Abstract
The Wake Forest nonhuman primate (NHP) Radiation Late Effects Cohort (RLEC) is a unique and irreplaceable population of aging NHP radiation survivors which serves the nation’s need to understand the late effects of radiation exposure. Over the past 16 years, Wake Forest has evaluated > 250 previously irradiated rhesus macaques (Macaca mulatta) that were exposed to single total body irradiation (IR) doses of 1.14–8.5 Gy or to partial body exposures of up to 10 Gy (5% bone marrow sparing) or 10.75 Gy (whole thorax). Though primarily used to examine IR effects on disease-specific processes or to develop radiation countermeasures, this resource provides insights on resilience across physiologic systems and its relationship with biological aging. Exposure to IR has well documented deleterious effects on health, but the late effects of IR are highly variable. Some animals exhibit multimorbidity and accumulated health deficits, whereas others remain relatively resilient years after exposure to total body IR. This provides an opportunity to evaluate biological aging at the nexus of resilient/vulnerable responses to a stressor. Consideration of inter-individual differences in response to this stressor can inform individualized strategies to manage late effects of radiation exposure, and provide insight into mechanisms underlying systemic resilience and aging. The utility of this cohort for age-related research questions was summarized at the 2022 Trans-NIH Geroscience Interest Group’s Workshop on Animal Models for Geroscience. We present a brief review of radiation injury and its relationship to aging and resilience in NHPs with a focus on the RLEC.
Keywords: Resilience, Biology of aging, Multimorbidity, Nonhuman primates, Animal models, Ionizing radiation
Introduction
Age is the strongest risk factor for multiple chronic diseases and conditions across many organ systems. In industrialized nations, modern medical care has increased lifespan, and as a result, people are surviving major health challenges and disease diagnoses that once would have proved fatal. The result is a rapidly rising population of older adults who increasingly manage multiple chronic diseases and conditions and frailty that may be accelerated or accentuated as a result of exposure to major stressors. Relying exclusively on humans as subjects in aging research is complicated by numerous issues, including ethical issues, long lifespan, and environmental and genetic influences [1]. The use of animal models may circumvent many of these issues. Thus in 2022, the Trans-NIH GeroScience Interest Group organized a workshop on Animal Models for Gerosicence to review progress and potential of model organisms for translational and preclinical research. One such model is the nonhuman primate (NHP), and the scientific potential of a specialized cohort of aging NHPs was summarized at the meeting and reviewed herein.
Though several mammalian species are promising models of aging, primate models uniquely recapitulate key features of human function, behavior, and the ability to observe multiple chronic conditions [2]. The rhesus macaque model is advantageous in that it shares approximately 95 to 97.5% genetic similarity to humans depending on the gene examined, with nearly identical anatomy, and is one of the few well-characterized laboratory animal species that lives in social groups, and has a 28-day menstrual cycle [3, 4]. Additionally, the rhesus spontaneously develops many conditions like humans as they age such as colorectal and mammary carcinomas, endometriosis, type 2 diabetes, heart disease, hypertension, glomerulopathies, obesity, sarcopenia, osteopenia, degenerative joint diseases, cataract formation, and cognitive decline [5]. Moreover, this coupled with their long lifespan makes them a natural model to investigate the late effects of exposure to stressors on aging health trajectories, frailty, and multimorbidity. Other animal models of aging, including marmosets and companion animals, are beyond the scope of this review and have been described elsewhere [6–9].
Aging, frailty, and resilience
Aging is a complex phenomenon in which genetics, epigenetics, the environment, and even chance play important roles. Aging also comprises the phenotypic effects of a lifetime of exposure to these variables. As aging progresses, the ability of the organism to deal with insults of equal magnitude decreases, which might underlie the increased prevalence of age-related multimorbidity. For example, data from the Framingham study indicate that being 70 years old is, by itself, a higher risk factor for cardiovascular disease than high cholesterol, high blood pressure, and obesity combined [10]. The reason for increased age-related multimorbidity is multifactorial, but is an accumulation of damage over time, and a reduced ability to mount robust defenses against homeostatic insults and challenges [11, 12]. Intimately linked are the concepts of frailty and resilience, which are often thought of as two sides of the same coin, though they are not strictly the reciprocal of one another [13].
Frailty is the decline in tissue and organism function that occurs with age, and it can be objectively measured without perturbation. Numerous methods have been proposed to define frailty, though most aging researchers agree that frailty is a state of physiological vulnerability from age-related decline in biological systems, and it manifests clinically as an increased risk of adverse outcomes. Operationally, it can be defined by either a phenotype or dynamic state characterized by the accumulation of health and functional deficits [14, 15]. Resilience is the dynamic property which enables cells, organs, and organisms to resist or recover from adverse effects of a stressor [13, 16–18]. Resilience is diminished with age, observable as accumulation of health deficits, multimorbidity, and greater risk of mortality [17]. However, the response to exposure is heterogeneous and influenced by the magnitude of the stressor and individual, age-related biologic processes [17]. If so, biomarkers of biologic aging may predict resilience and late effects of stressor exposure in survivors. A deeper understanding of the physiologic and molecular mechanisms underlying resilience to an exposure may identify novel protective factors and strategies to prevent or delay multimorbidity, disability, and death in aging survivors.
Translational modeling of resilience and frailty
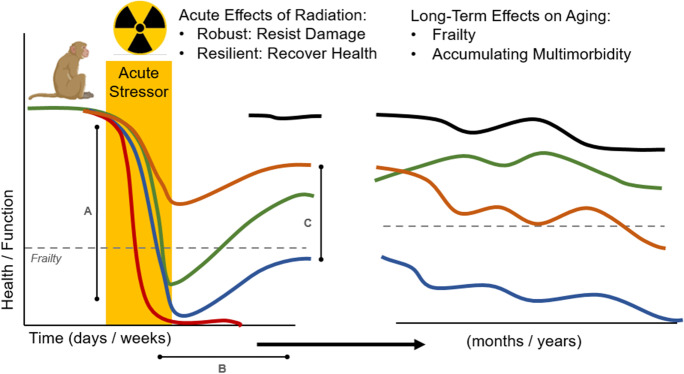
Translational models that reflect the interplay between the biology of aging, frailty, and resilience are of particular interest in the field of geroscience [12]. We expect that the hallmarks of aging (genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, progenitor cell exhaustion, and altered intercellular communication) would teleologically drive vulnerability to stressors and age-related frailty, and vice versa [19]. Animal models may be uniquely suited to disentangle this biology at the nexus of frailty and resilience, and extensive translational testing paradigms are being established for mice. Many translational experimental methods have been employed, including administering acute stressors including mild-to-moderate starvation, anesthesia, surgical stress, trauma, chemotherapy, and exposure to ionizing radiation [12, 20]. Following exposure to experimental stressor, investigators measure acute effects on resilience, including amplitude of response (degree of health or function lost), duration of sustained impairment, and recovery of health or function [12]. However, testing paradigms in mice and rodents rarely explore the long-term effects of acute major stressors such as ionizing radiation exposure on the trajectory of aging and frailty (Fig. 1).
Fig. 1.
Translational approach to study resilience as a short-term or long-term response to an acute stressor in animal models. An acute response to a stressor like radiation exposure can have several stages and elements, including A amplitude of response — which may be an index of the robustness of the organism, B duration of time spent in perturbed state or in ill health, and C degree of resolution after the acute stressor. These resilience measures can be complemented by long-term follow-up of health trajectories after major acute stressors like whole body ionizing radiation that have residual effects after the acute stressor
Characterizing the late- and long-term effects of an acute but severe stressor is important because there is often heterogeneous late-response to stress exposures observed clinically that may impact risk of frailty, multimorbidity, and death. For example, some older patients tolerate cancer or radiation therapy without significant adverse effects on health (highly resilient). Others experience major toxicities, and of these, some will return to their prior level of health or functioning (resilient) and others do not (nonresilient) [21]. Heterogeneity in response to a major acute stressor is thought to be influenced by pre-stressor biological determinants and the magnitude of the stressor itself. A related concept is hormesis, the evolutionarily conserved adaptive strategy, whereby exposure to stressors can lead to the development of acquired resilience in a dose-response fashion [22, 23]. Hormesis is best captured by the layman’s adage: “what doesn’t kill you makes you stronger.” In this case, there could be a negative effect of radiation on late-life outcomes and aging phenotypes, but there may be some survivors of early life radiation exposure, especially at low doses, that are paradoxically resilient to age-related dysfunction and disease accumulation [24]. The legacy effects of these heterogeneous responses are postulated to depend on the same age-related biologic processes that drive aging, frailty, and multimorbidity. If this is true, then biomarkers of aging may be candidate measures to predict resilience in older survivors of substantial acute stress exposures.
Radiation exposure as stressor
One of the experimental manipulations used to test resilience is exposure to ionizing radiation (IR). IR induces DNA and macromolecular damage via reactive oxygen species (ROS) which manifest broadly as chronic inflammation, multiorgan fibrosis, and shortened lifespan, recapitulating the basic mechanisms of aging on an accelerated timeline [25]. The acute effects of IR, known collectively as Acute Radiation Syndrome (ARS), have been widely explored and consist of hematopoietic, gastrointestinal, cutaneous, and cerebrovascular injury [26, 27]. More relevant to human aging are the late effects of radiation exposure on long-term health events such as incidence and progression of cancer, cardiovascular diseases, musculoskeletal decline, and multimorbidity. For example, studies of individuals subjected to moderate and high levels of IR from nuclear weapons (Hiroshima and Nagasaki) or occupational exposure (Chornobyl) have demonstrated an increased risk of neoplasia [28–30], and radiotherapy patients have a 1.7‐ to 2-fold increase in cardiovascular death and an up to 10-fold acceleration of bone loss leading to an elevated risk of fracture in already vulnerable older adults [31, 32]. However, it is difficult to identify clear biological mediators of resilient versus vulnerable survivors in epidemiologic studies given opportunistic data collection following exposures and complex variables related to underlying health status, environmental and occupational risk, and radiation dose, type, and exposure duration.
Biological aging and late effects of radiation
Following the development and use of nuclear weapons, investigators in the 1940s through to the 1960s vigorously pursued links between IR and longevity [33]. These were temporarily abandoned because radiation’s effects initially appeared to cause genetic damage and to affect dividing cells leading to neoplasms [34]. But in recent decades, our understanding of both IR and biological aging have undergone a renaissance, and as it does, we can better appreciate both differences and areas of convergence between processes. For instance, we now know that radiation-mediated aging appears to be associated with ROS tissue injury, double-stranded breaks (DSBs) and DNA damage response, apoptosis and cell cycle checkpoints, epigenetic alterations, and immune dysfunction and inflammation. These shared processes can induce further damage to cellular and molecular processes that are also implicated in biological aging, including DNA damage response, cell cycle checkpoints and cellular senescence, epigenetic alterations, immune dysfunction, and dysregulated nutrient sensing and metabolism, among many others [25]. Moreover, IR induces cellular senescence and is an experimental model to test the effects of senolytics, drugs that target clearance of senescent cells both in vitro and in vivo [35]. IR also induces widespread transient and persistent methylation changes across tissues, mirroring the global and gene-specific methylation changes seen in aging [36]. The persistence of these cellular and molecular changes cannot be evaluated in studies of ARS. Thus, animal models of radiation survival, such as the Radiation Late Effects Cohort (RLEC), are a unique and essential resource to characterize late and long-term effects of exposure to IR.
Wake Forest NHP RLEC
Animal models such as NHPs reveal the late and long-term effects of radiation exposure, including cellular, molecular, and metabolic changes leading to chronic inflammation, organ dysfunction and failure, fibrosis, and neoplasia. The RLEC is an unparalleled resource to identify and understand the late effects of radiation exposure and provide critical data regarding inter-survivor differences in tissue damage, recovery, and long-term outcomes. RLEC investigators at Wake Forest have assessed adverse effects of single-dose exposures of IR in male and female rhesus macaques (M. mulatta) observed for up to 16 years. At the time of publication, the cohort includes ~ 200 live animals (17% unirradiated controls) and ~ 120 deceased animals (15% controls). Irradiated NHPs were exposed to single total body irradiation (TBI) doses of 1.14–8.5 Gy of IR or to partial body exposures of 10 Gy (5% bone marrow sparing)–10.75 Gy (whole thorax). The LD50 for TBI is 6.75 Gy without supportive care. These single exposures were either from Cobalt-60 or linear accelerator sources. The cohort includes juvenile and adult exposures, and subsets of animals (39%) received post-IR mitigating treatments such as hematopoietic growth factors or antibiotics. The animals are pair-housed in indoor/outdoor pens and are fed a specially formulated high-fat, high-sodium chow diet designed to replicate a Western pattern diet. Importantly, these NHPs are well characterized clinically, with an annual cycle of veterinary examinations, imaging (ultrasound, CT, and MRI), complete blood counts, chemistry panels, bone marrow analyses, and cognitive assessments. When an experimental or humane endpoint is reached, the animal is euthanized and taken to necropsy by board-certified veterinary pathologists with extensive tissue examination and archiving.
Major findings in the RLEC include the development of skeletal muscle fibrosis with microvascular loss; myocardial fibrosis and systemic inflammation; monocyte polarization and adaptive immune system derangements, and “blind spots”; type 2 diabetes and insulin-resistance in adipose and skeletal muscle tissues; and adipose progenitor cell dysfunction [37–45]. Late effects of radiation are also observed within the central nervous system including disruption of the blood brain barrier, microvascular and white-matter injury, neuroinflammation, and cognitive impairment [46–50]. Bone is particularly sensitive to IR, and the RLEC reports substantial cortical thinning and structural changes in bone, and loss of bone mineral density [51]. These published findings in the RLEC are summarized in Table 1. Additional clinical findings include increased rates of cataracts, testicular atrophy, renal disease, and mesenchymal neoplasms [52–54]. Most conditions manifest 5–10 years post-exposure, making the RLEC animals a unique national resource as they age. Like aging, IR impacts nearly every major organ system — resulting in widespread multimorbidity and a shortened average health span and lifespan.
Table 1.
Age-related chronic diseases or conditions in the RLEC cohort that have been published
| Chronic disease or condition | Study design/comparison | Primary finding | Reference |
|---|---|---|---|
| Type II diabetes mellitus | 6.5–8.4 Gy TBI animals compared to age-matched unirradiated controls 5–9 years post-irradiation | Higher prevalence of T2DM in irradiated animals | Kavanagh et al. [43] |
| Adipose tissue insulin resistance | 4 Gy TBI animals compared to unirradiated controls 2 years post-irradiation | Irradiated animals exhibited decreased overall adipose tissue with increased insulin resistance, incidence of T2DM, and macrophage infiltration | Bacarella et al. [44] |
| Skeletal muscle fibrosis, microvascular changes, and insulin resistance | 6.5–8.4 Gy TBI animals compared to unirradiated controls 5–9 years post-irradiation | Skeletal muscle in irradiated animals exhibited increased levels of collagen IV and TGF-β1 with decreased microvascular density and insulin resistance | Fanning et al. [37] |
| Myocardial fibrosis and systemic inflammation | 6.5–8.4 Gy TBI animals compared to unirradiated controls 5–10 years post-irradiation | Increased histologic evidence of myocardial fibrosis & increased circulating biomarkers of inflammation in irradiated animals | DeBo et al. [38] |
| Bone structural changes and bone mineral density loss (osteopenia) | Animals received 10 Gy thorax only irradiation and were compared to unirradiated controls 8 months post-irradiation | DEXA, CT, and histopathology revealed cortical thinning, increased bony porosity, and decreased bone mineral density in the superior lumbar vertebrae of irradiated animals | Farris et al. [51] |
| Cerebrovascular injury | 1.14–8.5 Gy TBI animals followed longitudinally with MRI up to 11 years post-irradiation and compared to age-matched controls | Increased prevalence of MRI SWI lesions (cerebral microbleeds or necrosis) in irradiated animals with new lesion formation observed years after irradiation | Andrews et al. [46] |
| Cognitive impairment | 6.75–8.05 Gy TBI animals compared to age-matched unirradiated controls | Irradiated animals exhibited slower performance on cognitive reversal tests | Hanbury et al. [50] |
| Immune “blind spots” | 6.5–8.5 Gy TBI animals compared to age-matched controls 2 years post-irradiation | While irradiated animals produced antibodies to 10 out of 11 exposed antigens, they uniformly failed to produce antibodies to a pneumococcal antigen in contrast to the control animals | Hale et al. [45] |
Resilient and vulnerable NHP radiation survivors
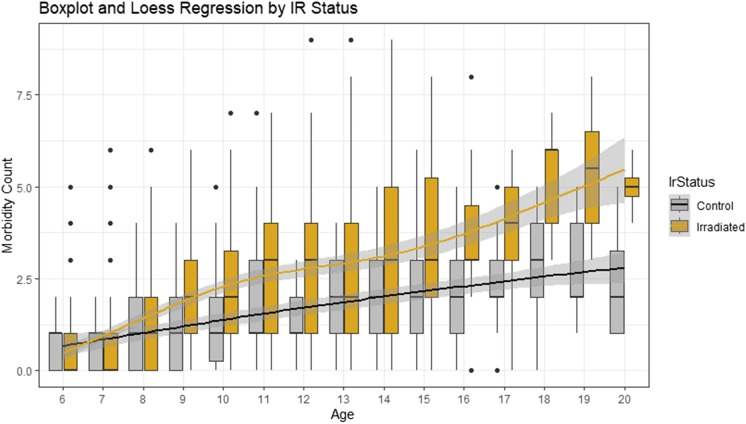
Despite an overall elevated risk of disease in radiation exposed compared to non-irradiated NHPs, there is marked heterogeneity in system-level response to radiation exposure in survivors. Multimorbidity and accumulation of health deficits are not consistently observed following IR, even in animals receiving doses that are typically lethal (Fig. 2). In our preliminary examination of multimorbidity, both IR and non-IR animals show increased multimorbidity with age. While the order of onset of diseases varies across animals, the first chronic condition is diagnosed at a mean age of 9.0 years (equal to 35% of median lifespan) and the second at age 10.7 years. However, 20% of animals that live to age 12 remain healthy, while others had as many as 9 comorbidities. In a Cox proportional-hazard model, each additional morbidity doubles likelihood of death (hazard ratio > 2) among animals 3–6 years of age with the hazard ratio declining from 1.9 to 1.4 from ages 6 to 15. IR animals have more comorbid conditions and at a younger age than non-IR animals. However, while animals exposed to higher doses have the most conditions on average, variability in multimorbidity in IR-exposed animals is not explained by dose alone, nor driven by any single disease process. This suggests that an intrinsic set of biological processes drives a resilient survivor phenotype in aging animals previously exposed to IR. The richness of the RLEC and the frequency of specific diseases of aging in the cohort enables study of tissue-specific resilience at a molecular level and progression of disease of aging including white matter brain legions, cognition, diabetes, cancer, osteoarthritis, and declining immune function. Ongoing studies are characterizing cellular senescence histologically in tissues from this cohort. We anticipate discovering new disease patterns and unanticipated mechanisms that will allow us to broaden our mechanistic understanding into new domains of inquiry and formation of new collaborative teams to facilitate additional research in this cohort by established RLEC researchers and new, geroscience-focused scientists.
Fig. 2.
Morbidity accumulation with age by irradiation status. Boxplot of distribution of morbidity count for animals of each age shows both an increase in the mean number of morbidities per animal (linear regression adjusted R2 = 0.24, p = 1.8 × 10−75) and an increase in variance (Breusch–Pagan test for heteroskedasticity p = 6.0 × 10−16). For animals over the age of 9, Welch’s t-tests show a significant difference in mean number of morbidities between control and irradiated animals (p < 0.05). Black dots represent individual outlier animals
Conclusions
The rhesus macaque’s genetic, epigenetic, physiologic, anatomic, and geropathologic similarities to humans make them one of the most well-characterized and valuable large animal models for the study of aging. The RLEC represents nearly 17 years of unique longitudinal data in an aging population of animals exposed to a stressor that decreases health span and lifespan. Utilizing ionizing radiation as a stressor is particularly valuable as the basic mechanisms of injury — ROS tissue injury in combination with DNA damage — are similar to those of biologic aging. The observed heterogenic response to irradiation provides rich territory for studying resilience and vulnerability in a large population of aging rhesus macaques.
Acknowledgements
We thank the laboratory and veterinary technicians and research assistants who conducted assessments. We also give special thanks to John Olson at WFSM for assistance with the animal cohort, and data retrieval, and members of the Register Laboratory for performing clinical biochemical assays.
Funding
This work was supported by the National Institutes of Health (NIH) program grant to Wake Forest University School of Medicine to support the NIAID-funded Radiation Survivor Cohort, U01 AI150578, (Drs. Cline and Schaaf), and the NIA-supported administrative supplement to evaluate aging outcomes and biological mechanism in this cohort. The authors were also supported by career development grants K01 AG059837 (Dr. Justice), and K01 AG056663 (Dr. Quillen).
Declarations
Ethics approval
All Radiation Late Effects Cohort experimental animal work is conducted at the Wake Forest University School of Medicine, an AAALAC-accredited institution, with IACUC approval.
Consent for publication
All authors consent to publication in GeroScience.
Conflict of interest
Drs. Cline and Schaaf have funding from Roche Pharmaceutical for work unrelated to this manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
George W. Schaaf and Jamie N. Justice made equal contributions to this manuscript.
References
- 1.Vanhooren V, Libert C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res Rev. 2013;12(1):8–21. doi: 10.1016/j.arr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Justice JN, Cesari M, Seals DR, Shively CA, Carter CS. Comparative approaches to understanding the relation between aging and physical function. J Gerontol a-Biol. 2016;71(10):1243–1253. doi: 10.1093/gerona/glv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers J, Gibbs RA. Comparative primate genomics: emerging patterns of genome content and dynamics. Nat Rev Genet. 2014;15(5):347–359. doi: 10.1038/nrg3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod. 1983;29(4):841–848. doi: 10.1095/biolreprod29.4.841. [DOI] [PubMed] [Google Scholar]
- 5.Simmons HA. Age-associated pathology in rhesus macaques (Macaca mulatta) Vet Pathol. 2016;53(2):399–416. doi: 10.1177/0300985815620628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829. [DOI] [PubMed] [Google Scholar]
- 7.Kaeberlein M, Creevy KE, Promislow DE. The dog aging project: translational geroscience in companion animals. Mamm Genome. 2016;27(7–8):279–288. doi: 10.1007/s00335-016-9638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freire-Cobo C, Rothwell ES, Varghese M, Edwards M, Janssen WGM, Lacreuse A, et al. Neuronal vulnerability to brain aging and neurodegeneration in cognitively impaired marmoset monkeys (Callithrix jacchus) Neurobiol Aging. 2023;123:49–62. doi: 10.1016/j.neurobiolaging.2022.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardif SD, Mansfield KG, Ratnam R, Ross CN, Ziegler TE. The marmoset as a model of aging and age-related diseases. ILAR J. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 11.Fraser HC, Kuan V, Johnen R, Zwierzyna M, Hingorani AD, Beyer A, et al. Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell. 2022;21(4):e13524. doi: 10.1111/acel.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkland JL, Stout MB, Sierra F. Resilience in aging mice. J Gerontol A Biol Sci Med Sci. 2016;71(11):1407–1414. doi: 10.1093/gerona/glw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitson HE, Cohen HJ, Schmader KE, Morey MC, Kuchel G, Colon-Emeric CS. Physical resilience: not simply the opposite of frailty. J Am Geriatr Soc. 2018;66(8):1459–1461. doi: 10.1111/jgs.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadley EC, Kuchel GA, Newman AB, Workshop S, Participants Report: NIA workshop on measures of physiologic resiliencies in human aging. J Gerontol A Biol Sci Med Sci. 2017;72(7):980–90. doi: 10.1093/gerona/glx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBrasseur NK. Physical resilience: opportunities and challenges in translation. J Gerontol A Biol Sci Med Sci. 2017;72(7):978–979. doi: 10.1093/gerona/glx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colon-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71(4):489–495. doi: 10.1093/gerona/glv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffman DM, Justice JN, Stout MB, Kirkland JL, Barzilai N, Austad SN. Evaluating health span in preclinical models of aging and disease: guidelines, challenges, and opportunities for geroscience. J Gerontol A Biol Sci Med Sci. 2016;71(11):1395–1406. doi: 10.1093/gerona/glw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sedrak MS, Gilmore NJ, Carroll JE, Muss HB, Cohen HJ, Dale W. Measuring biologic resilience in older cancer survivors. J Clin Oncol. 2021;39(19):2079–2089. doi: 10.1200/JCO.21.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese EJ. Hormesis mediates acquired resilience: using plant-derived chemicals to enhance health. Annu Rev Food Sci Technol. 2021;12:355–381. doi: 10.1146/annurev-food-062420-124437. [DOI] [PubMed] [Google Scholar]
- 23.Epel ES. The geroscience agenda: toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev. 2020;63:101167. doi: 10.1016/j.arr.2020.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese EJ, Baldwin LA. Radiation hormesis: its historical foundations as a biological hypothesis. Hum Exp Toxicol. 2000;19(1):41–75. doi: 10.1191/096032700678815602. [DOI] [PubMed] [Google Scholar]
- 25.Al-Jumayli M, Brown SL, Chetty IJ, Extermann M, Movsas B. The biological process of aging and the impact of ionizing radiation. Semin Radiat Oncol. 2022;32(2):172–178. doi: 10.1016/j.semradonc.2021.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 27.Dainiak N, Albanese J. Medical management of acute radiation syndrome. J Radiol Prot. 2022;42(3). 10.1088/1361-6498/ac7d18. [DOI] [PubMed]
- 28.Zablotska LB, Bazyka D, Lubin JH, Gudzenko N, Little MP, Hatch M, et al. Radiation and the risk of chronic lymphocytic and other leukemias among chornobyl cleanup workers. Environ Health Perspect. 2013;121(1):59–65. doi: 10.1289/ehp.1204996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ron E, Preston DL, Mabuchi K, Thompson DE, Soda M. Cancer incidence in atomic bomb survivors. Part IV: comparison of cancer incidence and mortality. Radiat Res. 1994;137(2 Suppl):S98–112. doi: 10.2307/3578894. [DOI] [PubMed] [Google Scholar]
- 30.Douple EB, Mabuchi K, Cullings HM, Preston DL, Kodama K, Shimizu Y, et al. Long-term radiation-related health effects in a unique human population: lessons learned from the atomic bomb survivors of Hiroshima and Nagasaki. Disaster Med Public Health Prep. 2011;5(Suppl 1 (0 1)):S122–33. doi: 10.1001/dmp.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooning MJ, Aleman BM, van Rosmalen AJ, Kuenen MA, Klijn JG, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Wakeford R. The cancer epidemiology of radiation. Oncogene. 2004;23(38):6404–6428. doi: 10.1038/sj.onc.1207896. [DOI] [PubMed] [Google Scholar]
- 33.Upton AC, Kimball AW, Furth J, Christenberry KW, Benedict WH. Some delayed effects of atom-bomb radiations in mice. Cancer Res. 1960;20((8)Pt2):1–60. [PubMed] [Google Scholar]
- 34.Strehler BL. Origin and comparison of the effects of time and high-energy radiations on living systems. Q Rev Biol. 1959;34(2):117–142. doi: 10.1086/402632. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miousse IR, Kutanzi KR, Koturbash I. Effects of ionizing radiation on DNA methylation: from experimental biology to clinical applications. Int J Radiat Biol. 2017;93(5):457–469. doi: 10.1080/09553002.2017.1287454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanning KM, Pfisterer B, Davis AT, Presley TD, Williams IM, Wasserman DH, et al. Changes in microvascular density differentiate metabolic health outcomes in monkeys with prior radiation exposure and subsequent skeletal muscle ECM remodeling. Am J Physiol Regul Integr Comp Physiol. 2017;313(3):R290–R297. doi: 10.1152/ajpregu.00108.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBo RJ, Lees CJ, Dugan GO, Caudell DL, Michalson KT, Hanbury DB, et al. Late effects of total-body gamma irradiation on cardiac structure and function in male rhesus macaques. Radiat Res. 2016;186(1):55–64. doi: 10.1667/RR14357.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalson KT, Macintyre AN, Sempowski GD, Bourland JD, Howard TD, Hawkins GA, et al. Monocyte polarization is altered by total-body irradiation in male rhesus macaques: implications for delayed effects of acute radiation exposure. Radiat Res. 2019;192(2):121–134. doi: 10.1667/RR15310.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.French MJ, Wuerker R, Dugan G, Olson JD, Sanders BR, Tooze JA, et al. Long-term immunological consequences of radiation exposure in a diverse cohort of rhesus macaques. Int J Radiat Oncol Biol Phys. 2022 doi: 10.1016/j.ijrobp.2022.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macintyre AN, French MJ, Sanders BR, Riebe KJ, Shterev ID, Wiehe K, et al. Long-term recovery of the adaptive immune system in rhesus macaques after total body irradiation. Adv Radiat Oncol. 2021;6(5):100677. doi: 10.1016/j.adro.2021.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggiero AD, Davis MA, Davis AT, DeStephanis D, Williams AG, Vemuri R, et al. Delayed effects of radiation in adipose tissue reflect progenitor damage and not cellular senescence. Geroscience. 2022 doi: 10.1007/s11357-022-00660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kavanagh K, Dendinger MD, Davis AT, Register TC, DeBo R, Dugan G, et al. Type 2 diabetes is a delayed late effect of whole-body irradiation in nonhuman primates. Radiat Res. 2015;183(4):398–406. doi: 10.1667/Rr13916.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bacarella N, Ruggiero A, Davis AT, Uberseder B, Davis MA, Bracy DP, et al. Whole body irradiation induces diabetes and adipose insulin resistance in nonhuman primates. Int J Radiat Oncol Biol Phys. 2020;106(4):878–886. doi: 10.1016/j.ijrobp.2019.11.034. [DOI] [PubMed] [Google Scholar]
- 45.Hale LP, Rajam G, Carlone GM, Jiang C, Owzar K, Dugan G, et al. Late effects of total body irradiation on hematopoietic recovery and immune function in rhesus macaques. PLoS One. 2019;14(2):e0210663. doi: 10.1371/journal.pone.0210663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews RN, Bloomer EG, Olson JD, Hanbury DB, Dugan GO, Whitlow CT, et al. Non-human primates receiving high-dose total-body irradiation are at risk of developing cerebrovascular injury years postirradiation. Radiat Res. 2020;194(3):277–287. doi: 10.1667/RADE-20-00051.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrews RN, Caudell DL, Metheny-Barlow LJ, Peiffer AM, Tooze JA, Bourland JD, et al. Fibronectin produced by cerebral endothelial and vascular smooth muscle cells contributes to perivascular extracellular matrix in late-delayed radiation-induced brain injury. Radiat Res. 2018;190(4):361–373. doi: 10.1667/Rr14961.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andrews RN, Dugan GO, Peiffer AM, Hawkins GA, Hanbury DB, Bourland JD, et al. White matter is the predilection site of late-delayed radiation-induced brain injury in non-human primates. Radiat Res. 2019;191(3):217–231. doi: 10.1667/RR15263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017;187(5):599–611. doi: 10.1667/Rr14616.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanbury DB, Peiffer AM, Dugan G, Andrews RN, Clinc JM. Long-term cognitive functioning in single-dose total-body gamma-irradiated rhesus monkeys (Macaca mulatta) Radiat Res. 2016;186(5):447–454. doi: 10.1667/Rr14430.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farris M, McTyre ER, Okoukoni C, Dugan G, Johnson BJ, Blackstock AW, et al. Cortical thinning and structural bone changes in non-human primates after single-fraction whole-chest irradiation. Radiat Res. 2018;190(1):63–71. doi: 10.1667/RR15007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Little MP, Brenner AV, Grant EJ, Sugiyama H, Preston DL, Sakata R, et al. Age effects on radiation response: summary of a recent symposium and future perspectives. Int J Radiat Biol. 2022;1–11. 10.1080/09553002.2022.2063962. [DOI] [PMC free article] [PubMed]
- 53.Sills WS, Tooze JA, Olson JD, Caudell DL, Dugan GO, Johnson BJ, et al. Total-body irradiation is associated with increased incidence of mesenchymal neoplasia in a radiation late effects cohort of rhesus macaques (Macaca mulatta) Int J Radiat Oncol Biol Phys. 2022;113(3):661–674. doi: 10.1016/j.ijrobp.2022.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen EP, Olson JD, Tooze JA, Bourland JD, Dugan GO, Cline JM. Detection and quantification of renal fibrosis by computerized tomography. PLoS One. 2020;15(2):e0228626. doi: 10.1371/journal.pone.0228626. [DOI] [PMC free article] [PubMed] [Google Scholar]