Abstract
Background
Right middle lobe (RML) syndrome is a recurrent or chronic obstruction of the RML causing atelectasis of the right middle lobe due to mechanical and nonmechanical etiologies. The consequences of untreated RML syndrome range from chronic cough to post‐obstructive pneumonia and bronchiectasis. We report here our bronchoscopy experience in patients with RML syndrome.
Methods
We conducted a retrospective study of adult patients who underwent bronchoscopy for RML syndrome at Rabin Medical Center from 2008 through 2022. Demographic data and medical history, bronchoscopy findings and procedures, and follow‐up results were collected.
Results
A total of 66 patients (57.6% male, mean age 63 ± 13 years) underwent bronchoscopy for RML syndrome during the study period. Bronchoscopy revealed a mechanical etiology in 49 (74.2%) cases, including endobronchial mass (21, 31.8%) and external compression (7, 10.6%). Malignancy was identified in 20 (30.3%) cases. In 62 patients (93.9%), the bronchoscopy resulted in partial or complete reopening of the RML bronchus. The therapeutic bronchoscopic procedures were balloon dilatation (19), laser ablation (17), mechanical debridement (12), endobronchial stent insertion (11), and cryoablation (6).
Conclusions
Malignancy was identified as the etiology of RML syndrome in approximately 25% of cases, suggesting bronchoscopy should be performed in every case of RML atelectasis. To our knowledge, this is the first reported series of endobronchial stenting of the RML bronchus in the context of RML syndrome.
Keywords: bronchoscopy, endobronchial stents, right middle lobe syndrome
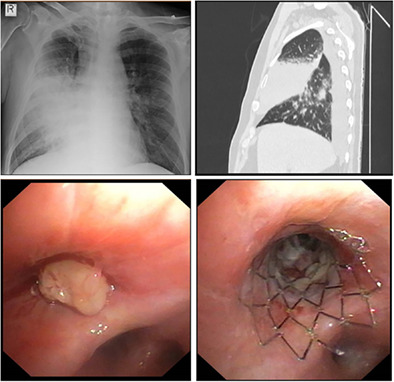
Treatment of right middle lobe (RML) obstruction with endobronchial stenting in the patient with post‐obstructive RML pneumonia (upper left and right) and a mass (lower left) that obstructed the lobar orifice. The stenosis remained severe even after the mass was removed, and eventually required insertion of an endobronchial stent (lower right).
INTRODUCTION
Right middle lobe (RML) syndrome was first described in 1948 as chronic or recurrent atelectasis of the middle lobe of the right lung due to enlarged lobar lymph nodes secondary to tuberculosis infection. 1 Subsequently, the etiology of RML syndrome has been divided into mechanical and nonmechanical causes. Mechanical obstruction of the RML can be caused by various endobronchial lesions including malignancy, foreign body, mucus plug, or hilar lymphadenopathy leading to extrinsic compression. 2 Nonmechanical causes of RML syndrome are associated with underlying inflammatory 3 or infectious 4 , 5 processes and are less well understood, often considered cryptogenic.
Clinical presentation of RML syndrome ranges from chronic cough and dyspnea to hemoptysis, fever, and weight loss. 6 The consequences of untreated RML syndrome can vary in severity, from recurrent hemoptysis secondary to bronchiectasis, post‐obstructive pneumonia and, in the context of malignancy, delay in treatment of the underlying malignant disease.
Chest radiography can assist in diagnosis in the presence of volume loss or collapse of the RML (see Figure 1a); however, x‐ray imaging can be nondiagnostic. Therefore, chest computed tomography (CT) is mandatory for the diagnosis of RML syndrome. The appropriate treatment depends on the underlying etiology and may consist of mechanical opening of right middle orifice stenosis with bronchoscopy techniques, antibiotic treatment of microbial organism isolated from cultures or anti‐inflammatory medication such as corticosteroids for inflammatory disease. Despite appropriate treatment, however, a small subset of patients may ultimately require surgical right middle lobectomy. 7 Patients with malignant cause can either undergo upfront surgery during the early stages with or without neoadjuvant/adjuvant therapy or be referred for endoscopic intervention until the effects of chemo‐, immuno‐, or radiotherapy take hold.
FIGURE 1.
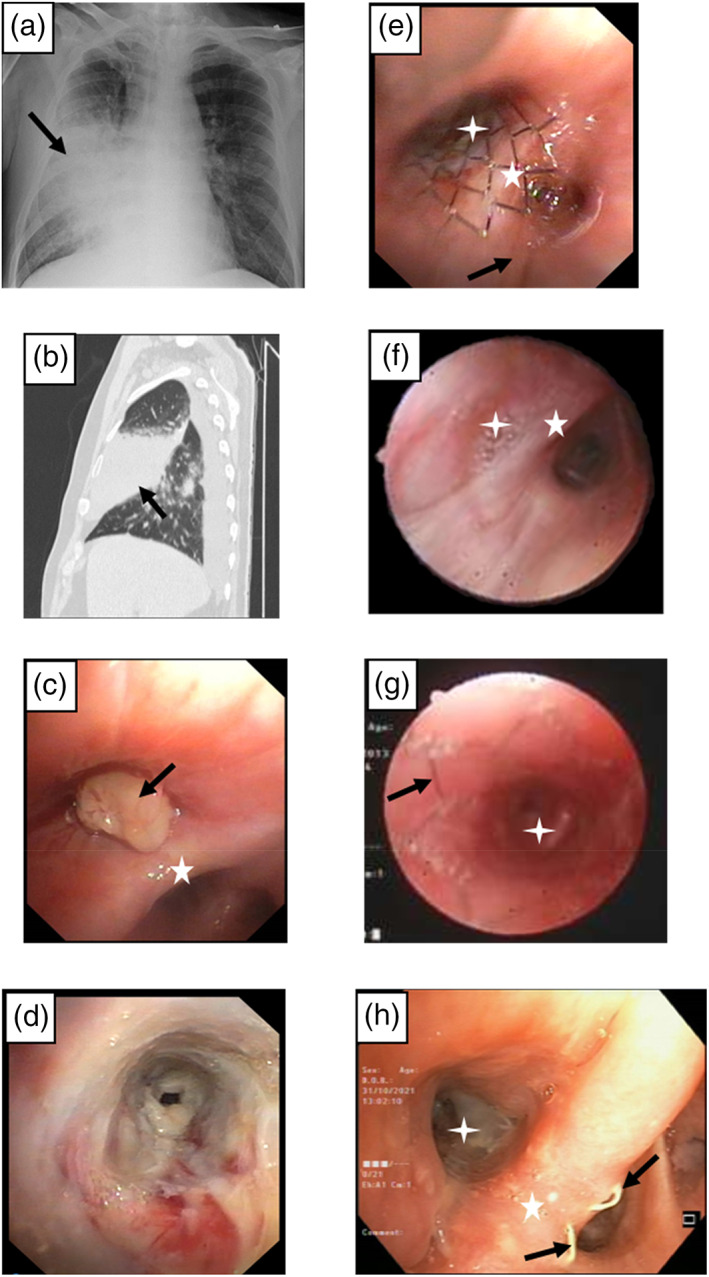
Treatment of right middle lobe (RML) obstruction with endobronchial stenting. (a–e) A 62‐year‐old man with a history of oropharynx cancer and pulmonary metastases treated with surgery and radiotherapy presented 5 years later with post‐obstructive RML pneumonia (black arrow on x‐ray and computed tomography [CT] scan, a, b). Bronchoscopy revealed a mass (black arrow, c) that obstructed the lobar orifice and airways covered by necrotic tissue (d). The stenosis remained severe even after the mass and necrotic tissue were removed, and eventually required insertion of an endobronchial stent (black arrow, e). (f–h) A 56‐year‐old woman presented with RML atelectasis caused by severe stenosis (f) of idiopathic origin. Treatment consisted of endobronchial stent (black arrow) insertion, with full lobe opening (g). On follow‐up bronchoscopy 8 years later, the stent was almost completely covered by endothelial tissue (h). Note the proximal end of the metal mesh (black arrow). The five‐pointed star indicates the carina between the RML and right lower lobe orifices. The four‐pointed star denotes the RML orifice.
The aim of this study was to report our 14‐year experience with the use of bronchoscopy for the evaluation and management of RML syndrome.
METHODS
The electronic database of a tertiary university center (Rabin Medical Center) was retrospectively searched for all adult patients diagnosed with RML syndrome who underwent flexible bronchoscopy study from 2008 through 2022. The following data were collected: demographics, medical history, bronchoscopy findings, pathology reports, and follow‐up results. The study was approved by the institutional ethics board (RMC# 0035‐23). Descriptive statistics were used for analysis. Continuous variables are summarized by mean and standard deviation, and categorical variables, by number and percentage.
RESULTS
The cohort consisted of 66 patients, 38 male (57.6%), and 28 females, of mean age 63 years (SD ± 13). Table 1 presents the demographic data. A total of 32 (48.5%) patients had a history of tobacco use, and 20 (30.3%) of all patients had known malignant disease prior to the bronchoscopy: lung cancer in seven and tumor of nonpulmonary origin in 13. Chest CT findings included bronchiectasis, bronchiolitis, atelectasis with or without obstructive pneumonia, and abscess, as well as hilar adenopathy or endobronchial mass 2 (see Figures 1b and 2a,b,e,i). The indications for bronchoscopy were atelectasis in 26 patients (39.4%), airway obstruction in 16 (24.2%), lung mass in 12 (18.2%), cough in four (6.1%), recurrent RML pneumonia in two (3%), maintenance of non‐RML endobronchial stent in three (4.5%), and other in three (4.5%).
TABLE 1.
Demographics and baseline characteristics of 66 patients with RML syndrome.
| Characteristics | Value |
|---|---|
| Sex, n (%) | |
| Men | 38 (57.6) |
| Women | 28 (42.4) |
| Age (years), mean ± SD | 63 ± 13 |
| Malignancy history, n (%) | 20 (30.3) |
| Lung cancer | 7 (35.0) |
| Other | 13 (65.0) |
| Lung transplant history, n (%) | 6 (9.1) |
| Smoking history, n (%) | 32 (48.5%) |
Abbreviation: RML, right middle lobe.
FIGURE 2.

Presentation and management of right middle lobe (RML) syndrome. (a–c) A 67‐year‐old man with a history of colon cancer presented with RML atelectasis (white arrow) on (a) sagittal (b) on axial computed tomography (CT) scan. Bronchoscopy revealed obstruction of the RML orifice by an endobronchial mass (black arrow, c), subsequently identified as colon cancer metastasis. (d) A 54‐year‐old man presented with infiltrated yellowish walls of the right bronchus intermedius caused by amyloidosis, resulting in RML stenosis. (e, h) A 51‐year‐old man presented with RML atelectasis (white arrow) on CT scan (e) due to severe bronchial stenosis (black arrow, f). Treatment consisted of balloon dilatation (g), with good results. Note the open RML orifice after the balloon procedure (h). Biopsy showed chronic inflammation of unknown origin. (i–l) A 64‐year‐old with chronic obstructive pulmonary disease and RML atelectasis (white arrow, i), caused by previously inserted endobronchial valves (red circle) for persistent air leak pneumothorax. Note the endobronchial valve (black arrow) in the RML orifice (j), extracted endobronchial valves (k), and resolution of the atelectasis on the follow‐up CT‐scan (l). The white star indicates the carina between RML and right lower lobe orifices.
Bronchoscopy identified mechanical causes of RML syndrome in 49 cases (74.2%). Table 2 presents the bronchoscopic findings. The more prevalent were endobronchial mass in 21 patients (31.8%), and bronchial stenosis in 17 (25.8%), followed by external compression in seven (10.6%). One patient (1.5%) had enlarged lymph nodes that led to external compression of RML bronchus and was positive for malignancy.
TABLE 2.
Bronchoscopic findings in 66 patients with RML syndrome.
| Endoscopic finding | Number of patients a |
|---|---|
| RML stenosis | 28 |
| Severe stenosis | 6 |
| Complete obstruction | 6 |
| Endobronchial mass | 21 |
| Purulent secretions | 11 |
| External compression | 7 |
| Anthracotic fibrosis | 2 |
| Previous inserted stent, valves | 2 |
| Tracheomalacia | 1 |
| Mucoid impaction | 1 |
| Cartilage protrusion | 1 |
| Foreign body | 1 |
Abbreviation: RML, right middle lobe.
Some patients had multiple findings.
Bronchoscopy succeeded in partial or complete reopening of the RML bronchus in the vast majority of patients (62, 93.9%), with only four cases (6.1%) remaining obstructed, including two cases with distal obstruction beyond endoscopic reach. Table 3 presents the types of therapeutic bronchoscopic procedures applied, including balloon dilatation in 19 patients (28.4% of all interventional procedures in first endoscopy), laser ablation in 17 (25%), mechanical debridement in 12 (18%) cryoablation in six (9%) and endobronchial stents in four (6%).
TABLE 3.
Procedures during bronchoscopy in 66 patients with RML syndrome.
| Procedure | Number of patients a |
|---|---|
| Therapeutic | |
| Balloon dilatation | 19 |
| Laser photoresection | 17 |
| Suction and other mechanical debridement | 12 |
| Endobronchial stent insertion | |
| At initial bronchoscopy | 4 |
| During follow‐up | 7 |
| Cryoablation | 6 |
| Removal of endobronchial valves, foreign body | 2 |
| Biopsy | |
| Endobronchial biopsy | 44 |
| Transbronchial biopsy | 9 |
| EBUS | 5 |
| Wang needle biopsy | 3 |
| Brushing | 1 |
Abbreviations: EBUS, endobronchial ultrasound; RML, right middle lobe.
Some patients were treated with several techniques. Two patients were not treated due to distal obstruction beyond endoscopic reach.
Complications were similar to those expected for any bronchoscopic biopsy procedures: mild hemoptysis in four patients (6.1%), pneumothorax requiring chest tube in four (6.1%) and one case of transient brain air emboli which resolved spontaneously without any clinical sequelae.
All patients underwent biopsy study. The pathology results are shown in Table 4. Malignancy was identified as the cause of RML syndrome in 20 cases (30.3%), of whom 14 had lung cancer. In eight cases (40% of cancer diagnoses) the malignant disease was already known prior to the bronchoscopy: two patients with non‐small cell lung cancer, one with small cell lung cancer, two with colorectal cancers, and one patient each with lymphoma, breast cancer, and pancreatic cancer. In terms of nonmechanical etiologies, pathology results identified various inflammatory conditions, including sarcoidosis, amyloidosis, and fungal infections.
TABLE 4.
Pathological diagnosis in 66 patients with RML syndrome.
| Diagnosis | Patients, n (%) |
|---|---|
| Normal lung tissue a | 26 (39.4) |
| Benign tumor | 2 (3.0) |
| Lung origin (hamartoma) | 1 |
| Other (lipoma) | 1 |
| Malignant tumor | 20 (30.3) |
| Lung origin | 14 |
| NSCLC | 7 |
| SCLC | 2 |
| Carcinoid | 5 |
| Metastatic disease | 6 |
| Colorectal | 2 |
| Breast | 2 |
| Sarcomatoid carcinoma of unknown primary | 1 |
| Metastatic adenocarcinoma of unknown primary | 1 |
| Chronic inflammation b | 9 (13.6) |
| Amyloidosis | 2 (3.0) |
| Sarcoidosis | 2 (3.0) |
| Fungus infection | 2 (3.0) |
| Aspergillus | 1 |
| Candida | 1 |
| Organizing pneumonia | 1 (1.5) |
Abbreviations: NSCLC, non‐small cell lung cancer; RML, right middle lobe; SCLC, small cell lung cancer.
In one case the diagnosis of follicular lymphoma was made by mediastinoscopy.
In one case the diagnosis of melanoma was made by computed tomography (CT)‐guided biopsy.
The follow‐up period ranged from 1 week to 11.6 years (median 1 year). A total of 20 patients (30.3%) required additional intervention in subsequent bronchoscopies during the follow‐up period, on average 180 days after the first bronchoscopy. One additional procedure was sufficient in 12 patients (18.2%) and 2–5 procedures were necessary in eight patients to achieve satisfactory results. The interventions were balloon dilatation in 14 patients (36% of all additional procedures), laser photoresection in 12 patients (31%), and endobronchial stent in seven patients (18%) who did not receive a stent during the first procedure (Figure 3). Two patients with carcinoid tumor (10% of all malignant cases) underwent lobectomy, while other 18 patients (90%) with malignant disease were not fit for surgery due to locally advanced disease with unsuitable anatomy or advanced (metastatic) form. They received radio‐, chemo‐, or immunotherapy along with endoscopic follow‐up.
FIGURE 3.
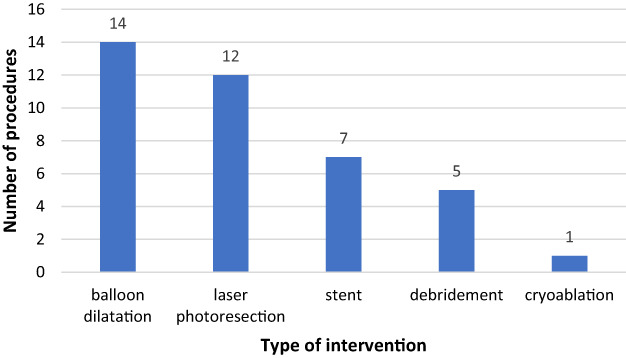
Type and number of additional procedures that were applied in 20 patients during 34 follow‐up bronchoscopies to ensure patency of the RML bronchus. Stent, insertion of endobronchial stent; debridement, suction and other mechanical debridement.
Figures 1 and 2 describe the pre‐ and post‐procedural endobronchial findings and management of RML syndrome in six representative cases.
DISCUSSION
Our experience with RML syndrome supports the use of bronchoscopy as a safe and effective tool for management of the obstruction. Partial or complete resolution was achieved in more than 90% of patients. Approximately 30% of our cohort underwent at least two bronchoscopies, pointing to the importance of post‐bronchoscopy surveillance for durable results.
In select cases in which balloon dilatation, laser photoresection, or mechanical debridement did not resolve the obstruction, we applied endobronchial stenting, either during the initial or a subsequent bronchoscopy procedure. All results were satisfactory. Stenting for RML syndrome was recently described by Gubin et al. 8 in a single patient case report. In our cohort, RML obstruction was successfully resolved in 11 patients by endoscopic stent insertion.
Biopsies obtained during bronchoscopy play a pivotal role in determining etiology. The presence of an endobronchial mass or extrinsic compression does not necessarily favor a malignant etiology, as these findings can be explained by other inflammatory (organizing pneumonia, sarcoidosis), infectious (fungal), or benign (lipoma, hamartoma, foreign body) processes. In the present study, in line with prior reports, 9 airway obstruction secondary to lung malignancy was the cause of RML syndrome in approximately one‐fourth of cases. In contrast, inflammation accounted for only 13% of findings compared to 47% in a retrospective review from 1983 by Wagner and Johnston. 10 Improvements in diagnostic tools and techniques in the interim 40 years may account for the discrepancy.
We found that bronchoscopy was relatively safe for the management of RML syndrome. The types and number of complications were comparable to those reported for other bronchoscopic procedures involving biopsy. We did have one case of brain air emboli which, despite the potential of serious health consequences, resolved without any clinical sequelae. While rare, air emboli are a known risk associated with interventional bronchoscopy. 9
The limitations of our study were its retrospective design and single‐center setting. However, to our knowledge, this is the first reported series of endobronchial stenting of the RML bronchus in the context of RML syndrome.
In conclusion, we present the experience of a tertiary medical center with bronchoscopy for the management of RML syndrome and identification of its etiology. We found bronchoscopy to be an effective and a relatively safe tool for the management of RML syndrome. In our experience, bronchoscopy is mandatory and plays an important role in the diagnosis and treatment of RML syndrome. From our perspective, endobronchial stenting has promising potential in the management of RML syndrome.
AUTHOR CONTRIBUTIONS
All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, Mordechai Reuven Kramer; Methodology, Mordechai Reuven Kramer and Shimon Izhakian; Data curation, Lev Freidkin; Formal Analysis, Barak Pertzov; Resources, Dror Rosengarten, Shai M. Amor, and Evgeni Gershman; Visualization, Lev Freidkin, Writing—Original draft, Lev Freidkin and Moshe Heching, Supervision, Mordechai Reuven Kramer.
FUNDING INFORMATION
No funding was received for this study.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare.
Freidkin L, Heching M, Rosengarten D, Pertzov B, Gershman E, Izhakian S, et al. Bronchoscopy for management and identification of etiology of right middle lobe syndrome: Analysis of 66 cases. Thorac Cancer. 2023;14(32):3226–3231. 10.1111/1759-7714.15113
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available in the article.
REFERENCES
- 1. Graham E, Burford T, Mayer J. Middle lobe syndrome. Postgrad Med. 1948;4(1):29–34. [DOI] [PubMed] [Google Scholar]
- 2. Kwon KY, Myers JL, Swensen SJ, Colby TV. Middle lobe syndrome: a clinicopathological study of 21 patients. Hum Pathol. 1995;26(3):302–307. [DOI] [PubMed] [Google Scholar]
- 3. Chen HA, Lai SL, Kwang WK, Liu JC, Chen CH, Huang DF. Middle lobe syndrome as the pulmonary manifestation of primary Sjögren's syndrome. Med J Aust. 2006;184(6):294–295. [DOI] [PubMed] [Google Scholar]
- 4. Rashid A, Nanjappa S, Greene JN. Infectious causes of right middle lobe syndrome. Cancer Control. 2017;24(1):60–65. [DOI] [PubMed] [Google Scholar]
- 5. Shah A, Behera SPC. Middle lobe syndrome: a rare presentation of allergic bronchopulmonary aspergillosis. Eur Ann Allergy Clin Immunol. 2014;46(4):147–151. [PubMed] [Google Scholar]
- 6. Bertelsen S, Struve‐Christensen E, Aasted A, Sparup J. Isolated middle lobe atelectasis: Aetiology, pathogenesis, and treatment of the so‐called middle lobe syndrome. Thorax. 1980;35(6):449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gudbjartsson T, Gudmundsson G. Middle lobe syndrome: a review of clinicopathological features, diagnosis and treatment. Respiration. 2012;84(1):80–86. [DOI] [PubMed] [Google Scholar]
- 8. Gubin S, Jamil AK, Kopita JM, Schwartz GS. Usefulness of endobronchial stenting for nonmalignant right middle lobe syndrome. Baylor Univ Med Cent Proc. 2021;34(4):503–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feller‐Kopman D, Shojaee S. Therapeutic bronchoscopy: interventional techniques. In: Broaddus C, Ernst JD, King TE, Lazarus SC, editors. Murray & Nadel's textbook of respiratory medicine. 7th ed. Philadelphia, PA: Elsevier; 2021. p. 388–398. [Google Scholar]
- 10. Wagner RB, Johnston MR. Middle Lobe Syndrome. Ann Thorac Surg. 1983;35(6):679–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available in the article.


