Abstract
A major barrier to the use of nitrogen-fixing inoculum strains for the enhancement of legume productivity is the inability of commercially available strains to compete with indigenous rhizobia for nodule formation. Despite extensive research on nodulation competitiveness, there are no examples of field efficacy studies of strains that have been genetically improved for nodulation competitiveness. We have shown previously that production of the peptide antibiotic trifolitoxin (TFX) by Rhizobium etli results in significantly increased nodule occupancy values in nonsterile soil in growth chamber experiments (E. A. Robleto, A. J. Scupham, and E. W. Triplett, Mol. Plant-Microbe Interact. 10:228–233, 1997). To determine whether TFX production by Rhizobium etli increases nodulation competitiveness in field-grown plants, seeds of Phaseolus vulgaris were inoculated with mixtures of Rhizobium etli strains at different ratios. The three nearly isogenic inoculum strains used included TFX-producing and non-TFX-producing strains, as well as a TFX-sensitive reference strain. Data was obtained over 2 years for nodule occupancy and over 3 years for assessment of the effect of the TFX production phenotype on grain yield. In comparable mixtures in which the test strain accounted for between 5 and 50% of the inoculum, the TFX-producing strain exhibited at least 20% greater nodule occupancy than the non-TFX-producing strain in both years. The TFX production phenotype had no effect on grain yield over 3 years; the average yields reached 2,400 kg/ha. These results show that addition of the TFX production phenotype significantly increases nodule occupancy under field conditions without adverse effects on grain yield. As we used common inoculation methods in this work, there are no practical barriers to the commercial adoption of the TFX system for agriculture.
Many bacterial genera within the α subdivision of the division Proteobacteria infect the roots, and occasionally the stems, of leguminous plants, resulting in the formation of nodules. Within these nodules, the bacteria differentiate to become nitrogen-fixing bacteroids that are provided carbon and energy sources by the host plant.
Inoculum strains of these nitrogen-fixing bacteria have been available for legume agriculture for more than 100 years. The strains isolated to date vary greatly in the amount of fixed N provided to the host plant. Some of this variation has been ascribed to specific phenotypes (13). However, inoculum strains with the best symbiotic properties often fail to infect legume roots in the presence of large indigenous populations of nodule bacteria.
This nodulation competitiveness problem has been reviewed recently (13, 21). Many solutions to this problem that include genetic enhancement of the competitiveness phenotype of nodule bacteria have been proposed. To date, no one has developed such a genetic improvement that is successful under agricultural conditions.
One of the best-characterized systems for genetic enhancement of nodulation competitiveness in root nodule bacteria is a cassette of genes that code for the production of a potent antirhizobial peptide, trifolitoxin (TFX). TFX is a ribosomally synthesized, posttranslationally modified peptide produced by Rhizobium leguminosarum bv. trifolii T24 (10). Workers have isolated and sequenced a set of genes that code for TFX production and resistance in every Rhizobium, Mesorhizobium, Sinorhizobium, Phyllobacterium, Brucella, and Agrobacterium strain tested to date (10, 20). TFX inhibits members of a specific clade of the α subdivision of the division Proteobacteria that includes legume symbionts, plant pathogens, and animal pathogens (20).
Under laboratory conditions, the TFX production phenotype has been shown to significantly increase the nodulation competitiveness of Rhizobium and Sinorhizobium strains (15, 17–19). Recently, it has been shown that TFX production enhances strain nodule occupancy in nonsterile soil despite the fact that TFX is broken down rapidly in nonsterile soil (15). In this work, we tested the efficacy of the TFX production system. In addition to testing the ability of TFX production to improve nodulation competitiveness, we also tested the effects of TFX production on grain yield. The effect of TFX production on host plant productivity is an important risk assessment question that must be addressed prior to any commercialization of this technology.
MATERIALS AND METHODS
Growth conditions, bacterial strains, and plasmids.
Bacterial cultures were kept as frozen stock solutions at −70°C in 15% glycerol. Cultures used for seed inoculation were streaked onto Bergensen’s synthetic medium plates (4) to obtain single colonies, which were resuspended in 1 ml of water and inoculated into yeast extract-mannitol broth (7). We grew cultures for 3 days at 28°C and 250 rpm; the cells in these cultures were centrifuged at 4,000 × g for 10 min, washed, resuspended in 0.1 volume of 15% glycerol, and stored at −70°C for later use. A 1-ml aliquot was used to estimate cell density.
All of the inoculant strains used in this work, CE3(pFPMZ), CE3(pT2TX3K), and CE3(pT2TFXK), are derivatives of Rhizobium etli CE3 (Table 1); these strains are referred to below as the TFX-sensitive (TFXs), non-TFX-producing (TFXnp), and TFX-producing (TFXp) strains, respectively. Antibiotics were used at the following concentrations: ampicillin, 100 μg/ml; cycloheximide, 100 μg/ml; kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; and tetracycline, 2.5 μg/ml.
TABLE 1.
Plasmids used in this study
Preparation of inoculum mixtures and seed coating.
Stock solutions having known cell densities were used to mix the TFXs strain with either the TFXnp or TFXp strain at different ratios. In 1995, seeds of Phaseolus vulgaris cv. Azteca received single-strain suspensions at concentrations varying from 0.5 × 107 to 4.6 × 107 cells/seed. For the 1996 experiment, in addition to the single-strain inoculations, we prepared five TFXs-TFXnp mixtures at ratios of 43:1, 17:1, 1.7:1, 1:5.7, and 1:12.8 and five TFXs-TFXp mixtures at ratios of 42:1, 21:1, 1.8:1, 1:5.3, and 1:11. For the 1997 experiment, we prepared five TFXs-TFXnp mixtures at ratios of 25:1, 10:1, 1:1, 1:10, and 25:1 and five TFXs-TFXp mixtures at the same ratios. The number of cells on each seed was 106 to 107.
In 1995 and 1996, seeds (1.02 kg) were coated by using 24 g of peat, 0.83 g of charcoal, 1.66 g of CaCO3, 15 ml of a 25% corn syrup solution, and 15 ml of bacterial suspension. In 1997, we used 280 g of seed/treatment, and the amounts of all of the other ingredients were adjusted based on the amount of seed used. Seeds and ingredients were placed in an Intermatic seed mixer (Gustafson Inc., Plano, Tex.) and spun for 5 min at 40 rpm.
Experimental plot and sites.
Experiments were conducted at the University of Wisconsin—Madison experimental station at Arlington, Wis. The soil characteristics are shown in Table 2. Seeds were planted in early June in 1995 and 1997 and on July 2 in 1996. Plants were harvested at maturity. The locations of the 1995, 1996, and 1997 sites were, respectively, 43°18.24′N, 89°20.26′W; 43°17.94′N, 89°20.79′W; and 43°19.60′N, 89°20.21′W. Seeds were planted with a seed planter (1995 and 1996) or by hand (1997) at a rate of 20 seeds/m in 2.4- by 4-m plots with four rows (1995 and 1996) or in 1.8- by 4-m plots with three rows (1997). Only the central row was inoculated in 1997. There were eight plots/treatment arranged in a randomized complete block design for 1996 and 1997, whereas only four replicates were used in 1995. Weeds were removed from the plots four times each year by hand.
TABLE 2.
Soil characteristics of Arlington sites used in this study in 1995, 1996, and 1997a
| Year | pH | % Organic matter | P concn (ppm) | K concn (ppm) | Density (g/cm3) | Texture | Previous crop | No. of bean-nodulating rhizobia/g of soilb | TFX phenotype |
|---|---|---|---|---|---|---|---|---|---|
| 1995 | 5.8 | 3.1 | 51 | 175 | 1.16 | Plano silt loam | Corn | 3.3 × 102 | Sensitive |
| 1996 | 6.8 | 3.0 | 56 | 270 | 1.16 | Plano silt loam | Corn | 2.3 × 102 | Sensitive |
| 1997 | 6.3 | 4.4 | 33 | 135 | 1.16 | Plano silt loam | Corn | <0.4 × 102 | Sensitive |
The residual N concentration from the previous crop varied between 60 and 70 kg/ha.
Estimated by the most-probable-number technique (11).
Nodule occupancy and yield.
Forty-eight nodules from five plants (1996 and 1995) and 96 nodules from 10 plants (1997) were recovered from each plot to assay for strain occupancy. The nodules were surface sterilized, placed in 96-well microtiter plates, crushed, and plated onto medium containing antibiotics (3). The yield was determined by weighing the dry seeds harvested from 1 m2 per plot per treatment.
Data analysis.
Nodule occupancy was determined as described by Amarger and Lobreau (1). Nodule occupancy values were obtained by using the following expression: TFXnp/(TFXnp + TFXs) or TFXp/(TFXp + TFXs), where each term represents the proportion of nodules occupied by strains with the indicated phenotypes.
The nodule occupancy values for treatments with mixtures containing TFXp or TFXnp strains at the same inoculum ratio were compared by using an f test. The yield was analyzed by using an f test and including the data for all treatments.
RESULTS
Nodule occupancy.
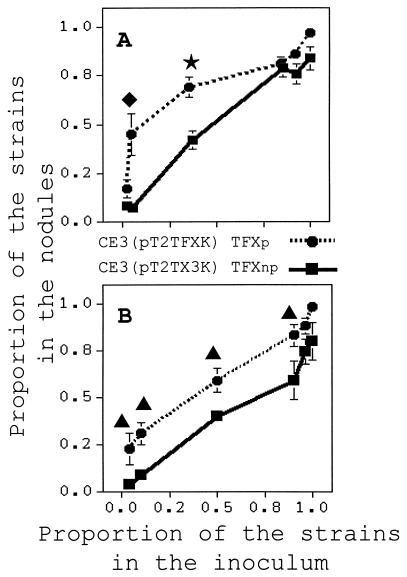
We determined the nodule occupancy values in 1996 for R. etli strains that differed only in TFX production with plants inoculated with the TFXs strain in Arlington, Wis., over two seasons. In 1996, TFX production increased nodulation competitiveness of R. etli under field conditions (Fig. 1A). Inoculation of plants with a 12:1 TFXs-TFXp strain mixture resulted in 55% of the nodules being occupied by the TFXs strain and 45% being occupied by the TFXp strain. Inoculation with a mixture containing 40% TFXp strain resulted in 70% of the nodules being occupied by this strain. In contrast, a comparable mixture containing the TFXs and TFXnp strains resulted in only 35% of the nodules being occupied by the TFXnp strain. The nodule occupancy values did not differ significantly for the TFXp and TFXnp strains when the plants were inoculated with a TFXs-TFXnp or TFXs-TFXp mixture at ratios of 40:1, 1:7, and 1:12 (Fig. 1A).
FIG. 1.
Nodulation competitiveness of nearly isogenic strains of R. etli CE3 derivatives that produce and do not produce TFX in 1996 (A) and 1997 (B) at Arlington, Wis. Seeds were coated with TFXs-TFXp or TFXs-TFXnp mixtures at different ratios. Each point represents eight replicates. Nodule occupancy values were estimated by using the model described by Amarger and Lobreau (1). ★, ⧫, and ▴, differences significant at P ≤ 0.01, P ≤ 0.05, and P ≤ 0.10, respectively.
In 1997, we observed that for all of the different inoculum ratios the nodule occupancy values for the TFXp strain were roughly 20% greater than the nodule occupancy values for the TFXnp strain (Fig. 1B). For example, inoculation of 25:1 and 10:1 TFXs-TFXnp and TFXs-TFXp mixtures resulted in nodule occupancy values of 4 and 9% for the TFXnp strain and 24 and 32% for the TFXp strain. The TFXp strain had significantly higher nodule occupancy values than the TFXnp strain with all of the treatment mixtures except when the inoculum contained 25-fold less TFXs strain.
TFX production increased the nodulation competitiveness of R. etli under field conditions (Fig. 1). TFX production was found to be most effective in increasing nodule occupancy when the inoculum contained 5 to 60% TFXp strain. Mixtures in which the inoculum contained an extremely low or extremely high number of TFXp cells compared to the number of TFXs (reference) strain cells resulted in nodule occupancy values that were not substantially different from those reached by the TFXnp strain.
Competition of the TFXp and TFXnp strains with the indigenous rhizobia is shown in Table 3. Over 3 years, the TFXp strain had a significantly higher nodule occupancy value than the TFXnp strain (95 and 83%, respectively). These results indicate that TFX-producing strains are effective against the indigenous rhizobia found in the sites used, as well as the reference strain.
TABLE 3.
Nodulation and yield of P. vulgaris cv. Aztec inoculated with single-strain suspensions of the TFXnp and TFXp strains in Arlington, Wis., from 1995 to 1997a
| Treatment | Nodule occupancy (%) | Yield (kg/ha) |
|---|---|---|
| No inoculation | 0 ± 3 c | 2,454 ± 85 a |
| TFXnp strain | 83 ± 4 b | 2,380 ± 80 a |
| TFXp strain | 95 ± 2 a | 2,400 ± 60 a |
Values are the averages ± standard errors based on data obtained in 1995, 1996, and 1997. Means followed by different letters are significantly different at P ≤ 0.05. Treatments were repeated four times in 1995 and eight times in 1996 and 1997.
Yield of beans inoculated with TFX-producing strains.
Table 3 shows the yields of bean plants inoculated with nearly isogenic strains of CE3 that differed in TFX production. The yield obtained with plants inoculated with either of the strains used did not vary significantly from the yield obtained with uninoculated plants. The average yields obtained in these trials, 2,400 kg/ha, are similar to the yields obtained when 110 kg of applied N per ha was used (5, 6, 12).
Plasmid transfer.
The disadvantage of having plasmid-borne tfx genes is the possibility that pT2TFXK will be transferred to ineffective or inefficient indigenous rhizobia. This would enhance the competitiveness of the indigenous strains. As pT2TFXK lacks the mobilization locus necessary for self-transmission of the plasmid, we expected the plasmid transfer rate to be very low. We were unable to detect any plasmid transfer in the field during the 3 years of the experiment (data not shown). However, the method used by us to examine transfer lacks the resolution necessary to observe transfer events having a frequency of less than 1 in 105. We tested many of the streptomycin-sensitive indigenous isolates obtained during the nodule occupancy work for the presence of tetracycline and kanamycin resistance. No indigenous isolates were found with the resistance markers on pT2TFXK.
DISCUSSION
A major barrier to the use of commercial legume inoculants is the lack of strains that occupy a high proportion of root nodules. In this paper we show that TFX production is an effective way to increase nodule occupancy by inoculum strains under field conditions. With most mixture inoculation treatments, the nodule occupancy value for the TFXp strain was at least 20% greater than the nodule occupancy value for the TFXnp strain (Fig. 1). Even though Bosworth et al. (9) reported that TFX is rapidly degraded under nonsterile soil conditions, the increase in nodule occupancy observed in this study may have been due to the inhibitory effect of TFX on TFX-sensitive strains in the rhizosphere, as previously suggested (15). A steady-state level of TFX production in the rhizosphere by the TFXp strain is apparently sufficient to reduce nodulation by the TFXs strain.
The improved nodulation competitiveness obtained from the TFX production phenotype was less pronounced in the field experiments described here than in the nonsterile soil growth chamber experiment described previously (15). The most likely explanation for this difference is that the soil used in the growth chamber experiment is known to support 10 to 15 times more TFX production than the Arlington soil 2 to 4 days after inoculation with a TFX-producing strain (9).
Other reports have described strains that exhibit increased nodulation competitiveness compared to the wild-type strain (2, 8, 14). In each case, the value of the data for resolving the nodulation competitiveness problem in the field was not determined since these studies were carried out either in synthetic medium or under growth chamber conditions. In one of these studies, a highly competitive strain was generated by tandem duplication of a specific DNA region in R. etli (14). It is not known whether this enhanced copy number of specific genes in tandem is stable.
The TFXp and TFXnp strains are nearly isogenic strains which differ only by the absence of tfxA and the 5′ end of tfxB in the TFXnp strain. Both strains are resistant to TFX, and the two strains differ only in TFX production. Both pT2TX3K and pT2TFXK are stable plasmids that are maintained during cell division in the absence of selection pressure. Thus, any differences observed in either grain yield or nodulation competitiveness can be attributed solely to the TFX production phenotype.
There are three justifications for adding tfx genes to CE3 by adding a broad-host-range plasmid rather than by chromosomally inserting these genes. First, we have learned that it is very difficult to identify a symbiotically silent site for chromosomal insertion of a cassette of genes. Scupham et al. (16) showed that an inositol utilization site previously thought to be symbiotically silent in Sinorhizobium meliloti was actually detrimental to host plant productivity.
Second, adding the tfx genes by plasmid transfer allows us to rapidly add the TFX production phenotype to any inoculum strain of any Rhizobium, Mesorhizobium, or Sinorhizobium species. This should allow much more rapid commercialization of this technology. Conjugation of pT2TFXK or a similar plasmid into any strain of root nodule bacteria can be done in 1 week.
Third, adding the tfx genes on a multicopy plasmid can result in a much higher level of TFX production than the level of production found in strains that possess one copy of the tfx genes on the chromosome (18, 20). The overproduction of TFX conferred by pT2TFXK greatly expands the range of species of root nodule bacteria inhibited by TFX and, as a result, expands the usefulness of this technology to other agriculturally important legumes (18, 20).
We also show here that a high level of nodule occupancy by a TFX-producing strain has no effect on the grain yield of Phaseolus vulgaris. This result addresses an important regulatory question. That is, does TFX production have any adverse effects on the growth of the host plant? Plants inoculated with either the TFXp or TFXnp strain showed no difference in grain yield over a 3-year period. This means that there can be commercial adoption of the TFX system without any fear that plant productivity will be affected.
In summary, we describe a specific genetic improvement to a Rhizobium strain, the addition of the TFX production phenotype, which increases nodulation competitiveness for a reference strain in the field. We found that it is possible to genetically improve the nodulation competitiveness of a strain and demonstrated its efficacy under agricultural conditions. This paper also shows that production of a peptide antibiotic, despite its apparent instability in nonsterile soil (9), can affect bacterial strain interactions with plant roots. The design of the field experiments in this study shows that the TFX system can be applied easily to legume agriculture.
ACKNOWLEDGMENTS
Funds for this work were provided by U.S. Department of Agriculture NRI grant 94-37-050767 and by Hatch project 5201.
We thank Paul Focke, Erika Vikstad, Jenifer Jansen, and Michelle Wjotasiak for technical assistance.
REFERENCES
- 1.Amarger N, Lobreau J P. Quantitative study of nodulation competitiveness in Rhizobium strains. Appl Environ Microbiol. 1982;44:583–588. doi: 10.1128/aem.44.3.583-588.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie G A, Handelsman J. Evaluation of a strategy for identifying nodulation competitiveness genes in Rhizobium leguminosarum biovar phaseoli. J Gen Microbiol. 1993;139:529–538. doi: 10.1099/00221287-139-3-529. [DOI] [PubMed] [Google Scholar]
- 3.Beattie G A, Handelsman J. A rapid method for isolation and identification of Rhizobium from nodules. J Microbiol Methods. 1989;9:29–33. [Google Scholar]
- 4.Bergensen F J. The growth of Rhizobium in synthetic media. Aust J Biol Sci. 1961;14:349–360. [Google Scholar]
- 5.Berglund D, Courneya T, Franzen D, Glogoza P, Hellevang K, Hofman V, Kuntz B, Lamey A, Scherer T, Zollinger R. Dry bean production guide. Publication A-1133. Fargo: North Dakota State University; 1997. [Google Scholar]
- 6.Berglund D, Grafton K. North Dakota dry bean performance testing. Publication A-654. Fargo: North Dakota State University Extension Service; 1997. [Google Scholar]
- 7.Beringer J E. R-factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974;84:188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwat A A, Keister D L. Identification and cloning of Bradyrhizobium japonicum genes expressed strain selectively in soil and rhizosphere. Appl Environ Microbiol. 1992;58:1490–1495. doi: 10.1128/aem.58.5.1490-1495.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosworth A H, Breil B T, Triplett E W. Production of the anti-rhizobial peptide, trifolitoxin, in sterile soils by Rhizobium leguminosarum bv. trifolii T24. Soil Biol Biochem. 1993;25:829–832. [Google Scholar]
- 10.Breil B T, Ludden P W, Triplett E W. DNA sequence and mutational analysis of genes involved in the production and resistance of the antibiotic peptide trifolitoxin. J Bacteriol. 1993;175:3693–3702. doi: 10.1128/jb.175.12.3693-3702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockwell J. Plant-infection counts of rhizobia in soils. In: Vincent J M, editor. Nitrogen fixation in legumes. Sydney, Australia: Academic Press; 1982. pp. 41–58. [Google Scholar]
- 12.Kelly J D, Copeland L O. Aztec, a new pinto bean. Extension bulletin E-2486. East Lansing: Michigan State University; 1994. [Google Scholar]
- 13.Maier R J, Triplett E W. Toward more productive, efficient, and competitive nitrogen-fixing symbiotic bacteria. Crit Rev Plant Sci. 1996;15:191–234. [Google Scholar]
- 14.Mavingui P, Flores M, Romero D, Martinez-Romero E, Palacios R. Generation of Rhizobium strains with improved symbiotic properties by random DNA amplification. Nat Biotechnol. 1997;15:564–569. doi: 10.1038/nbt0697-564. [DOI] [PubMed] [Google Scholar]
- 15.Robleto E A, Scupham A J, Triplett E W. Trifolitoxin production in Rhizobium etli strain CE3 increases competitiveness for rhizosphere growth and root nodulation of Phaseolus vulgaris in soil. Mol Plant-Microbe Interact. 1997;10:228–233. [Google Scholar]
- 16.Scupham A J, Bosworth A H, Ellis W R, Wacek T J, Albrecht K A, Triplett E W. Inoculation with Sinorhizobium meliloti RMBPC-2 increases alfalfa yield compared with inoculation with a nonengineered wild-type strain. Appl Environ Microbiol. 1996;62:4260–4262. doi: 10.1128/aem.62.11.4260-4262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triplett E W. Construction of a symbiotically effective strain of Rhizobium leguminosarum bv. trifolii with increased nodulation competitiveness. Appl Environ Microbiol. 1990;56:98–103. doi: 10.1128/aem.56.1.98-103.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triplett E W. Isolation of genes involved in nodulation competitiveness from Rhizobium leguminosarum bv. trifolii T24. Proc Natl Acad Sci USA. 1988;85:3810–3814. doi: 10.1073/pnas.85.11.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Triplett E W, Barta T M. Trifolitoxin production and nodulation are necessary for the expression of superior nodulation competitiveness by Rhizobium leguminosarum bv. trifolii T24. Plant Physiol. 1987;85:335–342. doi: 10.1104/pp.85.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triplett E W, Breil B T, Splitter G A. Expression of tfx and sensitivity to the rhizobial peptide antibiotic trifolitoxin in a taxonomically distinct group of α-proteobacteria including the animal pathogen Brucella abortus. Appl Environ Microbiol. 1994;60:4163–4166. doi: 10.1128/aem.60.11.4163-4166.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlassak K M, Vanderleyden J. Factors influencing nodule occupancy by inoculant rhizobia. Crit Rev Plant Sci. 1997;16:163–229. [Google Scholar]