Abstract
Senescent cells may have a prominent role in driving inflammation and frailty. The impact of cellular senescence on frailty varies depending on the assessment tool used, as it is influenced by the criteria or items predominantly affected by senescent cells and the varying weights assigned to these items across different health domains. To address this challenge, we undertook a thorough review of all available studies involving gain- or loss-of-function experiments as well as interventions targeting senescent cells, focusing our attention on those studies that examined outcomes based on the individual frailty phenotype criteria or specific items used to calculate two humans (35 and 70 items) and one mouse (31 items) frailty indexes. Based on the calculation of a simple “evidence score,” we found that the burden of senescent cells related to musculoskeletal and cerebral health has the strongest causal link to frailty. We deem that insight into these mechanisms may not only contribute to clarifying the role of cellular senescence in frailty but could additionally provide multiple therapeutic opportunities to help the future development of a desirable personalized therapy in these extremely heterogeneous patients.
Keywords: Aging, Frailty, Cellular senescence, Brain function, Musculoskeletal health
Introduction
Frailty is a complex age-related condition characterized by an increased individual’s vulnerability and an elevated risk of severe health outcomes [1, 2]. Geriatricians are aware that frailty frequently coexists with age-related chronic diseases like obesity, diabetes mellitus, heart disease, cancer, stroke, and musculoskeletal disorders, and that frailty can influence the effects of treatments for these conditions, acting as an effect modifier [3–6]. In clinical practice, the management of frail patients is complex mainly because of the inadequate evidence base for interventions to manage the condition [7] and endless debates on frailty assessment instruments [8, 10]. Indeed, there is no global standard assessment instrument for frailty, and hundreds of tools are used and cited in the literature [8]. Moreover, frailty can originate from a specific disease or be the consequence of various and multiple impairments, and even frail individuals identified with the same instrument are highly heterogeneous [11–13]. These problems hinder progress in discovering the biological hallmarks of frailty, which in turn could be helpful in the prevention and diagnosis of frailty and in providing the necessary accuracy for developing appropriate interventions.
Assuming that frailty is a condition of accelerated aging, it is possible to speculate that the same hallmarks indicated for the aging process (e.g., inflammation and altered intercellular communication, stem cell exhaustion, mitochondrial dysfunction, deregulated nutrient sensing, cellular senescence, telomere and genomic instability, epigenetic alterations, loss of proteostasis, compromised autophagy, microbiome disturbance, splicing dysregulation, and altered mechanical properties) [14, 15] are key factors of the frailty syndrome. However, the respective weight of these hallmarks in the aging process is still unclear; importantly, since frailty is measured with specific tools, these hallmarks will have different weights in different frailty models.
Indeed, recent studies addressed the research on frailty biomarkers in body fluids focusing on the identification of genes and factors modulated in aging and age-related diseases [16–19]. This approach has provided several panels of frailty biomarkers for future experimental validation in human and animal models. Among these panels, the low-grade chronic inflammation associated with aging (inflammaging) is likely the most studied and convincing factor implicated in the pathophysiology of frailty [20, 21]. Interestingly, in a subset of the population, initiating systemic inflammation during midlife may independently promote future pathophysiological changes underlying frailty [22, 23]. However, the cause and source of inflammaging in these conditions remain elusive. Understanding the processes underlying midlife inflammation, its consequences, and the mechanisms leading to the amplification of these signals could be influential for early therapeutic targeting. The immune system is notoriously the primary modulator of pro- and anti-inflammatory factor levels; however, to date, it is not the only source of these factors. Senescent cells are emerging as an essential source of inflammatory mediators, leading to the recent hypothesis that these cells’ accumulation may drive the biological phenomenon underlining frailty [24]. This is particularly significant as developing a new class of senescent cells targeting drugs, known as senotherapeutics, has paved the way for potential therapies for age-related diseases and frailty [25]. However, since senescent cells arise in response to damage, a lack of information on the sources of this damage could result in poor therapeutic efficacy. The source of this damage may not yet be active or may be a consequence of lifelong metabolic damage. Still, in the opposite case, these therapies may be similar to treating cancer by removing a consequent effect, i.e., the inflammation, without eliminating the tumoral mass.
This manuscript aims to review the current knowledge on the causal connection between cellular senescence and frailty (considering the different assessment tools in humans and preclinical models) while addressing prominent age-related factors and potential therapeutic targets that may contribute to the excessive accumulation of senescent cells in frailty.
Frailty assessment tools in humans and murine models
Frailty assessment in humans
The most widely used frailty assessment tools in humans are based on the adaptation of the physical frailty phenotype (PP) [26] or of the deficit accumulation frailty index (FI) [27], which are well validated in many populations and settings [1]. These two instruments entail profound differences in the conceptualization of the phenotype (physical vs. multidimensional) and how frailty is assessed (categorical vs. quantitative).
The PP is based on five criteria (i.e., shrinkness, weakness, exhaustion, slowness, and inactivity). Individuals are deemed frail if they meet at least three criteria, pre-frail if they fulfill one or two criteria, and not frail if no criteria are met, thus allowing the categorization of subjects into three distinct risk subgroups. Hence, PP is related to physical function and muscular performance, which are considered summary indices of overall body capacity, but leave out several areas affected by frailty, such as the cognitive status and the psychosocial components.
Conversely, the FI can be considered the practical implementation of the idea of “damage accumulation,” and it is calculated as the ratio between the number of deficits detected and the total number of multiple health deficits considered within an extensive list. This way, the FI is typically scored on a scale of 0 to 1, where higher scores indicate greater levels of frailty. The checklist items may be variable, including diseases, physical and cognitive impairments, psychosocial risk factors, and geriatric syndromes [27–30], to construct a picture as complete as possible of the adverse event risk. However, this frailty measure is principally applied in research settings; in contrast, the Clinical Frailty Scale (which considers only a limited number of health-related variables) is commonly used in clinical settings [31].
Frailty assessment in preclinical models
With a view to understanding the biological mechanisms underlying the onset of frailty, the reverse translation of PP and FI from humans to murine models has recently been emphasized [32]. In this regard, Liu and colleagues [33] translated the PP method to mice by emulating the measurement of four of the five original criteria. More recently, the original murine PP was modified to include body weight and an enhanced endurance analysis with a more established test [34, 35]. As in humans, it allows the categorization of mice into robust (none of the criteria), pre-frail (one or two criteria), and frail (three or more criteria). However, this dichotomous classification suffers from high sample size requirements and misclassification problems. Based on the monthly longitudinal non-invasive assessment of frailty in a large cohort of mice, we recently published an alternative scoring method, which we called the physical function score (PFS), proposed as a continuous variable that resumes into a unique function, one of the five criteria included in the PP [36].
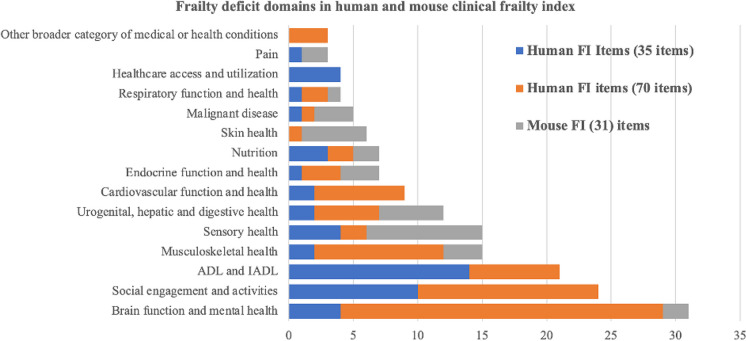
Similarly, Whitehead et al. [37] developed a non-invasive FI for mice based on a checklist of 31 easily observed deficits related to biological systems (integument, musculoskeletal system, auditory system, urogenital system, ocular/nasal system, respiratory system) and signs of discomfort. While the age-related pattern of the mouse FI closely resembles that observed in humans [38], its individual items give high emphasis to skin and sensory health but show inadequate representation of many other items of relevance in the human frailty index (Table 1). This is mainly due to the need to find the best compromise between wide-ranging coverage of frailty items and operational costs in mice [38]. The respective weight (based on the number of items) given to the clinical frailty domains in the human and mouse FI is reported in Fig. 1, considering two highly cited human frailty indexes based on 70 [29] and 35 items [30] as representative examples. Importantly, there are substantial differences in the weight given to different domains in the human and mouse FI, with a partial or complete lack of correspondence for most of them. For instance, while ADL and IADL, social engagement, and cardiovascular deficits are not included in the mouse FI items, they are important components of the human FI.
Table 1.
Items assigned to different frailty domains in the human and mouse clinical frailty index
| Frailty deficit domains | Human FI items (35 items) [30] | Human FI items (71 items) [29] | Mouse FI (31) items [37] | |||
|---|---|---|---|---|---|---|
| Items | n | Items | n | Items | n | |
| Cardiovascular function and health |
- Heart disease - Vascular disease |
2 |
- Cardiac problems - Myocardial infarction - Arrhythmia - Congestive heart failure - Cerebrovascular problems - Arterial hypertension - Peripheral pulses - Syncope or blackouts |
7 | - Not included | 0 |
| Endocrine function and health |
- Endocrine or metabolic disorder - Obesity |
1 |
- History of diabetes mellitus - History of thyroid disease - Thyroid problems |
3 |
- Body condition score - Weight - Temperature |
3 |
| Respiratory function and health | - Chronic lung disease | 1 |
- Lung problems - Respiratory problems |
2 | - Breathing problems | 1 |
| Brain function and mental health |
- Neurological disease - CDR -MMSE - Psychiatric disorder |
4 |
- Bradykinesia facial - Bradykinesia of the limbs - History of Parkinson’s disease - Seizures, partial complex - Seizures, generalized - Tremor at rest - Postural tremor - Intention tremor - Changes in general mental functioning - Clouding or delirium - Memory changes - Short-term memory impairment - Long-term memory impairment - Onset of cognitive symptoms - Paranoid features - History relevant to cognitive impairment or loss - Family history relevant to cognitive impairment or loss - Mood problems - Feeling sad, blue, and depressed - History of depressed mood - Depression - Sleep changes - Restlessness - Presence of snout reflex - Presence of palmomental reflex |
25 |
- Vestibular disturbance - Tremor |
2 |
| Urogenital, hepatic and digestive health |
- Renal, urological, or genital disorder - Gastrointestinal or liver disease |
2 |
- Urinary incontinence - Toileting problems - Rectal problems - Abdominal problems - Gastrointestinal problems |
5 |
- Diarrhea - Malocclusion - Rectal prolapse - Distended abdomen - Vaginal/penile prolapse |
5 |
| Musculoskeletal health |
- Osteoarticular disease - SPPB |
2 |
- Musculoskeletal problems - Falls - Poor limb coordination - Poor standing posture - Poor coordination, trunk - Irregular gait pattern - Impaired mobility - Bradykinesia of the limbs - Poor muscle tone in limbs - Poor muscle tone in the neck |
10 |
- Gait disorders - Kyphosis - Forelimb grip strength |
3 |
| Sensory health |
- Otorhinolaryngology or ophthalmology disorder - Visual impairment (near) - Visual impairment (distant) - HHIE (hearing impairment) |
4 |
- Impaired vibration - Head and neck problems |
2 |
- Hearing loss - Vestibular disturbance - Nasal discharge - Cataracts - Corneal opacity - Eye discharge/swelling - Microphthalmia - Vision loss - Menace reflex |
9 |
| Skin health | - Not included | 0 | - Skin problems | 1 |
- Alopecia - Loss of fur color - Dermatitis - Loss of whiskers - Coat condition - Tail stiffening |
5 |
| Malignant disease | - Cancer, malignant blood disease, or HIV | 1 | - Malignant disease | 1 |
- Tumor - Distended abdomen - Anal prolapse |
3 |
| ADL and IADL |
- Help bathing - Help dressing - Help using the toilet - Incontinence - Help eating - Help transferring from bed to chair - Help using telephone - Help shopping - Help with food preparation - Help with housekeeping - Help with laundry - Help with traveling - Help with medication use - Help with finances |
14 |
- Problems with bathing - Problems getting dressed - Impaired mobility - Falls - Toileting problems - Urinary incontinence - Sucking problems |
7 | - Not included | 0 |
| Healthcare access and utilization |
- Regular visit at home from a healthcare professional - Help with medication use - Help with traveling - Help with finances |
4 | - Not included | 0 | - Not included | 0 |
| Social engagement and activities |
- Psychiatric disorder - Help with bathing - Help with dressing - Help with using the toilet - Incontinence - Help with eating - Help with transferring from bed to chair - Help using the telephone - Help with shopping - Help with traveling |
10 |
- Changes in everyday activities - Problems going out alone - Mood problems - Feeling sad, blue, and depressed - Depression - Restlessness - History of depressed mood - Tiredness all the time - Problems getting dressed - Problems carrying out personal grooming - Bulk difficulties - Impaired mobility - Falls - Tiredness all the time |
14 | - Not included | 0 |
| Nutrition |
- MNA - Vitamin D - Obesity |
3 |
- Problems cooking - Sucking problems |
2 |
- Weight - Body condition score |
2 |
| Pain | - Headache | 1 | - Not included | 0 |
- Mouse grimace scale - Piloerection |
2 |
| Other broader category of medical or health conditions | - Not included | 0 |
- Other medical history - Family history of degenerative disease - Breast problems |
3 | - Not included | 0 |
Fig. 1.
Number of items attributed to different frailty domains in the human (70 and 35 items) and mouse (31 items) frailty index
Even if both PP and FI predicted adverse outcomes and death [36, 39, 40], when the two assessment tools were compared in the same population, they did not necessarily identify the same subject as frail [36, 41–43]. This discrepancy is related to the different rationale and design of the two instruments, which should be regarded as complementary rather than contradictory. In fact, in a recent study conducted in a large cohort of geriatric C57BL/6 J mice, we demonstrated that the use of a combined assessment tool, the so-called vitality score (VS), derived from the arithmetic mean of PFS and FI, displays a higher association with mortality compared to using individual assessment tools alone [36].
Cellular senescence in aging and frailty
Cellular senescence involves a complex series of processes that lead to a state of permanent cell cycle arrest, during which cells undergo distinct phenotypic changes. These alterations include profound changes in chromatin structure and cell morphology, an increase in lysosomal activity and levels of tumor suppressors, as well as significant modifications in the secretome [44]. The secretome produced by senescent cells, or senescence-associated secretory phenotype (SASP) [45], includes cytokines, chemokines, proteases, and growth factors, which functionally link cell senescence to physiological and pathological processes.
Albeit two major canonical pathways are known to mediate the cell cycle arrest during senescence, the p16/Rb and the p53/p21 pathways, senescent cells and their SASP are extremely heterogenous; they depend on the cellular origin (e.g., fibroblasts and endothelial cells), organism (mouse, humans, others), and temporal steps (early, full, and late senescence) [44, 46] as well as by the cause of the senescent state [47]. Due to this heterogeneity, there is no universal biomarker of cellular senescence. Its characterization is commonly performed through the combination of multiple biomarkers, such as the expression of the tumor suppressor genes p16 and p21, the presence of DNA damage response, and the activity of the senescence-associated beta-galactosidase (SA-β-gal) [48, 49].
Senescence has a natural physiological role as a protective response to damage. It represents one of the most important mechanisms for suppressing cancer [50] and repairing tissues [51] and may even be valuable for limiting viral infection [52]. Conversely, excessive accumulation of senescent cells has been demonstrated to play a deleterious role in aging and many age-related pathologies [44]. The senescent cells need to persist for a transient lapse of time necessary to drive the correct response by the tissues surrounding the origin of the damage and the immune system. Various cells of the immune system, mainly macrophages, NK cells, and helper T cells, are attracted by the SASP and orchestrate the removal of senescent cells [53].
The mechanisms involved in the accumulation of senescent cells in aging are still not completely elucidated; however, it is likely that the increased rate of senescence-inducing damage with the functional decline of the immune system [54] as well as the resistance to apoptosis displayed by some senescent cells [55] play a role in this process. Considering that senescence can spread to neighboring cells through a process known as the “bystander effect” [53, 56, 57], it is likely that once the mechanisms that keep senescence under control are bypassed, the rate of accumulation of senescent cells will become exponential. Soluble bioactive molecules of the SASP as well as miRNAs and other molecules secreted in extracellular vesicles are likely to mediate this process [58–60].
Although cellular senescence is not a primary hallmark of aging [61] [15], senescent cells can represent a source of damage that is able to rapidly propagate resembling a contagious process [62]. Indeed, senescence cells, at least partly through the SASP, can induce secondary senescence even in distant non-senescent cells [63–65]. This consideration may form a strong rationale for supporting cellular senescence among the critical underlining factors behind the increased vulnerability of frail individuals.
Since frailty is measured using various assessment tools, each assigning different weights to items or criteria across different health domains, the impact of cellular senescence will differ depending on the specific assessment tool and the criteria or items primarily affected by the accumulation of senescent cells.
To address this challenge, we undertook a thorough review of all available studies involving gain- or loss-of-function experiments as well as interventions targeting senescent cells, focusing our attention on those studies that examined outcomes based on the individual PP criteria or specific items used to calculate the FI.
Cellular senescence and its causal inference on frailty
Models and intervention to establish causal inference of cellular senescence
A handful of mouse models and interventions are currently available to investigate the causal mechanisms related to cellular senescence in different diseases.
The most common and appropriate models express a reporter gene and a conditional pro-apoptotic factor under key senescent-associated gene promoters, such as the p16-3MR or the INK-ATTAC mice [51, 66], which enable the elimination of senescent cells by administration of ganciclovir (GCV) or AP20187 (AP), respectively. Senescence can also be enhanced through transplantation [63] or modulated through the “classic” depletion or overexpression of genes (i.e., p16 and p21) which induces the core properties of cellular senescence.
Furthermore, a new class of drugs, named senotherapeutics, is emerging as a new possible approach to attenuate the accumulation of senescent cells. Two major senotherapeutic categories can be identified [67]: one that selectively eliminates senescent cells (senolytics) and the other that suppresses markers of senescence or components of the SASP (SASP suppressors) [68, 69]. Although both SASP suppressors and senolytics exhibit off-target effects, senolytics are largely used as complementary tools to provide evidence of causation between the accumulation of senescent cells and age-related pathologies.
Senolytics include a multitude of compounds of different natures, including dasatinib plus quercetin cocktail (D + Q), navitoclax, ABT-737, fisetin, proxofim, geldanamycin, tanespimycin, Foxo4-DRI, panobinostat, azithromycin, roxithromycin, and UBX0101, as well as SA-β-gal-activated prodrugs and nanoparticles.
Hence, both the results obtained in suitable animal models (Tables 2 and 3) and interventions with senolytics (Tables 4 and 5) may contribute to understanding the influence of (specific) senescent cells on the different PP criteria and domains of clinical frailty.
Table 2.
Evidence of a causal relationship between cellular senescence and individual criteria established for PP in mouse models
| Frailty criteria | Model | Method | Results | Role of cellular senescence | Reference |
|---|---|---|---|---|---|
| Shrinkness | INK-ATTAC BubR1 progeroid mouse | AP20187 treatment | Improved body weight, corrected kyphosis, and reduced fat loss | Detrimental | [66] |
| Mouse model of p21 overexpression (p21OE) | p21 induced senescence | Mice display reduced body weight and muscle atrophy | Detrimental | [70] | |
| Young and old (17 months) C57BL/6 mice | Senescent cells transplantation | No effect on body weight in transplanted young mice | No evidence | [63] | |
| Aged (27 months) INK-ATTAC mice | AP20187 treatment | No effect on body weight | No evidence | [71] | |
| Weakness | Young and old (17 months) C57BL/6 mice | Senescent cells transplantation | Decreased grip strength in a dose-dependent manner | Detrimental | [63]) |
| Young (3–6 months) and old (> 28 months) p16-3MR mice | GCV or SASP suppressor (CD36 antibody) treatment | Enhanced muscle force generation | Detrimental | [76] | |
| Mouse model of p21 overexpression (p21OE) | p21 induced senescence | Mice exhibit reduced grip strength | Detrimental | [70] | |
| Aged (20 months) p16-3MR mice | GCV treatment | Moderate beneficial effects on skeletal muscle function only in male mice | Detrimental | [75] | |
| Exhaustion | INK-ATTAC BubR1 progeroid mouse | AP20187 treatment | Treadmill distance travelled and work significantly increased | Detrimental | [66] |
| Young and old (17 months) C57BL/6 mice | Senescent cell transplantation | Hanging endurance decreased but no effect on treadmill performance | Detrimental | [63] | |
| Mouse model of p21 overexpression (p21OE) | p21 induced senescence | Significant reduction in treadmill performance | Detrimental | [70] | |
| Inactivity | INK-ATTAC aged (18 months) C57BL/6 mice and mixed background | AP20187 treatment | Prevented age-dependent reductions in both spontaneous activity and exploratory behavior | Detrimental | [84] |
| Young and old (17 months) C57BL/6 mice | Senescent cells transplantation | No effect on daily activity | No evidence | [63] | |
| Doxo-treated p16-3MR mice | GCV treatment | Alleviates impairment in running activity | Detrimental | [85] | |
| Diet obesity-induced p16‐3MR mice | GCV treatment | Did not affect activity | No evidence | [87] | |
| Aged (27 months) INK-ATTAC mice | AP20187 treatment | No effect on locomotor activity | No evidence | [71] | |
| Slowness | Young and old (17 months) C57BL/6 mice | Senescent cells transplantation | Impairs walking speed | Detrimental | [63] |
| Aged (27 months) INK-ATTAC mice | AP20187 treatment | No effect on the max speed at rotarod test | No evidence | [71] |
Table 3.
Evidence from mouse models of a causal relationship between cellular senescence and different frailty deficit domains*
| Frailty deficit domains | Model | Method | Results | Role of cellular senescence | Reference |
|---|---|---|---|---|---|
| Cardiovascular health | INK-ATTAC BubR1 progeroid mouse | AP20187 treatment | Reduced cardiomyocyte cross-sectional area without affecting heart rate, ventricular mass, and many other functional parameters | Detrimental | [66] |
| INK-ATTAC aged (18 months) C57BL/6 mice and mixed background | AP20187 treatment | Improved the adaptive cardiac response to stress | Detrimental | [84] | |
| INK-ATTAC aged (28 months) C57BL/6 mice | AP20187 treatment | Reduces fibrosis and activates resident cardiac progenitors | Detrimental | [92] | |
| INK-ATTAC aged (27 months) C57BL/6 mice | AP20187 treatment | Alleviated myocardial hypertrophy and fibrosis | Detrimental | [93] | |
| p16-CreERT2/R26-DTA mice (aged 18 months) | TAM treatment | Induces perivascular heart fibrosis, worsens blood vessel permeability, and increases plasma oxidized LDL | Beneficial | [99] | |
| LDL receptor-deficient p16-3MR mice | GCV treatment | Regression of atherosclerotic lesions | Detrimental | [95] | |
| Irradiated LDL receptor-deficient p16-3MR mice | GCV treatment | Reduced plaque necrosis and increased production of key intraplaque-resolving mediators | Detrimental | [96] | |
| Various vascular injury models in p16 overexpressing mice | p16 overexpression | Augmented venous thrombosis | Detrimental | [94] | |
| Myocardial infarction induced in male ICR mice | p16 overexpression and KO by adenovirus delivery | Overexpression of p16 protected, while knockdown of p16 worsened cardiac function | Beneficial | [91] | |
| p16-3MR ApoE − / − mice | GCV treatment | No effect on aortic root plaque size and induces inflammation | Beneficial | [97] | |
| Endocrine and metabolic health | INK-ATTAC BubR1 progeroid mouse | AP20187 treatment | Prevented loss of adipose tissue | Detrimental | [66] |
| INK-ATTAC aged (18 months) C57BL/6 mice | AP20187 treatment | Prevented age-related lipodystrophy | Detrimental | [84] | |
| Diet-induced obesity in p16‐3MR mice | GCV treatment | Alleviates metabolic and adipose tissue dysfunction | Detrimental | [87] | |
| Diet obesity-induced INK-ATTAC mice | AP20187 treatment | Alleviates metabolic and adipose tissue dysfunction | Detrimental | [87] | |
| Insulin resistance induced (pharmacologically or by high-fat diet) in INK-ATTAC mice | AP20187 treatment | Improved glucose metabolism and β-cell function | Detrimental | [104] | |
| High-fat diet in INK-ATTAC mice (12 months) | AP20187 treatment | Improved β-cell function and increased proliferative capacity in a subset of animals | Detrimental | [105] | |
| Naturally aged mice overexpressing p16 (Super-INK4a/ARF mice | p16 overexpression | Enhanced glucose tolerance and insulin responsiveness | Beneficial | [102] | |
| Beta cell-specific activation of p16INK4a in a transgenic mouse model of diabetes (Pdx1-tTA) | p16 overexpression | Enhances glucose-stimulated insulin secretion and improved glucose homeostasis | Beneficial | [103] | |
| Respiratory health | Induced emphysema in ARF‐DTR mice | DT treatment | Prevents lung dysfunction and emphysema-associated pathologies | Detrimental | [114] |
| Bleomycin-induced pulmonary fibrosis in INK-ATTAC mice | AP20187 treatment | Improves pulmonary function without effect on the fibrosis | Detrimental | [109] | |
| Pulmonary hypertension model derived from INK-ATTAC mice | AP20187 treatment | Worsen Pulmonary hemodynamic | Beneficial | [115] | |
| Cigarette smoke-induced lung senescence in p16-3MR mice | GCV treatment | Restore cellular homeostasis and reversed airspace enlargement | Detrimental | [112] | |
| Cigarette smoke-exposed p16 KO mice | p16 KO | Improved lung function | Detrimental | [113] | |
| Brain function and mental health | Aged (27 months) INK-ATTAC mice | AP20187 treatment | Improved cognitive function | Detrimental | [71] |
| Mouse model (PS19;ATTAC) of tau-mediated neurodegeneration | AP20187 treatment | Prevents hyperphosphorylation of tau and cognitive decline | Detrimental | [117] | |
| Paclitaxel-induced cerebrovascular senescence as cognitive impairment model in p16-3MR mice | GCV treatment | Improved cognitive performance | Detrimental | [120] | |
| Brain-irradiated p16-3MR mice | GCV treatment | Improved cognitive performance | Detrimental | [118] | |
| Diet-induced obesity in INK-ATTAC mice | AP20187 treatment | Alleviates neuropsychiatric disorders in obese mice | Detrimental | [119] | |
| Aged (19 months) p16 KO mice | p16 KO | Induced decline in subventricular zone proliferation, neurogenesis, and self-renewal | Detrimental | [121] | |
| Urogenital, hepatic, and digestive health | Chronic renal ischemia induced in INK-ATTAC and C57BL/6 mice | AP20187 treatment | Improved renal function and structure | Detrimental | [127] |
| Accelerated aging (XpdTTD/TTD mice) and naturally aged (27 months) p16-3MR mice | GCV treatment | Restored renal function | Detrimental | [128] | |
| INK-ATTAC aged [18 months] C57BL/6 mice and mixed background | AP20187 treatment | Prevented age-dependent impairment of kidney function | Detrimental | [84] | |
| p16-CreERT2/R26-DTA mice | TAM treatment | Induces perivascular kidney and liver fibrosis | Beneficial | [99] | |
| Aged (24 months) INK-ATTAC mice | AP20187 treatment | Reduces hepatic steatosis | Detrimental | [86] | |
| Partial hepatectomy in young p16-3MR mice | GCV treatment | Impairs liver regeneration | Beneficial | [126] | |
| Carbon tetrachloride (CCl4) induced liver fibrosis in p16 KO mice | p16 KO | Promote fibrogenesis | Detrimental | [129] | |
| Musculoskeletal health | Aged (20 months) p16-3MR male mice | GCV treatment | Mitigates intervertebral disc degeneration | Detrimental | [137] |
| Adult (12 months) INK-ATTAC mice | AP20187 treatment until natural death | Reduced age-related cartilage degeneration | Detrimental | [138] | |
| Osteoarthritis model by anterior cruciate ligament transection in p16-3MR mice | GCV treatment | Attenuates the development of post-traumatic osteoarthritis | Detrimental | [138] | |
| INK-ATTAC BubR1 progeroid mouse | AP20187 treatment | Correction of kyphosis | Detrimental | [66] | |
| Aged (21 months) INK-ATTAC mice | AP20187 treatment | Improved bone mass and strength as well as bone microarchitecture | Detrimental | [139] | |
| Aged (20 months) DMP1-Cre ± p16-LOX-ATTAC mice (model for specific elimination of senescent osteocytes) | AP20187 treatment | Prevented age-related bone loss at the spine, but not the femur, without affecting osteoclasts or marrow adipocytes | Detrimental | [65] | |
| Aged (20 months) β-actin–Cre ± p16-LOX-ATTAC | AP20187 treatment | Prevented bone loss at the spine and femur, bone formation, and reduced osteoclast and marrow adipocyte numbers | Detrimental | [65] | |
| C57BL/6 young mice | Murine senescent fibroblast transplantation | Caused bone loss | Detrimental | (65) | |
| Radiation-induced osteoporosis in p21-ATTAC and INK-ATTAC | AP20187 treatment | Prevented radiation-induced osteoporosis and increased marrow adiposity only in p21-ATTAC | Detrimental | [140] | |
| Aged (23 months) p16-3MR mice | GCV treatment | No effect on the age-related loss of bone mass | No evidence | [193] | |
| Estrogen deficiency-induced osteoporosis in p16 KO mice | p16 KO | Promote bone formation and prevent osteoporosis | Detrimental | [141] | |
| Model of fracture healing in p16 KO mice | p16 KO | Accelerate fracture healing | Detrimental | [142] | |
| Mouse tail suspension (TS)-induced intervertebral disc degeneration in p16 KO mice | p16 KO | Attenuates intervertebral disc degeneration | Detrimental | [143] | |
| Various murine models (see “Weakness,” “Exhaustion,” and “Slowness” criteria in Table 2) | Various treatments | Senescent cells negatively affect strength, endurance, and walking speed | Mostly (8 studies) detrimental | See Table 2 for ref | |
| Sensory health | INK-ATTAC BubR1 progeroid mouse (3 weeks of age) | Preventive AP20187 treatment | Delayed onset of cataracts | Detrimental | (66) |
| INK-ATTAC BubR1 progeroid mouse (aged 5 months) | AP20187 treatment | No effect on cataracts | No evidence | [66] | |
| INK-ATTAC aged (18 months) C57BL/6 mice | AP20187 treatment | Eye alterations occurred slowly | Detrimental | [84] | |
| INK-ATTAC aged (18 months) mixed background mice | AP20187 treatment | No effect on cataracts | No evidence | [84] | |
| Elevated intraocular pressure (glaucoma model) in adult p16-3MR or C57BL/6 mice | GCV treatment | Showed vision rescue and neuroprotective effect on retinal ganglion cells | Detrimental | [146] | |
| Skin health | Accelerated aging (XpdTTD/TTD mice) and naturally aged (27 months) p16-3MR mice | GCV treatment | Improved fur density | Detrimental | [128] |
| Wound healing model in p16-3MR mice | GCV treatment | Delayed wound healing | Beneficial | [51] | |
| Malignant diseases | C57BL/6 mice (6–8 weeks old) | Senescent inducible lymphoma cell transplantation | Promote tumor growth | Detrimental | [154] |
| Nude (nu/nu) mice (5 weeks old) | Human senescent fibroblast transplantation | Stimulate premalignant and malignant proliferation and promote tumors | Detrimental | [153] | |
| Athymic nude mice | Subcutaneous transplant of melanoma cells (previously exposed to senescent secretome) | Promotes metastatic properties in vivo | Detrimental | [155] | |
| p16-3MR mice transplanted with breast cancer cells and treated with doxorubicin | GCV treatment | Reduced cancer relapse after chemotherapy | Detrimental | [85] | |
| T cell acute lymphoblastic leukemia injected in INK-ATTAC mice pre-treated with doxorubicin | Pre-treatment with AP20187 | Accelerates the development of leukemia | Beneficial | [163] | |
| Squamous cell skin carcinoma induced in p16-3MR mice treated with doxorubicin | Pre-treatment with GCV | Prevented malignant tumor development | Detrimental | [156] | |
| Naturally aged mice overexpressing p16 (Super-INK4a/ARF mice | p16 overexpression | Manifest higher resistance to and have normal aging and lifespan | Beneficial | [151] | |
| Mouse model of p16 chronic overexpression in basal keratinocytes of the epidermis | p16 overexpression | Induces hyperplasia and dysplasia | Detrimental | [152] | |
| ADL and IADL | - | - | - | - | - |
| Healthcare access and utilization | - | - | - | - | - |
| Social engagement and activities | Various murine models (see “Inactivity” criteria in Table 2) | Various treatments | Senescent cells negatively affect daily activity | Detrimental (2 studies out of 4) | See Table 2 for ref |
| Nutrition | Various murine models (See “Shrinkness” criteria in Table 2) | Various treatments | Senescent cells negatively affect age-related shrinkness | Detrimental (2 studies out of 3) | See Table 2 for ref |
| Diet obesity-induced INK-ATTAC mice | AP20187 treatment | No effect on body weight and composition | No evidence | [119] | |
| Diet obesity-induced p16‐3MR mice | GCV treatment | Did not affect weight or food intake | No evidence | [87] | |
| Pain | Cisplatin-induced neuropathy in p16-3MR mice | GCV treatment | Improved mechanical and thermal sensitivity | Detrimental | [167] |
| Other broader category of medical or health conditions | - | - | - | - | - |
*Includes only experimental studies addressed to a causal relationship with senescent cells “in vivo”
Table 4.
Evidence of a causal-relationship between cellular senescence and individual criteria established for PP from intervention studies with senolytics
| Frailty criteria | Model | Senolytic | Results | Role of cellular senescence | Reference |
|---|---|---|---|---|---|
| Shrinkness | Aged (17 months) and young/adult C57BL/6 J mice | D + Q | No effect on body mass | No evidence | [73] |
| SAMP10 mice (9 month) | D + Q | No effect on body weight | No evidence | [74] | |
| Irradiated C57BL/6 mice | D + Q or navitoclax (starting early or late after irradiation) | Improved body condition and body weight loss over 1 year of follow-up | Detrimental | [72] | |
| Aged (27 months) INK-ATTAC mice | D + Q | No effect on body weight | No evidence | [71] | |
| Old (20 months) C57BL/6 mice | D + Q | No effect on body weight | Detrimental | [63] | |
| Weakness | Aged (20 months) C57BL/6 J mice | D + Q | Improve grip strength in mice | Detrimental | [77] |
| C57BL/6 mice beginning at 6 months | D + Q | Improve strength in mice after 5/6 months of treatment | Detrimental | [78] | |
| Young (3–6 months) and old (> 28 months) p16-3MR mice | D + Q | Enhanced muscle force generation | Detrimental | [76] | |
| Aged (20 months) and young/adult C57BL/6 J mice | D + Q | Grip strength was not improved | No evidence | [73] | |
| Human patients with Idiopathic pulmonary fibrosis | D + Q | Grip strength was not improved | No evidence | [80] | |
| Aged (20-month-old) C57BL/6 mice | SA-β-gal activable gemcitabine (SSK1) | Enhanced grip strength | Detrimental | [79] | |
| SAMP10 mice (9 month) | D + Q | Improved grip strength | Detrimental | [74] | |
| Old (20 months) C57BL/6 mice | D + Q | Improved grip strength | Detrimental | [63] | |
| Exhaustion | Aged (17-month) and young/adult C57BL/6 J mice | D + Q | Average time spent in rotarod increased in old mice (but not in young mice) | Detrimental | [73] |
| Aged (21 months) and irradiated C57BL/6 J mice | Photosensitive senolysis activated by SA-β-gal | Improved hanging endurance | Detrimental | [82] | |
| Leg-irradiated C57BL/6 male mice | D + Q | Alleviates impairment in treadmill exercise endurance | Detrimental | [83] | |
| Aged (20-month-old) C57BL/6 mice | SA-β-gal activable gemcitabine (SSK1) | Enhanced treadmill distance and rotarod time | Detrimental | [79] | |
| Old (20 months) C57BL/6 mice | D + Q | Improved hanging endurance | Detrimental | [63] | |
| Inactivity | Irradiated C57BL/6 young mice | Cycloastragenol | Alleviates impairment in locomotor activity and behavioral responses | Detrimental | [88] |
| Old (20 months) C57BL/6 mice | D + Q | Improved daily activity | Detrimental | [63] | |
| Aged (20-month-old) C57BL/6 mice | SA-β-gal activable gemcitabine (SSK1) | Enhanced rearing frequency | Detrimental | [79] | |
| SAMP10 mice (9 month) | D + Q | Improved total distance on locomotor activity | Detrimental | [74] | |
| Aged (27 months) INK-ATTAC mice | D + Q | No effect on locomotor activity | No evidence | [71] | |
| Slowness | Old (20 months) C57BL/6 mice | D + Q | Improved walking speed | Detrimental | [63] |
| Human patients with idiopathic pulmonary fibrosis | D + Q | Improved 6-min walk distance and 4-m gait speed | Detrimental | [80] | |
| Aged (24-month-old) C57BL/6 mice | Navitoclax | No effect on swimming speed | No evidence | [89] | |
| SAMP10 mice (9 months) | D + Q | No significant effect on average speed | No evidence | [74] | |
| Aged (27 months) INK-ATTAC mice | D + Q | No effect on max speed at rotarod after normalization for baseline | No evidence | [71] |
Table 5.
Evidence of a causal relationship between cellular senescence and different frailty deficit domains from intervention studies with senolytics
| Frailty deficit domains | Model | Senolytic | Results | Role of cellular senescence | Reference |
|---|---|---|---|---|---|
| Cardiovascular health | Aged (28 months) C57BL/6 mice | D + Q | Reduces fibrosis, activates resident cardiac progenitors, and increases proliferating cardiomyocytes | Detrimental | [92] |
| Aged (24-month-old) C57BL/6 mice | D + Q | Improved ventricular ejection fraction, shortening, and end‐systolic cardiac dimensions without effect on contractile response and cardiac mass | Detrimental | [83] | |
| Aged (23 months) C57BL/6 mice | Navitoclax | Reduced telomere‐associated foci-positive cardiomyocytes and alleviated myocardial hypertrophy and fibrosis | Detrimental | [93] | |
| Myocardial infarction in young C57BL/6 mouse | Navitoclax pre-infarction | Attenuates profibrotic protein expression and improves myocardial remodeling, diastolic function, and survival | Detrimental | [98] | |
| Aged (24 months) C57BL/6 mice | D + Q | Reduced aortic calcification and improved vasomotor function | Detrimental | [100] | |
| Aged (20 months) C57BL/6N mice | D + Q | Reduces detrimental effects of extracellular vesicles from old mice on endothelial cells | Detrimental | [101] | |
| p16-3MR ApoE − / − mice | Navitoclax | Reduced atherosclerosis lesion by senolysis-independent activity | No evidence | [97] | |
| Endocrine and metabolic health | Diet-induced obesity in C57BL/6 mice | D + Q | Improves glucose homeostasis and insulin sensitivity | Detrimental | [87] |
| Aged (22 months) C57BL/6 mice | FOXO4-DRI | Alleviated age-related testosterone secretion insufficiency | Detrimental | [108] | |
| Insulin resistance induced (pharmacologically or by high-fat diet) in INK-ATTAC mice | Navitoclax | Improved glucose metabolism and β-cell function | Detrimental | [104] | |
| Aged (21 months) male C57BL/6 mice | D + Q | Improved fasting blood glucose and glucose tolerance | Detrimental | [106] | |
| Retrospective study on dasatinib prescription in type 2 diabetes | D | Lowers serum glucose comparable to first-line diabetic medications | Detrimental | [107] | |
| Respiratory health | Bleomycin-induced pulmonary fibrosis in INK-ATTAC mice | D + Q | Improves pulmonary function without affecting lung fibrosis | Detrimental | [109] |
| Irradiated C57BL/6 mice | D + Q or navitoclax | Improved breathing rate | Detrimental | [72] | |
| Human patients with Idiopathic pulmonary fibrosis | D + Q | No effect on pulmonary function | No evidence | [80] | |
| Pulmonary hypertension model derived from INK-ATTAC mice | Navitoclax or FOXO4-DRI | Worsen pulmonary hemodynamic | Beneficial | [115] | |
| Bleomycin‐induced pulmonary fibrosis in C57BL/6 male mice | Galacto-encapsulated doxorubicin | Improve pulmonary function | Detrimental | [110] | |
| Lung fibrosis induced by intratracheal administration of senescent human cells (IMR90) in mice | Digoxin | Reduced fibrosis | Detrimental | [111] | |
| Aged (21 months) C57BL/6:FVB | Fisetin | No effect on composite lesion score in the lung | No evidence | [116] | |
| Brain function and mental health | Aged (27 months) INK-ATTAC mice | D + Q | Improved cognitive function | Detrimental | [71] |
| Mouse model (PS19;ATTAC) of tau-mediated neurodegeneration with inducible clearance of p16 + cells | Early with navitoclax | Prevents hyperphosphorylation of tau and cognitive decline | Detrimental | [117] | |
| Mouse model of Alzheimer (20 months old, tauNFT‐Mapt0/0) | D + Q | Reduction in total neurofibrillary tangles density, neuron loss, and ventricular enlargement | Detrimental | [124] | |
| SAMP10 mouse model of brain aging | D + Q | Improved working memory and exploratory behavior | Detrimental | [74] | |
| The motor neuron disease mouse model hSOD1-G93A | Navitoclax | Did not alter the disease progression | No evidence | [123] | |
| Young (3 months old) and aged (22 months old) rats | D + Q | Improvements in cognitive abilities observed in aged rats | Detrimental | [122] | |
| Brain-irradiated p16-3MR mice | Navitoclax | Improved cognitive performance | Detrimental | [118] | |
| Diet obesity-induced INK-ATTAC mice | D + Q | Alleviates neuropsychiatric dysfunction in obese mice | Detrimental | [119] | |
| Aged (21 months) C57BL/6:FVB | Fisetin | Reduced composite lesion score in the brain | Detrimental | [116] | |
| Pilot study in early-stage symptomatic Alzheimer’s disease patients | D + Q | No changes in cognitive and neuroimaging endpoints as well as in depression and neuropsychiatric outcomes | No evidence | [125] | |
| Urogenital, hepatic, and digestive health | Chronic renal ischemia induced in INK-ATTAC and C57BL/6 mice | D + Q | Improved renal function and structure | Detrimental | [127] |
| Diet obesity-induced C57BL/6 mice | D + Q | Reduces proteinuria | Detrimental | [87] | |
| Accelerated aging (XpdTTD/TTD mice) and naturally aged (27 months) p16-3MR mice | FOXO4-DRI | Restored renal function | Detrimental | [128] | |
| Renal ischemia/reperfusion injury and multiple-cisplatin-treatment in male C57BL/6 mice | D + Q | Ameliorate renal fibrosis | Detrimental | [130] | |
| Patients with diabetic kidney disease | D + Q | Preliminary evidence of decreased senescent cells in adipose tissue, tissue macrophage infiltration, crown-like structures, and circulating SASP factors | Detrimental | [131] | |
| Aged (21 months) and irradiated C57BL/6 J mice | Photosensitive senolysis activated by SA-β-gal | Attenuated age-related loss of renal function | Detrimental | [82] | |
| Aged (18 months) Prf1 − / − mice (model of accelerated aging) | ABT-737 | Reduce liver and kidney fibrosis | Detrimental | [54] | |
| Partial hepatectomy model in adult C57BL/6 J | ABT-737 | Improves liver regeneration | Detrimental | [134] | |
| Aged (24 months) INK-ATTAC mice | D + Q | Reduces hepatic steatosis | Detrimental | [86] | |
| CCL4- or diet-induced liver fibrosis | Senolytic CAR T cells | Restore tissue homeostasis | Detrimental | [135] | |
| Cu/Zn-superoxide dismutase null mice (Sod1KO) aged 6 months | D + Q | No effect on various markers of liver fibrosis | No evidence | [132] | |
| Partial hepatectomy in young p16-3MR mice | Navitoclax | Impairs liver regeneration | Beneficial | [126] | |
| Mouse models of nonalcoholic fatty liver disease (chemical and high-fat diet-induced) | D + Q | Worsened liver disease progression | Beneficial | [133] | |
| Aged (21 months) and irradiated C57BL/6 J mice | Photosensitive senolysis activated by SA-β-gal | Attenuated age-related loss of liver function | Detrimental | [82] | |
| Aged (21 months) C57BL/6:FVB | Fisetin | Reduced composite lesion score in the kidney but not in the liver | Detrimental | [116] | |
| Aged (18 months) female BALB/c mice | D + Q | Reduces intestinal inflammation related to changes in specific microbiota signatures | Detrimental | [136] | |
| Musculoskeletal health | Osteoarthritis model by anterior cruciate ligament transection in p16-3MR mice | intra-articular with UBX0101 | Attenuates the development of post-traumatic osteoarthritis | Detrimental | [138] |
| Aged (20 months) C57BL/6N mice | D + Q | Improved bone mass and microarchitecture | Detrimental | [139] | |
| Randomized, double-blind, placebo-controlled, study (Phase 2) in knee osteoarthritis patients | intra-articular with UBX0101 | No effect on knee pain and function | No evidence | [145] | |
| Zmpste24 − / − progeria mouse model | Fisetin | Reduced bone density loss | Detrimental | [144] | |
| Zmpste24 − / − progeria mouse model | D + Q | Did not mitigate trabecular bone loss | No evidence | [144] | |
| Human patients with Idiopathic pulmonary fibrosis | D + Q | Improved SPPB score | Detrimental | [80] | |
| Human patients with Idiopathic pulmonary fibrosis | D + Q | Grip strength was not improved | No evidence | [80] | |
| Various murine models (see “Weakness,” “Exhaustion,” and “Slowness” criteria in Table 4) | Various senolytics | Senescent cells negatively affect strength, endurance, and walking speed | Detrimental (13 studies out of 18) | See Table 4 for ref | |
| Sensory health | SAMP10 mice (9 months) | D + Q | Significant reduction of frailty index with particular improvements in eye discharge, vision loss, and microphthalmia | Detrimental | [74] |
| Retrospective cohort study examining medical record data for adult patients with glaucoma | History of senolytic drug exposure | No significant changes on progression of glaucomatous visual field | No effect | [147] | |
| Elevated intraocular pressure (glaucoma model) in adult p16-3MR or C57BL/6 mice | Dasatinib early | Showed vision rescue and neuroprotective effect on retinal ganglion cells | Detrimental | [146] | |
| Skin health | Accelerated aging (XpdTTD/TTD mice) and naturally aged (27 months) p16-3MR mice | FOXO4-DRI | Improved fur density | Detrimental | [128] |
| Female aged (18 months) albino hairless mice (Skh-1) | Navitoclax or ABT-737 intradermal | Ameliorate the aging skin phenotype (collagen deposition and epidermal proliferation) | Detrimental | [148] | |
| Aged (18 months) Prf1 − / − mice (model of accelerated aging) | ABT-737 | Reduce skin fibrosis | Detrimental | [54] | |
| Nude mice transplanted with skin grafts from aged humans | Glutaminase inhibitor (BPTES) | Increased collagen density and cell proliferation in the dermis | Detrimental | [149] | |
| Aged (20 months) male Bl6 mice | Anti-apolipoprotein D PDB-conjugate antibody | Improved the senescent skin phenotype | Detrimental | [150] | |
| Malignant diseases | C57BL/6 mice transplanted with senescence inducible lymphoma cells | Bafilomycin A1 | Extended the survival of mice bearing therapy-induced senescent tumors | Detrimental | [157] |
| Breast cancer cells xenograft induced to senescence by irradiation in ovariectomized female NCR NUNU mice | ABT-737 | Inhibited tumor growth | Detrimental | [158] | |
| Adenocarcinoma or triple-negative breast cancer cell xenograft induced to senescence by etoposide or doxorubicin in immunodeficient NSG mice | Navitoclax | Decreased tumor volume and prolonged tumor maintenance | Detrimental | [159] | |
| Ovarian and breast cancer cell xenograft induced to senescence by olaparib in immunodeficient NSG mice | Navitoclax | Decreased tumor size | Detrimental | [160] | |
| Adenocarcinoma cell xenograft induced to senescence by cisplatin in immunodeficient SCID mice | Navitoclax or galacto-conjugated navitoclax (Nav-Gal) | Inhibited tumor growth | Detrimental | [161] | |
| Xenografts of SK‐MEL‐103 melanoma cells induced to senescence by palbociclib in athymic female nude mice | Galacto-encapsulated doxorubicin | Inhibited tumor growth | Detrimental | [110] | |
| Obesity-induced hepatocellular carcinoma mouse model and | BET family protein degrader (ARV825) | Reduced liver cancer development | Detrimental | [162] | |
| Xenograft of colon cancer cells induced to senescence by doxorubicin in nude mice | BET family protein degrader (ARV825) | Reduced tumor size | Detrimental | [162] | |
| Lung cancer or breast cancer xenografts induced to senescence by gemcitabine or doxorubicin in immunodeficient nude NMRI nu/nu mice | Digoxin | Reduced tumor volume | Detrimental | [111] | |
| Mice harboring orthotopic lung adenocarcinomas induced to senescence by MEK and CDK4/6 inhibitors | Senolytic CAR T cells | Prolonged survival | Detrimental | [135] | |
| Cu/Zn-superoxide dismutase null mice (Sod1KO) aged 6 months | D + Q | Reduced the incidence of hepatocellular carcinoma | Detrimental | [132] | |
| Xenograft studies conducted with hepatic carcinoma cells induced or not to senescence with doxorubicin in athymic nude mice | D + Q | Ineffective in cleaning senescent cells and induced acute pro-tumorigenic effects in control mice | Beneficial | [164] | |
| T cell acute lymphoblastic leukemia injected in doxorubicin pre-treated C57BL/6 mice | Navitoclax | Accelerates the development of leukemia | Beneficial | [163] | |
| Mouse model of p16 chronic overexpression in basal keratinocytes of the epidermis | ABT-737 | Partial reversal of hyperplasia induced by p16 overexpression | Detrimental | [152] | |
| ADL and IADL | Pilot study in early-stage symptomatic Alzheimer’s disease patients | D + Q | No changes in informant-reported Lawton IADL | No evidence | [125] |
| Healthcare access and utilization | - | - | - | - | - |
| Social engagement and activities | Various murine models (see “Inactivity” criteria in Table 4) | Various senolytics | Senescent cells negatively affect daily activity | Detrimental (4 studies) | See Table 4 for ref |
| Nutrition | Various murine models (See “Shrinkness” criteria in Table 4) | Various senolytics | Senescent cells negatively affect age-related shrinking | Detrimental (2 study out of 4) | See Table 4 for ref |
| Dietary induced obesity in INK-ATTAC mice | D + Q | No effect on body weight and composition | No evidence | [119] | |
| Obese mouse model (ob/ob female mice) | D + Q | No effect on body weight and composition | No evidence | [166] | |
| Pain | Irradiated C57BL/6 mice | D + Q or navitoclax (starting early or late after irradiation) | Improved mouse grimace scale | Detrimental | [72] |
| SAMP10 mice (9 month) | D + Q | Improved mouse grimace scale | Detrimental | [74] | |
| Other broader category of medical or health conditions | Aged (19 months) mice infected with a sublethal dose of H1N1 and H3N2 influenza virus | D + Q | Did not improve overall influenza responses | No evidence | [194] |
| SARS-CoV-2-infected hamsters and mice | D + Q or navitoclax | Mitigated lung disease | Detrimental | [168] | |
| COVID-19 murine model K18-hACE2 | D + Q | Reduced mortality and other clinical symptoms | Detrimental | [169] | |
| Young and old (20 months) SARS-CoV-2-infected hamsters | Navitoclax | Ameliorated lung disease in aged animals | Detrimental | [170] |
Causal links between cellular senescence and physical frailty criteria
Available studies that investigated the causal relationship between cellular senescence and specific PP criteria using suitable murine models or senolytic interventions are reported in Tables 2 and 4, respectively.
Shrinkness
The first evidence that the clearance of p16INK4a-positive senescent cells antagonizes several processes associated with physical frailty was provided in a mouse model of accelerated aging (BubR1H/H) carrying the INK-ATTAC transgene [66]. Treatment with AP20187 in these mice improved body weight, corrected kyphosis, and reduced fat loss, thus demonstrating a detrimental effect of senescent cells on age-related shrinkage. These results are confirmed by the phenotype of the mouse model of p21 overexpression (p21OE mice), which displays an accelerated weight loss phenotype [70].
However, another study in normally aged INK-ATTAC mice [71] as well as the transplantation of senescent cells in young and old mice [63] did not report similar effects after a relatively short-term (1–2 months) follow-up.
Similarly, senolytic (D + Q or navitoclax) interventions showed a positive effect on body weight loss after one year of follow-up in irradiated C57BL/6 mice [72] but did not affect the body weight of normally [63, 71, 73] or accelerated aging mice [74] after a relatively shorter time of follow-up (approximately 4 months).
Hence, age-related shrinkage appears to be a long-lasting effect of accumulating senescent cells, which may be rarely observed in model or intervention studies with a relatively short follow-up.
Weakness
Removal of senescent cells in aged p16-3MR mice [75, 76], as well as senescent cell transplantation [63] and p21 overexpression [70] models, support a causal relationship between accumulating senescent cells and weakness. Interventions performed with D + Q in normally or accelerated aging mice, quite uniformly, reinforce this hypothesis [63, 74, 76–78]. A detrimental role of senescent cells on muscle weakness was also demonstrated using a completely different senolytic (SA-β-gal activable gemcitabine) [79]. A lack of significant effect of D + Q on grip strength was reported in one study in aged mice [73], as well as in a human pilot clinical trial [80]. Notably, both of these studies reported positive effects on other areas related to physical function performance. Remarkably, muscle weakness appears to be only partially related to the senescence of muscle satellite cells as these are extremely rare in mice aged below 27 months [81]. The presence of senescent cells in other tissues, such as adipose tissue, and circulating SASP factors are likely to be directly involved in this relationship.
Exhaustion
Only a few studies have investigated endurance performances in animals with manipulated levels of cellular senescence. Overexpression of p21, but not senescent cell transplantation [63], significantly reduces the endurance performance measured by the treadmill test [70]. However, the transplantation model affected hanging performances, another parameter related to exhaustion. As expected, the genetic removal of senescent cells from a model of accelerated aging improved treadmill distance and work [66].
In agreement, all senolytic treatments tested so far in aged and irradiated mice have been effective in ameliorating endurance performance [63, 73, 79, 82, 83].
It is likely that the burden of accumulating senescent cells may affect endurance performance through systemic inflammatory mediators secreted in the SASP.
Inactivity
Not only physical but also cognitive and psychological conditions contribute to physical inactivity. Senescent cells have the potential to affect all these components, and certain experimental models have indeed confirmed this relationship in aged [84] and chemotherapy-treated mice [85]. Consistent with the findings observed for the shrinkness criteria, experiments with a relatively lower follow-up (period 1–2 months) after modulating the senescent cell burden did not report significant improvement in the daily or locomotor activity of different mouse models [63, 86, 87]. Excluding one study with D + Q [86], an improvement in the activity has also been reported with at least three different senolytic interventions in normally [63, 79] and accelerated aging, as well as in irradiated mice [88]. Overall, it seems that senolytic interventions display greater efficacy in improving the activity of aged mice rather than transgenic or transplantation models. Whether the efficacy of these interventions is related only to the removal of senescent cells or to additional off-targets that can benefit the physical, cognitive, and psychological conditions of the mice is still unknown.
Slowness
At this moment, the major evidence that senescent cells casually contribute to physical frailty has been obtained after the transplantation of senescent cells in young and old mice [63]. Transplanting a few senescent cells (adipose-derived mesenchymal cells induced by ionizing radiation) by i.p. injection caused persistent physical dysfunction, including impaired walking speed, and a frailty-like phenotype with reduced survival in young and older recipients. However, senescent cell removal in aged INK-ATTAC mice did not affect the max speed of running recorded by a rotarod test [71]. Paradoxically, only one intervention [63] with senolytics (out of 4 retrieved from literature) reported an improvement of parameters related to the slowness criteria in normally and accelerated aging mice [71, 74, 89], whereas a pilot clinical trial with D + Q in patients with idiopathic pulmonary fibrosis reported an improvement in 6-min walk distance and 4-m gait speed [80]. This could be related to the challenge of getting a reliable recording of speed in mice considering the confounding impact of gender, walking style, gait, and neurodegenerative patterns [90].
Overall, these findings confirm that senescent cells can negatively affect the criteria of the PP related to muscle strength, endurance, and activity, while less involvement is recorded for the criteria related to speed and body condition.
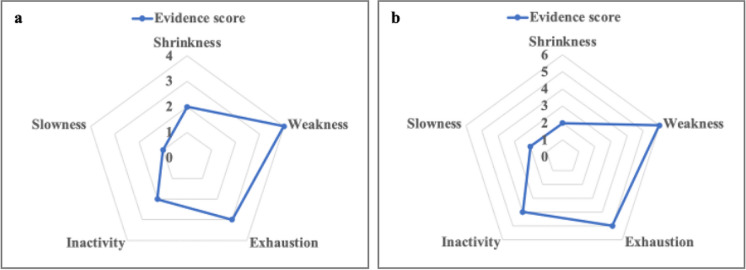
A schematic representation of the overall evidence of a causal impact of cellular senescence on each PP criteria can be visualized (Fig. 2) by calculating a simple score: Evidence score = no. of studies supporting a detrimental role of senescence – no. of studies supporting a beneficial role of senescence. Interestingly, the evidence score build based on the results of studies in animal models (suitable for modulation of cellular senescence) (Fig. 2a and Table 2) overlaps quite completely with the one build based on the results of senolytic interventions (Fig. 2b and Table 4).
Fig. 2.
Current evidence of a causal relationship between cellular senescence and physical frailty phenotype criteria. The evidence scores are computed as no. of studies supporting a detrimental role of senescence − no. of studies supporting a beneficial role of senescence. a Evidence score computed on the basis of the results of the studies on animal models reported in Table 2. b Evidence score computed on the basis of the results of the studies with senolytic interventions reported in Table 4
Causal links between cellular senescence and clinical frailty index domains
In both humans and mice, the clinical FI usually comprises more than 30 items involving domains related to comorbidities, symptoms, diseases, disabilities, and other health deficits. Therefore, it is not surprising that a huge number of studies have used outcomes related to these domains after modulating senescent cells in suitable animal models (Table 3) or after interventions with senolytics (Table 5).
Cardiovascular health
A wide range of cardiovascular factors contribute to the clinical frailty index in humans, but they have not been included in the mouse FI. However, cardiovascular aspects are one of the major focus of preclinical research around cellular senescence.
While an increased expression of p16 seems to play an important role in cardiac remodeling after myocardial infarction [91], the removal of senescent cells in normally aging INK-ATTAC mice [84, 92, 93], and to a lesser extent in a model of accelerated aging [66], leads to improvements in cardiac function. This may be consistent with the negative role of excessively accumulating senescent cells.
However, results related to vascular health in animal models are highly controversial. Overexpression of p16 is detrimental in multiple vascular injury models [94], and some studies in models of atherosclerosis derived from p16-3MR mice found that the removal of senescent cells favors the regression of atherosclerotic lesions [95, 96]. However, these results were not recently replicated in a very similar model, where GCV treatment was ineffective in reducing plaque size and induced inflammation [97]. In the same study, macrophages and vascular smooth muscle cells were shown to express p16, p21, and β-galactosidase activity without being senescent. Most importantly, navitoclax was shown to reduce atherosclerosis lesions by removing leukocytes and platelets rather than through senolysis, providing an alternative explanation to the previous beneficial effects observed in a myocardial infarction model [98]. Similar findings were obtained in the p16-CreERT2/R26-DTA mouse model, where senescent cells can be removed by administration of tamoxifen (TAM) [99]. TAM treatment in aged mice worsened blood-vessel permeability and induced perivascular fibrosis in the heart, liver, and kidney. These results suggest that some p16-positive cells (senescent or non-senescent) are structurally and functionally important and may have a specific beneficial effect related to the cardiovascular domain of the FI.
Hence, it might not be excluded that the beneficial effects of D + Q treatment on the cardiovascular function of aged mice [83, 92, 100, 101] could be related to off-target effects independent of senolysis.
Endocrine and metabolic health
An enhanced glucose tolerance and insulin responsiveness have been observed in systemic and pancreatic-specific models of p16 overexpression [102, 103], suggesting a beneficial role of senescent cells in metabolic function related to type 2 diabetes. Conversely, an improvement in metabolic (glucose homeostasis), endocrine (β-cell function), and adipose tissue function has been reported after the removal of senescent cells in all INK-ATTAC or p16-3MR models currently tested, including normal and accelerated aging as well as diet-induced obesity or insulin resistance [66, 84, 87, 104, 105]. This apparent discrepancy could be eventually explained by the beneficial role of p16-positive cells and the detrimental role of an excess of senescent cells.
Treatments with D + Q or navitoclax also support the detrimental effect of accumulating senescent cells on endocrine and metabolic deficits related to glucose homeostasis [87, 104, 106]. These results are further supported by a retrospective investigation on dasatinib prescription in patients with type 2 diabetes, which revealed a reduction in blood glucose levels comparable to first-line diabetic medications [107].
Further evidence of the detrimental impact of senescent cells on various endocrine functions comes from a study where treatment with FOXO4-DRI corrected the age-related decline in testosterone secretion in aged mice [108].
Respiratory health
The role of senescent cells on respiratory health seems to follow the same trend observed for cardiovascular health, with initial findings of a detrimental role and a more recent study suggesting a more complex picture.
The genetic [109] and senolytic (galacto-encapsulated doxorubicin) [110] removal of senescent cells in mouse models of bleomycin-induced pulmonary fibrosis led to various improvements in pulmonary function. Accordingly, D + Q or navitoclax treatments attenuated the deficits in the breathing rate of irradiated mice [72], whereas positive anti-fibrotic effects of a senolytic treatment with digoxin were reported in a particular model of lung fibrosis induced by intratracheal administration of senescent human cells [111]. A detrimental effect of senescent cells on lung function was additionally demonstrated in cigarette smoke-treated p16-3MR [112] and p16 KO mice [113], as well as in porcine pancreatic elastase‐induced emphysema ARF-DTR mice, which enabled the elimination of p19ARF-positive senescent cells through diphtheria toxin (DT) administration [114]. Conversely, a recent study in a different model of chronic lung disease reported that the elimination of pulmonary endothelial cells aggravates hypertension and worsens the general hemodynamic [115]. Moreover, a treatment in aged mice with fisetin (a natural compound with senolytic properties) did not report significant effects on a lung lesion score [116], neither there were positive results in a pilot clinical trial with D + Q in idiopathic pulmonary fibrosis [80]. Based on these last results, it remains challenging to gain a comprehensive understanding of the precise impact of senescent cells on respiratory health. Further research and a larger body of evidence are needed to draw more definitive conclusions in this regard.
Brain function and mental health
Removal of senescent cells by AP or GCV, using models derived from INK-ATTAC or p16-3MR mice, ameliorated cognitive deficits in several experimental models, including tau-mediated neurodegeneration [117], brain irradiation [118], diet-induced obesity [119], and in paclitaxel-induced cognitive impairment [120]. Aged p16 KO mice display preserved cognitive function and reduced decline in subventricular zone proliferation, olfactory bulb neurogenesis, and self-renewal potential of multipotent progenitors compared to the wild-type [121]. These results suggest that p16 expression contributes to aging by reducing progenitor function and neurogenesis in at least certain regions of the nervous system, but it remains unclear whether these effects are dependent on the induction of cellular senescence. In agreement with this hypothesis, treatment with D + Q improved the cognitive function of old mice and rats [71, 122], whereas fisetin ameliorated a lesion score in the brain of aged mice [116]. Excluding the report of a lack of efficacy of navitoclax in a motor neuron disease mouse model [123], all other interventions in experimental models are in agreement with the deleterious role of senescent cells in brain health. Attenuation of the pathology and cognitive improvement was reported by senolytic treatment (navitoclax or D + Q) in two models of neurodegenerative diseases [117, 124], in a mouse model of accelerated brain aging [74] as well as in brain-irradiated mice [118].
Furthermore, genetic and senolytic removal of senescent cells ameliorated the behavioral deficits related to psychiatric disorders in diet-induced obese mice [119].
Hence, regarding this frailty domain, experimental results are quite in agreement with the detrimental role of cellular senescence. Nevertheless, a pilot study in early-stage symptomatic Alzheimer’s disease patients, with 12-week intermittent orally-delivered D + Q, showed only decreased levels of circulating inflammatory factors without significant effects on cognitive and neuroimaging endpoints [125]. These preliminary data suggest that future randomized placebo-controlled trials with longer follow-up are required for a careful evaluation of the impact of D + Q on cognitive function.
Urogenital, hepatic, and digestive health
This frailty domain encloses the functional status of two important organs, the kidney and the liver, that are the objective of several studies focused on the role of cellular senescence.
The beneficial role of vascular senescent cells in kidney and liver dysfunctions was demonstrated in p16-CreERT2/R26-DTA mice [99], and a similar beneficial role of senescent cells in liver regeneration was observed in p16-3MR mice after partial hepatectomy [126].
Excluding these studies, all other models of chronic kidney or liver dysfunction derived from normally or accelerated aging models as well as induced fibrosis provided evidence of the detrimental role of senescent cells [127] [128] [86] [129]. This is also observed in INK-ATTAC models, where p16-expressing cells are not efficiently removed in the liver and colon [84], further supporting the notion that the removal of senescent cells in some tissues may affect the function of other distant tissues.
Almost all interventions with senolytics have consistently demonstrated a favorable impact on kidney function. In aged mice and other models of kidney dysfunction, D + Q, FOXO4-DRI, and photosensitive senolytics exhibited a notable amelioration of kidney function [82, 87, 127, 128, 130]. Notably, a clinical trial in humans has shown preliminary evidence that D + Q may be beneficial in reducing the inflammatory status of patients with diabetic kidney disease [131]. Additionally, treatment with fisetin was also found to reduce a lesion score in the kidney, but not in the liver, of aged mice [116].
Indeed, the results of senolytics on liver function are still controversial. D + Q reduced hepatic steatosis in aged mice [86], but had no impact on fibrosis markers in Sod1KO mice [132], and worsened the disease progression in a nonalcoholic fatty liver disease model [133]. These results could be partly explained by the inability of D + Q to remove senescent hepatocytes [133]. However, controversial results were also found with another class of senolytic compounds, those targeting Bcl-2-related pathways (ABT-737 and navitoclax). For instance, ABT-737 improved liver regeneration in a partial hepatectomy model [134] and reduced both hepatic and renal fibrosis in a mouse model of accelerated aging [54], whereas navitoclax impaired liver regeneration in a model of partial hepatectomy [126].
The use of advanced senolytic strategies, such as CAR T cells and photosensitive senolytics, has shown promising results in reducing off-target effects. Studies have demonstrated that these approaches hold the potential for restoring tissue homeostasis in liver fibrosis models [135] and improving liver function in aged and irradiated mice [82].
Despite these positive outcomes, the diverse findings also highlight that the impact of senescent cells on liver health is strongly influenced by the specific treatment and experimental models used. This raises the possibility of confounding off-target effects, meaning that the results may be influenced by factors other than the targeted senescent cells.
Interestingly, an intervention with D + Q in aged mice reduced intestinal inflammation and induced changes in specific microbiota signatures [136]. These data confirm that a major off-target effect of some senolytics could be just the intestinal microbiota.
Musculoskeletal health
Not surprisingly, most frailty studies, including those on physical performance outcomes (Tables 2 and 4), are focused on this domain. All these studies are characterized by consistent results on the beneficial effect of systemic [65, 66, 137–140] and specific senescent cell removal [65] on musculoskeletal function. These findings have further support from various p16 KO models related to osteoporosis [141], fracture healing [142], and intervertebral disc degeneration [143].
Overwhelming preclinical evidence from senolytic treatments also confirms the detrimental impact of senescent cells on musculoskeletal health. This is reported for various senolytics in different models [138, 139, 144] (see also Table 4 for outcomes related to physical performances).
Nevertheless, clinical trials in humans have only partly confirmed these results. A significant improvement in the short physical performance battery (SPPB), but not in pulmonary function, was reported in patients with idiopathic pulmonary fibrosis treated with D + Q [80], while the local injection of the senolytic UBX0101 failed to improve knee function in osteoarthritis patients [145].
Sensory health
Age-related eye dysfunctions can also be corrected by the elimination of senescent cells in INK-ATTAC models [66, 84]. Similar results were also observed in a model of glaucoma induced in p16-3MR mice [146]. Relatively few preclinical studies with senolytics in mice have focused on eye function and other aspects related to sensory health. D + Q or dasatinib alone have shown positive effects on the age-related vision loss of SAMP10 mice and in a glaucoma model, respectively [74, 146]. Interestingly, a retrospective study in humans examining medical records and exposure to senolytic drugs also suggested a potential positive effect of senolytics on glaucoma [147]. Albeit the results on glaucoma progression were not significant, the small sample size (n = 9 patients with available post-exposure data) and the confirmation of the safety profile of some senolytic drugs (such as tocilizumab, imatinib, or quercetin) warrant further investigation.
Skin health
While the role of transient senescent cells in wound healing was shown to be beneficial [51], a single study focused on the skin health of normally and accelerated aging p16-3MR mice provided evidence of the overall benefits of senescent cell removal on fur density [128].
Skin health is also ameliorated with different senolytic treatments both in normally and accelerated aging mouse models [54, 128, 148, 149], as well as in a mouse model of skin graft from aged humans [150].
Malignant diseases
Malignant diseases can significantly contribute to the clinical FI, both as a consequence of the disease itself or of the therapy.
Although studies in mice overexpressing p16 have clearly provided evidence that cellular senescence is a strong suppressor of tumor cells [151], chronic expression of p16 in specific tissues [152] and accumulating senescent cells during aging have an opposite pro-tumorigenic role. Indeed, the SASP stimulates pre-malignant and malignant cells to form tumors and drive a much more aggressive growth phenotype [153–155]. The detrimental effect of senescent cells on cancer development and progression has been demonstrated in various models of senescent cell transplantation [153–155], as well as in models of chemotherapy-induced senescence in p16-3MR mice [85, 156].
In murine xenograft, transplant, and spontaneous tumor models, both D + Q and several other senolytics have shown that the removal of senescent cells can have preventive effects on tumor formation and relapse [110, 111, 132, 135, 152, 157–162].
However, contrary to conventional expectations, chemotherapy-induced senescent cells were shown to be beneficial in leukemia [163] and hepatic carcinoma models [164]. It has been hypothesized that the increase of senescent cells, due to chemo- and radio-therapeutic treatment, induces SASP-mediated activation of the immune system that results in an overall antitumoral effect. On the other hand, an incomplete elimination of senescent cancer cells by the immune system and the consequent long-term inflammatory state caused by the SASP may promote tumor growth and relapse [165].
ADL and IADL
Only one pilot study, performed with D + Q in early-stage Alzheimer’s disease patients, evaluated an outcome (informant-reported Lawton IADL form) related to IADL without observing any significant change [125].
Healthcare access and utilization
Senolytic trials or retrospective studies in humans focused on healthcare utilization, as well as studies with similar outcomes back translated in mice, are still not available.
Social engagement and activities
While social aspects have not been specifically addressed, the genetic and pharmacological removal of senescent cells has been shown to ameliorate various aspects of behavioral activity in at least two studies performed in mice (see Tables 2 and 4 and the previous chapter for details).
Nutrition
Genetic or pharmacological removal of senescent cells in different models of obesity can ameliorate the consequences of this pathology but seems to display no effect on nutritional aspects (based on unchanged weight and body composition) [87, 119, 166].
Conversely, genetic or pharmacological removal of senescent cells seems to attenuate the age-related shrinkage after long-term treatment and follow-up (see Table 2 and previous chapter for details).
Pain
A single study in p16-3MR mice addressed the pain domain, after cisplatin-induced neuropathy, demonstrating a detrimental effect of senescent cells on mechanical and thermal sensitivity [167]. Limited studies addressed the effect of senolytics on pain in mice, with a general positive effect of systemic senolytics [72, 74]. It is noteworthy to highlight the failure to reduce knee pain in osteoarthritis patients after local administration of the senolytic UBX101 [145].
Other broader category of medical or health conditions
Since this category could be extensively expanded, we limited our attention to contagious respiratory illnesses that are of relevance for elderly care and health, namely, flu and COVID-19.
While D + Q was shown to not improve overall influenza responses in aged mice [145], it has demonstrated efficacy in improving the clinical symptoms of murine models of COVID-19 [168, 169]. Similarly, the use of navitoclax has also proven effective in these COVID-19 models [168], particularly in infected aged animals [170]. The type of cell death induced by different senolytics (i.e., apoptosis or necrosis) during viral infection is also likely to play an important and differential role in the host response to different viral infections [171].
Based on all the results above, the relationship of cellular senescence with clinical frailty cannot be generalized. It is likely that the detrimental effect on frailty is limited to specific senescent cells exerting their influence on specific domains of frailty. However, each FI is computed on the basis of the weight given to the items included in the domains of each FI instrument. Hence, the overall impact of cellular senescence on the FI also depends on the number of items within each domain.
A schematic representation of the overall evidence of a causal impact of cellular senescence on the human and mouse FI can be visualized (Fig. 3) by calculating a simple score separately for each FI instrument:
Fig. 3.
Current evidence of a causal relationship between cellular senescence and clinical frailty criteria. The evidence scores are computed as [(no. of studies supporting a detrimental role of senescence) − (no. of studies supporting a beneficial role of senescence)] × (no. of items assigned to the criteria in the respective FI instrument). a–c Evidence scores computed for each FI based on the results of the studies in animal models reported in Table 3. d–f Evidence score computed for each FI on the basis of the results of the studies with senolytic interventions reported in Table 5
Evidence scores = [(no. of studies supporting a detrimental role of senescence) − (no. of studies supporting a beneficial role of senescence)] × (no. of items assigned to the criteria in the respective FI instrument).
The number of items assigned to each criterion within each FI instrument can be retrieved from Table 1. Interestingly, the scores computed based on the results in animal models (suitable for modulation of cellular senescence—Table 3) as well as based on the results with senolytic interventions (Table 5) overlap only for the criteria of musculoskeletal health. Cellular senescence also has an impact on brain function and mental health which can highly contribute to the score of the FI in humans, but this domain of frailty is, unfortunately, still not considered in the mouse frailty index. Regarding mouse models, it is interesting to note the discrepancy between the results obtained in transgenic mice (Fig. 4c) vs. senolytic (Fig. 4f) interventions related to urogenital, hepatic, and digestive health deficits. This could be interpreted as a potential positive impact of senolytics due to off-target effects.
Fig. 4.
Relationship between stressors, senescent cell burden, and time to recover the physiological integrity in the face of stressors. In normal (robust) conditions, stressors may induce a transient raise of senescent cells, which likely exerts a beneficial role, and the physiological system integrity is rapidly recovered. When frailty occurs, the physiological system’s integrity cannot be recovered, and the accumulation rate of senescent cells is out of control. Given the substantial weight assigned to musculoskeletal and cerebral function-related items or criteria in various frailty assessment tools, coupled with evidence indicating the detrimental impact of accumulating senescent cells on these health domains, targeting factors that regulate the accumulation rate of senescent cells affecting musculoskeletal and cerebral function may represent a key therapeutic strategy
Hence, the impact of cellular senescence on the clinical frailty index depends mostly on the weight given to the items related to musculoskeletal health as well as brain function and mental health within each FI instrument.
Cellular senescence and frailty from observational studies in humans
Regarding human aging and frailty, studies on the expansion of senescent cells in various tissues are limited by the lack of circulating biomarkers that may be representative of the accumulation of senescent cells in different tissues and the type of senescence [172].
While recent studies support the existence of prostaglandin D2-related lipids as markers of senolysis [173], clinical studies using these biomarkers still have to be performed. Limited studies on biopsies from some tissues have provided evidence of an accumulation of senescent cells with human aging [174]. These studies cannot provide insight into a causal relationship but suggest that the accumulation of senescent cells is exacerbated in the presence of major chronic disease. For example, senescent astrocytes (p16-positive) have been observed to increase in the aging brain, with the highest levels in the brains of Alzheimer’s disease patients [175]. Senescent vascular cells accumulate in humans with sedentary aging [176] and in human atheroma tissues determining various dysfunction features [177]. Additional evidence of senescent cell accumulation has been provided in muscle satellite cells and myocytes from elderly individuals [81, 178], in cartilage and synovial fibroblasts isolated from patients with arthritis [179, 180], in cardiac progenitor cells isolated from older subjects with cardiovascular disease [92] as well as in the visceral and subcutaneous fat of elderly and obese individuals [181–183]. In vitro study performed on human fibroblasts also showed a correlation between oncogene-induced senescence and vitamin D receptor downregulation [184], a parameter included in some human FI.
Evocative evidence of the association between cellular senescence and age-related diseases has also been derived from studies aimed at identifying candidate genes for frailty; for example, the p16INK4a locus was among the most robust risk genes associated with primary open-angle glaucoma [185], while polymorphisms in genetic loci related to cellular senescence (e.g., the CDKN2A/B locus) are associated with an increased risk of developing type 2 diabetes [186].
In addition to the above, recent studies have also demonstrated the association between circulating components of the SASP and measures of physical function or FI in humans. For example, serum levels of the SASP-related factors ICAM-1 and TIMP-1 were found to be higher, while IL1-β and IL6 were lower in older adults with physical frailty and sarcopenia than in controls [187]. In contrast, a higher SASP index was correlated with worse cognitive functioning (MMSE) in older patients [188]. Similarly, another study conducted in 1377 older adults revealed significant associations between several SASP proteins and the short physical performance battery (SPPB), its subcomponents (gait speed, balance, and chair rise time), and 400-m walk time [189].
The evidence of an increasing accumulation of senescent cells in human aging and in chronic age-related diseases, combined with the observation that various components of inflammaging are included in the SASP and correlate with PP, seems to support the role of senescent cells in the pathophysiological changes underlying human frailty [24, 190]. Factors affecting the kinetics of the accumulation and spreading of senescent cells in aged organisms are still under investigation. However, we deem these to be pivotal to understanding the contribution of cellular senescence in frailty as well as to developing strategies to preserve health in geriatric age.
Frailty can be well graphically represented by the compromised time to recover physiological integrity after a stressor [191]. Based on available evidence, it can be argued that the senescent cell burden, mainly related to musculoskeletal and cerebral health, increases exponentially (Fig. 4) when this time is compromised.
Hence, a potential successful strategy to develop therapeutic interventions for frailty is to understand which systems work to keep under control the senescence accumulation rate.
Conclusions and therapeutic perspectives
Experimental pre-clinical models have convincingly demonstrated a causal role of cellular senescence in multiple aspects of frailty, mainly related to musculoskeletal and cerebral health deficits. Currently, these findings are impacting the development of therapeutic strategies for preventing and treating frailty based on senolytic compounds. Some clinical trials with these compounds are underway in patients with frailty (i.e., NCT02848131 and NCT04733534). Nevertheless, there is still a need to acquire more knowledge from murine models by utilizing frailty instruments that encompass brain health deficits as well. While certain senotherapeutics have been identified to target specific aspects of musculoskeletal frailty, which show a significant overlap between mice and human frailty assessment tools, comprehensive longitudinal studies examining frailty, including the brain health domain, in animal models treated with senotherapeutics still require further exploration.
Furthermore, it should be checked whether the increased emphasis on skin and otorhinolaryngology disorders given in the mouse frailty index, compared to the human indexes, could impact the translation of pre-clinical results into clinical applications. Despite these limitations, ongoing efforts are focused on improving the lifelong longitudinal screening of frailty in mice [36], and several treatments based on these new instruments are currently being tested.
Understanding the primary factors that contribute to the uncontrolled spreading of cellular senescence in response to endogenous and exogenous stressors is also crucial to understanding the different degrees of fragilization in elderly individuals and to optimizing experimental designs where senotherapeutics can be combined with treatments targeting the potential source of fast senescence spreading. Otherwise, senotherapeutics may have the effect of “trying to avoid sinking a boat with a bucket without closing the large hole that lets in a massive amount of water.”
This review identifies at least two major domains of frailty affected by senescence, “musculoskeletal” and “brain health.”
The most likely hypothesis is that a rapid and excessive accumulation of senescent cells affecting musculoskeletal and brain health occurs in frail individuals under various types of stress. Further insight into the underlying causes of the uncontrolled spreading of senescence burden may pave the way for combination therapies that target senescent cell accumulation at different levels. As pointed out by others [24, 192], identifying circulating biomarkers as a surrogate for tissue accumulation of senescent cells would greatly benefit this area of research.
Author contribution
MM and SM: conceptualization, writing—original draft, and writing—review and editing. GB, MEG, RG, FP, MC, DB, AS, AV, EN, and MP: writing—review and editing and supervision. FL, MP, EN, AV, and MM: funding acquisition.
Funding
This work was supported by the IRCCS Aging Network, projects: “Implementazione della RoadMap nella ricerca sull’Aging (IRMA)—grant number RCR-2019–23669121,” “Sinergie di Ricerca della Rete Aging (SIRI)—grant number RCR-2020–23670069,” and “PROMISING: aPpROccio alla MedIcina di preciSIoNe in Geriatria: dalle basi biomolecolari dell’invecchiamento e delle malattie età correlate ai modelli clinico-assistenziali—grant number RCR-2021–23671216” to FL; by Fondazione Cariplo (grant number 2016–1006) “Multicomponent Analysis of Physical Frailty Biomarkers” to EN, MP, and AV; by Ricerca Corrente funding from Italian Ministry of Health to Fondazione IRCCS Istituto Neurologico “Carlo Besta” to DB; and by Ricerca Corrente funding from Italian Ministry of Health to IRCCS-INRCA to MM and MP.
Data availability
No data was used for the research described in the article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 2.Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394:1376–1386. doi: 10.1016/S0140-6736(19)31785-4. [DOI] [PubMed] [Google Scholar]
- 3.Onder G, Vetrano DL, Marengoni A, Bell JS, Johnell K, Palmer K. Accounting for frailty when treating chronic diseases. Eur J Intern Med. 2018;56:49–52. doi: 10.1016/j.ejim.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Correa A, Rochlani Y, Khan MH, Aronow WS. Pharmacological management of hypertension in the elderly and frail populations. Expert Rev Clin Pharmacol. 2018;11(8):805–817. 10.1080/17512433.2018.1500896. [DOI] [PubMed]
- 5.Zazzara MB, Vetrano DL, Carfì A, Onder G. Frailty and chronic disease. Panminerva Med. 2019;61:486–492. doi: 10.23736/S0031-0808.19.03731-5. [DOI] [PubMed] [Google Scholar]
- 6.Fedecostante M, Cherubini A. Frailty and delirium: Unveiling the hidden vulnerability of older hospitalized patients. Eur J Intern Med. 2019;70:16–17. 10.1016/j.ejim.2019.10.020. [DOI] [PubMed]
- 7.Van der Elst M, Schoenmakers B, Duppen D, Lambotte D, Fret B, Vaes B, De Lepeleire J; D-SCOPE Consortium. Interventions for frail community-dwelling older adults have no significant effect on adverse outcomes: a systematic review and meta-analysis. BMC Geriatr. 2018;18(1):249. 10.1186/s12877-018-0936-7. [DOI] [PMC free article] [PubMed]
- 8.Faller JW, do Nascimento Pereira D, de Souza S, Nampo FK, de Souza Orlandi F, Matumoto S. Instruments for the detection of frailty syndrome in older adults: a systematic review. PLoS One. 2019;14:e0216166. doi: 10.1371/journal.pone.0216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Op het Veld LP, Beurskens AJ, de Vet HC, van Kuijk SM, Hajema KJ, Kempen GI, van Rossum E. The ability of four frailty screening instruments to predict mortality, hospitalization and dependency in (instrumental) activities of daily living. Eur J Ageing. 2019;16:387–394. doi: 10.1007/s10433-019-00502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Checa-López M, Oviedo-Briones M, Pardo-Gómez A, Gonzales-Turín J, Guevara-Guevara T, Carnicero JA, Alamo-Ascencio S, Landi F, Cesari M, Grodzicki T, Rodriguez-Mañas L. FRAILTOOLS study protocol: a comprehensive validation of frailty assessment tools to screen and diagnose frailty in different clinical and social settings and to provide instruments for integrated care in older adults. BMC Geriatr. 2019;19:86. doi: 10.1186/s12877-019-1042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aalen OO, Valberg M, Grotmol T, Tretli S. Understanding variation in disease risk: the elusive concept of frailty. Int J Epidemiol. 2015;44:1408–1421. doi: 10.1093/ije/dyu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Looman WM, Fabbricotti IN, Blom JW, Jansen APD, Lutomski JE, Metzelthin SF, Huijsman R. The frail older person does not exist: development of frailty profiles with latent class analysis. BMC Geriatr. 2018;18:84. doi: 10.1186/s12877-018-0776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Ortuno R, Scarlett S, O’Halloran AM, Kenny RA. Is phenotypical prefrailty all the same? A longitudinal investigation of two prefrailty subtypes in TILDA. Age Ageing. 2019;49:39–45. doi: 10.1093/ageing/afz129. [DOI] [PubMed] [Google Scholar]
- 14.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Schmauck-Medina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, Cao S, Soendenbroe C, Mansell E, Vestergaard MB, Li Z, Shiloh Y, Opresko PL, Egly JM, Kirkwood T, Verdin E, Bohr VA, Cox LS, Stevnsner T, Rasmussen LJ, Fang EF. New hallmarks of ageing: a 2022 Copenhagen ageing meeting summary. Aging (Albany NY) 2022;14:6829–6839. doi: 10.18632/aging.204248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, de Angelis MH, Guedes JR, Brito MA, Ortolano S, Pani G, Athanasopoulou S, Gonos ES, Schosserer M, Grillari J, Peterson P, Tuna BG, Dogan S, Meyer A, van Os R, Trendelenburg A-U. Towards frailty biomarkers: candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Sebastiani P, Thyagarajan B, Sun F, Schupf N, Newman AB, Montano M, Perls TT. Biomarker signatures of aging. Aging Cell. 2017;16:329–338. doi: 10.1111/acel.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.dos Gonçalves RS, Maciel ÁC, Rolland Y, Vellas B, de Souto Barreto P. Frailty biomarkers under the perspective of geroscience: a narrative review. Ageing Res Rev. 2022;81:101737. doi: 10.1016/j.arr.2022.101737. [DOI] [PubMed] [Google Scholar]
- 19.Gerosa L, Malvandi AM, Malavolta M, Provinciali M, Lombardi G. Exploring cellular senescence in the musculoskeletal system: any insights for biomarkers discovery? Ageing Res Rev. 2023;88:101943. doi: 10.1016/j.arr.2023.101943. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed]
- 21.Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017;36:1–10. doi: 10.1016/j.arr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Walker KA, Walston J, Gottesman RF, Kucharska-Newton A, Palta P, Windham BG. Midlife systemic inflammation is associated with frailty in later life: the ARIC study. J Gerontol A Biol Sci Med Sci. 2019;74:343–349. doi: 10.1093/gerona/gly045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olivieri F, Prattichizzo F, Lattanzio F, Bonfigli AR, Spazzafumo L. Antifragility and antiinflammaging: can they play a role for a healthy longevity? Ageing Res Rev. 2023;84:101836. doi: 10.1016/j.arr.2022.101836. [DOI] [PubMed] [Google Scholar]
- 24.Zampino M, Ferrucci L, Semba RD. Biomarkers in the path from cellular senescence to frailty. Exp Gerontol. 2020;129:110750. doi: 10.1016/j.exger.2019.110750. [DOI] [PubMed] [Google Scholar]
- 25.Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735. 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed]
- 26.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56. 10.1093/gerona/56.3.m146. [DOI] [PubMed]
- 27.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Mitnitski AB, MacKnight C. Some mathematical models of frailty and their clinical implications. Rev Clin Gerontol. 2002;12:109–117. doi: 10.1017/S0959259802012236. [DOI] [Google Scholar]
- 29.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. C Can Med Assoc J. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, Abellan van Kan G, Guyonnet S, Vellas B, Cesari M. Components of the frailty phenotype in relation to the frailty index: results from the Toulouse frailty platform. J Am Med Dir Assoc. 2015;16:855–859. doi: 10.1016/j.jamda.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:210–215. doi: 10.5770/CGJ.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat Aging. 2021;1:651–665. doi: 10.1038/s43587-021-00099-3. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69:1485–1491. doi: 10.1093/gerona/glt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann CW, Kwak D, Thompson LV. Assessing onset, prevalence and survival in mice using a frailty phenotype. Aging (Albany NY). 2018;10(12):4042-4053. 10.18632/aging.101692. [DOI] [PMC free article] [PubMed]
- 35.Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, Garcia-Garcia FJ, Olaso-Gonzalez G, Salvador-Pascual A, Tarazona-Santabalbina FJ, Viña J. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol A Biol Sci Med Sci. 2017;72:885–891. doi: 10.1093/gerona/glw337. [DOI] [PubMed] [Google Scholar]
- 36.Marcozzi S, Bigossi G, Giuliani ME, Giacconi R, Cardelli M, Piacenza F, Orlando F, Segala A, Valerio A, Nisoli E, Brunetti D, Puca A, Boschi F, Gaetano C, Mongelli A, Lattanzio F, Provinciali M, Malavolta M. Comprehensive longitudinal non-invasive quantification of healthspan and frailty in a large cohort (n = 546) of geriatric C57BL/6 J mice. Geroscience. 2023. 10.1007/s11357-023-00737-1. [DOI] [PMC free article] [PubMed]
- 37.Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Zglinicki T, Nieto IV, Brites D, Karagianni N, Ortolano S, Georgopoulos S, Cardoso AL, Novella S, Lepperdinger G, Trendelenburg AU, van Os R. Frailty in mouse ageing: a conceptual approach. Mech Ageing Dev. 2016;160:34–40. doi: 10.1016/j.mad.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62:738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 40.Kane AE, Huizer-Pajkos A, Mach J, Mitchell SJ, de Cabo R, Le Couteur DG, Howlett SE, Hilmer SN. A comparison of two mouse frailty assessment tools. J Gerontol A Biol Sci Med Sci. 2017;72:904–909. doi: 10.1093/gerona/glx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi: 10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogan DB, Freiheit EA, Strain LA, Patten SB, Schmaltz HN, Rolfson D, Maxwell CJ. Comparing frailty measures in their ability to predict adverse outcome among older residents of assisted living. BMC Geriatr. 2012;12:56. doi: 10.1186/1471-2318-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todorovic S, Loncarevic-Vasiljkovic N, Jovic M, Sokanovic S, Kanazir S, Mladenovic DA. Frailty index and phenotype frailty score: sex- and age-related differences in 5XFAD transgenic mouse model of Alzheimer’s disease. Mech Ageing Dev. 2020;185:111195. doi: 10.1016/j.mad.2019.111195. [DOI] [PubMed] [Google Scholar]
- 44.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muñoz-Espín D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M. Unmasking transcriptional heterogeneity in senescent cells. Curr Biol. 2017;27:2652–2660.e4. doi: 10.1016/j.cub.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28(6):436-453. 10.1016/j.tcb.2018.02.001. [DOI] [PubMed]
- 49.Ogrodnik M. Cellular aging beyond cellular senescence: markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell. 2021;20:1–19. doi: 10.1111/acel.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Childs BG, Baker DJ, Kirkland JL, Campisi J, van Deursen JM. Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep. 2014;15:1139–1153. doi: 10.15252/embr.201439245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge R-M, Vijg J, Van Steeg H, Dollé MET, Hoeijmakers JHJ, de Bruin A, Hara E, Campisi J. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baz-Martínez M, Da Silva-Álvarez S, Rodríguez E, Guerra J, El Motiam A, Vidal A, Garciá-Caballero T, González-Barcia M, Sánchez L, Munõz-Fontela C, Collado M, Rivas C. Cell senescence is an antiviral defense mechanism. Sci Rep. 2016;6:37007. doi: 10.1038/srep37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burton DGA, Stolzing A. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev. 2018;43:17–25. doi: 10.1016/j.arr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H, Krizhanovsky V. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hampel B, Malisan F, Niederegger H, Testi R, Jansen-Dürr P. Differential regulation of apoptotic cell death in senescent human cells. Exp Gerontol. 2004;39:1713–1721. doi: 10.1016/j.exger.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 56.da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, von Zglinicki T. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell. 2019;18:e12848. doi: 10.1111/acel.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowald A, Passos JF, Kirkwood TBL. On the evolution of cellular senescence. Aging Cell. 2020;19:1–12. doi: 10.1111/acel.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borghesan M, Fafián-Labora J, Eleftheriadou O, Carpintero-Fernández P, Paez-Ribes M, Vizcay-Barrena G, Swisa A, Kolodkin-Gal D, Ximénez-Embún P, Lowe R, Martín-Martín B, Peinado H, Muñoz J, Fleck RA, Dor Y, Ben-Porath I, Vossenkamper A, Muñoz-Espin D, O’Loghlen A. Small extracellular vesicles are key regulators of non-cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Rep. 2019;27:3956–3971.e6. doi: 10.1016/j.celrep.2019.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeon OH, Wilson DR, Clement CC, Rathod S, Cherry C, Powell B, Lee Z, Khalil AM, Green JJ, Campisi J, Santambrogio L, Witwer KW, Elisseeff JH. Senescence cell–associated extracellular vesicles serve as osteoarthritis disease and therapeutic markers. JCI Insight. 2019;4:e125019. doi: 10.1172/jci.insight.125019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waters DW, Schuliga M, Pathinayake PS, Wei L, Tan HY, Blokland KEC, Jaffar J, Westall GP, Burgess JK, Prêle CM, Mutsaers SE, Grainge CL, Knight DA. A senescence bystander effect in human lung fibroblasts. Biomedicines. 2021;9:1–17. doi: 10.3390/biomedicines9091162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogrodnik M, Salmonowicz H, Gladyshev VN. Integrating cellular senescence with the concept of damage accumulation in aging: Relevance for clearance of senescent cells. Aging Cell. 2019;18(1):e12841. 10.1111/acel.12841. [DOI] [PMC free article] [PubMed]
- 63.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeon OH, Mehdipour M, Gil T-H, Kang M, Aguirre NW, Robinson ZR, Kato C, Etienne J, Lee HG, Alimirah F, Walavalkar V, Desprez P-Y, Conboy MJ, Campisi J, Conboy IM. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat Metab. 2022;4:995–1006. doi: 10.1038/s42255-022-00609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farr JN, Saul D, Doolittle ML, Kaur J, Rowsey JL, Vos SJ, Froemming MN, Lagnado AB, Zhu Y, Weivoda M, Ikeno Y, Pignolo RJ, Niedernhofer LJ, Robbins PD, Jurk D, Passos JF, LeBrasseur NK, Tchkonia T, Kirkland JL, Monroe DG, Khosla S. Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J Clin Invest. 2023;133(8):e162519. 10.1172/JCI162519. [DOI] [PMC free article] [PubMed]
- 66.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Myrianthopoulos V, Evangelou K, Vasileiou PVS, Cooks T, Vassilakopoulos TP, Pangalis GA, Kouloukoussa M, Kittas C, Georgakilas AG, Gorgoulis VG. Senescence and senotherapeutics: a new field in cancer therapy. Pharmacol Ther. 2019;193:31-49. 10.1016/j.pharmthera.2018.08.006. [DOI] [PubMed]
- 68.Short S, Fielder E, Miwa S, von Zglinicki T. Senolytics and senostatics as adjuvant tumour therapy. EBioMedicine. 2019;41:683-692. 10.1016/j.ebiom.2019.01.056. [DOI] [PMC free article] [PubMed]
- 69.Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL. Reducing senescent cell burden in aging and disease. Trends Mol Med. 2020;26:630–638. doi: 10.1016/j.molmed.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Englund DA, Jolliffe A, Aversa Z, Zhang X, Sturmlechner I, Sakamoto AE, Zeidler JD, Warner GM, McNinch C, White TA, Chini EN, Baker DJ, van Deursen JM, LeBrasseur NK. p21 induces a senescence program and skeletal muscle dysfunction. Mol Metab. 2023;67:101652. doi: 10.1016/j.molmet.2022.101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogrodnik M, Evans SA, Fielder E, Victorelli S, Kruger P, Salmonowicz H, Weigand BM, Patel AD, Pirtskhalava T, Inman CL, Johnson KO, Dickinson SL, Rocha A, Schafer MJ, Zhu Y, Allison DB, von Zglinicki T, LeBrasseur NK, Tchkonia T, Neretti N, Passos JF, Kirkland JL, Jurk D. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell. 2021;20:e13296. doi: 10.1111/acel.13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fielder E, Wan T, Alimohammadiha G, Ishaq A, Low E, Weigand BM, Kelly G, Parker C, Griffin B, Jurk D, Korolchuk VI, von Zglinicki T, Miwa S. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. Elife. 2022;11:e75492. doi: 10.7554/eLife.75492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dungan CM, Murach KA, Zdunek CJ, Tang ZJ, VonLehmden GL, Brightwell CR, Hettinger Z, Englund DA, Liu Z, Fry CS, Filareto A, Franti M, Peterson CA. Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell. 2022;21:e13528. doi: 10.1111/acel.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ota H, Kodama A. Dasatinib plus quercetin attenuates some frailty characteristics in SAMP10 mice. Sci Rep. 2022;12:2425. doi: 10.1038/s41598-022-06448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guzman SD, Judge J, Shigdar SM, Paul TA, Davis CS, Macpherson PC, Markworth JF, Van Remmen H, Richardson A, McArdle A, Brooks SV. Removal of p16INK4 expressing cells in late life has moderate beneficial effects on skeletal muscle function in male mice. Front Aging. 2022;2:1–10. doi: 10.3389/fragi.2021.821904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moiseeva V, Cisneros A, Sica V, Deryagin O, Lai Y, Jung S, Andrés E, An J, Segalés J, Ortet L, Lukesova V, Volpe G, Benguria A, Dopazo A, Aznar-Benitah S, Urano Y, del Sol A, Esteban MA, Ohkawa Y, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature. 2023;613:169–178. doi: 10.1038/s41586-022-05535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X, Habiballa L, Aversa Z, Ng YE, Sakamoto AE, Englund DA, Pearsall VM, White TA, Robinson MM, Rivas DA, Dasari S, Hruby AJ, Lagnado AB, Jachim SK, Granic A, Sayer AA, Jurk D, Lanza IR, Khosla S, Fielding RA, Nair KS, Schafer MJ, Passos JF, LeBrasseur NK. Characterization of cellular senescence in aging skeletal muscle. Nat Aging. 2022;2:601–615. doi: 10.1038/s43587-022-00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Novais EJ, Tran VA, Johnston SN, Darris KR, Roupas AJ, Sessions GA, Shapiro IM, Diekman BO, Risbud MV. Long-term treatment with senolytic drugs dasatinib and quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat Commun. 2021;12:5213. doi: 10.1038/s41467-021-25453-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai Y, Zhou H, Zhu Y, Sun Q, Ji Y, Xue A, Wang Y, Chen W, Yu X, Wang L, Chen H, Li C, Luo T, Deng H. Elimination of senescent cells by β-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30:574–589. doi: 10.1038/s41422-020-0314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardí M, Ballestar E, González S, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 82.Shi D, Liu W, Gao Y, Li X, Huang Y, Li X, James TD, Guo Y, Li J. Photoactivatable senolysis with single-cell resolution delays aging. Nat aging. 2023;3:297–312. doi: 10.1038/s43587-023-00360-x. [DOI] [PubMed] [Google Scholar]
- 83.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, Alston S, Academia EC, Kilmarx S, Valdovinos A, Wang B, De Bruin A, Kennedy BK, Melov S, Zhou D, Sharpless NE, Muss H, Campisi J. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017;7:165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, Von Zglinicki T, Jurk D. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi: 10.1038/ncomms15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, Prata LG, van Dijk TH, Verkade E, Casaclang-Verzosa G, Johnson KO, Cubro H, Doornebal EJ, Ogrodnik M, Jurk D, Jensen MD, Chini EN, Miller JD, Matveyenko A, Stout MB, Schafer MJ, White TA, Hickson LJ, Demaria M, Garovic V, Grande J, Arriaga EA, Kuipers F, von Zglinicki T, LeBrasseur NK, Campisi J, Tchkonia T, Kirkland JL. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18:e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y, Gao D, Yuan Y, Zheng R, Sun M, Jia S, Liu J. Cycloastragenol: a novel senolytic agent that induces senescent cell apoptosis and restores physical function in TBI-aged mice. Int J Mol Sci. 2023;24:6554. doi: 10.3390/ijms24076554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyúl-Tóth Á, Mukli P, Toth P, Ahire C, Ungvari A, Benyo Z, Csiszar A, Ungvari Z. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/navitoclax improves functional hyperemia in aged mice. GeroScience. 2021;43:2427–2440. doi: 10.1007/s11357-021-00440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Broom L, Stephen J, Nayar V, VanderHorst VG. Shifts in gait signatures mark the end of lifespan in mice, with sex differences in timing. Front Aging Neurosci. 2021;13:716993. doi: 10.3389/fnagi.2021.716993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi J, Sun J, Liu L, Shan T, Meng H, Yang T, Wang S, Wei T, Chen B, Ma Y, Wang Q, Wang H, Liu J, Wang L. P16ink4a overexpression ameliorates cardiac remodeling of mouse following myocardial infarction via CDK4/pRb pathway. Biochem Biophys Res Commun. 2022;595:62–68. doi: 10.1016/j.bbrc.2022.01.077. [DOI] [PubMed] [Google Scholar]
- 92.Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, Clark JE, Punjabi PP, Awad W, Torella D, Tchkonia T, Kirkland JL, Ellison-Hughes GM. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. 2019;18:e12931. doi: 10.1111/acel.12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Anderson R, Lagnado A, Maggiorani D, Walaszczyk A, Dookun E, Chapman J, Birch J, Salmonowicz H, Ogrodnik M, Jurk D, Proctor C, Correia-Melo C, Victorelli S, Fielder E, Berlinguer-Palmini R, Owens A, Greaves LC, Kolsky KL, Parini A, Douin-Echinard V, LeBrasseur NK, Arthur HM, Tual-Chalot S, Schafer MJ, Roos CM, Miller JD, Robertson N, Mann J, Adams PD, Tchkonia T, Kirkland JL, Mialet-Perez J, Richardson GD, Passos JF. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 2019;38:100492. doi: 10.15252/embj.2018100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardenas JC, Owens AP, 3rd, Krishnamurthy J, Sharpless NE, Whinna HC, Church FC. Overexpression of the cell cycle inhibitor p16INK4a promotes a prothrombotic phenotype following vascular injury in mice. Arterioscler Thromb Vasc Biol. 2011;31:827–833. doi: 10.1161/ATVBAHA.110.221721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sadhu S, Decker C, Sansbury BE, Marinello M, Seyfried A, Howard J, Mori M, Hosseini Z, Arunachalam T, Finn AV, Lamar JM, Jourd'heuil D, Guo L, MacNamara KC, Spite M, Fredman G. Radiation-Induced Macrophage Senescence Impairs Resolution Programs and Drives Cardiovascular Inflammation. J Immunol. 2021;207(7):1812-1823. 10.4049/jimmunol.2100284. [DOI] [PMC free article] [PubMed]
- 97.Garrido AM, Kaistha A, Uryga AK, Oc S, Foote K, Shah A, Finigan A, Figg N, Dobnikar L, Jørgensen H, Bennett M. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc Res. 2022;118:1713–1727. doi: 10.1093/cvr/cvab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell. 2019;18:e12945. doi: 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grosse L, Wagner N, Emelyanov A, Molina C, Lacas-Gervais S, Wagner K-D, Bulavin DV. Defined p16(High) senescent cell types are indispensable for mouse healthspan. Cell Metab. 2020;32:87–99.e6. doi: 10.1016/j.cmet.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 100.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973-7. 10.1111/acel.12458. [DOI] [PMC free article] [PubMed]
- 101.Alibhai FJ, Lim F, Yeganeh A, DiStefano PV, Binesh-Marvasti T, Belfiore A, Wlodarek L, Gustafson D, Millar S, Li S-H, Weisel RD, Fish JE, Li R. Cellular senescence contributes to age-dependent changes in circulating extracellular vesicle cargo and function. Aging Cell. 2020;19:e13103. doi: 10.1111/acel.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.González-Navarro H, Vinué Á, Sanz MJ, Delgado M, Pozo MA, Serrano M, Burks DJ, Andrés V. Increased dosage of Ink4/Arf protects against glucose intolerance and insulin resistance associated with aging. Aging Cell. 2013;12:102–111. doi: 10.1111/acel.12023. [DOI] [PubMed] [Google Scholar]
- 103.Helman A, Klochendler A, Azazmeh N, Gabai Y, Horwitz E, Anzi S, Swisa A, Condiotti R, Granit RZ, Nevo Y, Fixler Y, Shreibman D, Zamir A, Tornovsky-Babeay S, Dai C, Glaser B, Powers AC, Shapiro AMJ, Magnuson MA, Dor Y, Ben-Porath I. p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med. 2016;22:412–420. doi: 10.1038/nm.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aguayo-Mazzucato C, Andle J, Lee TBJ, Midha A, Talemal L, Chipashvili V, Hollister-Lock J, van Deursen J, Weir G, Bonner-Weir S. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019;30:129–142.e4. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bahour N, Bleichmar L, Abarca C, Wilmann E, Sanjines S, Aguayo-Mazzucato C. Clearance of p16Ink4a-positive cells in a mouse transgenic model does not change β-cell mass and has limited effects on their proliferative capacity. Aging (Albany NY) 2023;15:441–458. doi: 10.18632/aging.204483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Islam MT, Tuday E, Allen S, Kim J, Trott DW, Holland WL, Donato AJ, Lesniewski LA. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell. 2023;22:e13767. doi: 10.1111/acel.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salaami O, Kuo C-L, Drake MT, Kuchel GA, Kirkland JL, Pignolo RJ. Antidiabetic effects of the senolytic agent dasatinib. Mayo Clin Proc. 2021;96:3021–3029. doi: 10.1016/j.mayocp.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang C, Xie Y, Chen H, Lv L, Yao J, Zhang M, Xia K, Feng X, Li Y, Liang X, Sun X, Deng C, Liu G. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging (Albany NY) 2020;12:1272–1284. doi: 10.18632/aging.102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Muñoz-Espín D, Rovira M, Galiana I, Giménez C, Lozano-Torres B, Paez-Ribes M, Llanos S, Chaib S, Muñoz-Martín M, Ucero AC, Garaulet G, Mulero F, Dann SG, VanArsdale T, Shields DJ, Bernardos A, Murguía JR, Martínez-Máñez R, Serrano M. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10(9):e9355. 10.15252/emmm.201809355. [DOI] [PMC free article] [PubMed]
- 111.Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, Pietrocola F, Llanos S, Rodilla V, Soprano E, Pedrosa P, Ferreirós A, Barradas M, Hernández-González F, Lalinde M, Prats N, Bernadó C, González P, Gómez M, Ikonomopoulou MP, Fernández-Marcos PJ, García-Caballero T, Del Pino P, Arribas J, Vidal A, González-Barcia M, Serrano M, Loza MI, Domínguez E, Collado M. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun. 2019;10:4731. doi: 10.1038/s41467-019-12888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kaur G, Muthumalage T, Rahman I. Clearance of senescent cells reverts the cigarette smoke-induced lung senescence and airspace enlargement in p16–3MR mice. Aging Cell. 2023;22:e13850. doi: 10.1111/acel.13850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cottage CT, Peterson N, Kearley J, Berlin A, Xiong X, Huntley A, Zhao W, Brown C, Migneault A, Zerrouki K, Criner G, Kolbeck R, Connor J, Lemaire R. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun Biol. 2019;2:307. doi: 10.1038/s42003-019-0532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mikawa R, Suzuki Y, Baskoro H, Kanayama K, Sugimoto K, Sato T, Sugimoto M. Elimination of p19ARF-expressing cells protects against pulmonary emphysema in mice. Aging Cell. 2018;17:e12827. doi: 10.1111/acel.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Born E, Lipskaia L, Breau M, Houssaini A, Beaulieu D, Marcos E, Pierre R, Do Cruzeiro M, Lefevre M, Derumeaux G, Bulavin DV, Delcroix M, Quarck R, Reen V, Gil J, Bernard D, Flaman J-M, Adnot S, Abid S. Eliminating senescent cells can promote pulmonary hypertension development and progression. Circulation. 2023;147:650–666. doi: 10.1161/CIRCULATIONAHA.122.058794. [DOI] [PubMed] [Google Scholar]
- 116.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD, Niedernhofer LJ. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi: 10.1016/j.ebiom.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 2018;562:578–582. doi: 10.1038/s41586-018-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fülöp GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, Sonntag WE, Schwartzman ML, Campisi J, Csiszar A, Ungvari Z. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. GeroScience. 2020;42:409–428. doi: 10.1007/s11357-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab. 2019;29:1061–1077.e8. doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, Tarantini A, Nagaraja R, Yan F, Tang Q, Mukli P, Csipo T, Yabluchanskiy A, Campisi J, Ungvari Z, Csiszar A. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell. 2023;22:e13832. doi: 10.1111/acel.13832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krzystyniak A, Wesierska M, Petrazzo G, Gadecka A, Dudkowska M, Bielak-Zmijewska A, Mosieniak G, Figiel I, Wlodarczyk J, Sikora E. Combination of dasatinib and quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile. Aging (Albany NY) 2022;14:572–595. doi: 10.18632/aging.203835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torres P, Anerillas C, Ramírez-Núñez O, Fernàndez A, Encinas M, Povedano M, Andrés-Benito P, Ferrer I, Ayala V, Pamplona R, Portero-Otín M. A motor neuron disease mouse model reveals a non-canonical profile of senescence biomarkers. Dis Model Mech. 2022;15(8):dmm049059. 10.1242/dmm.049059. [DOI] [PMC free article] [PubMed]
- 124.Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17:e12840. doi: 10.1111/acel.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Orr M, Gonzales M, Garbarino V, Kautz T, Palavicini J, Lopez-Cruzan M, Dehkordi SK, Mathews J, Zare H, Xu P, Zhang B, Franklin C, Habes M, Craft S, Petersen R, Tchkonia T, Kirkland J, Salardini A, Seshadri S, Musi N. Senolytic therapy to modulate the progression of Alzheimer's Disease (SToMP-AD) - Outcomes from the first clinical trial of senolytic therapy for Alzheimer's disease. Res Sq [Preprint]. 2023;24:rs.3.rs-2809973. 10.21203/rs.3.rs-2809973/v1.
- 126.Cheng N, Kim K-H, Lau LF. Senescent hepatic stellate cells promote liver regeneration through IL-6 and ligands of CXCR2. JCI insight 7, 2022. 10.1172/jci.insight.158207. [DOI] [PMC free article] [PubMed]
- 127.Kim SR, Puranik AS, Jiang K, Chen X, Zhu X-Y, Taylor I, Khodadadi-Jamayran A, Lerman A, Hickson LJ, Childs BG, Textor SC, Tchkonia T, Niewold TB, Kirkland JL, Lerman LO. Progressive cellular senescence mediates renal dysfunction in ischemic nephropathy. J Am Soc Nephrol. 2021;32:1987–2004. doi: 10.1681/ASN.2020091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JH, Campisi J, de Keizer PJ. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell. 2017;169:132–147.e16. doi: 10.1016/j.cell.2017.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lv F, Li N, Kong M, Wu J, Fan Z, Miao D, Xu Y, Ye Q, Wang Y. CDKN2a/p16 antagonizes hepatic stellate cell activation and liver fibrosis by modulating ROS levels. Front cell Dev Biol. 2020;8:176. doi: 10.3389/fcell.2020.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li C, Shen Y, Huang L, Liu C, Wang J. Senolytic therapy ameliorates renal fibrosis postacute kidney injury by alleviating renal senescence. FASEB J Off Publ Fed Am Soc Exp Biol. 2021;35:e21229. doi: 10.1096/fj.202001855RR. [DOI] [PubMed] [Google Scholar]
- 131.Hickson LTJ, Langhi Prata LGP, Bobart SA, Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q, Jordan KL, Kellogg TA, Khosla S, Koerber DM, Lagnado AB, Lawson DK, LeBrasseur NK, Lerman LO, McDonald KM, McKenzie TJ, Passos JF, Pignolo RJ, Pirtskhalava T, Saadiq IM, Schaefer KK, Textor SC, Victorelli SG, Volkman TL, Xue A, Wentworth MA, Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thadathil N, Selvarani R, Mohammed S, Nicklas EH, Tran AL, Kamal M, Luo W, Brown JL, Lawrence MM, Borowik AK, Miller BF, Van Remmen H, Richardson A, Deepa SS. Senolytic treatment reduces cell senescence and necroptosis in Sod1 knockout mice that is associated with reduced inflammation and hepatocellular carcinoma. Aging Cell. 2022;21:e13676. doi: 10.1111/acel.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Raffaele M, Kovacovicova K, Frohlich J, Lo Re O, Giallongo S, Oben JA, Faldyna M, Leva L, Giannone AG, Cabibi D, Vinciguerra M. Mild exacerbation of obesity- and age-dependent liver disease progression by senolytic cocktail dasatinib + quercetin. Cell Commun Signal. 2021;19:44. doi: 10.1186/s12964-021-00731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ritschka B, Knauer-Meyer T, Gonçalves DS, Mas A, Plassat J-L, Durik M, Jacobs H, Pedone E, Di Vicino U, Cosma MP, Keyes WM. The senotherapeutic drug ABT-737 disrupts aberrant p21 expression to restore liver regeneration in adult mice. Genes Dev. 2020;34:489–494. doi: 10.1101/gad.332643.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amor C, Feucht J, Leibold J, Ho Y-J, Zhu C, Alonso-Curbelo D, Mansilla-Soto J, Boyer JA, Li X, Giavridis T, Kulick A, Houlihan S, Peerschke E, Friedman SL, Ponomarev V, Piersigilli A, Sadelain M, Lowe SW. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583:127–132. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saccon TD, Nagpal R, Yadav H, Cavalcante MB, Nunes ADDC, Schneider A, Gesing A, Hughes B, Yousefzadeh M, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD, Masternak MM. Senolytic combination of dasatinib and quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol - Ser A Biol Sci Med Sci. 2021;76:1895–1905. doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patil P, Dong Q, Wang D, Chang J, Wiley C, Demaria M, Lee J, Kang J, Niedernhofer LJ, Robbins PD, Sowa G, Campisi J, Zhou D, Vo N. Systemic clearance of p16INK4a-positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18:e12927. doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jeon OH, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chandra A, Lagnado AB, Farr JN, Doolittle M, Tchkonia T, Kirkland JL, LeBrasseur NK, Robbins PD, Niedernhofer LJ, Ikeno Y, Passos JF, Monroe DG, Pignolo RJ, Khosla S. Targeted clearance of p21- but not p16-positive senescent cells prevents radiation-induced osteoporosis and increased marrow adiposity. Aging Cell. 2022;21:e13602. doi: 10.1111/acel.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li J, Karim MA, Che H, Geng Q, Miao D. Deletion of p16 prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Am J Transl Res. 2020;12:672–683. [PMC free article] [PubMed] [Google Scholar]
- 142.Ding Q, Liu H, Liu L, Ma C, Qin H, Wei Y, Ren Y. Deletion of p16 accelerates fracture healing in geriatric mice. Am J Transl Res. 2021;13:11107–11125. [PMC free article] [PubMed] [Google Scholar]
- 143.Che H, Li J, Li Y, Ma C, Liu H, Qin J, Dong J, Zhang Z, Xian CJ, Miao D, Wang L, Ren Y. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9:e52570. 10.7554/eLife.52570. [DOI] [PMC free article] [PubMed]
- 144.Hambright WS, Mu X, Gao X, Guo P, Kawakami Y, Mitchell J, Mullen M, Nelson A-L, Bahney C, Nishimura H, Hellwinkel J, Eck A, Huard J. The senolytic drug fisetin attenuates bone degeneration in the Zmpste24 (-/-) progeria mouse model. J Osteoporos. 2023;2023:5572754. doi: 10.1155/2023/5572754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lane N, Hsu B, Visich J, Xie B, Khan A, Dananberg J. A phase 2, randomized, double-blind, placebo-controlled study of senolytic molecule UBX0101 in the treatment of painful knee osteoarthritis. Osteoarthr Cartil. 2021;29:S52–S53. doi: 10.1016/j.joca.2021.02.077. [DOI] [Google Scholar]
- 146.Rocha LR, Nguyen Huu VA, Palomino La Torre C, Xu Q, Jabari M, Krawczyk M, Weinreb RN, Skowronska-Krawczyk D. Early removal of senescent cells protects retinal ganglion cells loss in experimental ocular hypertension. Aging Cell. 2020;19:e13089. doi: 10.1111/acel.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.El-Nimri NW, Moore SM, Zangwill LM, Proudfoot JA, Weinreb RN, Skowronska-Krawczyk D, Baxter SL. Evaluating the neuroprotective impact of senolytic drugs on human vision. Sci Rep. 2020;10:21752. doi: 10.1038/s41598-020-78802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim H, Jang J, Song MJ, Kim G, Park C-H, Lee DH, Lee S-H, Chung JH. Attenuation of intrinsic ageing of the skin via elimination of senescent dermal fibroblasts with senolytic drugs. J Eur Acad Dermatol Venereol. 2022;36:1125–1135. doi: 10.1111/jdv.18051. [DOI] [PubMed] [Google Scholar]
- 149.Takaya K, Ishii T, Asou T, Kishi K. Glutaminase inhibitors rejuvenate human skin via clearance of senescent cells: a study using a mouse/human chimeric model. Aging (Albany NY) 2022;14:8914–8926. doi: 10.18632/aging.204391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Takaya K, Asou T, Kishi K. New Senolysis Approach via Antibody-Drug Conjugate Targeting of the Senescent Cell Marker Apolipoprotein D for Skin Rejuvenation. Int J Mol Sci. 2023;24(6):5857. 10.3390/ijms24065857. [DOI] [PMC free article] [PubMed]
- 151.Matheu A, Pantoja C, Efeyan A, Criado LM, Martín-Caballero J, Flores JM, Klatt P, Serrano M. Increased gene dosage of Ink4a/Arf results in cancer resistance and normal aging. Genes Dev. 2004;18:2736–2746. doi: 10.1101/gad.310304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Azazmeh N, Assouline B, Winter E, Ruppo S, Nevo Y, Maly A, Meir K, Witkiewicz AK, Cohen J, Rizou SV, Pikarsky E, Luxenburg C, Gorgoulis VG, Ben-Porath I. Chronic expression of p16(INK4a) in the epidermis induces Wnt-mediated hyperplasia and promotes tumor initiation. Nat Commun. 2020;11:2711. doi: 10.1038/s41467-020-16475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Milanovic M, Fan DNY, Belenki D, Däbritz JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA, Mendoza-Parra MA, Kanashova T, Metzner M, Pardon K, Reimann M, Trumpp A, Dörken B, Zuber J, Gronemeyer H, Hummel M, Dittmar G, Lee S, Schmitt CA. Senescence-associated reprogramming promotes cancer stemness. Nature. 2018;553:96–100. doi: 10.1038/nature25167. [DOI] [PubMed] [Google Scholar]
- 155.Ohanna M, Giuliano S, Bonet C, Imbert V, Hofman V, Zangari J, Bille K, Robert C, Bressac-de Paillerets B, Hofman P, Rocchi S, Peyron JF, Lacour JP, Ballotti R, Bertolotto C. Senescent cells develop a parp-1 and nuclear factor-κB-associated secretome (PNAS) Genes Dev. 2011;25:1245–1261. doi: 10.1101/gad.625811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alimirah F, Pulido T, Valdovinos A, Alptekin S, Chang E, Jones E, Diaz DA, Flores J, Velarde MC, Demaria M, Davalos AR, Wiley CD, Limbad C, Desprez P-Y, Campisi J. Cellular senescence promotes skin carcinogenesis through p38MAPK and p44/42MAPK signaling. Cancer Res. 2020;80:3606–3619. doi: 10.1158/0008-5472.CAN-20-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dörr JR, Yu Y, Milanovic M, Beuster G, Zasada C, Däbritz JHM, Lisec J, Lenze D, Gerhardt A, Schleicher K, Kratzat S, Purfürst B, Walenta S, Mueller-Klieser W, Gräler M, Hummel M, Keller U, Buck AK, Dörken B, Willmitzer L, Reimann M, Kempa S, Lee S, Schmitt CA. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature. 2013;501:421–425. doi: 10.1038/nature12437. [DOI] [PubMed] [Google Scholar]
- 158.Wu H, Schiff DS, Lin Y, Neboori HJR, Goyal S, Feng Z, Haffty BG. Ionizing radiation sensitizes breast cancer cells to Bcl-2 inhibitor, ABT-737, through regulating Mcl-1. Radiat Res. 2014;182:618–625. doi: 10.1667/RR13856.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saleh T, Carpenter VJ, Tyutyunyk-Massey L, Murray G, Leverson JD, Souers AJ, Alotaibi MR, Faber AC, Reed J, Harada H, Gewirtz DA. Clearance of therapy-induced senescent tumor cells by the senolytic ABT-263 via interference with BCL-X(L) -BAX interaction. Mol Oncol. 2020;14:2504–2519. doi: 10.1002/1878-0261.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fleury H, Malaquin N, Tu V, Gilbert S, Martinez A, Olivier M-A, Sauriol A, Communal L, Leclerc-Desaulniers K, Carmona E, Provencher D, Mes-Masson A-M, Rodier F. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat Commun. 2019;10:2556. doi: 10.1038/s41467-019-10460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.González-Gualda E, Pàez-Ribes M, Lozano-Torres B, Macias D, Wilson JR, 3rd, González-López C, Ou H-L, Mirón-Barroso S, Zhang Z, Lérida-Viso A, Blandez JF, Bernardos A, Sancenón F, Rovira M, Fruk L, Martins CP, Serrano M, Doherty GJ, Martínez-Máñez R, Muñoz-Espín D. Galacto-conjugation of navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell. 2020;19:e13142. doi: 10.1111/acel.13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wakita M, Takahashi A, Sano O, Loo TM, Imai Y, Narukawa M, Iwata H, Matsudaira T, Kawamoto S, Ohtani N, Yoshimori T, Hara E. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat Commun. 2020;11:1935. doi: 10.1038/s41467-020-15719-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Y, Gundelach J, Lindquist LD, Baker DJ, van Deursen J, Bram RJ. Chemotherapy-induced cellular senescence suppresses progression of Notch-driven T-ALL. PLoS ONE. 2019;14:e0224172. doi: 10.1371/journal.pone.0224172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kovacovicova K, Skolnaja M, Heinmaa M, Mistrik M, Pata P, Pata I, Bartek J, Vinciguerra M. Senolytic cocktail dasatinib+quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front Oncol. 2018;8:459. doi: 10.3389/fonc.2018.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Marcozzi S, Beltrami AP, Malavolta M. Molecular mechanisms to target cellular senescence in aging and disease. Cells. 2022;11:3732. doi: 10.3390/cells11233732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hense JD, Garcia DN, Isola JV, Alvarado-Rincón JA, Zanini BM, Prosczek JB, Stout MB, Mason JB, Walsh PT, Brieño-Enríquez MA, Schadock I, Barros CC, Masternak MM, Schneider A. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. GeroScience. 2022;44:1747–1759. doi: 10.1007/s11357-022-00573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Acklin S, Zhang M, Du W, Zhao X, Plotkin M, Chang J, Campisi J, Zhou D, Xia F. Depletion of senescent-like neuronal cells alleviates cisplatin-induced peripheral neuropathy in mice. Sci Rep. 2020;10:14170. doi: 10.1038/s41598-020-71042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee S, Yu Y, Trimpert J, Benthani F, Mairhofer M, Richter-Pechanska P, Wyler E, Belenki D, Kaltenbrunner S, Pammer M, Kausche L, Firsching TC, Dietert K, Schotsaert M, Martínez-Romero C, Singh G, Kunz S, Niemeyer D, Ghanem R, Salzer HJF, Paar C, Mülleder M, Uccellini M, Michaelis EG, Khan A, Lau A, Schönlein M, Habringer A, Tomasits J, Adler JM, Kimeswenger S, Gruber AD, Hoetzenecker W, Steinkellner H, Purfürst B, Motz R, Di Pierro F, Lamprecht B, Osterrieder N, Landthaler M, Drosten C, García-Sastre A, Langer R, Ralser M, Eils R, Reimann M, Fan DNY, Schmitt CA. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature. 2021;599:283–289. doi: 10.1038/s41586-021-03995-1. [DOI] [PubMed] [Google Scholar]
- 169.Pastor-Fernández A, Bertos AR, Sierra-Ramírez A, Del Moral-Salmoral J, Merino J, de Ávila AI, Olagüe C, Villares R, González-Aseguinolaza G, Rodríguez MÁ, Fresno M, Gironés N, Bustos M, Smerdou C, Fernandez-Marcos PJ, von Kobbe C. Treatment with the senolytics dasatinib/quercetin reduces SARS-CoV-2-related mortality in mice. Aging Cell. 2023;22:e13771. doi: 10.1111/acel.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Delval L, Hantute-Ghesquier A, Sencio V, Flaman JM, Robil C, Angulo FS, Lipskaia L, Çobanoğlu O, Lacoste A-S, Machelart A, Danneels A, Corbin M, Deruyter L, Heumel S, Idziorek T, Séron K, Sauve F, Bongiovanni A, Prévot V, Wolowczuk I, Belouzard S, Saliou J-M, Gosset P, Bernard D, Rouillé Y, Adnot S, Duterque-Coquillaud M, Trottein F. Removal of senescent cells reduces the viral load and attenuates pulmonary and systemic inflammation in SARS-CoV-2-infected, aged hamsters. Nat aging. 2023;3:829–845. doi: 10.1038/s43587-023-00442-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F. Exploring the relevance of senotherapeutics for the current sars-cov-2 emergency and similar future global health threats. Cells. 2020;9:909. doi: 10.3390/cells9040909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hornsby PJ. Cellular senescence and tissue aging in vivo. J Gerontol A Biol Sci Med Sci. 2002;57(7):B251-6. 10.1093/gerona/57.7.b251. [DOI] [PubMed]
- 173.Wiley CD, Sharma R, Davis SS, Lopez-Dominguez JA, Mitchell KP, Wiley S, Alimirah F, Kim DE, Payne T, Rosko A, Aimontche E, Deshpande SM, Neri F, Kuehnemann C, Demaria M, Ramanathan A, Campisi J. Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis. Cell Metab. 2021;33:1124–1136.e5. doi: 10.1016/j.cmet.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M. Survey of senescent-cell markers with age in human tissues. Aging (Albany NY) 2020;12:4052–4066. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE. 2012;7:e45069. doi: 10.1371/journal.pone.0045069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 178.Perez K, Ciotlos S, McGirr J, Limbad C, Doi R, Nederveen JP, Nilsson MI, Winer DA, Evans W, Tarnopolsky M, Campis J, Melov S. Single nuclei profiling identifies cell specific markers of skeletal muscle aging, frailty, and senescence. Aging (Albany NY) 2022;14:9393–9422. doi: 10.18632/aging.204435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jeon OH, David N, Campisi J, Elisseeff JH. Senescent cells and osteoarthritis: a painful connection. J Clin Invest. 2018;128(4):1229–1237. 10.1172/JCI95147. [DOI] [PMC free article] [PubMed]
- 180.Del Rey MJ, Valín Á, Usategui A, Ergueta S, Martín E, Municio C, Cañete JD, Blanco FJ, Criado G, Pablos JL. Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immun Ageing. 2019;16:29. doi: 10.1186/s12979-019-0169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gustafson B, Nerstedt A, Smith U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun. 2019;10:1–9. doi: 10.1038/s41467-019-10688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen MD, Kirkland JL. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rouault C, Marcelin G, Adriouch S, Rose C, Genser L, Ambrosini M, Bichet JC, Zhang Y, Marquet F, Aron-Wisnewsky J, Poitou C, André S, Dérumeaux G, Guerre-Millo M, Clément K. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia. 2021;64:240–254. doi: 10.1007/s00125-020-05307-0. [DOI] [PubMed] [Google Scholar]
- 184.Graziano S, Johnston R, Deng O, Zhang J, Gonzalo S. Vitamin D/vitamin D receptor axis regulates DNA repair during oncogene-induced senescence. Oncogene. 2016;35:5362–5376. doi: 10.1038/onc.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wiggs JL, Yaspan BL, Hauser MA, Kang JH, Allingham RR, Olson LM, Abdrabou W, Fan BJ, Wang DY, Brodeur W, Budenz DL, Caprioli J, Crenshaw A, Crooks K, DelBono E, Doheny KF, Friedman DS, Gaasterland D, Gaasterland T, Laurie C, Lee RK, Lichter PR, Loomis S, Liu Y, Medeiros FA, McCarty C, Mirel D, Moroi SE, Musch DC, Realini A, Rozsa FW, Schuman JS, Scott K, Singh K, Stein JD, Trager EH, VanVeldhuisen P, Vollrath D, Wollstein G, Yoneyama S, Zhang K, Weinreb RN, Ernst J, Kellis M, Masuda T, Zack D, Richards JE, Pericak-Vance M, Pasquale LR, Haines JL. Common variants at 9p21 and 8q22 are associated with increased susceptibility to optic nerve degeneration in glaucoma. PLoS Genet. 2012;8:e1002654. doi: 10.1371/journal.pgen.1002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bao XY, Xie C, Yang MS. Association between type 2 diabetes and CDKN2A/B: a meta-analysis study. Mol Biol Rep. 2012;39:1609–1616. doi: 10.1007/s11033-011-0900-5. [DOI] [PubMed] [Google Scholar]
- 187.Picca A, Calvani R, Coelho-Júnior HJ, Marini F, Landi F, Marzetti E. Circulating inflammatory, mitochondrial dysfunction, and senescence-related markers in older adults with physical frailty and sarcopenia: a BIOSPHERE exploratory study. Int J Mol Sci. 2022;23:14006. doi: 10.3390/ijms232214006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Seitz-Holland J, Mulsant BH, Reynolds CF, III, Blumberger DM, Karp JF, Butters MA, Mendes-Silva AP, Vieira EL, Tseng G, Lenze EJ, Diniz BS. Major depression, physical health and molecular senescence markers abnormalities. Nat Ment Heal. 2023;1:200–209. doi: 10.1038/s44220-023-00033-z. [DOI] [Google Scholar]
- 189.Fielding RA, Atkinson EJ, Aversa Z, White TA, Heeren AA, Achenbach SJ, Mielke MM, Cummings SR, Pahor M, Leeuwenburgh C, LeBrasseur NK. Associations between biomarkers of cellular senescence and physical function in humans: observations from the lifestyle interventions for elders (LIFE) study. GeroScience. 2022;44:2757–2770. doi: 10.1007/s11357-022-00685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fried LP, Cohen AA, Xue Q-L, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat aging. 2021;1:36–46. doi: 10.1038/s43587-020-00017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ipson BR, Fletcher MB, Espinoza SE, Fisher AL. Identifying exosome-derived microRNAs as candidate biomarkers of frailty. J frailty aging. 2018;7:100–103. doi: 10.14283/jfa.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim H-N, Chang J, Iyer S, Han L, Campisi J, Manolagas SC, Zhou D, Almeida M. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell. 2019;18:e12923. doi: 10.1111/acel.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Torrance BL, Cadar AN, Martin DE, Panier HA, Lorenzo EC, Bartley JM, Xu M, Haynes L. Senolytic treatment with dasatinib and quercetin does not improve overall influenza responses in aged mice. Front aging. 2023;4:1212750. doi: 10.3389/fragi.2023.1212750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.