Abstract
Parthenium hysterophorous, a widespread weed in India, contributes a substantial amount of lignocellulosic biomass. The key objective of this study is to evaluate the feasibility of producing xylanase enzyme from P. hysterophorus weed biomass using the fungus Aspergillus niger. The impact of various physiological factors was confirmed through a two-step approach: first, a one-factor-at-a-time (OFAT) investigation, and subsequently, employing the RSM-based CCD method in statistical design. This research revealed that the RSM-based model led to the optimization of enzyme activity, resulting in a value of 2098.08 IU/gds for xylanase. This was achieved with an incubation time of 4.5 days, a medium pH of 6, and a cultivation temperature of 32.5 °C. Additionally, a pretreatment involving 1% NaOH and a 30-min autoclave treatment was found to alter the chemical composition of lignocellulose substrates (cellulose 43.87% and xylan 28.7%), thereby enhancing the efficiency of enzymatic hydrolysis. Moreover, fermentable sugars were produced by autoclave-assisted alkali pretreatment (NaOH-1.0% w/v) at rates of 219.6 ± 2.05 mg/gds−1 by utilizing the crude xylanase from A. niger and 291.3 ± 1.2 mg/gds−1 from commercial xylanase enzyme. Our study revealed that P. hysterophorus served as a viable and affordable substrate for fermentable sugar liberation, and xylanase is a rate-limiting enzyme in enzymatic saccharification.
Keywords: Xylanase, Parthenium hysterophorus, Aspergillus niger, OVAT, Response surface methodology, Enzymatic saccharification
Introduction
In nature, lignocellulosic biomass is widely distributed, and xylan (a polysaccharide) is the hemicellulose component of holocellulose and an essential part of the weed. Xylan is an important source of renewable biomass and serves as raw material for manufacturing various goods, including biofuels, inexpensive sources of energy for fermentation, and enhanced feed for animals. Most bioconversion steps involve the conversion of xylan to xylose and xylo-oligosaccharides. Acid hydrolysis or xylanolytic enzymes (xylanases) that break down hemicellulose by deconstructing plant structural components can convert xylan into xylose (Bhardwaj et al. 2019). According to the Brundtland Commission, environmental sustainability is defined as growth that scales up to satisfy current demands without harming the potential to meet future needs (Hajian and Kashani 2021). Concerns about energy security are another motivator for nations looking for environmentally sustainable methods of energy production. Various recent research reports showed a significant change in environmental research from conventional technologies to environmentally friendly, sustainable, and cost-effective technologies known as "green technologies" (Shan et al. 2021). Enzyme technology, for example, is a sustainable and effective method that has been employed in a variety of manufacturing processes as a substitute for chemical catalysis, with benefits in terms of efficiency and sustainability (Loi et al. 2021).
P. hysterophorus, one of the most harmful invasive weeds in the world, is widespread in Asia, Africa, the United States, and Australia (Saini et al. 2014). This weed has proven challenging to control using conventional physical, chemical, biological, and integrative approaches. However, there are several microorganisms that produce the enzymes that can penetrate the cell walls of the lignocellulosic components and utilize biomass as a food source. This approach provides an efficient method for eliminating this weed. Recently, researchers have tried to use weeds as a bioresource for various purposes in an effort to take an innovative approach to weed management. For the production of xylanase (Bharti et al. 2018), β-Glucosidase (Kumar et al. 2022), endoglucanase (Saini and Aggarwal 2019), and cellulase (Saini et al. 2017), Parthenium weed has the potential to be an affordable substrate. The manufacturing of paper, textiles, pharmaceuticals, and food are just a few examples of the many uses for xylanases, which can break down xylan, the second-most abundant polymer after cellulose (Bhardwaj et al. 2019). Xylanases are hydrolytic enzymes that cleave the β-1,4-bonds that make up xylans. They are composed of d-Xylose monomers, occasionally with some branches of d-Mannose, l-Arabinose, or d-Galactose (Gupta et al. 2019).
Non-food lignocellulosic biomass is a highly environmentally friendly carbon source for renewable energy sources, biochemicals, biological materials, and sustainable goods. In contrast to 1G bioethanol production from food-based sources, 2G bioethanol generated from non-food lignocellulosic material will not put food crops in competition (Huang et al. 2019). SSF and submerged fermentation (SmF) are used in commercial and industrial applications to produce enzymes. However, due to its significant applications over SmF in current years, SSF has taken over as the preferred option. SSF gained this trust because of its minimal water usage, low risks of contamination, low energy requirements, and effectiveness of the process, including the downstream process step where a product of superior quality is obtained and easily separable (El-shishtawy et al. 2014). Furthermore, because a large amount of biomass is present and catabolic repression is less during SSF, a higher enzyme production can be achieved (Kar et al. 2013). For the synthesis of xylanases by SSF utilizing various agricultural wastes, the genus Aspergillus, especially A. fumigatus, A. oryzae, A. niger, and A. ibericus, has been used (Amin et al. 2021).
The cost-effective manufacture of xylanase in high quantities is necessary for its industrial uses. Using inexpensive agricultural waste as a carbon source and superior microbial strains can lower manufacturing costs. The OFAT technique is commonly used to optimize parameters for enzyme synthesis, but it does not account for variable interaction (Kumar et al. 2012). The optimal design of the culture medium for enzyme production using statistical models like RSM is an alternative that offers the fewest trials for a large number of process parameters and modelling of their interactions. RSM has been used effectively to increase production while lowering the cost and time required for biotechnological process development. RSM is a collection of statistical methods for designing and simulating the progression of experiments, examining the effects of various variables, and identifying the ideal conditions for achieving the desired outcomes. The predicted outcome of interactions between different process variables is also made possible by this methodology (Kumar et al. 2023a, b).
In this study, we used the one factor at a time (OFAT) and CCD approaches to optimize the xylanase production of A. niger strain isolated from decayed organic waste cultivated in SSF with P. hysterophorus as a substrate. The applications of crude and commercial xylanase enzymes were experiential to liberate reducing sugars from alkali-pretreated P. hysterophorus biomass by an enzymatic saccharification process.
Materials and methods
Microorganism
The fungal strain used in this study, Aspergillus niger (accession no. OP270219), was isolated from decaying organic matter and soil samples, purified, grown, and stored at 30 °C on PDA plates. It was obtained from the collection of cultures at the Enzyme and Fermentation Laboratory, Department of Microbiology, MDU, Rohtak.
Substrate collection and processing
Parthenium hysterophorus is an annual weed obtained from the university campus of Kurukshetra University, Kurukshetra, Haryana, India. These weeds further used as a substrate for the synthesis of xylanase in the current study. Water was used to cleanse any leftover contaminants prior to its usage. To remove moisture, the collected P. hysterophorus was dried in a hot air oven at 60 °C for 48 h. After washing and drying, it was crushed with a lab grinder and stored in poly bags for further examination.
Enzyme production
Parthenium hysterophorus was used as the primary carbon source substrate for stationary cultivation. The isolate was grown for 7 days at 30 °C on the PDA slant until complete sporulation was achieved for inoculum formation. P. hysterophorus (5 g) and distilled water (10 ml) are both present in the production medium. To produce xylanase, the flasks were allowed to incubate at 30 °C for five days under static conditions after being injected with 2 × 106 spores/ml. The flasks were carefully tapped at the bottom at regular intervals to stimulate air exchange. Enzyme harvesting was done using phosphate buffer containing 0.1% Tween 80. The contents were centrifuged at 4 °C for 20 min at 10,000 rpm to extract the enzyme. First, a muslin cloth separated the liquid culture filtrate from the solid mycelial portion. The clear supernatant was filtered using Whatman filter paper no. 1 and used as a crude enzyme source in subsequent tests.
Enzyme assay
The DNS test for reducing sugars was used to evaluate xylanase activity in accordance with Miller's method (1959). Beechwood xylan (1 g in 100 ml of citrate buffer, pH 5) and the enzyme were introduced to a reaction that contained 1% (w/v) beechwood xylan. After 15 min of incubation at 55 °C in a water bath, the reaction mixture was stopped by adding 1 ml of the 3,5-dintrosalicylic acid (DNS) reagent and further heated for 5 min in a water bath at 100 °C. A spectrophotometer-Shimadzu UV-1900, Japan was used to measure the absorbance at 540 nm to determine the quantity of sugar liberated by the enzyme. “Under the specified assay conditions, one unit (U) of xylanase was defined as the quantity of enzyme that liberated 1 µmol xylose as reducing sugar equivalents per minute”.
Optimization of xylanase production by OFAT approach
The impact of different process factors that included incubation period (1–6 days), cultivation temperature (20–40 °C), substrate concentration (5–20 g), substrate-to-moisture ratio w/v (1:0.5–1:2.5), inoculum size (2.0 × 106 to 5.0 × 106), initial medium pH (3–8), and nitrogen source on xylanase production by A. niger was studied under SSF using the OFAT method.
Experimental design
Xylanase production optimization was achieved by performing response surface methodology (RSM). The incubation period, pH, and temperature, three independent variables, were further optimized based on the outcomes of OFAT optimization. RSM optimized the effect of three variables on xylanase production: incubation duration (A, Days), pH (B), and temperature (C, °C). The CCD was used for statistical evaluations in this investigation. Table 1 describes the CCD levels and their coded values. A '2-Order Polynomial Model' was fitted to the experimental results using multiple regression equations.
| 1 |
“Where Y is the predicted response, β0 is the intercept, n is the number of factors analyzed, βi, βii, and βij are the linear (main effect), quadratic, or interactive model coefficients, respectively. Accordingly, Xi and Xj indicate the levels of the independent parameters”. For the RSM design, the statistical software ‘Design Expert 13.0’ Software, Stat-Ease, Inc., USA, was utilized for the investigation.
Table 1.
Factors and levels of variation in central composite design (CCD)
| Study type | Response Surface | ||||
|---|---|---|---|---|---|
| Design type | Central composite | Runs 20 | |||
| Design mode | Quadratic | Blocks—No blocks | |||
| Factor | Name | Units | Type | Minimum | Maximum |
| A | Incubation period | Days | Numeric | 1.98 | 7.02 |
| B | pH | Numeric | 4.32 | 7.68 | |
| C | Temperature | °C | Numeric | 19.89 | 45.11 |
| Response | Name | Units | Obs | Analysis |
|---|---|---|---|---|
| R1 | Xylanase | IU/gds | 20 | Polynomial |
Autoclave-assisted NaOH pretreatment
In an autoclave-assisted NaOH pretreatment process, one gram of weed powder was combined with 10 mL of a 1% NaOH solution, maintaining a solid-to-liquid ratio of 1:10 (w/v). These mixtures were then subjected to autoclaving at a temperature of 121 °C and a pressure of 15 psi for a duration of 30 min. This step aimed to break down the biomass through hydrolysis. Following the treatment, the solid residues were separated by filtration using a Rocker 300 vacuum pump. A porcelain crucible was used as a filter to separate the liquid portion from the solid part of the slurry. The solid portion was then washed many times with distilled water until the pH was stable or no more Na + was detected (Saroj and Korrapati 2018). The resulting solid waste was subsequently dried until it reached a consistent weight and then stored at room temperature for compositional analysis and enzyme hydrolysis.
Compositional analysis
Estimation of cellulose
To ascertain the cellulose content in 1 g of oven-dried biomass (whether it was untreated or subjected to pre-treatment), it was mixed with a solution containing 10 ml of 80% acetic acid and 1.5 ml of nitric acid for a duration of 20 min. This process served to dissolve the lignin and hemicellulose components present in the biomass (Updegraff 1969). The remaining cellulose content in the solid portion was determined through gravimetric analysis (Ahmed et al. 2010). The mixture was then filtered through pre-weighed filtering crucibles (w1) using a vacuum pump (specifically, a Rocker 300). These crucibles were subsequently subjected to oven drying at 105 °C until a constant weight (w2) was reached. The cellulose content (%w/w) was calculated using the following formula:
Estimation of Xylan
Xylan extraction from lignocellulosic biomass was carried out using a modified procedure based on Hauli et al. (2013) and Ipsit et al. (2013). The lignocellulosic powders (derived from P. hysterophorus) were first immersed in a 10% NaOH solution (1:10 ratio) and left to soak with continuous agitation at 60 °C overnight. Subsequently, they were subjected to steam treatment at 100 °C for 3 h. Following the alkaline treatment, the liquid phase was separated through centrifugation at 10,000 rpm for 15 min and then acidified to a pH of 5.0 using 12N HCl. Next, 1.5 times the volume of 95% ethanol was added to precipitate the xylan. After another round of centrifugation, the xylan was air-dried initially and then further dried in a hot air oven at 55 °C for 4 h. The resulting pellets were weighed, pulverized using a mixer, and stored at room temperature for subsequent analysis. The actual xylan content was determined using the following formula:
Estimation of lignin
The method developed by Yao et al. (2010) was used to calculate the lignin content. To perform this assessment, the dried biomass (referred to as "wo") underwent a two-hour hydrolysis process using 72% sulfuric acid at a temperature of 20 °C, maintaining a 1:15 bath ratio. During this procedure, both cellulose and hemicellulose were subjected to hydrolysis (Bhagia et al. 2016). Glass crucibles were utilized to separate the components, and their initial weight was recorded as "w1." The solid residue collected in the crucible was rinsed with hot water and subsequently dried in an oven at 105 °C until it reached a consistent weight (labeled "w2"). The difference in weight before and after acid hydrolysis was utilized to estimate the percentage of lignin content (w/w):
Hydrolysis of P. hysterophorus from crude and commercial xylanase enzyme
Enzymatic hydrolysis of alkali-pretreated P. hysterophorus was conducted in a 100 mL flask with 10% solid loading, 5, 10, 15, and 20 U of crude fungal xylanase and commercial xylanase, 50 mM buffer (Na3C6H5O7, pH 4.8), maintaining an overall volume of 25 mL, having 0.001% (w/v) NaN3, and incubated at 50 °C for 96 h with a shaking speed of 120 rpm. Aliquots of the sample were taken out at intervals of 24, 48, 72, and 96 h. The collected samples were centrifuged for 10 min at 10,000 rpm and the supernatant was used for estimating fermentable sugars using Miller's DNSA procedure (Miller 1959). The absorbance values were calculated at 540 nm using the spectrophotometer 'SHIMADZU UV-1900 UV–VIS Spectrophotometer'.
Analysis of reducing sugars by high-pressure liquid chromatography
High-Performance Liquid Chromatography (HPLC) is a powerful analytical technique used to separate, identify, and quantify various compounds in a mixture. In the context of fermentable sugars, HPLC is commonly employed to analyze the concentration of sugars produced during fermentation processes. The enzymatic hydrolysate of P. hysterophorus, pretreated with autoclave-assisted NaOH (1%), was examined for the existence of different reducing sugars using HPLC (High-Performance Liquid Chromatography) with an Agilent instrument and an Aminex HPX87 column from BioRad, USA. Distilled water was employed as the eluent, and the flow rate was set at 0.3 mL/min. The oven temperature was maintained at room temperature, and a refractive index (RI) detector was used for analysis.
Results and discussion
Xylanase optimization using OFAT approach
In this context, P. hysterophorus is potentially used as a substrate for the manufacturing of xylanase by A. niger. Furthermore, various operational parameters were optimized for xylanase enzymes in SSF, including incubation period, cultivation temperature, substrate concentration, substrate-to-moisture ratio, inoculum size, initial medium pH, and nitrogen source.
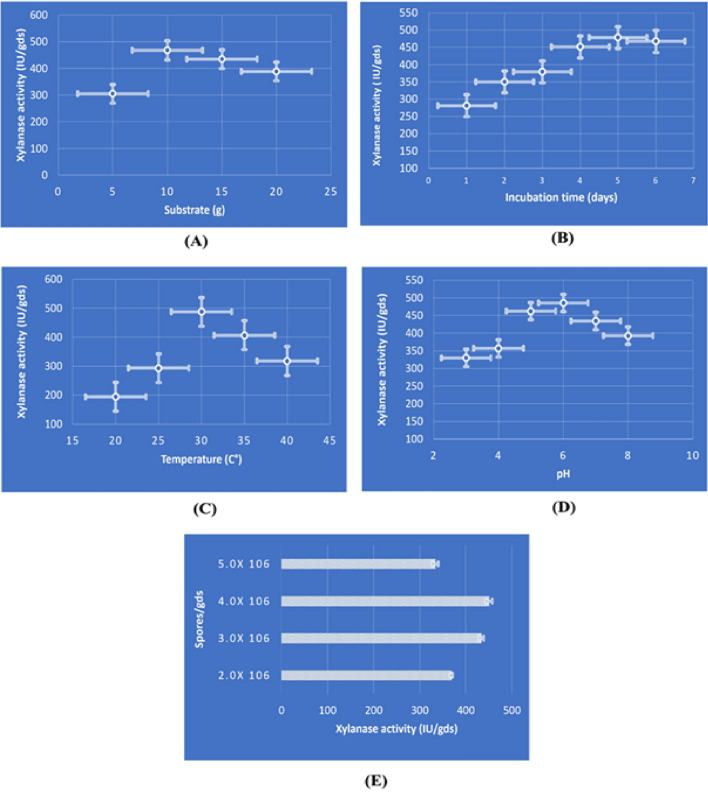
The ideal substrate concentration was also evaluated by varying the concentration from 5 to 20 g in a 250 ml flask. The carbon source P. hysterophorus (10 g) showed the highest level of xylanase activity (468 ± 8.7 IU/gds) (Fig. 1A). The primary substrate for the synthesis of the xylanase enzyme is xylan. However, the costly price of model base materials makes them unsuitable for industrial use. Lignocellulosic materials are suitable as substrates for commercial enzyme production due to their wide availability and affordable cost. Hemicelluloses, which serve as xylanase inducers, are present in the complex structure of lignocellulosic materials. According to a chemical composition analysis, P. hysterophorus contains the following percentages of cellulose, hemicelluloses, and lignin: 48.79, 28.66, and 14.71%, respectively (Kumar et al. 2023a).
Fig. 1.
Enzyme activity under OFAT conditions: A substrate, B incubation period, C temperature, D pH, E inoculum size
In this study, A. niger was incubated for varying periods, ranging from 1 to 6 days, to determine the impact of the incubation period on the synthesis of the enzyme. Under stationary conditions, maximum xylanase activity (478 ± 12.5 IU/gds) was achieved on the 5th day of growth (Fig. 1B). Enzyme activity decreases as incubation times are increased above the optimal time. The reasons may be a lack of nutrition in the medium used for fermentation and the formation of harmful compounds that interfere with spore development and xylanase synthesis (kar et al. 2013).
Enzymes are often sensitive to harsh temperature conditions; therefore, finding the optimal process parameters for the highest enzyme activity will improve the efficiency of the enzymatic processes. The temperature effect on xylanase enzyme production by A. niger was studied by raising the cultivation temperature from 20 to 40 °C at 5 °C gaps. Maximum enzyme activity (487 ± 7.6 IU/gds) was observed at 30 °C by A. niger (Fig. 1C). The activity of enzymes further decreased as the incubation temperature was raised.
Experiments were conducted at varied pH levels of the medium, ranging from 3 to 8, to determine the ideal initial medium pH for enzyme synthesis. When the starting pH of the medium was fixed at 6.0 under static conditions, the fungus exhibited its maximum xylanase activity (485 ± 7.8 IU/gds) (Fig. 1D). Changes in pH can affect the stability and rate of synthesis of fungus enzymes (Prasanna et al. 2016). The optimal pH for xylanase produced by A. oryzae LC1 was found to be 5.0 (Bhardwaj et al. 2019), but A. fumigatus SK1 gave superior results at a pH of 4.0 (Ang et al. 2013).
Findings in Fig. 1E indicated the effect of different inoculum sizes on enzyme synthesis by A. niger in SSF using P. hysterophorus as a substrate. Outcomes reported that the highest xylanase synthesis was observed with a 4.0 × 106 inoculum size, yielding an enzyme activity of 451 ± 6.5 IU/gds.
In the SSF process, moisture content plays an important role. The synthesis process involved varying the substrate-to-moisture ratio from 1:1 to 1:6 and measuring the enzyme. Table 2 contains the data, showing that the most xylanase was produced at a ratio of 1:1.5 (423 ± 11.6 IU/gds). When initial moisture is decreased from 33 to 50%, the corresponding enzyme activity is significantly reduced. Moisture has a direct impact on the synthesis of enzymes by affecting nutrient availability and gas flow throughout the course of fermentation (da Silva Menezes et al. 2018).
Table 2.
Effect of substrate-to-moisture ratio on the production of enzyme by A. niger
| Substrate: Moisture (w/v) | Xylanase activity (IU/gds) |
|---|---|
| 1:0.5 | 183 ± 12.09 |
| 1:1.0 | 280 ± 8.5 |
| 1:1.5 | 423 ± 11.6 |
| 1:2.0 | 379 ± 9.07 |
| 1:2.5 | 316 ± 6.42 |
Bold values indicate the maximum xylanase activity
Various nitrogen sources were studied for their impact on xylanase production under static conditions. Peptone was the optimum nitrogen source for the fungus's increased enzyme synthesis under stationary fermentation conditions. It was found that peptone was most effective at 1.5% (w/v) in inducing the highest levels of xylanase synthesis (464 ± 7.3 IU/gds) when trying to determine the ideal peptone concentration for maximizing xylanase output (Table 3). With the addition of 5 g/l of peptone, the highest xylanase (3069 U mg−1) synthesis by A. niger was also reported by Javed et al. (2017).
Table 3.
Effect of nitrogen sources on xylanase production by A. niger
| Nitrogen sources | Conc. (%) | Xylanase activity (IU/gds) |
|---|---|---|
| Ammonium sulphate | 0.5 | 333 ± 7.3 |
| 1.0 | 392 ± 4.7 | |
| 1.5 | 368 ± 8.3 | |
| 2.0 | 357 ± 5.6 | |
| Urea | 0.5 | 186 ± 7.7 |
| 1.0 | 236 ± 7.5 | |
| 1.5 | 457 ± 8.5 | |
| 2.0 | 312 ± 6.5 | |
| Peptone | 0.5 | 383 ± 7.09 |
| 1.0 | 458 ± 13.4 | |
| 1.5 | 464 ± 7.3 | |
| 2.0 | 421 ± 3.7 | |
| Yeast extract | 0.5 | 240 ± 8.08 |
| 1.0 | 445 ± 5.5 | |
| 1.5 | 457 ± 8.5 | |
| 2.0 | 312 ± 6.5 | |
| Control | - | 182 ± 6.1 |
“All the experiments were carried out in triplicate and results were presented as mean ± standard deviation.”
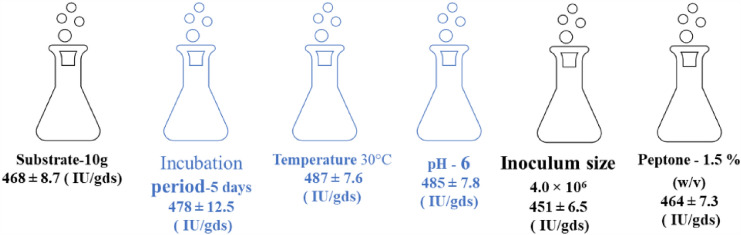
Three factors (incubation period, pH, and temperature) chosen from the OFAT techniques were applied to RSM under optimum conditions for further statically optimizing xylanase production (Fig. 2).
Fig. 2.
Factors chosen from the OFAT condition
Statistical analysis of xylanase production by CCD
According to the outcomes of the OFAT technique, the incubation period (A), pH (B), and temperature (C) were selected to be optimized by RSM to produce the most xylanase. The CCD of RSM determined ideal levels for these variables. For enzyme production optimization, 20 runs of experimentation are carried out utilizing various CCD combinations of factors. Table 4 shows the range of xylanase produced under specific experimental conditions from 1632.42 IU/gds to 2098.08 IU/gds. ANOVA was used to determine the equation's statistical significance, and the results are shown in Table 5.
Table 4.
Results of CCD of xylanase production (IU/gds)
| Factor 1 | Factor 2 | Factor 3 | Response | |
|---|---|---|---|---|
| Run no | A: Incubation period | B: pH | C: Temperature | Xylanase |
| (Days) | (°C) | (IU/gds) | ||
| 1 | 1.98 | 4.32 | 19.89 | 1856.56 |
| 2 | 7.02 | 4.32 | 19.89 | 1775.78 |
| 3 | 1.98 | 7.68 | 19.89 | 1842.46 |
| 4 | 7.02 | 7.68 | 19.89 | 1711.52 |
| 5 | 1.98 | 4.32 | 45.11 | 1854.76 |
| 6 | 7.02 | 4.32 | 45.11 | 1707.56 |
| 7 | 1.98 | 7.68 | 45.11 | 1878.92 |
| 8 | 7.02 | 7.68 | 45.11 | 1761.58 |
| 9 | 0.2 | 6 | 32.5 | 1781.82 |
| 10 | 8.7 | 6 | 32.5 | 1632.42 |
| 11 | 4.5 | 3.1 | 32.5 | 1998.01 |
| 12 | 4.5 | 8.8 | 32.5 | 1996.09 |
| 13 | 4.5 | 6 | 11.2 | 1829.28 |
| 14 | 4.5 | 6 | 53.7 | 1859.76 |
| 15 | 4.5 | 6 | 32.5 | 2098.08 |
| 16 | 4.5 | 6 | 32.5 | 2049.06 |
| 17 | 4.5 | 6 | 32.5 | 2048.1 |
| 18 | 4.5 | 6 | 32.5 | 2078.81 |
| 19 | 4.5 | 6 | 32.5 | 2036.08 |
| 20 | 4.5 | 6 | 32.5 | 2034.09 |
Table 5.
One-way analysis of variance for quadratic model of CCD
| Source | Sum of Squares | df | Mean Square | F-value | p-value | ||
|---|---|---|---|---|---|---|---|
| Model | 48.77 | 9 | 5.42 | 44.18 | < 0.0001 | Significant | |
| A-Incubation period | 5.49 | 1 | 5.49 | 44.80 | < 0.0001 | ||
| B-pH | 0.0001 | 1 | 0.0001 | 0.0010 | 0.9751 | ||
| C-Temperature | 0.0448 | 1 | 0.0448 | 0.3649 | 0.5592 | ||
| AB | 0.0070 | 1 | 0.0070 | 0.0571 | 0.8160 | ||
| AC | 0.0478 | 1 | 0.0478 | 0.3898 | 0.5464 | ||
| BC | 0.4332 | 1 | 0.4332 | 3.53 | 0.0896 | ||
| A2 | 33.44 | 1 | 33.44 | 272.69 | < 0.0001 | ||
| B2 | 1.55 | 1 | 1.55 | 12.64 | 0.0052 | ||
| C2 | 12.83 | 1 | 12.83 | 104.60 | < 0.0001 | ||
| Residual | 1.23 | 10 | 0.1226 | ||||
| Lack of Fit | 0.8310 | 5 | 0.1662 | 2.10 | 0.2172 | Not significant | |
| Pure Error | 0.3955 | 5 | 0.0791 | ||||
| Cor Total | 49.99 | 19 |
The model's F-value of 44.18 indicates that it is significant. A "Lack of Fit F-value" of 2.10 indicates that the lack of fit is insignificant in comparison to the pure error. A "lack of fit F-value" of this magnitude has a 21.72% chance of occurring due to noise. As a result, a non-significant lack of fit is desirable. The "Pred R-Squared" of 0.8592 is in reasonable agreement with the "Adj R-Squared" of 0.9534. Three linear factors (A, B, and C), all quadratic factors (A2, B2, and C2), and three interaction factors (AB, AC, and BC) were all significant.
| 2 |
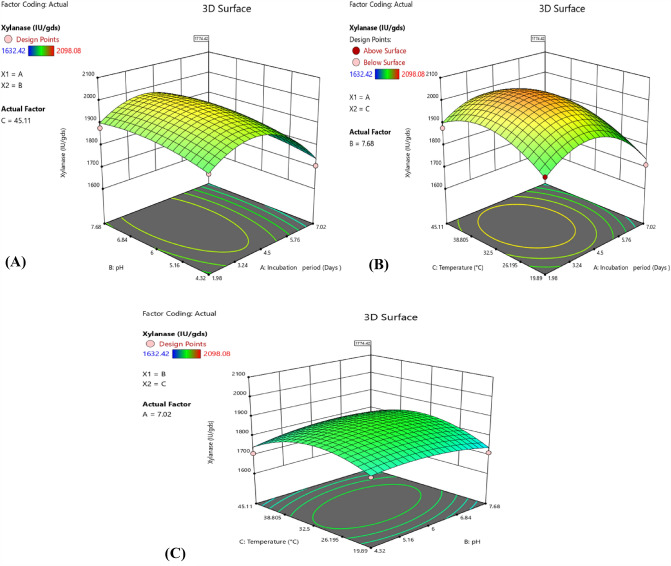
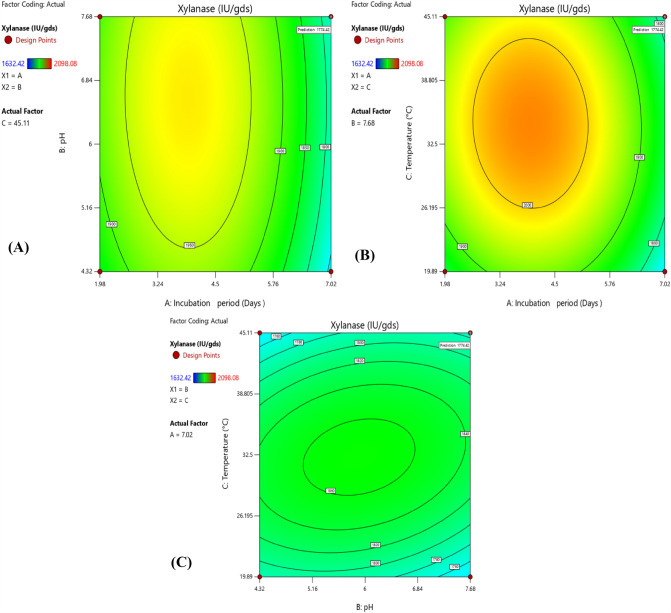
The 3D models (Fig. 3) and contour graphs (Fig. 4), which are represented by a 2nd-order polynomial equation, were created to explore the interactions between factors and an ideal level of factors for enzyme synthesis.
Fig. 3.
3D plot showing the effect and interaction of: a pH vs. incubation period; b Temperature vs. incubation period; c Temperature vs. pH on fungal xylanase production
Fig. 4.
Contour plot showing the effect and interaction of: a pH vs. incubation period; b Temperature vs. incubation period; c Temperature vs. pH on fungal xylanase production
Validation of the developed models
By evaluating the model's validity using a random set of 20 experiments, xylanase production was demonstrated to be accurate. The outcomes demonstrate that the model was successfully validated because the actual values were extremely similar to the predicted ones. Furthermore, these validation trials revealed. As a result, it was determined that the CCD-based RSM models could accurately and reliably predict A. niger ability to produce xylanase. The xylanase enzyme was produced in SSF in the represented study, and similar studies are given in Table 6.
Table 6.
Comparison of solid-state fermentation xylanase enzyme production from several fungi
| Microorganisms | Substrate | Xylanase (IU gds−1) | Fermentation conditions | References |
|---|---|---|---|---|
| A. niger CECT 2700 | Brewery spent grain | 1400.80 | SSF | Moran-Aguilar et al. (2021) |
| A. tubingensis JP-1 | Wheat straw | 6887 ± 16 | SSF | Pandya and Gupte (2012) |
| A. niger CECT 2700 | Corncob | 2926 | SSF | Pérez-Rodríguez et al. (2014) |
| A.niger CCUG33991 | Wheat bran | 2919 ± 174 | SSF | Khanahmadi et al. (2018) |
| A. niger BG | Wheat bran | 5427.51 ± 4.4 | SSF | Azzouz et al. (2020) |
| A. niger VSRK09 | P. hysterophorus | 2098.08 | SSF | Present study |
Autoclave-assisted NaOH pretreatment
The compositional analysis of untreated weed biomass was found to contain 39.46% cellulose, 29.2% xylan, and 23.26% lignin contents, as shown in Fig. 5. After treatment using 1% NaOH + 30-min autoclave, the biomass had 43.87% cellulose, 28.7% xylan, and 20.64% lignin (Fig. 5).
Fig. 5.
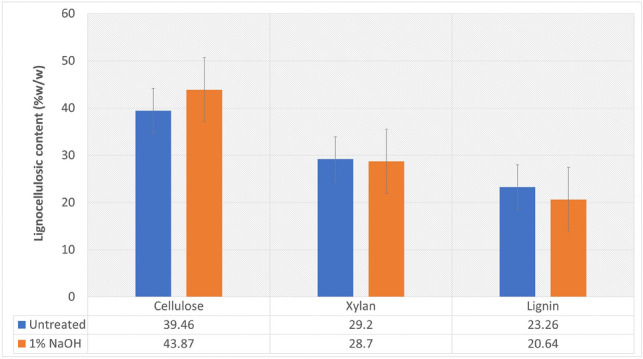
Effect of 1% NaOH concentration on P. hysterophorus biomass
Saccharification of alkali-treated P. hysterophorus biomass with crude and commercial xylanase
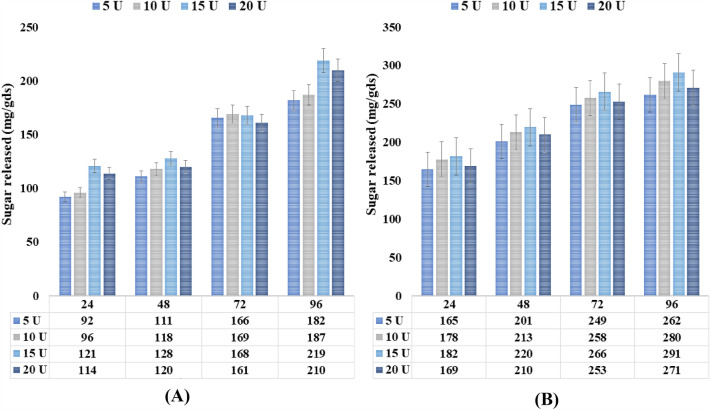
In modern biotechnology, the use of enzymatic saccharification to liberate sugar from agricultural leftovers is of particular interest, primarily for bioethanol production. In the current research, we attempted the enzymatic hydrolysis of alkali-pretreated (NaOH-1.0% w/v) weed biomass using crude xylanase produced by A. niger using P. hysterophorus under SSF and commercial xylanase. Each pretreatment technique has a unique impact on the cellulose, lignin, and xylan contents of the biomass. The essential substrate for the production of bioethanol is cellulose; hence, it should be preserved as much as possible from the biomass matrix after pretreatment by carefully selecting the suitable pretreatment techniques and conditions (Tsegaye et al. 2019). Alkali primarily interacts with lignin, which results in effective delignification. The ether and ester linkages, in particular, that bind lignin to hemicellulose are broken by sodium hydroxide (Moodley and Kana 2017). Because pretreatment of biomass improves enzymatic saccharification, it is extremely important before hydrolysis (Kucharska et al. 2020). Total liberated sugars showed that fungal xylanase enzymes from A. niger strains work effectively in the P. hysterophorus substrate. The efficiency of hydrolysis significantly increased during 48 to 96 h, with releases in fermentable sugar ranging from 128.3 ± 6.1 to 219.6 ± 2.05 mg/gds−1, respectively. The maximum conversion was observed during the 48 to 96 h period, as shown in Fig. 6a.
Fig. 6.
Enzymatic hydrolysis of P. hysterophorus by (A) crude xylanase from A. niger and (B) commercial xylanase at temperature 50 °C, pH 4.8 for 96 h. Enzyme doses varied from 5 to 20 U/g of substrate
In the case of commercial xylanase, the efficiency of hydrolysis significantly increased during 24 to 72 h with release in fermentable-sugar 182.6 ± 0.9 to 291.3 ± 1.2 mg/gds−1, respectively. The maximum conversion was observed during 72 to 96 h as shown in Fig. 6b. Figure 6 shows that boosting enzyme dosage from 5 to 15 U/g of P. hysterophorus improved fermentable sugar liberation with respect to each hydrolysis period. The plots shown between sugars liberated during saccharification vs. hydrolysis periods for enzyme dosages of 15 and 20 U/g overlap, demonstrating that the enzyme dose of 20 U/g of P. hysterophorus had no effect on fermentable sugar liberation. Table 7 summarizes earlier studies of lignocellulosic saccharification mediated by xylanase.
Table 7.
Overview of xylanase-mediated saccharification of various lignocellulosic biomasses
| Substrates | Pretreatment condition | Reducing sugar (mg/g) | References |
|---|---|---|---|
| Corn cob | 2 N NaOH, 30 °C, 20 h/thermal treatment, 90 min at 121 °C | 63.40 | Damaso et al. (2004) |
| Wheat straw | 15% ammonium, 121 °C for 1 h | 193.86 | Chapla et al. (2010) |
| Rice straw | Alkaline H2O2, 25 h | 126.89 | Kumar et al. (2017) |
| P. hysterophorus | NaOH-1.0% w/v | 219.6 ± 2.05 | Present study |
| 291.3 ± 1.2 |
Analysis of sugars using HPLC
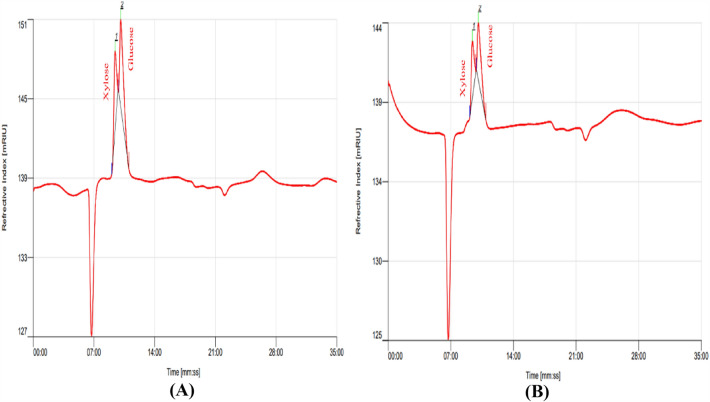
The hydrolysis products resulting from the saccharification process of alkali-pretreated P. hysterophorus using crude and commercial xylanase were examined using HPLC. The HPLC profile clearly showed that the crude enzymatic hydrolysate (Fig. 7A) and commercial enzymatic hydrolysate (Fig. 7B) both contained a better peak of glucose compared to xylose (Fig. 7). The xylose yield was quantified using HPLC; crude enzymatic hydrolysate showed 200 ± 0.1 mg/gds−1 of xylose sugar, while commercial enzymatic hydrolysate showed 250 ± 0.6 mg/gds−1 of xylose sugar. Bala and Singh (2019) observed a similar HPLC profile in the enzymatic hydrolysate of Saccharum munja and sugarcane bagasse. In the case of corncob hydrolysate, HPLC analysis revealed the presence of xylose, glucose, and cellobiose (Ghaffar et al., 2017). Similarly, Birhade et al. (2017) reported the presence of glucose and cellobiose in the enzymatic hydrolysate of ammonia-pretreated wheat straw.
Fig. 7.
High performance liquid chromatography profile of crude enzymatic hydrolysate (A) and commercial enzymatic hydrolysate (B) of alkali pretreated P. hysterophorus biomass
Conclusions
The two main reasons why these optimization studies were important were the value-added xylanase production using economic carbon sources, like P. hysterophorus as a substrate, and an improvement in enzyme production with a reduced fermentation period. In this investigation, A. niger was found to be effective in producing fungal xylanase when grown in solid-state conditions on P. hysterophorus. The current work used the response surface methodology via the CCD to increase A. niger production of xylanase. The RSM technique was used to optimize the cultural conditions, which resulted in a 4.3-fold increase in the production of xylanase from A. niger. Additionally, because RSM assumes random errors and reduces the number of experiments, it is a superior experimental technique to OFAT. OFAT, on the other hand, is considered a time-consuming and challenging technique. According to the findings reported here, the filamentous fungus A. niger can be an effective tool for the efficient valorization of lignocellulosic by-products through synthesizing precious hemicellulolytic enzymes like xylanases. Saccharification results indicated the release in reducing sugar of 219.6 ± 2.05 mg/gds−1 from crude fungal xylanase and 291.3 ± 1.2 mg/gds−1 from commercial xylanase. The result of this study depicts the high hydrolytic potential of optimized fungal xylanase and makes this bio-process suitable for scale studies. Importantly, valorizing weed biomass for xylanase production can be used as an effective method for controlling P. hysterophorus.
Acknowledgements
The authors thank Kurukshetra University, Kurukshetra, for providing laboratory facilities for this research.
Authors contributions
NK: conceptualization, investigation, methodology, validation, formal analysis, resources, data curation, writing original draft, writing review and editing; RS: writing review and editing, formal analysis; VS: writing review and editing, formal analysis, data curation, resources; Anita Yadav: validation, formal analysis, data curation; NKA: writing review and editing, visualization, supervision, project administration, funding acquisition.
Funding
The authors acknowledge the Haryana State Council for Science, Innovation, and Technology (HSCSIT), Panchkula (HSCSIT/R&D/2019/1309) for providing financial support during the tenure of this research work.
Data availability
Data will be made available on request.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Ahmed I, Zia MA, Iqbal HM. Bioprocessing of proximally analyzed wheat straw for enhanced cellulase production through process optimization with Trichoderma viride under SSF. Cellulose. 2010;2:100. [Google Scholar]
- Amin M, Bhatti HN, Sadaf S, Bilal M. Enhancing lipase biosynthesis by aspergillus melleus and its biocatalytic potential for degradation of polyester Vylon-200. Catal Lett. 2021;151:2257–2271. doi: 10.1007/s10562-020-03476-6. [DOI] [Google Scholar]
- Ang SK, Shaza EM, Adibah Y, Suraini AA, Madihah MS. Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation. Process Biochem. 2013;48:1293–1302. doi: 10.1016/j.procbio.2013.06.019. [DOI] [Google Scholar]
- Azzouz Z, Bettache A, Djinni I, Boucherba N, Benallaoua S. Biotechnological production and statistical optimization of fungal xylanase by bioconversion of the lignocellulosic biomass residues in solid-state fermentation. Biomass Convers Biorefin. 2020;11:1–3. doi: 10.1007/s13399-020-01018-z. [DOI] [Google Scholar]
- Bala A, Singh B. Development of an environmental-benign process for efficient pretreatment and saccharification of Saccharum biomasses for bioethanol production. Renew Energy. 2019;130:12–24. doi: 10.1016/j.renene.2018.06.033. [DOI] [Google Scholar]
- Bhagia S, Nunez A, Wyman CE, Kumar R. Robustness of two-step acid hydrolysis procedure for composition analysis of poplar. Biores Technol. 2016;216:1077–1082. doi: 10.1016/j.biortech.2016.04.138. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Kumar B, Verma P. A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess. 2019;6:1–36. doi: 10.1186/s40643-019-0276-2. [DOI] [Google Scholar]
- Bharti AK, Kumar A, Kumar A, Dutt D. Exploitation of Parthenium hysterophorous biomass as low-cost substrate for cellulase and xylanase production under solid-state fermentation using Talaromyces stipitatus MTCC 12687. J Radiat Res Appl Sci. 2018;11:271–280. doi: 10.1016/j.jrras.2018.01.003. [DOI] [Google Scholar]
- Birhade S, Pednekar M, Sagwal S, Odaneth A, Lali A. Preparation of cellulase concoction using differential adsorption phenomenon. Prep Biochem Biotechnol. 2017;47:520–529. doi: 10.1080/10826068.2016.1275009. [DOI] [PubMed] [Google Scholar]
- Chapla D, Divecha J, Madamwar D, Shah A. Utilization of agro-industrial waste for xylanase production by Aspergillus foetidus MTCC 4898 under solid state fermentation and its application in saccharification. Biochem Eng J. 2010;49:361–369. doi: 10.1016/j.bej.2010.01.012. [DOI] [Google Scholar]
- da Silva MB, Rossi DM, Squina F, Ayub MA. Comparative production of xylanase and the liberation of xylooligosaccharides from lignocellulosic biomass by Aspergillus brasiliensis BLf1 and recombinant Aspergillus nidulans XynC A773. Int J Food Sci Technol. 2018;53:2110–2118. doi: 10.1111/ijfs.13798. [DOI] [Google Scholar]
- Damaso MC, De Castro AM, Castro RM, Andrade CM, Pereira N (2004) Application of xylanase from Thermomyces lanuginosus IOC-4145 for enzymatic hydrolysis of corncob and sugarcane bagasse. In: Proceedings of the Twenty-Fifth Symposium on biotechnology for fuels and chemicals held May 4–7, 2003, in Breckenridge, CO, pp 1003–1012. Humana Press. 10.1007/978-1-59259-837-3_81. [DOI] [PubMed]
- El-Shishtawy RM, Mohamed SA, Asiri AM, Gomaa AB, Ibrahim IH, Al-Talhi HA. Solid fermentation of wheat bran for hydrolytic enzymes production and saccharification content by a local isolate Bacillus megatherium. BMC Biotechnol. 2014;14:1–8. doi: 10.1186/1472-6750-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar A, Yameen M, Aslam N, Jalal F, Noreen R, Munir B, Mahmood Z, Saleem S, Rafiq N, Falak S, Tahir IM, Noman M, Farooq MU, Qasim S, Latif F. Acidic and enzymatic saccharification of waste agricultural biomass for biotechnological production of xylitol. Chem Cent J. 2017;11:97. doi: 10.1186/s13065-017-0331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PK, Choudhary S, Chandrananthi C, Sharon Eveline J, Sushmitha SP, Hiremath L, Srivastava AK, Narendra Kumar S. Fungal biodiversity producing xylanase enzymes involved in efficient uses of xylanolysis. Mycodegrad Lignocelluloses. 2019;1:51–63. doi: 10.1007/978-3-030-23834-6_4. [DOI] [Google Scholar]
- Hajian M, Kashani SJ (2021) Evolution of the concept of sustainability. From Brundtland Report to sustainable development goals. In: Sustainable resource management. Elsevier, pp 1–24. 10.1016/B978-0-12-824342-8.00018-3.
- Hauli I, Sarkar B, Mukherjee T, Chattopadhyay A, Mukhopadhyay SK. Alkaline extraction of xylan from agricultural waste, for the cost effective production of xylooligosaccharides, using thermoalkaline xylanase of thermophilic Anoxybacillus sp. Ip-c Int J Pure Appl Biosci. 2013;1:126–131. [Google Scholar]
- Huang J, Zhu Y, Liu T, Sun S, Ren J, Wu A, Li H. A novel wet-mechanochemical pretreatment for the efficient enzymatic saccharification of lignocelluloses: small dosage dilute alkali assisted ball milling. Energy Convers Manag. 2019;194:46–54. doi: 10.1016/j.enconman.2019.04.078. [DOI] [Google Scholar]
- Ipsit H, Bidisha S, Anindita R, Mukhopadhyay SK. Ethanol production from xylose and enzymatic hydrolysate of hemicelluloses by a newly isolated yeast strain. J Microbiol Biotechnol Res. 2013;3:54–58. [Google Scholar]
- Javed U, Aman A, Qader SA. Utilization of corncob xylan as a sole carbon source for the biosynthesis of endo-1, 4-β xylanase from Aspergillus niger KIBGE-IB36. Bioresour Bioprocess. 2017;4:1–7. doi: 10.1186/s40643-017-0149-5. [DOI] [Google Scholar]
- Kar S, Sona Gauri S, Das A, Jana A, Maity C, Mandal A, Das Mohapatra PK, Pati BR, Mondal KC. Process optimization of xylanase production using cheap solid substrate by Trichoderma reesei SAF3 and study on the alteration of behavioral properties of enzyme obtained from SSF and SmF. Bioprocess Biosyst Eng. 2013;36:57–68. doi: 10.1007/s00449-012-0761-x. [DOI] [PubMed] [Google Scholar]
- Khanahmadi M, Arezi I, Amiri MS, Miranzadeh M. Bioprocessing of agro-industrial residues for optimization of xylanase production by solid-state fermentation in flask and tray bioreactor. Biocatal Agric Biotechnol. 2018;13:272–282. doi: 10.1016/j.bcab.2018.01.005. [DOI] [Google Scholar]
- Kucharska K, Słupek E, Cieśliński H, Kamiński M. Advantageous conditions of saccharification of lignocellulosic biomass for biofuels generation via fermentation processes. Chem Pap. 2020;74:1199–1209. doi: 10.1007/s11696-019-00960-1. [DOI] [Google Scholar]
- Kumar A, Gupta R, Shrivastava B, Khasa YP, Kuhad RC. Xylanase production from an alkalophilic actinomycete isolate Streptomyces sp. RCK-2010, its characterization and application in saccharification of second generation biomass. J Mol Catal B Enzym. 2012;74:170–177. doi: 10.1016/j.molcatb.2011.10.001. [DOI] [Google Scholar]
- Kumar V, Chhabra D, Shukla P. Xylanase production from Thermomyces lanuginosus VAPS-24 using low cost agro-industrial residues via hybrid optimization tools and its potential use for saccharification. Biores Technol. 2017;243:1009–1019. doi: 10.1016/j.biortech.2017.07.094. [DOI] [PubMed] [Google Scholar]
- Kumar N, Sharma R, Aggarwal NK, Yadav A. Parthenium hysterophorus weed as a novel substrate for β-glucosidase production by Penicillium citrinum NAF5: application of the crude extract to biomass saccharification. Lett Appl NanoBioSci. 2022;12:1. doi: 10.33263/LIANBS121.013. [DOI] [Google Scholar]
- Kumar N, Mittal M, Yadav A, Saini DK, Aggarwal NK. Statistical optimization of enzymatic saccharification of sodium hydroxide pretreated parthenium hysterophorus biomass using response surface methodology. J Wood Chem Technol. 2023;43(1):1–2. doi: 10.1080/02773813.2022.2145312. [DOI] [Google Scholar]
- Kumar N, Saharan V, Yadav A, Aggarwal NK. Ultrasound-assisted alkaline pretreatment of Parthenium hysterophorus for fermentable sugar production using a response surface approach. Sustain Chem Clim Action. 2023;4:100027. doi: 10.1016/j.scca.2023.100027. [DOI] [Google Scholar]
- Loi M, Glazunova O, Fedorova T, Logrieco AF, Mulè G. Fungal laccases: the forefront of enzymes for sustainability. Journal of Fungi. 2021;7:1048. doi: 10.3390/jof7121048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Moodley P, Kana EG. Development of a steam or microwave-assisted sequential salt-alkali pretreatment for lignocellulosic waste: effect on delignification and enzymatic hydrolysis. Energy Convers Manag. 2017;148:801–808. doi: 10.1016/j.enconman.2017.06.056. [DOI] [Google Scholar]
- Moran-Aguilar MG, Costa-Trigo I, Calderón-Santoyo M, Domínguez JM, Aguilar-Uscanga MG. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem Eng J. 2021;172:108060. doi: 10.1016/j.bej.2021.108060. [DOI] [Google Scholar]
- Pandya JJ, Gupte A. Production of xylanase under solid-state fermentation by Aspergillus tubingensis JP-1 and its application. Bioprocess Biosyst Eng. 2012;35:769–779. doi: 10.1007/s00449-011-0657-1. [DOI] [PubMed] [Google Scholar]
- Pérez-Rodríguez N, Oliveira F, Pérez-Bibbins B, Belo I, Torrado Agrasar A, Domínguez JM. Optimization of xylanase production by filamentous fungi in solid-state fermentation and scale-up to horizontal tube bioreactor. Appl Biochem Biotechnol. 2014;173:803–825. doi: 10.1007/s12010-014-0895-1. [DOI] [PubMed] [Google Scholar]
- Prasanna HN, Ramanjaneyulu G, Rajasekhar Reddy B. Optimization of cellulase production by Penicillium sp. 3 Biotech. 2016;6:1. doi: 10.1007/s13205-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A, Aggarwal NK. Enhanced endoglucanase production by soil inhabiting Streptomyces sp. strain NAA9 using lignocellulosic biomass. Energy Sources Part A Recov Utiliz Environ Effects. 2019;41:1630–1639. doi: 10.1080/15567036.2018.1549138. [DOI] [Google Scholar]
- Saini A, Aggarwal NK, Sharma A, Kaur M, Yadav A. Utility potential of Parthenium hysterophorus for its strategic management. Adv Agric. 2014 doi: 10.1155/2014/381859. [DOI] [Google Scholar]
- Saini A, Aggarwal NK, Yadav A. Cost-effective cellulase production using Parthenium hysterophorus biomass as an unconventional lignocellulosic substrate. 3 Biotech. 2017;7:1–1. doi: 10.1007/s13205-017-0604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saroj P, Korrapati N. Ultrasound-assisted alkaline pretreatment to intensify enzymatic saccharification of Crotalaria juncea using a statistical method. Biomass Convers Biorefin. 2018;8:659–668. doi: 10.1007/s13399-018-0324-8. [DOI] [Google Scholar]
- Shan S, Genç SY, Kamran HW, Dinca G. Role of green technology innovation and renewable energy in carbon neutrality: a sustainable investigation from Turkey. J Environ Manag. 2021;294:113004. doi: 10.1016/j.jenvman.2021.113004. [DOI] [PubMed] [Google Scholar]
- Tsegaye B, Balomajumder C, Roy P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers Manag. 2019;186:82–92. doi: 10.1016/j.enconman.2019.02.049. [DOI] [Google Scholar]
- Updegraff DM. Semimicro determination of cellulose inbiological materials. Anal Biochem. 1969;32:420–424. doi: 10.1016/S0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Yao S, Wu G, Xing M, Zhou S, Pu J. Determination of lignin content in Acacia spp using near-infrared reflectance spectroscopy. BioResources. 2010;5:556–562. doi: 10.15376/biores.5.2.556-562. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.