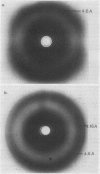
Abstract
Axes of soybean seeds are tolerant to dehydration at 6 hours of imbibition, but susceptible to dehydration injury if dried at 36 hours of imbibition. Smooth microsomal membranes were isolated from axes imbibed for 6 hours (dehydration tolerant state) and 36 hours (dehydration susceptible state) before and after dehydration treatment. The phase properties and the lipid composition of the membrane fraction were investigated. Wide angle x-ray diffraction patterns of microsomal membranes from axes imbibed for 6 or 36 hours indicated a liquid-crystalline to gel phase transition at approximately 7°C. Membranes from axes dehydrated at 6 or 36 hours of imbibition and rehydrated for 2 hours exhibited a phase transition at 7°C and 47°C, respectively. Changes in fatty acid saturation did not account for the changes in phase properties. However, the increased phase transition temperature of the membranes from dehydration injured axes was associated with an increase in free fatty acid:phospholipid molar ratio and a decrease in phospholipid:sterol ratio. These results suggests that dehydration prompted a deesterification of the linkage between glycerol and fatty acid side chains of the phospholipid molecules in the membrane. The resultant increase in free fatty acid content in the membrane is thought to alter the fluidity and phase properties of the membrane and contribute to dehydration injury.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldassare J. J., Saito Y., Silbert D. F. Effect of sterol depletion on LM cell sterol mutants. Changes in the lipid composition of the plasma membrane and their effects on 3-O-methlglucose transport. J Biol Chem. 1979 Feb 25;254(4):1108–1113. [PubMed] [Google Scholar]
- Chia L. S., Thompson J. E., Dumbroff E. B. Simulation of the effects of leaf senescence on membranes by treatment with paraquat. Plant Physiol. 1981 Mar;67(3):415–420. doi: 10.1104/pp.67.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Craft C. C., Audia W. V., Wilcox M. S. Biochemical Studies of Chilling Injury in Sweetpotatoes. Plant Physiol. 1958 Sep;33(5):307–311. doi: 10.1104/pp.33.5.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- McKersie B. D., Lepock J. R., Kruuv J., Thompson J. E. The effects of cotyledon senescence on the composition and physical properties of membrane lipid. Biochim Biophys Acta. 1978 Apr 4;508(2):197–212. doi: 10.1016/0005-2736(78)90325-5. [DOI] [PubMed] [Google Scholar]
- McKersie B. D., Stinson R. H. Effect of Dehydration on Leakage and Membrane Structure in Lotus corniculatus L. Seeds. Plant Physiol. 1980 Aug;66(2):316–320. doi: 10.1104/pp.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Lipid crystallization in senescent membranes from cotyledons. Plant Physiol. 1977 May;59(5):803–807. doi: 10.1104/pp.59.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Phase Behavior of Chloroplast and Microsomal Membranes during Leaf Senescence. Plant Physiol. 1978 Apr;61(4):639–643. doi: 10.1104/pp.61.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. In vitro simulation of senescence-related membrane damage by ozone-induced lipid peroxidation. Nature. 1980 Jan 31;283(5746):504–506. doi: 10.1038/283504a0. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Senaratna T., McKersie B. D. Characterization of Solute Efflux from Dehydration Injured Soybean (Glycine max L. Merr) Seeds. Plant Physiol. 1983 Aug;72(4):911–914. doi: 10.1104/pp.72.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna T., McKersie B. D. Dehydration Injury in Germinating Soybean (Glycine max L. Merr.) Seeds. Plant Physiol. 1983 Jul;72(3):620–624. doi: 10.1104/pp.72.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Microviscosity parameters and protein mobility in biological membranes. Biochim Biophys Acta. 1976 Apr 16;433(1):133–149. doi: 10.1016/0005-2736(76)90183-8. [DOI] [PubMed] [Google Scholar]
- Stewart R. R., Bewley J. D. Stability and Synthesis of Phospholipids during Desiccation and Rehydration of a Desiccation-Tolerant and a Desiccation-Intolerant Moss. Plant Physiol. 1982 Mar;69(3):724–727. doi: 10.1104/pp.69.3.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Sakura J. D. Raman analysis of the thermotropic behavior of lecithin-fatty acid systems and of their interaction with proteolipid apoprotein. Biochemistry. 1980 Feb 5;19(3):574–579. doi: 10.1021/bi00544a028. [DOI] [PubMed] [Google Scholar]