the study, 29 (59%) underwent PD and 20 (41%) underwent DP. A total of 17 patients (34%) developed fatty liver after surgery. The entire cohort developed significant weight loss, 72.1 versus 69.4 kg (P < 0.01). Postoperatively, there was significant increase in LFSF, 1.3% versus 9.6% following PD (P < 0.01), and 2.1% versus 9.4% following DP (P = 0.01).
Conclusion
Partial pancreatectomy increases the risk of NAFLD independent of chemotherapy-induced hepatotoxicity. The underlying mechanism remains unclear and possibly related to pancreatic exocrine insufficiency and malnutrition.
Keywords: IPMN, hepatic steatosis, partial pancreatectomy
Graphical abstract
Highlights
-
•
Study focused on the impact of partial pancreatectomy on NAFLD in IPMN patients without chemotherapy.
-
•
Reviewed 49 patients, tracking their liver fat signal fraction (LFSF) using MRI data.
-
•
34% of patients developed fatty liver post-surgery, and all experienced significant weight loss.
-
•
Postoperative LFSF increased significantly after partial pancreatectomy.
-
•
Findings point to a higher risk of NAFLD post-pancreatectomy, signaling the need for further research.
Intraductal papillary mucinous neoplasms (IPMNs) are common cystic pancreatic lesions that have malignant potential.1, 2, 3 Surgical resection is commonly recommended for IPMNs with various high-risk stigmata.4,5 Depending on lesion location, either pancreaticoduodenectomy (PD) or distal pancreatectomy with or without splenectomy (DP) is performed for head or tail lesions, respectively.6, 7, 8
Nonalcoholic fatty liver disease (NAFLD) has been observed in patients after partial pancreatectomy,9, 10, 11, 12 particularly in patients with pancreatic cancer who underwent surgical resection and received adjuvant chemotherapy.10,13 Chemotherapy has a known association with NAFLD and is thought to induce the formation of oxygen radicals that indirectly damage hepatocytes.14, 15, 16 Some chemotherapeutic agents are implicated in the development of simple hepatic steatosis, while others are associated with nonalcoholic steatohepatitis (NASH), which is a more severe and inflammatory form of NAFLD.17, 18, 19, 20
In addition to chemotherapy related hepatotoxicity, there have been suggestions that partial pancreatectomy independently contributes to hepatic steatogenesis in these patients.9,12,21, 22, 23, 24 The underlying mechanism is likely multifactorial and perhaps related to alteration of the gastrointestinal tract alignment, pancreatic exocrine insufficiency, and malnutrition.11,25, 26, 27, 28, 29, 30 Unfortunately, no studies thus far have examined these variables independently.
Our study was designed to determine whether partial pancreatectomy (either PD or DP), independent of chemotherapy, can induce NAFLD, using a cohort of patients with pancreatic IPMNs, which were resected and followed by serial imaging.
Materials and methods
Following IRB approval, we performed a retrospective search for patients who had pancreatic IPMNs and underwent PD or DP from 2006 to 2014 in a single tertiary care center. A total of 63 patients were identified, including 42 who had PD and 21 who had DP. Patients with preoperative hepatic steatosis (n = 3), diabetes mellitus (n = 1), obesity defined as having BMI >30 (n = 2), on steroid therapy (n = 0), history of malignancy (n = 1), or incomplete data (n = 7) were excluded from the study (n = 14) (Figure 1). The three patients with preexisting hepatic steatosis had either stable or worsened steatosis 1–2 years after surgery. Although no patient had iron deposition disease, attention was paid to exclude these patients as this may cause magnetic susceptibility resulting in underestimation of liver fat concentration. No patient received chemotherapy before or after surgery.
Figure 1.
Patient selection.
Pre- and postoperative MRI of the abdomen, separated by 12–24 months, was evaluated to measure hepatic steatosis, expressed as liver fat signal fraction (LFSF). Each patient had pre- and postoperative imaging on 1.5 T MR systems. Six patients were scanned in different MR scanners before and after surgery. T1 weighted in-phase (IP) and out-of-phase (OP) images were acquired with 7 mm thickness with 8 mm spacing, and TEs of 4.6 and 2.3 ms, respectively. MR images were reviewed on a Philips iSite picture archiving and communication system (Philips, Best, the Netherlands) by a radiologist. Four circular region of interests (ROIs), all with area greater than 2 cm2, were placed, 2 on the right lobe and 2 on the left lobe, at the level of the main portal vein at similar locations on IP and OP images (Figure 2). The mean signal intensities were computed from the four ROIs drawn on both IP and OP images. LFSF was computed using the Dixon method following this equation31:
where IP and OP denote the mean signal intensities on in-phase and out-of-phase imaging, respectively.
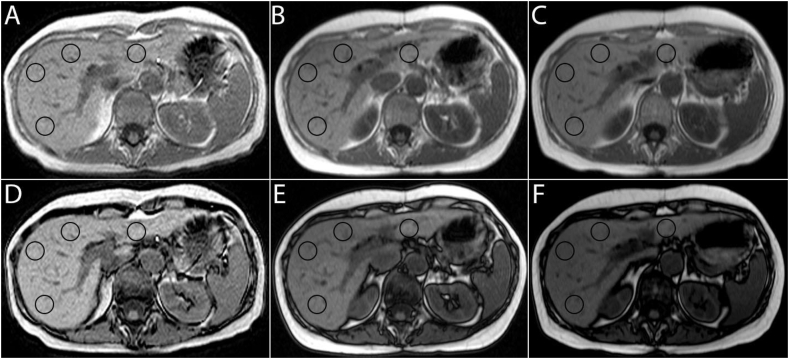
Figure 2.
T1-weighted in-phase (A, B, C) and out-of-phase (D, E, F) images: This is an example of a patient who developed mild hepatic steatosis with liver fat fraction of 8% one year out from pancreaticoduodenectomy (B, E) and 11% after 2 years (C, F). The circles on the images denote the 4 regions of interest used in liver fat fraction computation.
All images were reviewed carefully to ensure no fat-water cancellation artifacts adjacent to vessels, which is typically seen in severe steatosis (LFSF > 50%) to avoid underestimation of liver fat content using this technique. The Dixon method is dependent on fat-water signal cancellation, and this effect is maximum at 50% liver fat-water content; fat-water signal cancellation is inversely related to fat content at LFSF> 50%.31
Patient characteristics were compared between those who developed hepatic steatosis and those who did not after pancreatic IPMN resections (Table 1). Independent student t-tests were performed for continuous variables and chi-square tests for nominal variables. Using matched t-tests, comparisons were made between pre- and postoperative LFSF, weight (kg), BMI, fasting serum blood glucose (FBG), alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (T. Chol), and total triglycerides (T. Trig) (Table 2). Results were tabulated with P-values less than 0.05, indicating statistical significance. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, N.Y., USA).
Table 1.
Baseline Patient Characteristics: Comparison Between Patients Who Developed Hepatic Steatosis and Those Who did Not After Pancreatic IPMN Resections.
| With steatosis | Without steatosis | P-value | |
|---|---|---|---|
| Female | 68% | 32% | 0.76 |
| Male | 62% | 38% | |
| Age (years) | 63 ± 11.1 | 65 ± 9.3 | 0.89 |
| IPMN size (cm) | 2.7 ± 1.3 | 2.3 ± 1.4 | 0.68 |
| PD | 69% | 31% | 0.55 |
| DP | 60% | 40% | |
| Weight0 (kg) | 71.9 ± 14.5 | 72.3 ± 16.5 | 0.55 |
| BMI0 (kg/m2) | 24.7 ± 3.3 | 25.2 ± 4.3 | 0.14 |
| FBG0 (mg/dL) | 94.9 ± 10.1 | 96.9 ± 9.9 | 0.58 |
| ALP0 (IU/L) | 79.9 ± 21.0 | 76.8 ± 24.6 | 0.20 |
| AST0 (U/L) | 28.8 ± 12.2 | 26.1 ± 7.8 | 0.05 |
| ALT0 (IU/L) | 26.3 ± 13.9 | 26.9 ± 13.4 | 0.60 |
| T. Chol0 (mg/dL) | 189.4 ± 34.5 | 188.4 ± 31.2 | 0.41 |
| T. Trig0 (mg/dL) | 140.3 ± 42.1 | 120.9 ± 43.3 | 0.61 |
| LFSF0 | 1.5% ± 1.0% | 1.7% ± 1.3% | 0.16 |
| LFSF1 | 21.9% ± 14.6% | 2.5% ± 1.2% | <0.01a |
DP, distal pancreatectomy; LFSF, liver fat signal fraction; PD, pancreaticoduodenectomy; Subscript 0: before surgery; Subscript 1: after surgery.
Statistical significance.
Table 2.
Before and After Partial Pancreatectomy.
| Before surgery | After surgery | P-value | |
|---|---|---|---|
| Weight (kg) | 72.1 ± 15.0 | 69.4 ± 14.3 | <0.01∗ |
| BMI (kg/m2) | 24.9 ± 3.6 | 24.1 ± 3.5 | <0.01∗ |
| FBG (mg/dL) | 95.6 ± 9.9 | 96.7 ± 15.0 | 0.45 |
| ALP (IU/L) | 78.8 ± 22.1 | 88.6 ± 25.1 | 0.01∗ |
| AST (U/L) | 27.9 ± 10.9 | 30.2 ± 15.3 | 0.22 |
| ALT (IU/L) | 26.5 ± 13.6 | 31.9 ± 21.6 | 0.06 |
| T. Chol (mg/dL) | 189.1 ± 33.1 | 188.8 ± 34.5 | 0.79 |
| T. Trig (mg/dL) | 133.6 ± 43.1 | 137.8 ± 43.4 | 0.21 |
| LFSFPD | 1.3% ± 1.1% | 9.6% ± 13.6% | <0.01∗ |
| LFSFDP | 2.1% ± 1.0% | 9.4% ± 11.9% | 0.01∗ |
∗ indicates statistical significance or P-value <0.05.
LFSF, liver fat signal fraction; Subscript PD: pancreaticoduodenectomy; Subscript DP: distal pancreatectomy.
The degree of steatosis was converted from a continuous scale to an ordinal scale based on LFSF: (1) 0–5% was defined as within normal range, (2) 5–10% as mild steatosis, (3) 10–30% as moderate steatosis, and (4) >30% as severe steatosis (Figure 3). Nonparametric, Wilcoxon signed-rank test was used to determine whether the increase in hepatic fat content was significant after partial pancreatectomy.
Figure 3.
Comparison of varying degree of hepatic steatosis after partial pancreatectomy: pancreaticoduodenectomy vs. distal pancreatectomy. Note that significant proportion of patients developed fatty liver after the operation. Interestingly, the surgical approach, PD or DP, does not affect the outcome of hepatic steatosis.
Results
Patient Characteristics
Of the 49 patients included in our study, 21 patients (43%) were male, and mean age was 64 years. Mean IPMN sizes in patients who developed hepatic steatosis and those did not were 2.7 cm and 2.3 cm, respectively, with no significant difference (P = 0.68). Twenty-nine patients (59%) underwent PD for pancreatic head and neck IPMNs, while 20 patients (41%) underwent DP for pancreatic body and tail IPMNs. None of the patients included had preexisting hepatic steatosis, with mean liver fat signal fraction of 1.6% (range 0–4.6%) on MRI. Seventeen out of the 49 patients (34%) developed fatty liver after surgery. The two groups, those with postoperative fatty liver and those without, had no significant differences in gender, age, IPMN size, weight, BMI, serum fasting blood glucose, ALP, AST, ALT, total cholesterol, and total triglyceride levels (table 1).
Weight Loss After Partial Pancreatectomy
Following surgery, the entire cohort developed significant weight loss, with preoperative and postoperative mean weight of 72.1 kg (BMI 24.9) and 69.4 kg (BMI 24.1), respectively (P < 0.01) (table 2). There was also significant increase in serum ALP level, 70 U/L before, and 89 U/L after surgery (P < 0.01) although it remained within normal limits. Serum fasting blood glucose, AST, ALT, total cholesterol, and total triglyceride levels were not significantly different before and after surgery.
Comparing patients who developed postoperative steatosis and those who did not, there was a similar degree of weight loss (Table 3). For patients who developed steatosis, their mean weight was 71.9 kg (BMI 24.7) and 69.4 kg (BMI 24.0) before and after surgery, respectively. For patients who did not develop steatosis, their mean weight was 72.3 kg (BMI 25.2) and 69.3 kg (BMI 24.2) before and after surgery, respectively. Laboratory values were not significantly different after surgery except alkaline phosphatase (ALP), which is slightly higher after surgery but still within normal range.
Table 3.
Differences Between Pre- and Post-operative Weight, BMI and Laboratory Values According to Presence and Absence of Postoperative Steatosis.
| Postop steatosis absent (n = 32) |
Postop steatosis present (n = 17) |
|||||
|---|---|---|---|---|---|---|
| Before surgery | After surgery | P-value | Before surgery | After surgery | P-value | |
| Weight (kg) | 71.9 ± 14.5 | 69.4 ± 14.1 | <0.01∗ | 72.3 ± 16.4 | 69.4 ± 14.9 | <0.01∗ |
| BMI (kg/m2) | 24.7 ± 3.3 | 24.0 ± 3.2 | <0.01∗ | 25.2 ± 4.3 | 24.2 ± 4.1 | <0.01∗ |
| FBG (mg/dL) | 94.9 ± 10.1 | 97.7 ± 15.9 | 0.17 | 96.9 ± 9.9 | 94.9 ± 13.3 | 0.33 |
| ALP (IU/L) | 79.9 ± 20.9 | 88.2 ± 21.8 | <0.01∗ | 76.8 ± 24.6 | 89.6 ± 30.9 | 0.04∗ |
| AST (U/L) | 28.8 ± 12.2 | 30.4 ± 17.0 | 0.54 | 26.1 ± 7.8 | 29.8 ± 11.6 | 0.13 |
| ALT (IU/L) | 26.3 ± 13.9 | 30.9 ± 23.7 | 0.25 | 26.9 ± 13.3 | 33.8 ± 17.5 | 0.03 |
| T. Chol (mg/dL) | 189.4 ± 34.5 | 190.4 ± 35.0 | 0.33 | 188.4 ± 31.2 | 186.0 ± 34.3 | 0.22 |
| T. Trig (mg/dL) | 140.3 ± 42.1 | 140.5 ± 41.3 | 0.93 | 120.9 ± 43.3 | 132.6 ± 47.8 | 0.15 |
| LFSF | 1.5% ± 1.0% | 2.5% ± 1.2% | <0.01∗ | 1.7% ± 1.3% | 21.9% ± 14.6% | <0.01∗ |
∗ indicates statistical significance or P-value <0.05.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; FBG, fasting serum blood glucose; LFSF, Liver fat signal fraction; T. Chol, total cholesterol; T. Trig, total triglycerides.
Hepatic Steatogenesis Following Partial Pancreatectomy
Nine of the 29 patients in the PD group and 8 of the 20 patients in the DP group developed hepatic steatosis (Table 2). In the PD group, there was significant increase in LFSF on MRI after surgery, 1.3% versus 9.6% (P < 0.01). Among the nine patients with steatosis following PD, three (10%) had mild steatosis (LFSF between 5% and 10%), two (7%) had moderate steatosis (LFSF between 10% and 30%), and four (14%) had severe steatosis (LFSF greater than 30%). Similarly, in the DP group, there was also significant increase in LFSF on MRI after surgery, 2.1% versus 9.4% (P = 0.01). Among the eight patients with steatosis following DP, four (20%) had mild steatosis, three (15%) had moderate steatosis, and one (5%) had severe steatosis. The surgical approach, PD or DP, was not a significant predictor of postoperative hepatic steatosis (P = 0.55) (Table 1).
To minimize the effect of random measurement errors, liver fat fraction was converted into a discrete, four-level ordinal scale. The score of 0 was assigned to LFSF is less than 5% (normal), score of 1 to LFSF of 5–10%, score of 2 to LFSF of 10–30%, and score of 3 to LFSF greater than 30% (Figure 3). Using Wilcoxon signed-ranks test for matched, pre- and postoperative liver fat fractions, the increase in liver fat was shown to be significant in the overall, PD, and DP groups (P < 0.01, P < 0.01, P = 0.01, respectively).
Discussion
NAFLD is a spectrum of varying degrees of fatty accumulation in the liver ranging from simple steatosis to nonalcoholic steatohepatitis (NASH).32,33 NASH can potentially lead to cirrhosis and is an independent risk factor for hepatocellular carcinoma.34, 35, 36, 37 Typically, NAFLD is associated with metabolic derangements and thus more commonly seen in patients with obesity, hyperlipidemia and insulin resistance.38, 39, 40, 41
NAFLD is increasingly recognized in patients after partial pancreatectomy.9, 10, 11, 12 Paradoxically, these patients often have malnutrition after surgery and do not exhibit the stigmata of metabolic syndrome seen in typical NAFLD.9 Prior studies have shown hepatic steatosis in patients with pancreatic cancer following partial pancreatectomy and chemotherapy and have attributed this to the concomitant use of chemotherapy in these patients.10,13 Nishikawa et al. showed significantly higher incidence of NAFLD in patients who received adjuvant chemotherapy compared to those did not.13 Chemotherapy-related hepatotoxicity is a well-known entity, with an underlying mechanism related to chemotherapy-related oxidative stress on hepatocytes. For example, 5FU and leucovorin are associated with hepatic steatosis and postoperative infection. Irinotecan has been implicated in steatohepatitis, hepatic insufficiency and poor surgical outcome.42,43
A few studies have suggested that pancreatic resection and associated anatomical alteration can contribute to the development of NAFLD.10,12,22,23,26,44 Nomura et al. argued that partial pancreatectomy is an independent risk factor for NAFLD, as 14 out of 42 patients in their study developed postoperative NAFLD.10 However, more patients who developed NAFLD received adjuvant chemotherapy (43% vs. 18%) compared to those did not, confounding the causal factors associated with NAFLD. Without isolating the effect of partial pancreatectomy, it remained unclear whether surgical alteration of anatomy alone contributes to the development of NAFLD.
By studying patients with IPMNs, we were able to show significant liver fat deposition after pancreatic resection, with liver fat signal fraction of 1.3% in the pancreaticoduodenectomy group (PD) and 2.1% in the distal pancreatectomy group (DP) before and 9.6% and 9.4%, after surgery, respectively. The selection of this cohort of patients is ideal to investigate the independent effect of surgery without the confounding effect of chemotherapy. Interestingly, hepatic steatogenesis was similar in both PD and DP groups, implying that alteration of gastrointestinal tract alignment alone cannot explain this phenomenon.
Pancreatic exocrine insufficiency (PEI) and malnutrition are likely major factors contributing to the development of steatosis following partial pancreatectomy.9,28,29,45 The underlying mechanism is unclear, but a few studies have shown that pancreatic enzyme supplementation prevents or improves NAFLD.46, 47, 48, 49, 50 In contrast to typical NAFLD associated with metabolic syndrome, these patients have weight loss and malnutrition as evidenced by decreased BMI, serum protein, and lipoprotein levels. In our study, we did observe significant weight loss after surgery without significant change in serum lipid panel. However, a similar degree of weight loss was seen in those who developed postoperative steatosis and those who did not (table 3), suggesting against a direct causal relationship. Nonetheless, this perhaps increases the susceptibility of postoperative steatosis in patients with PEI. Incidental note was made of slight increase in alkaline phosphatase level which was still within normal range. One possible explanation is mildly impeded bile drainage following hepaticojejunostomy in the PD group.
There are several limitations in our study. The sample size was small. A few patients were scanned in different MR scanners before and after surgery. There are limitations inherent with respective studies. We were not able to directly measure liver fat fraction, as proton density fat fraction or MR spectroscopy was not available when this cohort underwent MR imaging. Our categories of hepatic steatosis severity were somewhat arbitrary, and not directly related to histological categorization of steatosis severity. Although none of the patients had hepatic iron overload, subtle iron deposition may cause magnetic susceptibility and result in underestimation of liver fat fraction. Unfortunately, we were unable to provide a detailed timeline regarding the exact time points at which NAFLD developed within the 1- to 2-year postsurgery time frame. This may restrict our understanding of the disease's dynamics following surgery. Lastly, diet and sedentary lifestyle, well-known as risk factors for NAFLD, were not included in our analysis, due to the challenges associated with quantifying such subjective variables and our focus on the 1- to 2-year postsurgery time frame. These limitations may have resulted in an incomplete understanding of the various factors influencing NAFLD outcomes in our study population. We encourage future research to consider these factors and explore their interaction with surgical interventions to provide a more comprehensive understanding of post-partial-pancraetectomy NAFLD development and management.
In conclusion, we have shown the development of NAFLD in patients with IPMNs who underwent partial pancreatectomy. A larger study is needed to verify and expand on our observations. The physiology of postoperative hepatic steatogenesis following partial pancreatectomy is yet to be elucidated. As long-term survival from partial pancreatectomy improves, the prevalence of postoperative NAFLD will likely rise. We need to be cognizant of this potential complication and investigate the underlying mechanisms in order to effectively manage these patients.
Credit authorship contribution statement
The authorship of this article is as follows: Zhenteng Li conducted the research, analyzed the data, and wrote the manuscript. Jonathan Weinstein, Ellen Redstone, and Donald G. Mitchell provided valuable contributions to the study design, data collection, and critical review of the manuscript. All authors have approved the final version of the manuscript and agree to its submission to the Journal of Clinical and Experimental Hepatology. The authors are fully responsible for the content and accuracy of the manuscript.
Conflicts of interest
The authors declare that they have following conflict of interest:
Zhenteng Li, Jonathan Weinstein, Ellen Redstone, and Donald G. Mitchell have no conflicts of interest or financial ties to disclose.
Acknowledgements
None.
Funding
None.
Ethics statement
This study was approved by institutional review board.
References
- 1.Laffan T.A., Horton K.M., Klein A.P., et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802–807. doi: 10.2214/AJR.07.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X.M., Mitchell D.G., Dohke M., Holland G.A., Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1148/radiol.2232010815. [DOI] [PubMed] [Google Scholar]
- 3.Do R.K., Katz S.S., Gollub M.J., et al. Interobserver agreement for detection of malignant features of intraductal papillary mucinous neoplasms of the pancreas on MDCT. AJR Am J Roentgenol. 2014;203:973–979. doi: 10.2214/AJR.13.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka M., Fernandez-del Castillo C., Adsay V., et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Heckler M., Michalski C.W., Schaefle S., Kaiser J., Buchler M.W., Hackert T. The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN - a meta-analysis on their accuracy. Pancreatology. 2017;17:255–262. doi: 10.1016/j.pan.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Cameron J.L., He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. doi: 10.1016/j.jamcollsurg.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 7.Jang J.Y., Kim S.W., Lee S.E., et al. Treatment guidelines for branch duct type intraductal papillary mucinous neoplasms of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 8.Vullierme M.P., Giraud-Cohen M., Hammel P., et al. Malignant intraductal papillary mucinous neoplasm of the pancreas: in situ versus invasive carcinoma surgical resectability. Radiology. 2007;245:483–490. doi: 10.1148/radiol.2451060951. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N., Horiuchi A., Yokoyama T., et al. Clinical characteristics of de novo nonalcoholic fatty liver disease following pancreaticoduodenectomy. J Gastroenterol. 2011;46:758–768. doi: 10.1007/s00535-011-0370-5. [DOI] [PubMed] [Google Scholar]
- 10.Nomura R., Ishizaki Y., Suzuki K., Kawasaki S. Development of hepatic steatosis after pancreatoduodenectomy. AJR Am J Roentgenol. 2007;189:1484–1488. doi: 10.2214/AJR.07.2809. [DOI] [PubMed] [Google Scholar]
- 11.Sim E.H., Kwon J.H., Kim S.Y., et al. Severe steatohepatitis with hepatic decompensation resulting from malnutrition after pancreaticoduodenectomy. Clin Mol Hepatol. 2012;18:404–410. doi: 10.3350/cmh.2012.18.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato H., Isaji S., Azumi Y., et al. Development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: proposal of a postoperative NAFLD scoring system. J Hepatobiliary Pancreat Sci. 2010;17:296–304. doi: 10.1007/s00534-009-0187-2. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa M., Aosasa S., Moriya T., Noro T., Hase K., Yamamoto J. The impact of postoperative adjuvant chemotherapy on the development of nonalcoholic fatty liver disease after pancreatoduodenectomy. J Surg Res. 2016;205:127–135. doi: 10.1016/j.jss.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.Z., Morris-Stiff G., Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg. 2009;16:137–144. doi: 10.1007/s00534-008-0016-z. [DOI] [PubMed] [Google Scholar]
- 15.Selzner M., Rudiger H.A., Sindram D., Madden J., Clavien P.A. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner D.E., Brunt E.M., Van Natta M., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Vauthey J.N., Pawlik T.M., Ribero D., et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 18.Nemoto Y., Saibara T., Ogawa Y., et al. Tamoxifen-induced nonalcoholic steatohepatitis in breast cancer patients treated with adjuvant tamoxifen. Intern Med. 2002;41:345–350. doi: 10.2169/internalmedicine.41.345. [DOI] [PubMed] [Google Scholar]
- 19.Ganeshan D.M., Salem U., Viswanathan C., et al. Complications of oncologic therapy in the abdomen and pelvis: a review. Abdom Imaging. 2013;38:1–21. doi: 10.1007/s00261-012-9899-x. [DOI] [PubMed] [Google Scholar]
- 20.Oien K.A., Moffat D., Curry G.W., et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353:36–37. doi: 10.1016/S0140-6736(05)74872-8. [DOI] [PubMed] [Google Scholar]
- 21.Ohgi K., Okamura Y., Yamamoto Y., et al. Perioperative computed tomography assessments of the pancreas predict nonalcoholic fatty liver disease after pancreaticoduodenectomy. Medicine. 2016;95:e2535. doi: 10.1097/MD.0000000000002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo D.G., Jung B.H., Hwang S., et al. Prevalence analysis of de novo hepatic steatosis following pylorus-preserving pancreaticoduodenectomy. Dig Surg. 2014;31:359–365. doi: 10.1159/000368381. [DOI] [PubMed] [Google Scholar]
- 23.Yu H.H., Shan Y.S., Lin P.W. Effect of pancreaticoduodenectomy on the course of hepatic steatosis. World J Surg. 2010;34:2122–2127. doi: 10.1007/s00268-010-0636-8. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y., Kenmochi T., Shibutani S., et al. Evaluation of predictive factors in patients with nonalcoholic fatty liver disease after pancreaticoduodenectomy. Am Surg. 2014;80:500–504. [PubMed] [Google Scholar]
- 25.Fish J.C., Smith L.B., Williams R.D. Digestive function after radical pancreaticoduodenectomy. Am J Surg. 1969;117:40–45. doi: 10.1016/0002-9610(69)90283-9. [DOI] [PubMed] [Google Scholar]
- 26.Kang C.M., Lee J.H. Pathophysiology after pancreaticoduodenectomy. World J Gastroenterol. 2015;21:5794–5804. doi: 10.3748/wjg.v21.i19.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitamura T., Anaguchi-Hirao R., Kouhara H. Combination of type 2 diabetes and malnutrition worsened by anastomotic stenosis and pancreas atrophy following resection of pancreas head. Intern Med. 2008;47:1225–1230. doi: 10.2169/internalmedicine.47.0233. [DOI] [PubMed] [Google Scholar]
- 28.Murata Y., Mizuno S., Kato H., et al. Nonalcoholic steatohepatitis (NASH) after pancreaticoduodenectomy: association of pancreatic exocrine deficiency and infection. Clin J Gastroenterol. 2011;4:242–248. doi: 10.1007/s12328-011-0226-9. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa N., Murakami Y., Uemura K., et al. Nonalcoholic fatty liver disease after pancreatoduodenectomy is closely associated with postoperative pancreatic exocrine insufficiency. J Surg Oncol. 2014;110:720–726. doi: 10.1002/jso.23693. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M., Nakata K., Matsumoto H., et al. Acyl/free carnitine ratio is a risk factor for hepatic steatosis after pancreatoduodenectomy and total pancreatectomy. Pancreatology. 2017;17:135–138. doi: 10.1016/j.pan.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Dixon W.T. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 32.Adams L.A., Lymp J.F., St Sauver J., et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Yeh M.M., Brunt E.M. Pathological features of fatty liver disease. Gastroenterology. 2014;147:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 34.Bugianesi E., Leone N., Vanni E., et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka Y., Hashimoto E., Yatsuji S., et al. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. J Gastroenterol. 2004;39:1215–1218. doi: 10.1007/s00535-004-1475-x. [DOI] [PubMed] [Google Scholar]
- 36.Diehl A.M., Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med. 2017;377:2063–2072. doi: 10.1056/NEJMra1503519. [DOI] [PubMed] [Google Scholar]
- 37.Feldstein A.E., Charatcharoenwitthaya P., Treeprasertsuk S., Benson J.T., Enders F.B., Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balkau B., Lange C., Vol S., Fumeron F., Bonnet F., Group Study DESIR Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 2010;10:56. doi: 10.1186/1471-230X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725 e716. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchesini G., Brizi M., Bianchi G., et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 42.Mahli A., Saugspier M., Koch A., et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut. 2017 doi: 10.1136/gutjnl-2016-312485. [DOI] [PubMed] [Google Scholar]
- 43.Makowiec F., Mohrle S., Neeff H., et al. Chemotherapy, liver injury, and postoperative complications in colorectal liver metastases. J Gastrointest Surg. 2011;15:153–164. doi: 10.1007/s11605-010-1368-7. [DOI] [PubMed] [Google Scholar]
- 44.Miura H., Ijichi M., Ando Y., et al. A rapidly progressive and fatal case of nonalcoholic steatohepatitis following pancreaticoduodenectomy. Clin J Gastroenterol. 2013;6:470–475. doi: 10.1007/s12328-013-0421-y. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya T., Tanaka N., Kimura T., et al. Mechanism of the development of nonalcoholic steatohepatitis after pancreaticoduodenectomy. BBA Clin. 2015;3:168–174. doi: 10.1016/j.bbacli.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kishi Y., Shimada K., Nara S., Esaki M., Kosuge T. Administration of pancrelipase as effective treatment for hepatic steatosis after pancreatectomy. Pancreas. 2015;44:983–987. doi: 10.1097/MPA.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 47.Nagai M., Sho M., Satoi S., et al. Effects of pancrelipase on nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2014;21:186–192. doi: 10.1002/jhbp.14. [DOI] [PubMed] [Google Scholar]
- 48.Sato T., Matsuo Y., Shiga K., Morimoto M., Miyai H., Takeyama H. Factors that predict the occurrence of and recovery from non-alcoholic fatty liver disease after pancreatoduodenectomy. Surgery. 2016;160:318–330. doi: 10.1016/j.surg.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Satoi S., Sho M., Yanagimoto H., et al. Do pancrelipase delayed-release capsules have a protective role against nonalcoholic fatty liver disease after pancreatoduodenectomy in patients with pancreatic cancer? A randomized controlled trial. J Hepatobiliary Pancreat Sci. 2016;23:167–173. doi: 10.1002/jhbp.318. [DOI] [PubMed] [Google Scholar]
- 50.Yamazaki S., Takayama T., Higaki T., et al. Pancrelipase with branched-chain amino acids for preventing nonalcoholic fatty liver disease after pancreaticoduodenectomy. J Gastroenterol. 2016;51:55–62. doi: 10.1007/s00535-015-1077-9. [DOI] [PubMed] [Google Scholar]