Abstract
Methionine restriction (MR) extends lifespan in various model organisms, and understanding the molecular effectors of MR could expand the repertoire of tools targeting the aging process. Here, we address to what extent the biochemical pathway responsible for redox metabolism of methionine plays in regulating the effects of MR on lifespan and health span. Aerobic organisms have evolved methionine sulfoxide reductases to counter the oxidation of the thioether group contained in the essential amino acid methionine. Of these enzymes, methionine sulfoxide reductase A (MsrA) is ubiquitously expressed in mammalian tissues and has subcellular localization in both the cytosol and mitochondria. Loss of MsrA increases sensitivity to oxidative stress and has been associated with increased susceptibility to age-associated pathologies including metabolic dysfunction. We rationalized that limiting the available methionine with MR may place increased importance on methionine redox pathways, and that MsrA may be required to maintain available methionine for its critical uses in cellular homeostasis including protein synthesis, metabolism, and methylation. Using a genetic mutant mouse lacking MsrA, we tested the requirement for this enzyme in the effects of MR on longevity and markers of healthy aging late in life. When initiated in adulthood, we found that MR had minimal effects in males and females regardless of MsrA status. MR had minimal effect on lifespan with the exception of wild-type males where loss of MsrA slightly increased lifespan on MR. We also observed that MR drove an increase in body weight in wild-type mice only, but mice lacking MsrA tended to maintain more stable body weight throughout their lives. We also found that MR had greater benefit to males than females in terms of glucose metabolism and some functional health span assessments, but MsrA generally had minimal impact on these metrics. Frailty was also found to be unaffected by MR or MsrA in aged animals. We found that in general, MsrA was not required for the beneficial effects of MR on longevity and health span.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00857-8.
Keywords: Methionine restriction (MetR), Lifespan, Frailty, Methionine sulfoxide reductase A (MsrA), Glucose metabolism, Health span
Introduction
Dietary approaches to increase longevity in laboratory animal models have been used to better understand the process of aging. For example, the gold standard intervention for longevity, calorie restriction (CR), has been shown to have a myriad of effects associated with improved health with age in addition to extension of lifespan. Importantly, CR has also been shown to alter regulation of a number of pro-longevity molecular pathways [1]. Understanding the longevity and health span effects of restricting specific dietary components like amino acids has refined our understanding of the functional requirement and contribution of these components to the aging process.
Dietary restriction of the essential amino acid methionine, without reduction of caloric intake, has been well-established as a means to increase lifespan among various laboratory rodent models [1–3]. Methionine restriction (MR) also has been shown to beneficially impact multiple functions associated with aging health including metabolism [4–8], oxidative stress [9–12], and mitochondrial function [9, 11, 13, 14]. While many of the effects of MR are phenotypically similar to CR, there is growing evidence that CR and MR are at least partially distinct in terms of molecular effectors. For example, CR has been reported to reduce mTOR signaling while MR has been reported to have little effect [1, 5]. CR and MR appear to share some overlap given that supplementation of essential amino acids, including methionine, to mice undergoing CR blunts its effects suggesting some interaction between methionine and CR [15]. Similarly, supplementation of methionine to yeast undergoing glucose restriction negates the effect of this intervention in regard to lifespan extension [16]. However, the molecular mechanisms by which MR promotes longevity have yet to be clearly identified, and their delineation could inform potential molecular targets that could assist in translating the benefits of MR without a restrictive diet.
Several downstream effectors of MR have been identified, including endocrine signaling through hormones such as growth hormone and IGF1 [3, 17–20], or the metabolic regulator FGF21 [6, 17, 20–22]. However, the regulatory mechanisms by which MR drives these physiological outcomes have yet to be clarified. Methionine has many critical roles that may play a part in regulating the proposed pillars of aging including regulating protein translation, serving as a methyl donor for DNA methylation, use as biochemical intermediates, regulating production of glutathione, and others. Moreover, methionine itself may have a large role in maintaining cellular redox homeostasis by serving as a target of oxidation [23]. Methionine contains a pro-chiral thioether group which is sensitive to oxidation and can form epimers of methionine sulfoxide [24]. Oxidation of methionine to methionine sulfoxide (MetO) causes a change in hydrophobicity that could limit the availability of this amino acid for methionine metabolism pathways through steric hindrance [25]. MetO formation at key residues has also been shown to directly alter protein function [26–28]. Aerobic organisms have evolved enzymes, methionine sulfoxide reductases, which reduce methionine sulfoxides and thus participate in the redox metabolism of this amino acid [29, 30]. Given the limitation of available methionine under MR, and the reported reduction in oxidative stress with MR, we reasoned that the regulatory processes that maintain methionine redox may play a crucial role in the beneficial outcomes of this intervention.
In this study, we tested this idea using a mouse model lacking methionine sulfoxide reductase A (MsrA) which is ubiquitously found in mammals and reduces the S-epimer of methionine sulfoxide in both free and protein-bound forms [31, 32]. The lack of MsrA in mice has been shown to have little impact on lifespan under normal conditions, though has significant impact on sensitivity to physiological challenges including oxidative and metabolic challenges [28, 33, 34]. In a recent report, we showed that lack of MsrA does not block metabolic benefits of MR including on glucose metabolic and endocrine function [35]. Here, we present overall findings suggesting a lack of functional requirement of MsrA for the benefits of MR on longevity and healthy aging in mice. However, more specifically, our study shows potentially complex interactions regarding the potential of sex, duration of MR, and presence of MsrA across the aging life course in mice.
Methods
Animal usage and ethical procedures
All animal experiments were approved by the Institutional Animal Care and Use Committees and UTHSA (Animal Protocol 20170190AR) and have been reported following ARRIVE guidelines. All methods were conducted in accordance with international ethical standards and guidelines.
Animal care
The background of all mice used in this study was C57BL/6J, and both male and female were used. MsrA knock-out (KO) mice were generated as previously reported [33, 34] and genotype confirmed via PCR from tail clips prior to study enrollment. Males were housed 2–4 per cage while females were housed 4–5 per cage. All mice were housed in a specific-pathogen free animal vivarium with temperature maintained at 23.8 ± 1.67 °C and a light cycle of 12 h:12 h light/dark. Prior to enrollment in study, mice were maintained on standard NIH31-based chow after weaning (Envigo 7192). Prior to study, mouse cages were assigned to each experimental group based on sex, genotype, and diet resulting in 8 total groups. Group assignments were first randomized and then balanced using body weight and ages within each sex/genotype combination. This resulted in male groups containing 22–29 mice and female groups containing 24–34 mice. Mice were median age of 9 months (range 7 and 12 months) of age at time of enrollment in the study with both sexes being tested in parallel. Both control (CD) and methionine-restricted (MR) diets were based off of AIN-76A from TestDiet and breakdown as energy from calories to 12.7% from protein, 18.2% from fat, and 69.1% from carbohydrate. Control diet for this study was based off TestDiet 578F with a final content of 0.86% Met and 0% Cys of total amino acids. Methionine-restricted diet had an identical base with a final content of 0.15% Met and 0% Cys of total amino acids. Additional diet composition information can be found in Supplemental Table 1. All food and water were provided ad libitum except during fasting. Mice were weighed and body composition measured (EchoMRI) prior to transitioning to test diets. All mice were then weighed and food consumption measured weekly. Body composition was measured monthly.
Lifespan
Cages were checked daily for deceased mice at which point necropsy was performed and carcasses stored in 10% paraformaldehyde following gross pathology assessment. Mice were euthanized when necessary due to veterinary recommendation but otherwise allowed to die from natural causes. Criteria for euthanasia included mice that were ill, presented with excessive weight loss, moribund, presented with lesions or tumors, or had prolapse. Carbon dioxide asphyxiation was used for euthanasia.
Glucose homeostasis
Mice were fasted overnight with water provided (17:00–10:00; 17 h) at which time tail bleeds were initiated via venous cut at the distal portion of the tail to measure fasting glucose using a handheld AimStrip Plus digital glucose meter. Blood (15–30 μl) was also directly collected in EDTA washed tubes and temporarily stored on ice. Collected blood was allowed to warm to room temperature before HbA1c measurement using Siemens Vantage DCA Analyzer. Samples were censored if they were outside the reading range for either system.
Frailty index
Frailty index was quantified based on methods established by Whitehead et al. [36] Clinical assessments were performed by evaluating 27 criteria for signs of deterioration/deficits including alopecia, loss of fur color, dermatitis, loss of whiskers, coat condition, piloerection, cataracts, eye discharge/swelling, microphthalmia, corneal opacity, nasal discharge, rectal prolapse, penile prolapse (male), vaginal prolapse (female), diarrhea, body condition score, tumors, kyphosis, gait disorders, tremor, breathing rate and depth, menace reflex, tail stiffening, vestibular disturbance, vision loss (visual placing), distended abdomen, malocclusions, and righting reflex. Assessments were performed at approximately the same time every day. Mice were briefly observed in their home cage and then taken to an assessment room. Each mouse was weighed and then assessed. Body temperature assessment with rectal thermometer was omitted due to concerns with rectal prolapse from treatment. Assessments were performed by individuals blinded to the study.
Rota-rod
Mice were placed on a revolving rod approximately 2 feet from the bottom of the chute. Foot-high spacers separated 4 mice from each other. The rod was slowly accelerated until the mice fell or begin riding the rod. This is a test for motor coordination that lasts for no more than 5 min. The test started at 4 rpm and accelerated to 40 rpm and hold. Most older mice fell or let themselves down from the rod around 30–35 rpm. Mice were trained 4 sessions per day, for 4 days and tested in day 5. There were at least 30 min between tests so the mice could rest. Adhesive-backed washable soft vinyl shelf liner was placed at the bottom of the chutes to cushion the fall, as some older or sick mice could be unduly stressed, injured or briefly stunned if they fall on hard metal. The rotarod was cleaned by removing feces and urine and wiping down with 70% ETOH between cages to prevent cage-to-cage transmission of pathogens. Methods are based on Dunham and Miya [37] and Crawley [38]. Assessments were performed by individuals blinded to the groups in the study.
Grip strength
Mice were measured on a Chatillon Ametek grip strength meter. Each animal was placed onto a wire grid to grip with either the forepaws or all four paws. The mouse was then gently and steadily pulled away from the grid by the tail. The maximal force produced (in grams) prior as well as the duration of the hold to the animal’s release of the grid will be recorded from 5 trials. The average grip strength was determined for both the forepaw and all four paws. The method is based on the International Mouse Phenotyping Consortium [39]. Assessments were performed by individuals blinded to the groups in the study.
Rearing
Mice were individually placed in a 1-L beaker cleaned with 70% ethanol in the center of a HEPA filter work station with its air circulation running to generate background noise. Mice were recorded by video for approximately 7 min (Pentax W90 camera) at which point they were returned to their cage, the beaker cleaned with 70% ethanol, and allowed to dry. The individual administering the test was hidden from the test mouse’s view behind a mouse cage rack not containing mice of this study. The test was restarted if the test was disrupted. During analysis, the first 20 s were censored to allow the test administrator to exit the testing area, and the following 5 min of the recording were analyzed for rearing events. A rearing event was defined as a mouse transitioning from all four paws on the base of the beaker to standing on its hind legs such that its front paws exceeded the height of the mouse’s back when on all four paws, and then proceeded to touch the wall of the beaker with at least one paw. All four limbs must then contact the base of the beaker to complete the rearing event. At any point during a test, if the room was entered, the test was restarted. Mice were tested on two separate days, either consecutive or with a 1 day gap, schedule permitting.
Statistics
Statistics were completed using Prism 8. Lifespan was analyzed with multiple log-ranked Kaplan-Meyer survival between pairs of groups. Maximum lifespan was also assessed by the Wang-Allision method [40]. Glucose metabolism, frailty, rotarod, grip-strength, and rearing were analyzed with two-way ANOVA within each sex with post hoc tests to assess diet effect within genotype using Sidac correction. Line graphs were presented with SEM to declutter the error bars and improve the interpretability of the data. All other graphs used SD to better represent the spread of the data.
Results
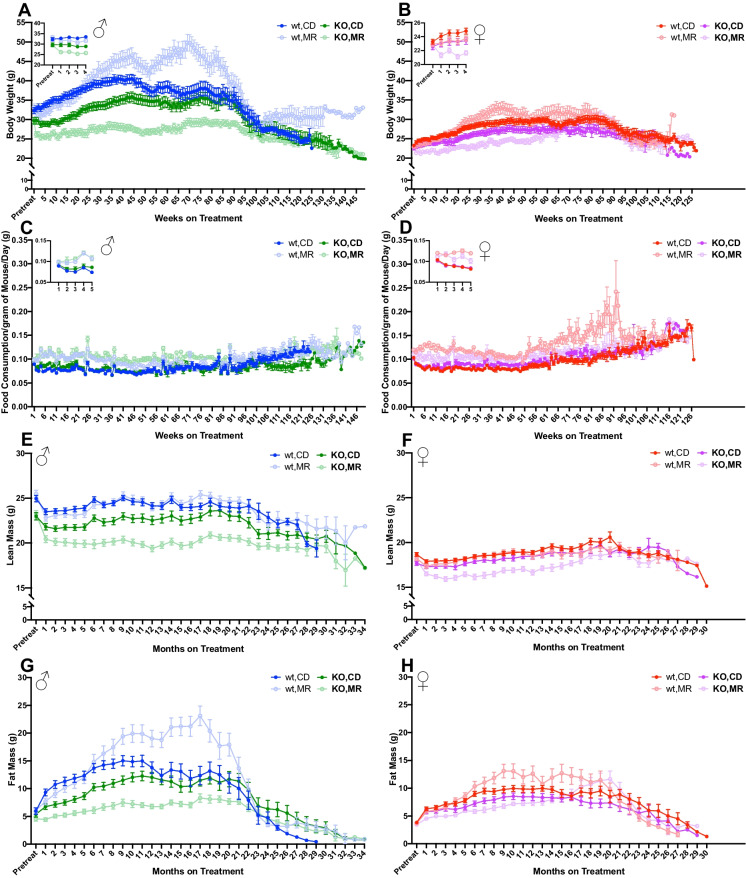
In this study, MR was started when mice had reached a median age of 9 months, an age at which we considered mice to be adult and no longer in the growth and developmental stage. In this large cohort of mice, both male and female MsrA knock-out (KO) mice were lighter in weight than age-matched wild-type (WT) control animals prior to MR (Fig. 1A, B). As expected, based on reported effects, WT mice under short-term MR rapidly lost body weight compared to those on control diet (CD), as seen in the punch-outs on the respective subfigures. We found that this decrease was primarily due to loss of fat mass (Fig. 1G,H). Similarly, KO mice also lost body weight under short-term MR. In contrast to WT mice, KO mice lost both fat mass and lean mass while under MR with this loss of lean mass persisting throughout their lifespan. Surprisingly, after approximately 6 months of treatment, we found that both male and female WT mice under MR began to regain weight and eventually became heavier than their WT CD counterparts (Fig. 1E, F). In comparison, KO mice under MR remained lighter compared to those fed CD throughout the majority of the lifespan study. It is possible the difference in body weight gain in WT mice may be due to an increase in food consumption of the MR diet relative to CD (Fig. 1C, D). MR food consumption in KO mice was also increased relative to CD suggesting a potential role for MsrA in the metabolic regulation responsible for these differences.
Fig. 1.
MR effects on physiological measures changes with duration of treatment. Body weight (A, B) and normalized food consumption (C, D) were measured weekly. Punch-outs represent an enhanced view of the first 5 timepoints for clarity. Body composition (E–H) was measured every month. Graphs represent mean ± SEM. Group sizes in Table 1
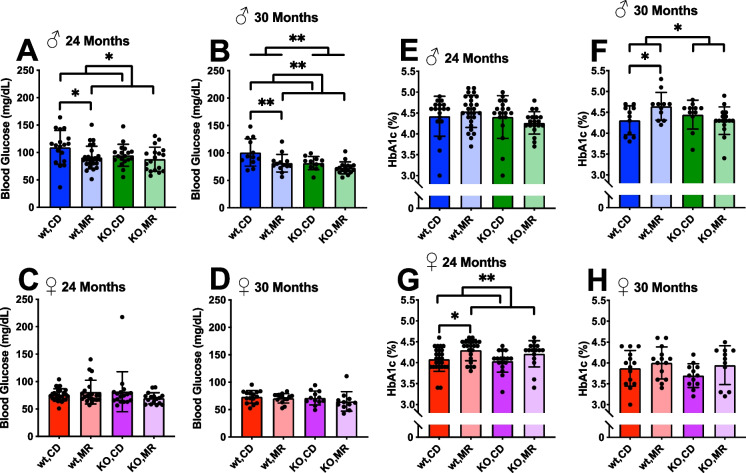
A goal of this study was to determine the extent to which MsrA is required for the beneficial effects of MR on aging. To this goal, we assessed the age-related changes to a number of relatively non-invasive health span assessments of physiological function during the transition from mid- to late-life in these cohorts of animals. Previously, others have shown that glucose metabolism is improved with MR [4, 5, 7, 17, 22, 41–43] in adult mice, and we recently showed that this effect did not require the expression of MsrA [35]. We report here that chronic MR (between 9 and 24 months of age) significantly reduces fasting blood glucose in male mice, but not females (Fig. 2A, C). In line with our previous report, the presence of MsrA is not required for this outcome as KO mice responded similarly to WT mice on MR. Importantly, MR reduced blood glucose concentrations in WT mice despite the unexpected outcome that WT mice under MR at this point were significantly heavier than their CD counterparts (Fig. 1A, B). In female mice, but not males, we found that MR was associated with an increase in HbA1c at 24 months of age (Fig. 2E, G). While unexpected, it is possible these results suggest that sex-hormones may have significant regulator roles on these markers of glucose metabolism. At 30 months of age, we re-assessed the surviving animals for these same markers of glucose metabolism. At 30 months, the effects of MR on fasting blood glucose remained similar to those at 24 months. Male mice at 30 months showed reduced fasting glucose with MR — post hoc analysis shows MR significantly reducing this marker in WT males compared to CD (Fig. 2B). Interestingly, at 30 months, the difference in HbA1c observed in the females was no longer significant (Fig. 2H). However, we found an interaction effect between diet and genotype in male HbA1C, with the WT mice on MR having significantly increased HbA1c (Fig. 2F).
Fig. 2.
MR effects on glucose metabolism are sex-dependent. Fasting glucose (A–D) and HbA1c (E–H) collected at 24 and 30 months of age. Analysis was within each sex via two-way ANOVA for main effects. Post hoc analysis was performed with Sidac multiple comparisons correction to assess the diet effect within each genotype. Graphs represent means ± SD. Group sizes for each timepoint are in Table 1 (*p < 0.05; **p < 0.01)
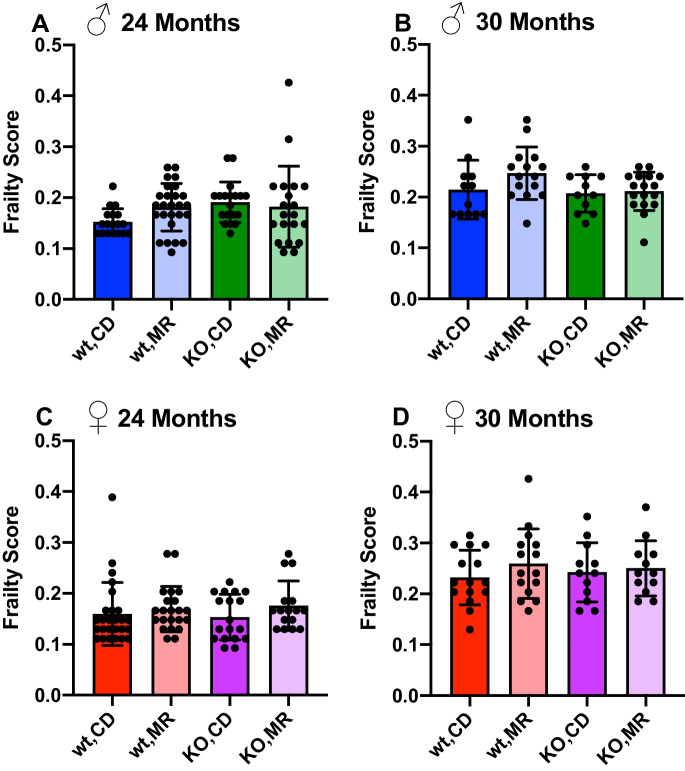
Physical frailty has been shown to increase with age and be predictive of future mortality [44]. In addition, interventions to extend longevity have been shown to prevent the development of frailty including dietary modifications such as calorie restriction and restriction of dietary intake of branched chain amino acids [45, 46]. To our surprise, we found few previous reports regarding the effects of MR on frailty in rodent models [47–49] . Here, we used a verified assessment of murine frailty to determine the impact of MR and requirement of MsrA during aging in old mice. As expected, we found an increase in frailty index outcomes between 24 and 30 months of age in both male and female WT mice on CD (Fig. 3). To our surprise, we found that MR had no effect on frailty at 24 or 30 months in comparison to CD despite mice having undergone this dietary intervention for approximately 15 and 21 months at respective times of testing. In addition, the lack of MsrA had no effect on frailty in either CD or MR mice, suggesting neither reduction of dietary methionine intake in adult mice nor modulation of methionine redox metabolism is sufficient to delay the occurrence of physical frailty in these mice. However, some of the individual metrics used to determine frailty score were altered by MR in sex- and MsrA-dependent ways (Supplementary Figure 1, Supplementary Table 2).
Fig. 3.
Long-term MR does not alter frailty score. Frailty assessment performed at 24 (A, C) and 30 months (B, D) of age. Analysis was within each sex via two-way ANOVA for main effects. Post hoc analysis was performed with Sidac multiple comparisons correction to assess the diet effect within each genotype. Graphs represent means ± SD. Group sizes for each timepoint are in Table 1
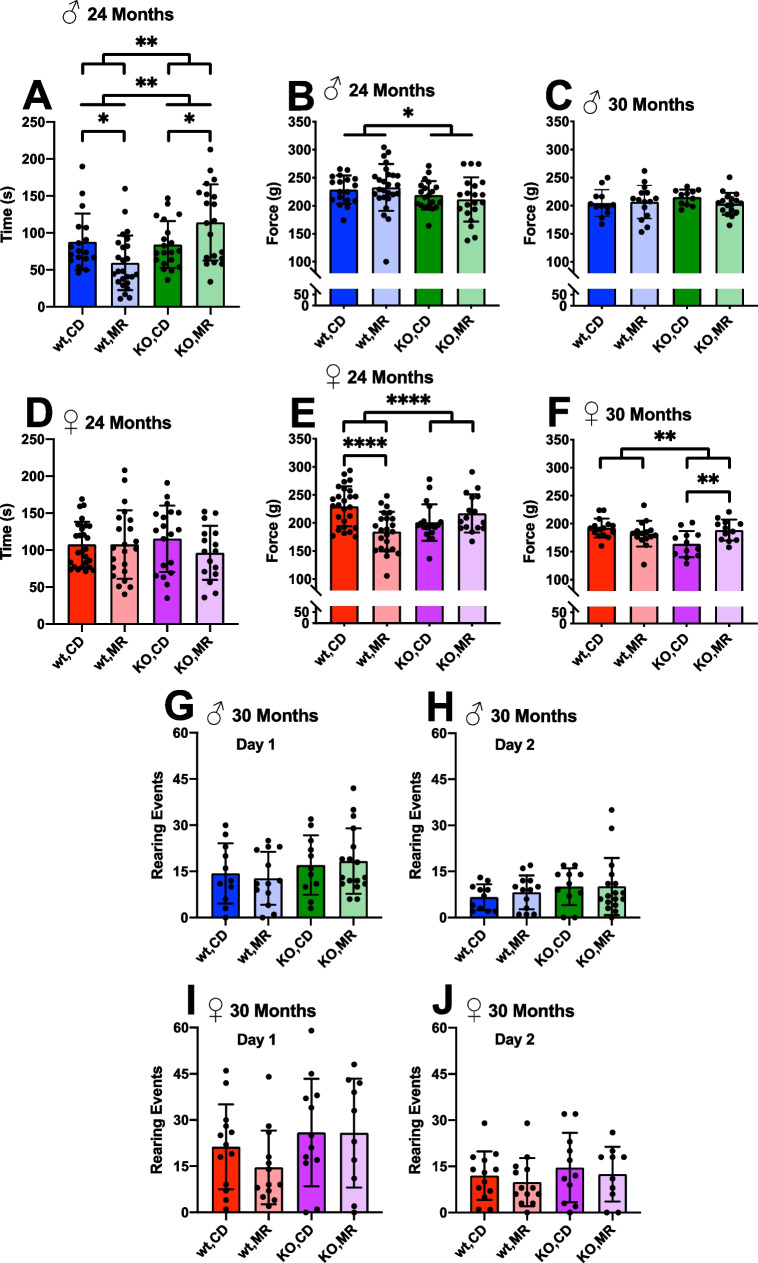
Loss of muscle function begins to occur during mid-age which could impact performance on various musculoskeletal evaluations [50, 51]. We used rotarod as a functional assessment of muscle coordination, grip strength, and measurements of rearing ability to delineate the effects of MR on musculoskeletal changes with age. Rotarod performance at 24 months of age showed KO males were able to stay on the rotating rod for a longer period of time than WT males, but we found no significant effect of diet overall (Fig. 4A). However, a detailed examination of these results shows that while there is no difference between WT and KO males on CD, there is a disparate response to MR in these mice. WT mice performed worse with MR compared to CD, and KO mice performing better with MR compared to CD. In part, this might be due to the associated disparate changes in body weight in these groups (Fig. 1A). However, we found no relationship in female mice which showed a similar, though blunted, pattern of weight changes (Fig. 4D).
Fig. 4.
MR effects on health span metrics. Physiological assessments of adult mice on long-term MetR. Rotarod was performed at 24 months, and graphs represent day 5 testing results for both sexes (A, D). All limb grip strength force was tested at 24 (B, E) and 30 months (C, F) for both sexes. Rearing assay was performed at 30 months of age on two separate days (G–J). Analysis was within each sex via two-way ANOVA for main effects. Post hoc analysis was performed with Sidac multiple comparisons correction to assess the diet effect within each genotype. Graphs represent means ± SD. Group sizes for each timepoint are in Table 1. (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001)
We also tested muscle function more directly by testing grip strength in aging mice. In general, all limb grip strength force slightly decreased between 24 months and 30 months of age. In males, we found a significant effect of genotype where KO males generated slightly lower force at 24 months than did WT males (Fig. 4B); however, this difference did not persist at 30 months (Fig. 4C). Diet had no effect on all limb grip strength in males. Females showed a persistent interaction effect with grip strength at both time points with MR causing decreased force generation in WT females and increased force generation in KO females, both compared to respective CD (Fig. 4E, F). Forelimb force and grip time showed no change with either diet or genotype, but did have some interaction effects. All limb grip time did show some effects of MR mainly in females (Supplementary Figure 2).
Mouse rearing has been used as a test of social anxiety in rodents in response to novel environments, but also measures physical ability to raise and lower using hindlimb strength [52–54]. At 30 months of age, we found no effect of either diet or genotype on the outcomes of this rearing test suggesting neither affects the physical function required to perform this test. In addition, we found a decline in rearing overall on the second day of testing suggesting reduction in anxiety with this new environment as expected. Diet and genotype had no significant impact on this outcome in either male or female mice (Fig. 4G–J).
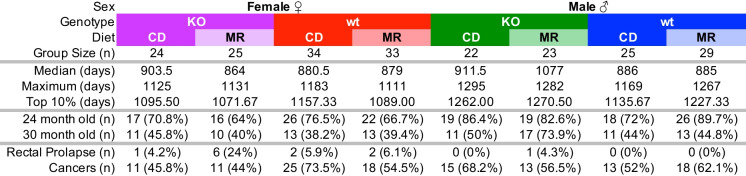
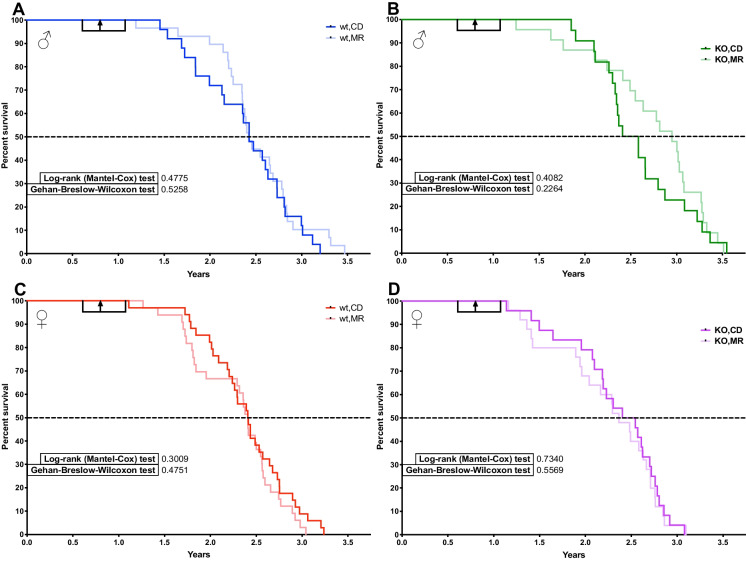
The main goal of this study was to determine the requirement of MsrA for the longevity outcomes of MR. We previously reported that lack of MsrA has no effect on lifespan in male mice [33]. We confirm again here that the lifespan of KO males is similar to WT and additionally show that lack of MsrA does not affect lifespan in female mice (Table 1). In females, MR can cause increased mortality prior to median lifespan in WT mice under certain circumstances [3]. Mortality was partially driven by increased incidences of rectal prolapse in our cohort with MR, predominantly in females and generally occurring before median lifespan. Lack of MsrA appeared to exacerbate this issue (Table 1). However, we found no effect of MR on lifespan overall in either genotype of females (Fig. 5C, D). In males, we also surprisingly saw no statistically significant effect of lifespan caused by MR when analyzed by Log Rank (Fig. 5A, B). We found no evidence for increased mortality caused by MR in males, but potentially a reduction of mortality through median lifespan in KO mice. Pair-wise analysis of groups showed no significant changes in lifespan with the exception of MR in males comparing KO to WT with p = 0.04 (Supplemental Table 3). We also found no evidence for any significant change in maximum lifespan caused by MR as measured by the Wang-Allison method [40]. Necropsies were performed when possible, and gross pathology was noted. While not conclusive, our observations suggest MR may have decreased solid cancer incidence in WT females and resulted in higher incidence in WT males. KO mice appeared to have similar incidence regardless of diet (Table 1).
Table 1.
MR study metrics
Metrics of study groups including group size, median, maximum, and oldest 10% lifespan from survival curves. Group sizes for 24 and 30 months represent the number of mice that completed all testing at that age timepoint. Total incidence of rectal prolapse for each group, as well as number of mice determined to have cancers based on gross pathology at time of autopsy
Fig. 5.
Long-term MR does not improve lifespan when started in adult mice. Survival of adult mice started on MetR at 7–12 months of age with a median and mean start time of 9 months. Start time and range indicated by black arrow and bracket. Males (A, B) and females (C, D), wt (A, C), and MsrA-KO (B, D). All groups were 22–34 mice. Kaplan-Meyer survival curves with log-ranked Mantel-Cox test. Group sizes in Table 1
Discussion
In this study, we set out to test the idea that there is a functional requirement for MsrA in the effects of MR on lifespan, glucose homeostasis, and health span metrics. Despite significant study, the mechanisms by which MR functions are still unclear. We rationalized that given methionine’s sensitivity to oxidation and its limited availability from the diet, protection and quality control of this resource would be of greater importance during MR. Moreover, MR has been shown to reduce oxidative conditions, including increasing antioxidant expression and reducing free radical generation, suggesting a key role in its health benefits [9–12]. Given the roles that methionine plays in protein translation, methylation, and generation of various biochemicals, there is then biological reason for focusing on aspects of methionine metabolism as a central mediator of MR.
Overall, our results suggest that MsrA has a complicated interaction with MR that is dependent on sex as well as the duration of the intervention. For example, one of the most interesting findings of this study was the change in weight in short-term versus long-term MR (Fig. 1A, B). For approximately the first 6 months of intervention, MR consistently reduced body weight and fat mass compared to CD, as has been demonstrated in numerous other studies [3, 5, 6, 12, 19–21]. However, after this point, WT mice on MR regained weight and fat, resulting in them being heavier in weight than CD. In contrast, KO mice generally retained the reduced body weight and fat mass with MR throughout the study. This suggests that MsrA is somehow responsible for controlling body weight under MR. We previously showed no effect of MsrA on changes associated with several metabolic hormones, including leptin and adiponectin, suggesting perhaps non-endocrinological regulation [35]. Of note, KO mice also lost lean mass with MR which we did not find to occur in WT mice (Fig. 1E, F). While one might conclude this loss of lean mass may also be associated with reduced muscle function, we found that MR did not significantly reduce muscle strength (Fig. 4B, C, E, F, Supplemental Fig 2) suggesting that the loss of lean mass may not be generally detrimental. More in-depth investigation into the changes in muscle structure and function that might occur under MR without functional MsrA would be warranted to understand this outcome. It is also worth noting that these physiological changes occurred despite increased food consumption (Fig. 1C, D). This increase in food consumption has been reported in other MR studies [12, 19, 21]. While the exact cause of this behavior is not well understood, our results suggest that MsrA may not play a significant role and that its effects are conserved with long-term treatment.
Improvements to glucose metabolism with MR have also been well established [4, 8, 17, 41], and these were also observed in this cohort (Fig. 2). Measures of fasting blood glucose showed a sex-specific effect with only males having reducing fasting glucose with MR in old age. It is important to note that this reduction in fasting glucose exists despite an increase in body weight with MR in WT males (Fig. 1A) suggesting a phenotype similar to metabolically normal obesity. However, we also show little benefit of MR in KO males suggesting MsrA may be involved in regulating glucose metabolism. This is in line with previous work showing MsrA’s involvement insulin function [34]. Interestingly, fasting glucose was unchanged by diet or genotype in females suggesting a sex-specific effect of MR on this outcome. We see a contradictory sex effect in HbA1c assessments with males showing no effect and females showing a significant diet effect (Fig. 2E–H). The reasons for this are unclear; however, HbA1c in general provides an average representation of blood glucose concentrations over weeks-months whereas fasting glucose is more representative of glucose regulation in short-term metabolism. This might suggest MR differentially affects these metabolic phenotypes dependent on sex-specific factors including sex hormones. More in-depth studies on the role of sex and MR are warranted from these results.
Surprisingly, there have been few examples of data that outline how MR impacts health span, particularly in old age, in mice. We performed a number of assessments of functional health across metabolism, musculoskeletal function, and physical condition in old animals under chronic MR since adulthood. In general, our results suggest that MR had little overall impact on these outcomes beyond metabolic function. In particular, muscle function seems to be minimally affected with no or negative impact on grip strength, rotarod, and rearing (Fig. 4). In addition, we found no effect of MR on frailty in WT or KO mice suggesting that MR does not contribute to the accumulation of deficits that contribute to decline in physiological function with aging (Fig. 3). It is unclear why some of these results do not correspond with the few other reports of MR on physical function [47–49]. One possibility is that the duration of treatment in our study was longer and that some beneficial effects of MR are lost over time, compared to other studies that looked at aged mice on treatment for a few months.
MR was first shown in 1992 in rats to significantly extend lifespan [55], and later studies suggest more modest effects in mice. For example, Miller et al. reported ~ 10% extension of maximum lifespan in female CB6F1 mice when MR was begun early in life [3]. Sun et al. reported that MR begun at 12 months of age extended lifespan in male CB6F1 mice by 7% [1]. While there are differences between those studies and ours — including background strain, time of MR, and diet formulation — it was surprising that we found no significant effect of MR on WT lifespan in our study (Fig. 5). However, MR interventions started in adult rodents can have a more limited effect on lifespan [56]. It is possible that we may not have been sufficiently powered to detect a significant effect even though our survival curves suggest some benefit of MR on longevity at least in males. This would be in line with a previous report of sexually dimorphic lifespan results with late life MR [49]. When we considered all males regardless of genotype, that is pooling WT and KO, we still find no significant effect of MR though the p value for log rank in this case reached ~ p = 0.2 suggesting that sample size may indeed be a consideration in our results. The increase in lifespan observed in the male KO could have been due to a sex-specific interaction with autophagy since MR and MsrA KO have been independently shown to increase autophagy [14, 57, 58]. In addition, we started in older adult animals at around 9 months of age, similar to the approach used by Sun et al. [1]. The alternative approach to start early in life raises the question of to what extent this intervention affects development. In this regard, it is interesting to note that MsrA and MsrB are required for growth when tested using a methionine growth assay [59]. On the other hand, translational approaches of MR are likely to start later in life, highlighting the importance of understanding these effects.
While the lack of effect of MR is an unexpected result, work concerning CR may provide some insight. Studies investigating the effects of genotype on CR response utilizing the ILSXISS mouse model showed that the majority of genotypes tested had no effect or a negative effect from CR [60, 61]. This is surprising considering how CR is considered a robust method for lifespan extension. Further work with this model has shown that the genetic background can have an effect on metabolism [62, 63]. This may help explain the lack of an observed effect on lifespan with MR (Fig 5) — some strains may not respond to MR, which may include the C57Bl/6J mice tested here. A mouse strain effect may also be the cause for the increased fat mass and body weight observed in the MR males with age (Fig. 1A, G).
Studies in young mice started on MR have shown that it can result in rectal prolapse and increased mortality [3], although the mechanism is unclear. We observed cases of rectal prolapse predominantly in the MR groups during the study which were far more prevalent in female mice (Table 1) resulting in some early deaths and that this is exacerbated by the lack of MsrA. It is possible that the increased food consumption places increased stress on the digestive tract resulting in this outcome, but closer investigation would be required to verify this. MR has been shown to decrease tumor size and prevalence in certain models [18, 20, 64]. In our cohort, MR did appear to potentially have an effect on cancers as determined by gross pathology during autopsy, but more thorough pathological assessments would need to be performed to verify these findings.
The results presented in this work in combination with results from other studies point towards a complex conclusion. While it is well documented that there are significant improvements to metabolic health and function with either short-term MR of a few months or MR initiated very early in life, the data presented here expands on this understanding. Given the limited effect of MR on health span metrics as well as the unexpected changes in body weight and composition after a delayed period, there may be a significant age/development component to the effects of MR. This suggests that MR exerts its benefits in the short term upon which the body can adapt thereby negating some of the benefits in the long term. When MR is enacted in young animals, the benefits appear long term and more significant possibly due to IGF-1 and/or GH effects of MR which are known to have significant impacts on these health outcomes [3, 17–20, 43]. Indeed, other studies have shown reduced effects of MR in IGF-1/GH mutant/impaired strains like Ames dwarf mice [42]. These conclusions indicate that care must be taken when considering how MR will impact the health of an organism, and that additional research is needed to untangle its mechanisms. In general, we conclude that MsrA is largely dispensable for the beneficial effects of MR on longevity and health span.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would also like to acknowledge the experimental assistance of Yuhong Liu throughout this study and Dr. Jonathan Dorigatti for experimental assistance and helpful discussions. We would also like to acknowledge Jodie Cropper and Dr. Wenbo Qi for their assistance in monitoring the mice during the lifespan study. We would like to acknowledge Dr. Catherine Cheng for her assistance in analyzing the lifespan data. Health span measurements were performed by the Integrated Physiology of Aging Core at the San Antonio Nathan Shock Center (P30 AG013319) [65].
Author contribution
Study was formulated by ABS and KMT. Data was collected and analyzed by KMT. Figures and manuscript drafts were prepared by KMT. ABS and KMT reviewed, edited, and approved the manuscript.
Funding
This research was funded in part by R01 AG050797, R01 AG057431, T32 AG021890 and the San Antonio Area Foundation. AS is partially supported by the Geriatric Research, Education and Clinical Center of the South Texas Veterans Health Care System. This material is the result of work supported with resources and the use of facilities at South Texas Veterans Health Care System, San Antonio, Texas.
Data availability
The data presented in the work are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in Middle Age. J Gerontol A: Biol Sci Med Scis. 2009;64A:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richie Jr John P, et al. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- 3.Miller Richard A, et al. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerra Beatriz A, et al. Dietary sulfur amino acid restriction upregulates DICER to confer beneficial effects. Molec Metab. 2019;29:124–135. doi: 10.1016/j.molmet.2019.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant Louise, et al. Methionine restriction improves renal insulin signalling in aged kidneys. Mech Aging Dev. 2016;157:35–43. doi: 10.1016/j.mad.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Lees Emma K, et al. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forney LA, Wanders D, Stone KP, Pierse A, Gettys TW. Concentration-dependent linkage of dietary methionine restriction to the components of its metabolic phenotype: concentration dependence of methionine restriction. Obesity. 2017;25:730–738. doi: 10.1002/oby.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caro P, et al. Forty percent and eighty percent methionine restriction decrease mitochondrial ROS generation and oxidative stress in rat liver. Biogerontology. 2008;9:183–196. doi: 10.1007/s10522-008-9130-1. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Roman I, et al. Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J Bioenerget Biomembr. 2011;43:699–708. doi: 10.1007/s10863-011-9389-9. [DOI] [PubMed] [Google Scholar]
- 11.Sanz A, et al. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, et al. Dietary methionine restriction ameliorated fat accumulation, systemic inflammation, and increased energy metabolism by altering gut microbiota in middle-aged mice administered different fat diets. J Agric Food Chem. 2020;68:7745–7756. doi: 10.1021/acs.jafc.0c02965. [DOI] [PubMed] [Google Scholar]
- 13.Patil Yuvraj N, Dille Kelly N, Burk David H, Cortez Cory C, Gettys Thomas W. Cellular and molecular remodeling of inguinal adipose tissue mitochondria by dietary methionine restriction. J Nutr Biochem. 2015;26:1235–1247. doi: 10.1016/j.jnutbio.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozieł Rafał, et al. Methionine restriction slows down senescence in human diploid fibroblasts. Aging Cell. 2014;13:1038–1048. doi: 10.1111/acel.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S, et al. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell. 2018;17:e12796. doi: 10.1111/acel.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou K, et al. Life span extension by glucose restriction is abrogated by methionine supplementation: Cross-talk between glucose and methionine and implication of methionine as a key regulator of life span. Sci. Adv. 2020;6:eaba1306. doi: 10.1126/sciadv.aba1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS ONE. 2012;7:e51357. doi: 10.1371/journal.pone.0051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown-Borg HM, et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell. 2014;13:1019–1027. doi: 10.1111/acel.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elshorbagy Amany K, et al. Cysteine supplementation reverses methionine restriction effects on rat adiposity: signifi cance of stearoyl-coenzyme A desaturase. J Lipid Res. 2011;52:104–112. doi: 10.1194/jlr.M010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hens JR, et al. Methionine-restricted diet inhibits growth of MCF10AT1-derived mammary tumors by increasing cell cycle inhibitors in athymic nude mice. BMC Cancer. 2016;16:349. doi: 10.1186/s12885-016-2367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forney LA, et al. Sexually dimorphic effects of dietary methionine restriction are dependent on age when the diet is introduced. Obesity. 2020;28:581–589. doi: 10.1002/oby.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanders Desiree, et al. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes. 2017;66:858–867. doi: 10.2337/db16-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scislowski PWD, Davis EJ. Sulfur oxidation of free methionine by oxygen free radicals. FEBS Lett. 1987;224:177–181. doi: 10.1016/0014-5793(87)80443-X. [DOI] [PubMed] [Google Scholar]
- 24.Bentley Ronald. Methionine and derivatives: exploring chirality at sulfur. Biochem Molec Biol Educ. 2005;33:274–276. doi: 10.1002/bmb.2005.49403304274. [DOI] [Google Scholar]
- 25.Chao C-C, Ma Y-S, Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Nat Acad Sci. 1997;94:2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Regulation of voltage-dependent K + channels by methionine oxidation: effect of nitric oxide and vitamin C. FEBS Lett. 1999;442:48–52. doi: 10.1016/S0014-5793(98)01616-0. [DOI] [PubMed] [Google Scholar]
- 27.Fricke TC, et al. Oxidation of methionine residues activates the high-threshold heat-sensitive ion channel TRPV2. Proc Natl Acad Sci U S A. 2019;116:24359–24365. doi: 10.1073/pnas.1904332116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brennan LA, Lee W, Giblin FJ, David LL, Kantorow M. Methionine sulfoxide reductase A (MsrA) restores α-crystallin chaperone activity lost upon methionine oxidation. Biochim Biophys Acta (BBA) - General Subjects 2009;1790:1665–1672. [DOI] [PMC free article] [PubMed]
- 29.Zhang X-H, Weissbach H. Origin and evolution of the protein-repairing enzymes methionine sulphoxide reductases. Biol Rev. 2008;83:249–257. doi: 10.1111/j.1469-185X.2008.00042.x. [DOI] [PubMed] [Google Scholar]
- 30.Delaye L, Becerra A, Orgel L, Lazcano A. Molecular evolution of peptide methionine sulfoxide reductases (MsrA and MsrB): on the early development of a mechanism that protects against oxidative damage. J Mol Evol. 2007;64:15–32. doi: 10.1007/s00239-005-0281-2. [DOI] [PubMed] [Google Scholar]
- 31.Peng-Fei Wu, et al. A specific and rapid colorimetric method to monitor the activity of methionine sulfoxide reductase A. Enzyme Microbial Technol. 2013;53:391–397. doi: 10.1016/j.enzmictec.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Kwak Geun-Hee, Hwang Kwang Yeon, Kim Hwa-Young. Analyses of methionine sulfoxide reductase activities towards free and peptidyl methionine sulfoxides. Arch Biochem Biophys. 2012;527:1–5. doi: 10.1016/j.abb.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Salmon AB, et al. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. The FASEB Journal. 2009;23:3601–3608. doi: 10.1096/fj.08-127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Styskal J, et al. Methionine sulfoxide reductase A affects insulin resistance by protecting insulin receptor function. Free Radical Biol Med. 2013;56:123–132. doi: 10.1016/j.freeradbiomed.2012.10.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyne KM, Salmon AB. Metabolic benefits of methionine restriction in adult mice do not require functional methionine sulfoxide reductase A (MsrA) Sci Rep. 2022;12:5073. doi: 10.1038/s41598-022-08978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitehead JC, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol: A. 2014;69:621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice**College of Pharmacy, University of Nebraska, Lincoln 8. J Am Pharm Assoc (Sci ed) 1957;46:208–209. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 38.Crawley JN. Behavioral phenotyping of rodents. Comp Med. 2003;53:140–146. [PubMed] [Google Scholar]
- 39.International Mouse Phenotyping Consortium. Grip Strength Protocol – IMPReSS. International Mouse Phenotyping Consortium https://www.mousephenotype.org/impress/ProcedureInfo?action=list&procID=1130.
- 40.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Swaminathan A, Fokin A, Venckūnas T, Degens H. Methionine restriction plus overload improves skeletal muscle and metabolic health in old mice on a high fat diet. Sci Rep. 2021;11:1260. doi: 10.1038/s41598-021-81037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanders D, et al. Role of GCN2-independent signaling through a noncanonical PERK/NRF2 pathway in the physiological responses to dietary methionine restriction. Diabetes. 2016;65:1499–1510. doi: 10.2337/db15-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malloy VL, et al. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell. 2006;5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood K, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. 2017;7:43068. doi: 10.1038/srep43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kane AE, et al. Impact of longevity interventions on a validated mouse clinical frailty index. GERONA. 2016;71:333–339. doi: 10.1093/gerona/glu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson NE, et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and life span in mice. Nat Aging. 2021;1:73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz MB, et al. Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun. 2020;11:4618. doi: 10.1038/s41467-020-18446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell S, MacArthur M, Kane A, Torrence M, Manning B. Improved healthspan and lifespan with late onset pharmacological or dietary interventions in mice. Innovation in Aging. 2019;3:S875. doi: 10.1093/geroni/igz038.3207. [DOI] [Google Scholar]
- 49.Mitchell S, et al. Late-onset pharmacological or dietary interventions improve healthspan and lifespan in male and female mice. Innovation in Aging. 2020;4:125. doi: 10.1093/geroni/igaa057.412. [DOI] [Google Scholar]
- 50.del Campo A, et al. Muscle function decline and mitochondria changes in middle age precede sarcopenia in mice. Aging. 2018;10:34–55. doi: 10.18632/aging.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sayed RKA, et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Experiment Gerontol. 2016;83:22–30. doi: 10.1016/j.exger.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/S0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 53.Chinta SJ, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Reports. 2018;22:930–940. doi: 10.1016/j.celrep.2017.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willard AM, Bouchard RS, Gittis AH. Differential degradation of motor deficits during gradual dopamine depletion with 6-hydroxydopamine in mice. Neuroscience. 2015;301:254–267. doi: 10.1016/j.neuroscience.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. J Nutr. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- 56.Nichenametla SN, Mattocks DAL, Malloy VL. Age-at-onset-dependent effects of sulfur amino acid restriction on markers of growth and stress in male F344 rats. Aging Cell. 2020;19:e13177. [DOI] [PMC free article] [PubMed]
- 57.Ruckenstuhl C, et al. Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. 2014;10:e1004347. doi: 10.1371/journal.pgen.1004347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pennington SM, et al. Defective protein repair under methionine sulfoxide A deletion drives autophagy and ARE-dependent gene transcription. Redox Biol. 2018;16:401–413. doi: 10.1016/j.redox.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao H, Kim G, Levine RL. Methionine sulfoxide reductase contributes to meeting dietary methionine requirements. Arch Biochem Biophys. 2012;522:37–43. doi: 10.1016/j.abb.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rikke BA, Liao C-Y, McQueen MB, Nelson JF, Johnson TE. Genetic dissection of dietary restriction in mice supports the metabolic efficiency model of life extension. Experiment Gerontol. 2010;45:691–701. doi: 10.1016/j.exger.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rikke BA, Battaglia ME, Allison DB, Johnson TE. Murine weight loss exhibits significant genetic variation during dietary restriction. Physiol Genom. 2006;27:122–130. doi: 10.1152/physiolgenomics.00068.2006. [DOI] [PubMed] [Google Scholar]
- 63.Mulvey L, et al. Strain-specific metabolic responses to long-term caloric restriction in female ILSXISS recombinant inbred mice. Molec Cell Endocrinol. 2021;535:111376. doi: 10.1016/j.mce.2021.111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao X, et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature. 2019;572:397–401. doi: 10.1038/s41586-019-1437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salmon AB, et al. San Antonio Nathan Shock Center: your one-stop shop for aging research. GeroScience. 2021;43:2105–2118. doi: 10.1007/s11357-021-00417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in the work are available from the corresponding author upon request.