Abstract
The prevailing assumption has been that the human spermatozoon provides only one centriole to the zygote: the proximal centriole, with a canonical, cylinder-like shape. This overly simplistic view has come under challenge since discovering that the human spermatozoon provides a second, atypical centriole to the zygote. The study of human zygotes is challenging for ethical reasons, and bovine zygotes provide an important model due to a similarity in centrosome embryonic inherence and function. Detailed ultrastructural analyses by Uzbekov and colleagues identify the persistence of atypical centrioles in bovine early embryos, raising questions about the original single-centriole model. Whether the parental origin of nascent atypical centrioles or their wide structural diversity and deviation from the canonical centriolar form in blastomeres constitutes sufficient evidence to warrant a reconsideration of the single-centriole model is discussed herein. Because previous human studies identified only one canonical centriole in the zygote, atypical centrioles are likely present in the early human embryo; therefore, it is time to rethink the role of paternal centrioles in human development.
Keywords: Embryogenesis, Sperm, Centriole, Centrosome, Infertility, Miscarriage
The enigma of centriole inheritance during sexual reproduction
In non-rodent mammals, only one canonical centriole was observed in spermatozoa [1], and up to three centrioles were observed in the cleaving zygote [2], creating the enigma of centriole inheritance during sexual reproduction. However, recent studies suggest that this enigma arises because embryonic centrioles are atypical in structure, composition, and function and evolve rapidly (reviewed in [3]). Therefore, determining the origin of embryonic centrioles requires flexibility in defining the term “centriole,” with a focus on their functions rather than their structure. We use the term “atypical centriole” as a broad category for centrioles of varying structures but similar functionality, as atypical centrioles can differ structurally in a species-, cell-type-, or cell-phase-specific manner (reviewed in [3]). Here, we will review recent progress in the study of spermatozoa and embryonic centrioles [4, 5]. We will compare early embryonic centrioles to dividing somatic centrioles, examining the hypothesis that though most zygotic and some blastomeric centrioles are structurally atypical, they maintain most canonical centriolar functions, including acting as a platform for the formation of procentrioles in precise quantities.
Human and bovine spermatozoa have two centrioles: one canonical and one atypical
Based on strict structural criteria, human and bovine spermatozoa possess only one centriole, the proximal centriole [6] (Fig. 1). A canonical centriole comprises nine triplet microtubules arranged in a radially symmetric circle along most of its length. As a result, the canonical centriole has a cylindrical shape. However, human and bovine spermatozoa possess a second, structurally atypical centriole, the spermatozoan distal centriole [4], which comprises nine doublet microtubules (not triplets as in the canonical centriole) along its length. The nine doublet microtubules splay, curve to the spermatozoon’s left and right sides, and have a flattened funnel shape, resulting in an oval cross-section. A similar combination of canonical proximal centriole and atypical distal centriole was found in the spermatozoa of other mammals [7]. The spermatozoan distal centriole is asymmetric, with the right microtubules oriented more rostrally than the left [8]. Unlike canonical centrioles, the spermatozoan distal centriole has two central microtubules through its center (the central pair), a characteristic typically found only in motile cilia or flagella. The spermatozoan centrioles are surrounded by segmented columns that are thought to be a modified pericentriolar material [4, 5, 8, 9].
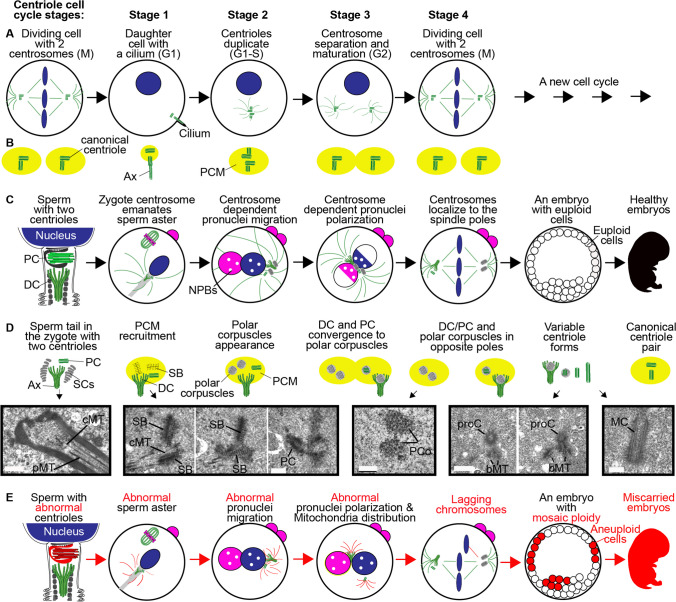
Fig 1.
A model of centriolar structure and function post-dertilization. A, B The centriole cell cycle comprises four main stages in dividing somatic cells. C, D The centriole cell cycle in the early embryo. C In bovines, humans, and many other mammals, sperm have two centrioles: the proximal centriole (PC) and the atypical distal centriole (DC), which is attached to the axoneme (Ax). During fertilization, spermatozoan centrioles are brought into the oocyte, forming the large sperm aster or the zygotic centrosome, which helps in pronuclear migration. The initial, single zygotic centrosome breaks into two centrosomes that move to the junction between the two pronuclei, where they polarize the paternal (blue) and maternal (pink) genomes as well as the nucleolus precursor bodies (NPBs). This localization leads to embryonic euploidy, and healthy pregnancy. D Sperm atypical centrioles and other forms of atypical centrioles, such as polar corpuscles, that can be found in the zygote and blastomeres and that recruit pericentriolar material (PCM), form centrosomes, and act as a platform to guide centriolar formation (“centriole duplication”). SC, striated columns; SB, striated body. Examples of electron microscopy pictures were obtained by Rustem Uzbekov. Black boxes surround a single section or a series of successive sections. cMT, central microtubules of flagellum; pMT, peripheral microtubules of flagellum; PCo, polar corpuscles; PC, proximal centriole; bMT, bundles of the atypical centriole microtubules, proC, procentriole; MC, mature centriole. E In sperm with abnormal centrioles (red), abnormal asters form post-fertilization. The pronuclear genomes and nucleolus precursor bodies are incorrectly polarized, resulting in embryonic aneuploidy/mosaicism (red) and miscarriage
Despite its very atypical structure, the distal centriole possesses some signature centriolar characteristics [4, 5, 8, 9]. First, it has centriole-specific proteins, such as Centrin, POC5, FAM161A, and POC1B (Fig. 2 in [4]). Second, it is found at the base of the spermatozoan tail, a type of cilia (Fig. 2 in [4]). Furthermore, after fertilization, the spermatozoan axoneme base—the attachment site of the distal centriole—acts like a centriole, recruiting pericentriolar material, forming a centrosome, and serving as a platform for the production of a single new centriole per cell cycle (Fig. 6 in [4]).
Bovine zygotes have four centrioles, and most or all are atypical
During fertilization, the sperm fuses with the egg, forming the zygote. Together, the spermatozoan canonical proximal centriole, atypical distal centriole, striated columns, and axoneme base, while still associated with each other, recruit the egg’s pericentriolar material proteins, forming a centrosome that produces a large microtubule aster (Fig. 2 in [5]) (Fig. 6 in [4]). This zygotic stage resembles Stage 1 of somatic cell division (Fig. 1A, B). The aster facilitates the union of the male and female pronuclei [10] [11].
Later, while still associated, the proximal centriole, distal centriole, and axoneme base associate with two novel structures referred to as “striated bodies” (Fig. 3 in [5]) (Fig. 1D). They are made of “a dense strand approximately 400 nm long and 50 nm in diameter, surrounded by granular material arranged in rows” [Page 7 in 5]. The striated bodies resemble the granular material observed in the spermatozoan striated columns, suggesting that the striated bodies are derived from the striated columns. These striated bodies appear to be the precursors of the new atypical centrioles formed in the zygote (see below). Also, the distal centriole is further modified, losing its microtubules (Fig. 4 in [5]). These four connected centrioles, acting as a centrosome with astral microtubules, were also observed later in the junction between the two pronuclei (Fig. 4 in [5]). The four-centriole configuration (proximal centriole, distal centriole, and two striated bodies) is the equivalent of a linked diplosome of the somatic cell cycle (Stage 3 in Fig. 1A, B). The centrosomes found in the junction of the two pronuclei were recently suggested to redistribute the chromosomes within the pronuclei, a process that is essential for accurate chromosome segregation during cleavage [12].
During cleavage, the still-associated proximal and distal centrioles (i.e., the axoneme base) are located near one of the zygotic spindle poles, forming a microtubule aster (as expected from functional centrioles) (Fig. 6 in [5]). The pair of new atypical centrioles, called polar corpuscles, are observed as para-crystallin, electron-dense material and are found near the other spindle pole, forming a second aster (Fig 7 in [5]). This zygotic stage parallels Stage 4 of somatic cell division, but unlike Stage 2 of somatic cell division, the original (i.e., inherited from the previous “cell cycle”) mother and daughter centrioles remain together. The role of the centrioles in zygotic spindle formation was recently challenged by the discovery that the mammalian zygote, unlike somatic cells, possesses two parallel male and female spindles [13]. However, a double spindle is present in Drosophila and other insect zygotes (called the monomeric spindle), and still, centrioles are critical for normal Drosophila zygotic division [14, 15]. Therefore, a double spindle by itself does not render the centriole dispensable.
As the zygotic cell cycle progresses, the proximal centriolar structure becomes atypical, changing from its canonical form in the spermatozoon. Later, the proximal centriole becomes shorter and, finally, takes on the para-crystallin, electron-dense appearance of a polar corpuscle (Fig. 8 in [5]). Similarly, the distal centriole found at the tail base takes on a para-crystallin, electron-dense appearance like that observed in the polar corpuscle (Fig. 8 in [5]). At the late cleavage stage, in some cases, all four centrioles are atypical and appear as two pairs of polar corpuscles. Yet, there are examples in which a centriole is present in a two-cell blastomere, suggesting some variations in the changes to the proximal centriole post-fertilization (see below). Altogether, with the exceptions of centriolar structure and the absence of a primary cilium, the above observations reflect both similarities and differences between the centriolar cell cycles of dividing somatic cells and zygotes.
Some bovine blastomeric centrioles are atypical
Blastomeres have two centrioles during interphase and four during mitosis. However, each blastomere has a distinct combination of canonical and atypical centrioles and their intermediates.
In a two-cell blastomere embryo (blastomere after the first cleavage), one cell, at the interphase stage, had a short canonical procentriole and one atypical centriole appearing as electron-dense material with microtubule bundles (Fig. 11 in [5]). The other cell, at the mitotic phase, had short canonical procentriole, one atypical centriole, and a striated body (Fig. 12 in [5]). In another two-cell blastomere, both cells had an unusually long canonical centriole associated with a procentriole (Fig. 9 in [5]). Atypical centrioles were also observed in the cells of a two-blastomere embryo during the second cleavage. In these cells, centrosome composition ranged from a canonical centriole to an electron-dense material without (Fig. 13 in [5]) or with irregular microtubules (Figs 14, 15, and 16 in [5]).
Classic centrosomes with two canonical centrioles appear only after the embryo’s third cleavage. However, the mother centriole in a centriolar pair is often atypically long (650–1050 nm) (Fig 17 in [5]). Interestingly, these later-stage embryos had two blastomeres among their ~8–14 blastomeres with atypical centrioles. In one of these blastomeres, the centrosome had a canonical procentriole associated with an atypical distal centriole marked by the axoneme base (Fig 18 in [5]). In other blastomeres of the same embryo, the centrosome had a canonical procentriole associated with an atypical centriole without an axoneme, suggesting that this centrosome is derived from the spermatozoan proximal centriole or from one of the polar corpuscles. These findings suggest that the spermatozoan centrioles retain function in the embryo while maintaining an atypical structure.
What about human embryos?
Due to ethical constraints, few studies have been performed on normal embryos produced from the fertilization of an egg by one sperm [16, 17]. In these studies, only one canonical centriole was observed in zygotes or two-cell-stage blastomeres. Therefore, it can be safely stated that the full range of centriolar variants and their adjunct structures are incompletely analyzed. With this limitation in mind, here we compare observations in bovines to those from the studies on humans.
Critical considerations
It is important to note that studying the centrioles in bovine embryos has several challenges.
First, zygotic centrosome anomalies are common, and ~65% of zygotic centrosomes have abnormal localization (Fig 4A in [12]), leading to a high rate of miscarriages in bovine (up to 48%) [18]. A similarly high miscarriage rate is observed in humans (26–70%) [19–21]. Therefore, it is unclear which centriole configuration, as observed with electron microscopy, describes a healthy or a pathological centrosome.
Second, the axoneme is an elongated structure that may sometimes break from the sperm shortly after fertilization (Figs S7 and S8 in [5]).
Third, the zygote cleaves into two-cell blastomeres that cleave into four-cell blastomeres, and so on. These cell divisions are often nonsynchronous, so blastomere quantities may not precisely reflect the number of cell cycles post-fertilization [22]. Also, the early embryo can tolerate some cell death, generating morulae with distinct numbers of blastomeres, making it challenging to determine the precise blastomere stage being studied. This difficulty in identifying the blastomere stage may explain some of the variation observed in the centriolar structure.
Another important note is that the canonical centriolar structure is dispensable for the formation of a functional centrosome but is essential for the formation of a functional cilium. Since cilia appear much later in embryonic development [23], atypical centrioles are, at least in principle, sufficient for normal early embryonic development. Therefore, it is possible that the centriolar structure is not strictly “enforced” in the zygote and blastomeres because it has no effect on centriolar function at this stage of embryogenesis.
Finally, the appearance of a centriole of the canonical form in parthenogenetically activated embryos already at the stage of 4 blastomeres (Fig 20 in [5]) allows us to conclude that, like in rodents, in the genome of other mammals, there is also a “reserve” program for constructing de novo centrioles, that is, without a previous centriolar matrix.
Considering these notes and challenges, the above hypothesis attempts to reconstruct the centriole cycle in the bovine zygote and blastomeres.
Conclusion
Mounting evidence from clinical assisted reproductive technologies has established the widespread nature and common occurrence of genomic and genetic instability in human embryos. Over 20 years have passed since the original solitary centriole model was proposed by Sathananthan and Trounson, with little, if any, modification or inclusion of new data despite the growing awareness of error-prone early divisions in human embryos [16, 17]. Here, we review convincing work in humans and bovines that calls into question these original ideas and provides a new framework for delving into the role of centrioles and centrosomes in early development that may have fundamental value in treating infertility.
In summary, the proposed origin of bovine embryonic centrioles is as follows:
Mature oocytes have no centrioles.
Spermatozoa provide the zygote with two centrioles: one that is structurally canonical (the proximal centriole) and one that is structurally atypical and is attached to the axoneme base (the spermatozoan distal centriole).
The structures of the spermatozoan proximal and distal centrioles are modified in the zygote, and two new structurally atypical zygotic centrioles are formed.
Blastomeres can have a variety of centriolar structures, including the atypical distal centriole attached to the axoneme base and the modified proximal centriole. New centrioles form during blastomere cell cycles and can be canonical with irregular length or atypical with disorganized microtubules embedded in electron-dense material. The sperm-tail-attached distal centriole is maintained in some embryos up to at least the 14 blastomere stage, where it is associated with a procentriole (with triplet microtubules) and astral microtubules, suggesting that the spermatozoan distal centriole or its derivative remains functional despite its atypical structure.
New canonical centrioles start appearing after the third cleavage.
Acknowledgements
We thank Derek Kluczynski, Katerina Turner, Luke A. Achinger, Nahshon Puente, and David Albertini for editing and commenting on the manuscript.
Funding
This project was supported by Agriculture and Food Research Initiative Competitive Grant number OHOW-2020-02790 from the USDA National Institute of Food and Agriculture and Grant number 1R15HD110863 from NIH-NICHD.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Highlights
• Most early embryonic centrioles are structurally atypical.
• The atypical spermatozoan distal centriole remains atypical in the embryo while “duplicating” new centrioles.
• The canonical spermatozoan proximal centriole changes in the embryo, sometimes acquiring an atypical structure.
• The first two new centrioles formed in the zygote are structurally atypical.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manandhar G, Simerly C, Schatten G. Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum Reprod. 2000;15(2):256–263. doi: 10.1093/humrep/15.2.256. [DOI] [PubMed] [Google Scholar]
- 2.Crozet N. Behavior of the sperm centriole during sheep oocyte fertilization. Eur J Cell Biol. 1990;53(2):326–332. [PubMed] [Google Scholar]
- 3.Avidor-Reiss T. Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cells. 2018;7(7):67. doi: 10.3390/cells7070067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fishman EL, et al. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018;9(1):2210. doi: 10.1038/s41467-018-04678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uzbekov R, et al. Centrosome formation in the bovine early embryo. Cells. 2023;12(9):1335. doi: 10.3390/cells12091335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatten H, et al. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci U S A. 1986;83(1):105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung MR, et al. The multi-scale architecture of mammalian sperm flagella and implications for ciliary motility. EMBO J. 2021;40(7):e107410. doi: 10.15252/embj.2020107410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanal S, et al. A dynamic basal complex modulates mammalian sperm movement. Nat Commun. 2021;12(1):3808. doi: 10.1038/s41467-021-24011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amargant F, et al. The human sperm basal body is a complex centrosome important for embryo preimplantation development. Mol Hum Reprod. 2021;27(11);gaab062. [DOI] [PMC free article] [PubMed]
- 10.Nakamura S, et al. Human sperm aster formation and pronuclear decondensation in bovine eggs following intracytoplasmic sperm injection using a Piezo-driven pipette: a novel assay for human sperm centrosomal function. Biol Reprod. 2001;65(5):1359–1363. doi: 10.1095/biolreprod65.5.1359. [DOI] [PubMed] [Google Scholar]
- 11.Simerly C, et al. The paternal inheritance of the centrosome, the cell's microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1(1):47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- 12.Cavazza T, et al. Parental genome unification is highly error-prone in mammalian embryos. Cell. 2021;184(11):2860–2877 e22. doi: 10.1016/j.cell.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider I, et al. Dual spindles assemble in bovine zygotes despite the presence of paternal centrosomes. J Cell Biol. 2021;220(11):e202010106. [DOI] [PMC free article] [PubMed]
- 14.Loppin B, Dubruille R, Horard B. The intimate genetics of Drosophila fertilization. Open Biol. 2015;5(8):150076. [DOI] [PMC free article] [PubMed]
- 15.Kawamura N. Fertilization and the first cleavage mitosis in insects. Dev Growth Differ. 2001;43(4):343–349. doi: 10.1046/j.1440-169x.2001.00584.x. [DOI] [PubMed] [Google Scholar]
- 16.Sathananthan AH, et al. The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum Reprod. 1996;11(2):345–356. doi: 10.1093/HUMREP/11.2.345. [DOI] [PubMed] [Google Scholar]
- 17.Sathananthan AH, et al. Centrioles in the beginning of human development. Proc Natl Acad Sci U S A. 1991;88(11):4806–4810. doi: 10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reese ST, et al. Pregnancy loss in beef cattle: a meta-analysis. Anim Reprod Sci. 2020;212:106251. doi: 10.1016/j.anireprosci.2019.106251. [DOI] [PubMed] [Google Scholar]
- 19.Quenby S, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658–1667. doi: 10.1016/S0140-6736(21)00682-6. [DOI] [PubMed] [Google Scholar]
- 20.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the 'black box' of early pregnancy loss. Hum Reprod Update. 2002;8(4):333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 21.ACOG. ACOG Practice bulletin No. 200: Early pregnancy loss. Obstet Gynecol. 2018;132(5):e197–e207. [DOI] [PubMed]
- 22.Koyama H, et al. Analysis of polarity of bovine and rabbit embryos by scanning electron microscopy. Biol Reprod. 1994;50(1):163–170. doi: 10.1095/biolreprod50.1.163. [DOI] [PubMed] [Google Scholar]
- 23.Bangs FK, et al. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17(2):113–122. doi: 10.1038/ncb3091. [DOI] [PMC free article] [PubMed] [Google Scholar]