Abstract
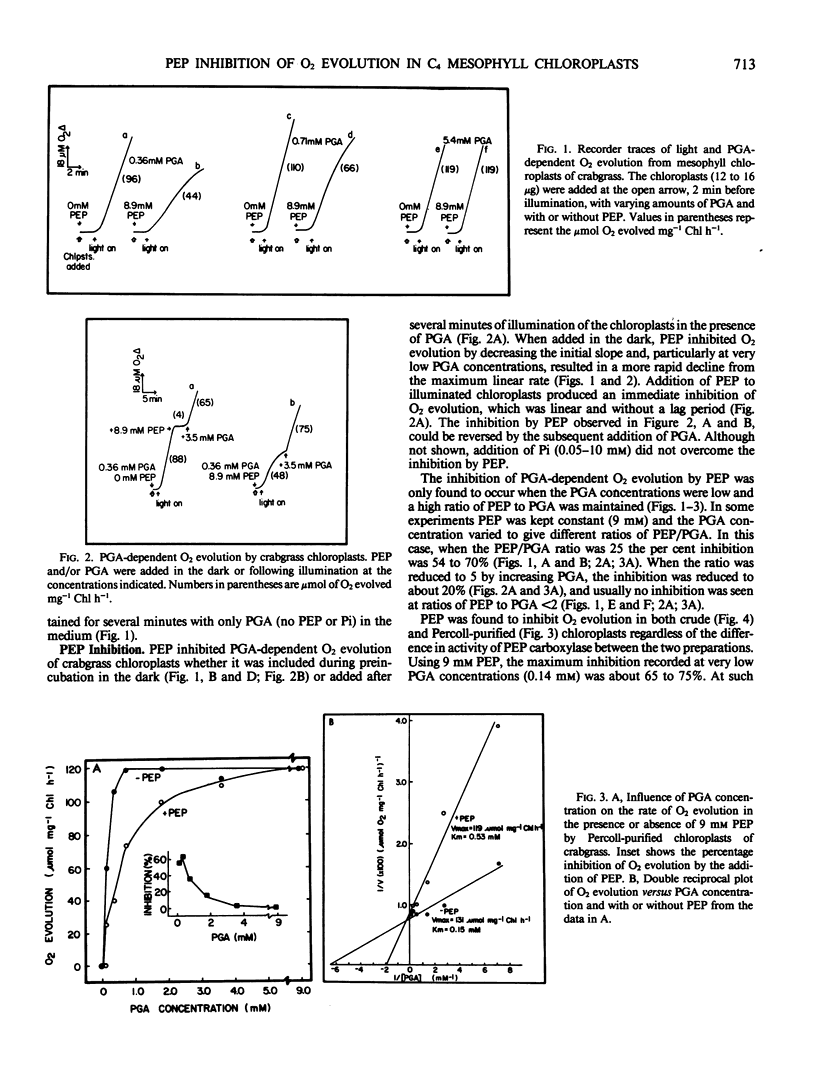
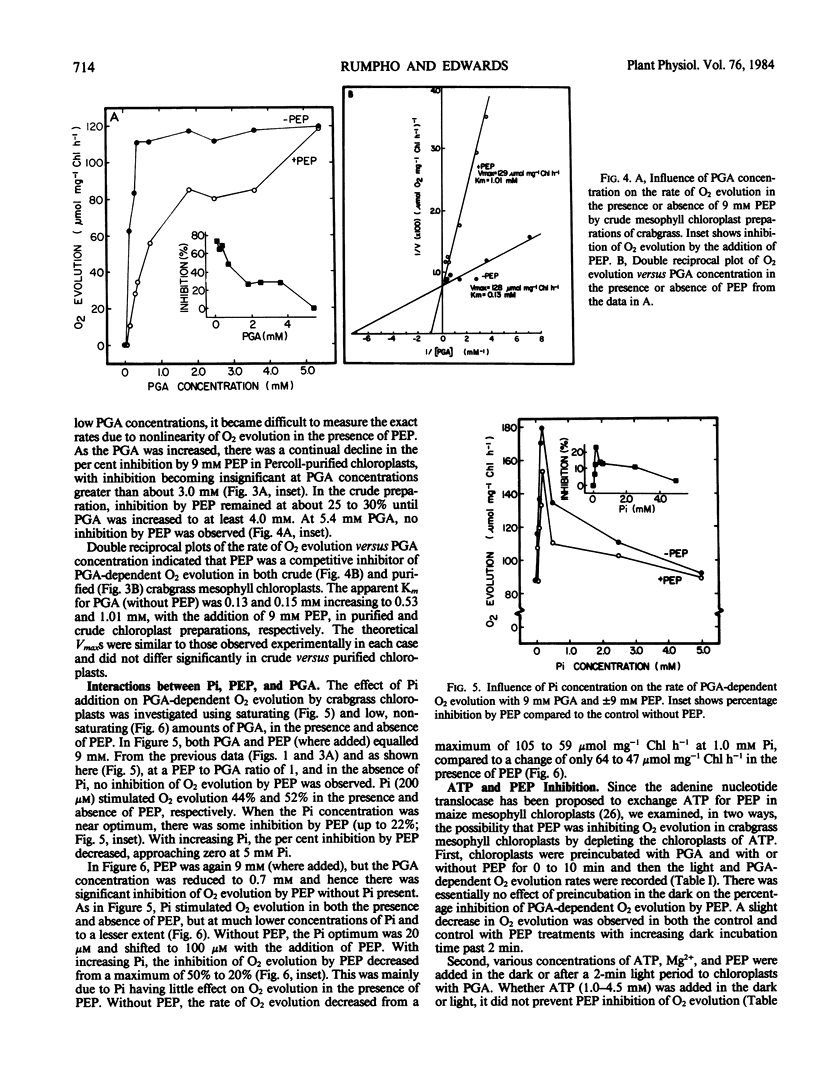
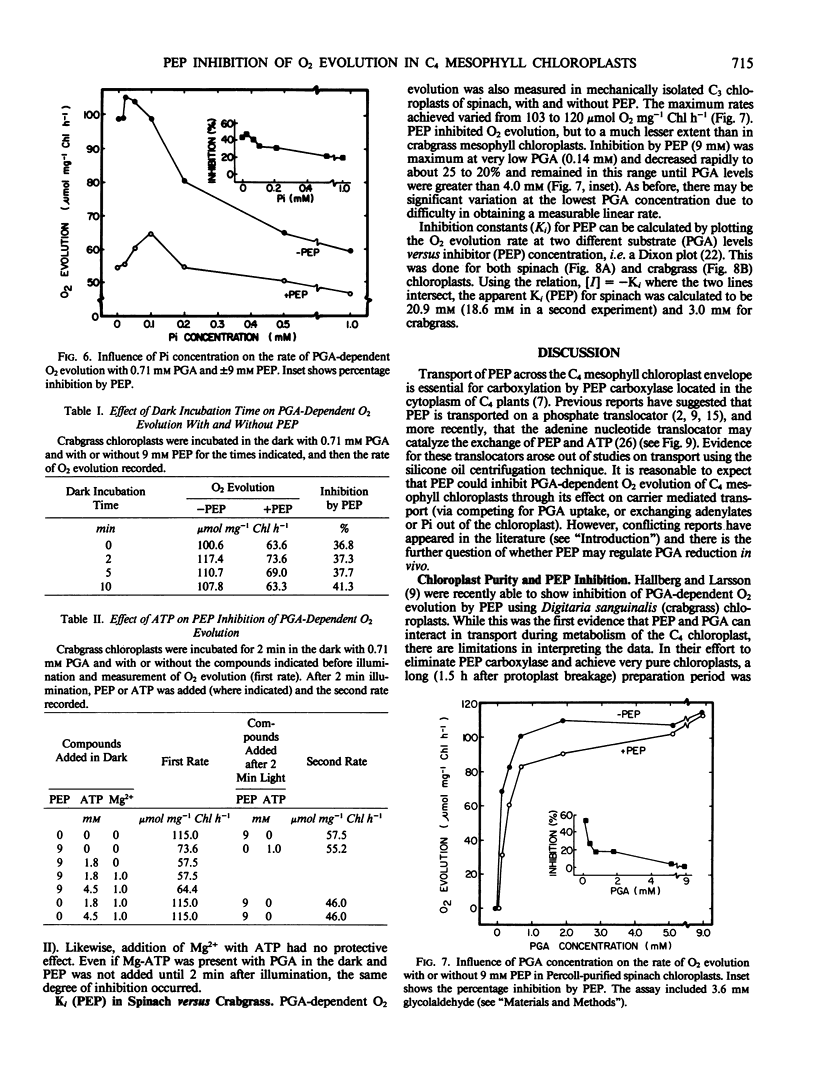
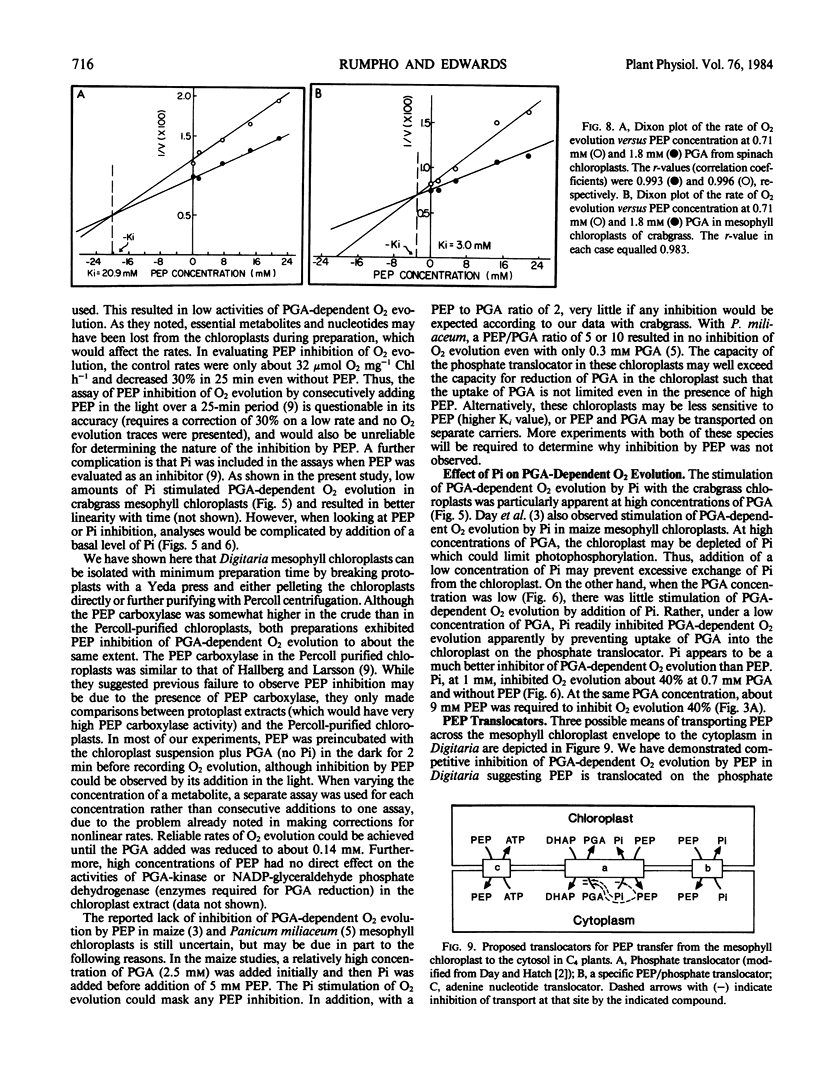
The effects of phosphoenolpyruvate (PEP), inorganic phosphate (Pi), and ATP on 3-phosphoglycerate (PGA)-dependent O2 evolution by chloroplasts of Digitaria sanguinalis (L.) Scop. (crabgrass) were evaluated relative to possible mechanisms of PEP transport by the C4 mesophyll chloroplast. Crude and Percoll purified chloroplast preparations exhibited rates of PGA-dependent O2 evolution in the range of 90 to 135 micromoles O2 per milligram chlorophyll per hour, and up to 180 micromoles O2 per milligram chlorophyll per hour at optimal Pi concentrations (approximately 0.2 millimolar at 9 millimolar PGA). Higher concentrations of Pi were inhibitory. PEP inhibited O2 evolution (up to 70%) in both chloroplast preparations when the PEP to PGA ratio was high (i.e. 9 millimolar PEP to 0.36 millimolar PGA). Usually no inhibition was seen when the PEP to PGA ratio was less than 2. PEP acted as a competitive inhibitor and, at a concentration of 9 millimolar, increased the apparent Km (PGA) from 0.15 to 0.53 millimolar in Percoll purified chloroplasts. A low concentration of PGA and high ratio of PEP to PGA, which are considered unphysiological, were required to detect any inhibition of O2 evolution by PEP. Similar results were obtained from crude versus Percoll purified preparations. Neither the addition of Pi nor ATP could overcome PEP inhibition. As PEP inhibition was competitive with respect to PGA concentration, and as addition of ATP or Pi could not prevent PEP inhibition of PGA-dependent O2 evolution, the inhibition was not due to PEP exchange of adenylates or Pi out of the chloroplast. Analysis of the effect of Pi and PEP, separately and in combination, on PGA-dependent O2 evolution suggests interactions between PEP, Pi, and PGA on the same translocator in the C4 mesophyll chloroplast. C3 spinach chloroplasts were also found to be sensitive to PEP, but to a lesser extent than crabgrass chloroplasts. The apparent Ki values (PEP) were 3 and 21 millimolar for crabgrass and spinach, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day D. A., Hatch M. D. Dicarboxylate transport in maize mesophyll chloroplasts. Arch Biochem Biophys. 1981 Oct 15;211(2):738–742. doi: 10.1016/0003-9861(81)90510-5. [DOI] [PubMed] [Google Scholar]
- Day D. A., Hatch M. D. Transport of 3-phosphoglyceric acid, phosphoenolpyruvate, and inorganic phosphate in maize mesophyll chloroplasts,, and the effect of 3-phosphoglyceric acid on malate and phosphoenolpyruvate production. Arch Biochem Biophys. 1981 Oct 15;211(2):743–749. doi: 10.1016/0003-9861(81)90511-7. [DOI] [PubMed] [Google Scholar]
- Edwards G. E. Isolation of Intact and Functional Chloroplasts from Mesophyll and Bundle Sheath Protoplasts of the C(4) Plant Panicum miliaceum. Plant Physiol. 1979 May;63(5):821–827. doi: 10.1104/pp.63.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. A highactivity ATP translocator in mesophyll chloroplasts of Digitaria sanguinalis, a plant having the C-4 dicarboxylic acid pathway of photosynthesis. Biochim Biophys Acta. 1976 Sep 13;440(3):675–687. doi: 10.1016/0005-2728(76)90050-5. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Regulation of oxaloacetate, aspartate, and malate formation in mesophyll protoplast extracts of three types of c(4) plants. Plant Physiol. 1975 Aug;56(2):324–331. doi: 10.1104/pp.56.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts characterization of the pyruvate carrier. Biochim Biophys Acta. 1977 Dec 23;462(3):583–602. doi: 10.1016/0005-2728(77)90103-7. [DOI] [PubMed] [Google Scholar]
- Huber S. C., Edwards G. E. Transport in C4 mesophyll chloroplasts: evidence for an exchange of inorganic phosphate and phosphoenolpyruvate. Biochim Biophys Acta. 1977 Dec 23;462(3):603–612. doi: 10.1016/0005-2728(77)90104-9. [DOI] [PubMed] [Google Scholar]
- Mbaku S. B., Fritz G. J., Bowes G. Photosynthetic and Carbohydrate Metabolism in Isolated Leaf Cells of Digitaria pentzii. Plant Physiol. 1978 Oct;62(4):510–515. doi: 10.1104/pp.62.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumpho M. E., Edwards G. E., Loescher W. H. A pathway for photosynthetic carbon flow to mannitol in celery leaves : activity and localization of key enzymes. Plant Physiol. 1983 Dec;73(4):869–873. doi: 10.1104/pp.73.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K., Foster J. G., Edwards G. E., Holtum J. A. Intracellular Localization of Enzymes of Carbon Metabolism in Mesembryanthemum crystallinum Exhibiting C(3) Photosynthetic Characteristics or Performing Crassulacean Acid Metabolism. Plant Physiol. 1982 Feb;69(2):300–307. doi: 10.1104/pp.69.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis G., Voss S., Shrago E., Werner-Washburne M., Keegstra K. An adenine nucleotide-phosphoenolpyruvate counter-transport system in C3 and C4 plant chloroplasts. Biochem Biophys Res Commun. 1983 Nov 15;116(3):945–951. doi: 10.1016/s0006-291x(83)80233-2. [DOI] [PubMed] [Google Scholar]


