Abstract
Purpose
To provide agreed-upon guidelines on the management of a hyper-responsive patient undergoing ovarian stimulation (OS)
Methods
A literature search was performed regarding the management of hyper-response to OS for assisted reproductive technology. A scientific committee consisting of 4 experts discussed, amended, and selected the final statements. A priori, it was decided that consensus would be reached when ≥66% of the participants agreed, and ≤3 rounds would be used to obtain this consensus. A total of 28/31 experts responded (selected for global coverage), anonymous to each other.
Results
A total of 26/28 statements reached consensus. The most relevant are summarized here. The target number of oocytes to be collected in a stimulation cycle for IVF in an anticipated hyper-responder is 15–19 (89.3% consensus). For a potential hyper-responder, it is preferable to achieve a hyper-response and freeze all than aim for a fresh transfer (71.4% consensus). GnRH agonists should be avoided for pituitary suppression in anticipated hyper-responders performing IVF (96.4% consensus). The preferred starting dose in the first IVF stimulation cycle of an anticipated hyper-responder of average weight is 150 IU/day (82.1% consensus). ICoasting in order to decrease the risk of OHSS should not be used (89.7% consensus). Metformin should be added before/during ovarian stimulation to anticipated hyper-responders only if the patient has PCOS and is insulin resistant (82.1% consensus). In the case of a hyper-response, a dopaminergic agent should be used only if hCG will be used as a trigger (including dual/double trigger) with or without a fresh transfer (67.9% consensus). After using a GnRH agonist trigger due to a perceived risk of OHSS, luteal phase rescue with hCG and an attempt of a fresh transfer is discouraged regardless of the number of oocytes collected (72.4% consensus). The choice of the FET protocol is not influenced by the fact that the patient is a hyper-responder (82.8% consensus). In the cases of freeze all due to OHSS risk, a FET cycle can be performed in the immediate first menstrual cycle (92.9% consensus).
Conclusion
These guidelines for the management of hyper-response can be useful for tailoring patient care and for harmonizing future research.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02918-5.
Keywords: Hyper-response, Ovarian hyperstimulation syndrome, Ovarian stimulation
Introduction
A hyper-response to ovarian stimulation (OS) with gonadotropins in assisted reproductive technology (ART) is often associated with poorer outcomes related both to the increased risk of ovarian hyperstimulation syndrome (OHSS) and a lower live birth rate (LBR) if a fresh transfer is attempted [1–5]. While there are many recommendations and guidelines for the prevention and management of OHSS, many of these guidelines are based on studies done before to the common use of a GnRH agonist (GnRHa) trigger for final oocyte maturation. The GnRHa trigger and freeze-all approach practically eliminate the risk of severe OHSS [6, 7], perhaps making some of the recommendations in these guidelines obsolete. For example, the ASRM guidelines state that there is fair evidence that aspirin treatment during OS can reduce the risk of OHSS [8]; however, this aspect of aspirin treatment may be less relevant with a GnRH antagonist cycle and a GnRHa trigger. Moreover, if the risk of OHSS is lower, the management of a hyper-responder patient can focus on reproductive outcomes such as cumulative LBR rather than on OHSS. Unfortunately, to the best of our knowledge, no guidelines on the management of a hyper-responder, apart from OHSS prevention, exist.
We recently conducted a Delphi consensus (the HERA—Hyper-response Risk Assessment) to better define the hyper-responder patient [9] (Table 1). Seeing that there is a growing need for updated recommendations regarding the management of a hyper responder, a Delphi consensus was conducted with the aim of developing agreed-upon recommendations on the management of hyper-response in patients undergoing IVF. The Delphi technique was selected because it is a systematic way of determining expert consensus and helps answer questions not amenable to experimental and epidemiological methods.
Table 1.
The consensus statements from the HERA study. No consensus was reached regarding the number of growing follicles ≥ 10 mm that would define a hyper-response. OS, ovarian stimulation
| Definitions of a hyper-response | |
| Hyper-response is characterized by the collection of ≥ 15 oocytes | |
| A history of a hyper-response or OHSS in a prior cycle is not required to define a hyper-responder | |
| OHSS is not relevant for the definition of hyper-response if the number of collected oocytes is above a threshold (≥15). | |
| Recognition of pending hyper-response during ovarian stimulation | |
| The most important factor in defining a hyper-response during the ovarian stimulation is the number of follicles ≥ 10 mm in mean diameter | |
| Risk factors for a hyper-response—risk factors prior to OS | |
| I consider the serum Anti Mullerian Hormone (AMH) levels when assessing the risk for a possible hyper-response | |
| I consider the antral follicular count (AFC) when assessing the risk for a possible hyper-response | |
| I consider the patient’s age when assessing the risk for a possible hyper-response | |
| I do not consider the ovarian volume when assessing the risk for a possible hyper-response | |
| In a patient without a previous ovarian stimulation, the most important risk factor for a hyper-response is the antral follicular count (AFC). | |
| In a patient without a previous ovarian stimulation, when AMH and AFC are discordant, with one suggesting a hyper-response and the other not, AFC is the more reliable marker | |
| Hyper-response can occur at any age | |
| The lowest serum AMH value that would put a woman at risk for a hyper-response is ≥2 ng/ml (14.3 pMol/L). | |
| The lowest AFC that would put a woman at risk for a hyper-response is ≥18 | |
| A patient with a history of hyper-response in a previous stimulation cycle would be considered at high risk for another hyper-response if there was no significant change in the serum AMH levels and the AFC (±3 follicles). | |
| Women with polycystic ovarian syndrome (PCOS) as per Rotterdam criteria are at a higher risk of hyper-response than women without PCOS with equivalent follicle counts and gonadotropin doses used to stimulate | |
| Risk factors for a hyper-response—risk factors for hyper-response during ovarian stimulation | |
| The lowest number of growing follicles ≥ 14 mm that would characterize a hyper-response to ovarian stimulation is ≥15 follicles | |
| The lowest peak estradiol level that would indicate a hyper-response to ovarian stimulation is ≥3000 pg/ml (11,013 pMol/l). |
Materials and methods
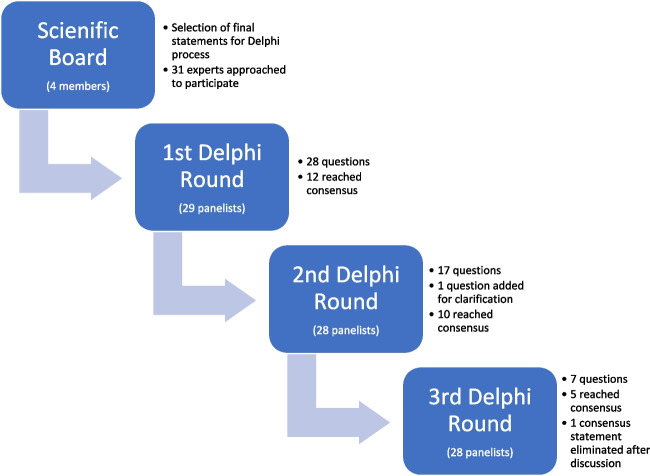
The consensus coordinators (consisting of Michael H. Dahan and Ido Feferkorn) performed a literature search on commonly used approaches for the management of hyper-responders. Based on these approaches, an initial list of defining questions was developed. This list was disseminated to a scientific board consisting of Samuel Dos Santos-Ribeiro, Filippo Maria Ubaldi, and Juan Garcia Velasco under the chairmanship of Dr. Dahan. The scientific board was selected for its expertise as well as significant experience in writing national and international guidelines and publications in the field. The scientific board discussed, amended, and selected the final statements in the questionnaire for the first round of the Delphi consensus (figure 1s). The board also discussed and agreed upon the method of disseminating the questionnaire, processing the results, and establishing a threshold for accepting a statement as a consensus. Each member of the scientific board was able to suggest experts who would participate in the Delphi consensus expert panel. The facilitator of the Delphi consensus was Dr. Ido Feferkorn.
Consensus participants
A group of 31 professionals in the field of reproductive medicine (with representation selected for global coverage) were approached by email and offered to participate as panelists. Each was provided with information regarding the study’s purpose and methods. Twenty-nine experts (including the board members) agreed to participate as panelists, and each remained anonymous to the others throughout the process. This anonymity was necessary to prevent the dominance of a specific member and enable a change in opinion in the following Delphi round. The geographic representation of the panelists is presented in Table 2. Overall, the panelists have published a total of 6522 peer-reviewed articles per Scopus, with a median publication of 193.5 documents per author (IQR 114.75–261 documents, Table 1s).
Table 2.
Geographic representation of the Delphi participant
| Continent | Country of origin | Number of panelists |
|---|---|---|
| Europe | Italy | 3 |
| Europe | Spain | 3 |
| Europe | Belgium | 1 |
| Europe | Denmark | 1 |
| Europe | Greece | 1 |
| Europe | Portugal | 1 |
| Europe | UK | 1 |
| Europe | Switzerland | 1 |
| Europe | France | 1 |
| Asia | Turkey | 2 |
| Asia | Israel | 2 |
| Asia | United Arab Emirates | 2 |
| North America | USA | 3 |
| North America | Canada | 2 |
| Oceania | Australia | 2 |
| South America | Brazil | 2 |
The consensus process
The consensus process is depicted in Fig. 1. After selecting the final statements for voting, the scientific board determined that a consensus would be reached when at least 66% of experts agreed and that up to three rounds would be used to obtain this consensus.
Fig. 1.
The Delphi process
In the first round, an online survey (created using “Google Forms”) consisting of 28 questions and statements was circulated to the Delphi participants. The survey included seven statements on which the experts could agree or disagree and 21 multiple-choice questions, seven of which the experts could choose the option “other” and add an opinion in free text.
In the second and third rounds, panelists were given the results from the previous Delphi round. The survey started with questions for which a consensus was not reached. These questions were revised according to the feedback and results from the previous round. This part of the survey was followed by a second part where all panelists had the option to discuss a statement that reached a consensus. If two or more panelists opted to discuss a statement, the statement would be reintroduced in the following stage of the Delphi process. If no strong objection was raised to an achieved consensus, the agreed-upon statement would not be reintroduced in the subsequent round.
The definitions of a hyper-response and an anticipated hyper-response used in the consensus process were those from the previous HERA study (Table 1) acknowledging that there are limitations to these definitions as well (for example the lack of consensus on the number of growing follicles ≥ defining a hyper-response and that the number of follicles defining a hyper-response change as per stage of stimulation—from AFC, to growing follicles to the final oocytes collected).
Results
Overall, 97% (28/29) of experts completed all three rounds of the Delphi. A total of 26/28 statements reached a consensus. The consensus statements are presented in Table 3. No consensus was reached regarding the preferred starting dose in the first IVF stimulation cycle of an anticipated hyper-responder weighing above 80 kg. No consensus was reached regarding hospitalizing vs. management as an outpatient in cases of a patient with significant ascites.
Table 3.
The consensus statements. IVF, in vitro fertilization; OHSS, ovarian hyperstimulation syndrome; PCOS, polycystic ovarian syndrome; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; FET, frozen embryo transfer. No consensus was reached regarding the preferred starting dose in the first IVF stimulation cycle of an anticipated hyper-responder, weighing above 80 kg. No consensus was reached regarding hospitalizing vs. management as an outpatient in the cases of a patient with significant ascites
| Stimulation goals in a hyper-responder | |
| 1. The target number of oocytes to be collected in a stimulation cycle for IVF in an anticipated hyper-responder is 15–19. | |
| 2. For a potential hyper-responder, I would rather achieve a hyper-response and freeze all than aim for a fresh transfer | |
| Treatment protocol in an anticipated hyper-responder | |
| 3. GnRH agonists should be avoided for pituitary suppression in anticipated hyper-responders performing IVF. | |
| 4. I do not use any specific algorithm to estimate the starting dose for an IVF cycle | |
| 5. The preferred starting dose in the first IVF stimulation cycle of an anticipated hyper-responder of average weight is 150 IU/day. | |
| 6. Body weight should be considered to determine the daily gonadotropin dosage for an anticipated hyper-responder undergoing IVF. | |
| 7. Increasing the starting gonadotropin dose in the first IVF cycle of an anticipated hyper-responder is recommended if her weight is above 80 kg | |
| Cycle modifications in response to a hyper-response | |
| 8. I decrease gonadotropin dosage in the middle of an IVF cycle if the patient seems to hyper-respond based on serum estradiol levels and/or number of growing follicles > 10mm. | |
| 9. Under the risk of hyper-response during stimulation, I would not trigger one to two days before the patient reaches my usual trigger criteria. | |
| 10. I do not use coasting to decrease the risk of OHSS. | |
| Use of adjuvants during the stimulation of a hyper-responder | |
| 11. I add metformin before/during ovarian stimulation to anticipated hyper-responders only if the woman has PCOS and is insulin resistant. | |
| 12. I do not add any adjuvants from the first day of stimulation to a potential hyper-responder. | |
| 13. During ovarian stimulation, I do not add any adjuvant if the patient seems to hyper-respond based on serum estradiol levels and/or number of growing follicles > 10 mm. | |
| Choice of trigger in a hyper-responder | |
| 14. On the day of ovulation trigger, if the number of follicles > 10 mm and the estradiol levels are discordant, I would decide to use a GnRH agonist trigger alone based on the number of follicles > 10 mm. | |
| 15. There is no minimal estradiol level on the day of trigger that would require agonist trigger alone | |
| 16. On the day of ovulation trigger, the minimal number of follicles > 10 mm that would require agonist trigger alone (in an antagonist cycle) is 18 | |
| OHSS prevention and the use of dopaminergic agent | |
| 17. In the case of a hyper-response, a dopaminergic agent should be used only if hCG will be used as a trigger (including dual/double trigger) with or without a fresh transfer. | |
| 18. For maximal effectiveness, cabergoline 0.5 mg for OHSS prevention should be started on the day of trigger. | |
| 19. For maximal effectiveness in OHSS prevention, cabergoline 0.5 mg should be continued for 7 days. | |
| 20. In a woman with a hyper-response, triggered with hCG, the only interventions I would add to prevent OHSS are freeze all and the use of cabergoline. | |
| 21. In a woman with a hyper-response, triggered with GnRH agonist the only interventions I would add to prevent OHSS is freeze all. | |
| 22. If a hyper-response occurred after a trigger that included hCG I would always freeze all | |
| 23. After using a GnRH agonist trigger due to a perceived risk of OHSS, I would not consider a luteal phase rescue with hCG and attempt a fresh transfer regardless of the number of oocytes collected. | |
| FET protocol in a hyper-responder | |
| 24. My choice of FET protocol is not influenced by the fact that the patient is a hyper-responder. | |
| 25. In cases of freeze all due to an OHSS risk, I perform an FET cycle in the immediate first menstrual cycle. | |
| OHSS management | |
| 26. I would recommend admission following a hyper-response after the diagnosis of severe OHSS. |
Discussion
Choice of protocol and gonadotropin dose
Studies assessing the association between the number of oocytes collected and live birth differ with respect to the outcome measured. Studies focusing on live birth after fresh transfer found that the LBR increases with the number of oocytes collected; however, a plateau was reached when >15 oocytes were collected [1, 10]; and a decline in LBR after fresh transfer was noted when more than 20 oocytes were collected [1]. When the outcome measured was cumulative LBR after the transfer of fresh and frozen embryos, the positive association between the number of oocytes collected and LBR continued [11, 12]; however, some studies found no plateau, and the cumulative live birth rates (CLBR) continued to rise with the increase in the number of oocytes collected [11]. While these results may be biased by the overall better response expected from hyper-responders, it seems that for this group, at least in terms of CLBR, more may be better. Of course, one must balance the benefit of recruiting more oocytes with the risks associated with hyperstimulation, of which OHSS is the most apparent. However, this risk is very low with GnRHa trigger and freeze-all cycles. It should be noted that while OHSS is probably the most significant risk of hyperstimulation, it is not the only adverse effect. Patient discomfort, risks associated with multiple ovarian punctures related to a collection of a large follicle cohort, and risk of thromboembolism associated with high estradiol levels [12] are all factors that need to be considered. Most studies focused on OHSS as the adverse event, and data are lacking on these aspects balancing efficacy and safety in hyper-responders. At the time being, the Delphi panel aims for a stimulation yielding a mild hyper-response (statement 1) while acknowledging that it is likely better to err to the side of a hyper-response than a hypo-response (statement 2). This is based on the freezing techniques currently available and the good prognosis for LBR in FET cycles in hyper-responders [3, 4, 13, 14]. Whether these results of improved LBR in frozen cycles are driven by patients with PCOS or could be generalized to the hyper-responder group has not been studied. The consensus of 15–19 collected oocytes as the target to aim for stems from an attempt to balance all of the above factors as well as other factors such as the cost of a stimulation cycle, the potential for a second child from the same stimulation cycle, the side effects from hyper-stimulation (not exclusively those related to OHSS), the ethics and costs related to storage of excess oocytes, and the pregnancy complications and neonatal outcomes related to fresh vs. frozen embryo transfer.
Since hyper-responders are at an increased risk of developing OHSS, the choice of a protocol that minimizes this risk is preferable. The GnRH antagonist protocol is associated with a reduced risk for OHSS [15] and enables the use of a GnRHa trigger, further decreasing the risk to almost negligible [6, 7]. Indeed, the Delphi panel (statement 3), as do both the ASRM and the ESHRE guidelines, recommends the GnRH antagonist protocol for predicted hyper-responders [8, 16].
The optimal gonadotropin dose for ovarian stimulation is a result of the number of oocytes one aims to collect while trying to balance LBR and patient safety. Several studies have assessed the outcomes of a reduced gonadotropin dose (usually defined as lower than 150 units/day) in hyper-responders. The OPTIMIST study group conducted a randomized control trial in predicted hyper-responders (defined as women with an AFC > 15) and found that CLBR and rates of severe OHSS were comparable between a gonadotropin dose of 150 IU/day and a 100 IU/day dose[17]. “Any grade” of OHSS was higher with the 150 IU/day dose whereas cancellation rates (for poorer response than expected) were higher in the 100 IU/day dose. Of note, only 26% of the study population were treated with the GnRH antagonist protocol, and all women were triggered with hCG making conclusions regarding OHSS less relevant to the current practice of GnRH antagonist protocols as the first-line treatment for predicted hyper-responders. Regarding cost analysis, no significant difference was noted between the groups. It is also worth noting that women with PCOS were excluded from the study and that the mean BMI in the study group was 23.8 making generalizability of the findings difficult. In a sub-analysis on individualized dosing vs. standard dosing in IVF in anticipated hyper-responders, a Cochrane review came to similar conclusions [18]. Overall, the cancellation rate was higher with the individualized (lower dose), and fewer women in the individualized dose had at least one embryo to transfer. The fact that these differences did not translate into a lower LBR might be secondary to the studies being underpowered to detect smaller differences which may still be clinically important. In an RCT comparing individualized vs. standard gonadotropin dose in women treated with the GnRH antagonist protocol, 38% of anticipated hyper-responders treated with 100 IU/day of gonadotropins had a low oocyte yield (<5 oocytes) compared to 6% in the group of women treated with 150 IU/day of gonadotropins [19]. These results suggest that in some women, a threshold for follicular recruitment exists. Seeing the lower risk of cycle cancellation secondary to OHSS risk with the GnRH antagonist protocol, it seems reasonable not to use gonadotropin doses lower than 150 IU/day even for hyper-responders, and the Delphi panel believes that the recommended starting dose for expected hyper-responders should be 150 IU/day (statement 5). Data are lacking regarding the a priori use of higher gonadotropin doses in expected hyper-responders, and it is plausible that in the future, further fine-tuning of the dose according to patient age and AFC would be possible. It is important to emphasize that the Delpho recommended starting dose of 150 IU/day is related to treatment naïve patients without a previous history of gonadotropin treatment and that information from previous insemination cycles for example is invaluable in assigning the starting dose for IVF. It is also worth emphasizing that specific patient populations, such as those with hypogonadotropic hypogonadism, may deserve different considerations; however, this was beyond the scope of this Delphi consensus.
It is known that the pharmacodynamics of gonadotropins are affected by body weight through the volume of distribution and drug clearance [20–23], which raises the question of dose adjustment in a predicted hyper-responder with high body weight. In a secondary analysis of data from the OPTIMIST trial described above, a reduction of the gonadotropin dose resulted in a reduction in LBR in young women and possibly in women with a relatively high body weight [24]. Increasing the dose above 150 IU/day in women with higher body weight was not studied in the OPTIMIST trial. A prospective observational cohort study comparing women below and above a BMI of 25 kg/m2, triggered with 0.2 mg triptorelin due to a hyper-response, found that women in the higher BMI category required higher doses of gonadotropins and had fewer mature oocytes [25]. There are no studies focusing on gonadotropin dose adjustment based on body weight in anticipated hyper responders. The Delphi participants recommend increasing the dose when the woman’s weight is above 80 kg (statements 6 and 7). No consensus was reached regarding the preferred starting dose in women weighing above 80 kg, perhaps because this too depends on the magnitude of increased body weight. Whether the dose of GnRHa trigger should be adjusted in women with higher weight was not addressed by the Delphi panel and requires further study.
While there are some algorithms taking into account different patient factors, such as AMH, when choosing the initial gonadotropin dose, there are no studies on the efficacy of such algorithms in hyper-responder patients. As such, at the moment, the panel does not recommend the use of a specific algorithm to estimate the starting dose of gonadotropin stimulation (statement 4).
OHSS prevention and management
There is limited data on dose adjustment during ovarian stimulation of a hyper-responder. While the ESHRE guidelines on ovarian stimulation state that adjustment (increase or decrease) of the gonadotropin dose in the mid-stimulation phase during OS is probably not recommended, this statement is based mainly on studies on dose increments in poor responders [16], and on one study in hyper-responders where a midcycle decrease in the dose of gonadotropins was associated with a shorter duration of coasting and cycle cancellation [26]. Indeed, dose adjustments during stimulation are rather common. A systematic review of studies published between 2007 and 2017 that allowed dose adjustment within the study protocol found that the pooled point estimated for unspecified dose adjustments was 45.3% and for dose decrease was 9.5% [27]. Dose adjustments were more frequent in protocols using the GnRHa than those using the GnRH antagonist. In a retrospective analysis of data from medical records of 39 clinics in the USA, dose adjustments during stimulation occurred in 40.7% of cycles; of them, 62.4% were dose decreases [28]. This higher incidence coming from real-world data as compared to those in clinical trials likely represents the limitations on dose adjustments in controlled trials. To the best of our knowledge, there are no studies assessing a fixed dose vs. dose adjustment in hyper-responders undergoing the GnRH antagonist protocol. Potential benefits of adjusting the dose midcycle are avoiding a hyper-response or at least decreasing its magnitude, decreasing patient discomfort associated with enlargement of the ovaries, reducing complications, and avoiding cycle cancellation and financial aspects related to gonadotropin costs. How these potential benefits translate into clinical practice and their effect on LBR is a matter of further study. At this time, the Delphi panel believes that when a hyper-response occurs during treatment, gonadotropin dose decrease could be attempted and acknowledges the need for further study on the matter (statement 8).
Another possible intervention to decrease complications related to hyper-response and patient discomfort is earlier triggering and collection. In a hyper-responder, earlier triggering could potentially still allow for a sufficient number of oocytes to be collected to achieve a live birth. While this approach has potential merits, there are no studies on its efficacy, and considering the low likelihood of OHSS with the GnRHa trigger and the overall good prognosis with FET of thawed vitrified embryos, the Delphi panel does not support earlier triggering in cases of a hyper-response (statement 9).
The ASRM guidelines on the prevention and treatment of OHSS state that “there is insufficient evidence to recommend coasting for the prevention of OHSS” [8]. This recommendation is based mainly on a Cochrane review from 2011 that found no evidence for the benefit of coasting to prevent OHSS [29]. This Cochrane review has since been updated and states that there is low-quality evidence that coasting reduces the rates of mild and severe OHSS, with too few data to determine the effect on other treatment outcomes [30]. With that in mind, most studies on coasting included an hCG trigger. The value of coasting with the use of a GnRHa trigger (in a GnRH antagonist cycle) has not been studied sufficiently, but considering the low likelihood of developing severe OHSS, it is unlikely to be beneficial. Indeed, the Delphi panel also does not support coasting to prevent OHSS (statement 10). The ESHRE guidelines too support GnRHa trigger over coasting in patients at risk for OHSS [16].
Use of adjuvants during stimulation
Metformin is an insulin-sensitizing agent that both reduces hyperinsulinemia and suppresses the production of androgens in the ovary [31]. This reduction of ovarian hyperandrogenism possibly reduces the number of preovulatory follicles and, thus, the risk of OHSS [8]. In a Cochrane review on the subject, metformin treatment before and during ART in women with PCOS was associated with a reduction in OHSS but only in the long GnRHa protocols [32]. When addressing clinical pregnancy rate (CPR) and LBR, the meta-analysis found that metformin use in the long GnRHa protocol was possibly associated with a higher CPR (RR 1.32, CI 1.08–1.63) but not LBR. Metformin use in the GnRH antagonist protocol was not associated with a difference in CPR; however, a reduction in LBR was found (RR 0.48, CI 0.29–0.79). In another meta-analysis on the role of metformin in women with PCOS undergoing IVF, metformin was associated with a decrease in OHSS rates but with no difference in CPR [33]. In a subgroup analysis, the reduction in OHSS was found to be significant only in women with a BMI > 26 [33]. This subgroup of women also had a higher CPR when taking metformin (OR 1.71, CI 1.12–2.60). A trend towards a higher LBR was also found in women with a BMI > 26, but this did not reach statistical significance (OR 1.55 CI 0.96–2.49). Considering the negligible likelihood of OHSS with a GnRHa trigger, the role of metformin in ovarian stimulation cycles in hyper-responders needs to be revisited. Indeed, the ESHRE guidelines state that routine use of metformin during ovarian stimulation is not recommended in the GnRH antagonist protocol for women with PCOS [16]. Whether there is a subset of women who do benefit from metformin is a matter of further study. Currently, the Delphi panels believe that metformin should be reserved for patients with PCOS and insulin resistance (statement 11).
There is only limited data on the role of other adjuvants during ovarian stimulation in hyper-responders. Most studies on the use of adjuvants prior to or during stimulation focus on the low-responder group. A Cochrane on the use of Aspirin in women undergoing ART found no benefit for its use in the general IVF population [34]. While aspirin may be useful for decreasing the rate of OHSS [8], it is likely unnecessary for reducing OHSS when using the GnRH antagonist protocols, tGnRHa trigger, and freeze-all. Similarly, the ESHRE guidelines do not support the use of any adjuvant during ovarian stimulation [16]. Considering the overall good prognosis hyper-responders have, the Delphi panel believes that no adjuvant treatment is needed during the stimulation of a hyper-responder patient (statements 12 and 13).
Choice of trigger in a hyper-responder
Only a few studies compare the predictive value of estradiol versus the number of developing follicles as a risk factor for OHSS. However, there are some factors that favor the number of follicles. Estradiol levels many times overlap between women undergoing IVF with and without OHSS [35]. While increased capillary permeability, the hallmark of OHSS, is many times associated with high levels of estradiol, it is likely mediated by a systemic inflammatory response [36, 37], including excessive vascular endothelial growth factor (VEGF) production, which does not correlate with estradiol levels [36]. One such example is women with defective estradiol synthesis (e.g., 17,20 desmolase deficiency) or very low estradiol levels, which can still have OHSS [38, 39]. Lastly, in a prospective study, the number of follicles ≥ 11 mm was superior to estradiol levels in predicting OHSS [40], and in a retrospective study on data from 2982 women undergoing 5493 cycles, the number of follicles ≥ 10 mm in diameter was the best predictor for OHSS when compared to estradiol levels [41].
In a prospective cohort study, the optimal threshold for predicting the risk for OHSS was ≥ 13 follicles ≥ 11 mm, and for predicting severe OHSS, the optimal threshold was ≥ 18 follicles [40]. Another prospective study found that the number of medium (10–15 mm) and large (>15 mm) follicles was the only independent predictor of OHSS prior to hCG trigger, with the optimal threshold, again, being 18 [42]. In a retrospective analysis of participants’ response to OS from 3 large phase III trials, the optimal threshold for prediction of OHSS was ≥19 follicles ≥ 11 mm [43]. While the positive predictive value of 18 follicles is relatively low (around 15.8% on one study [42]), its high sensitivity (~82%) and specificity (~79%), combined with the low risk (no reduction in LBR) when using a GnRHa trigger and freeze all policy, makes it a useful threshold. The above findings support the Delphi consensus to base the decision regarding the GnRHa trigger on the number of growing follicles rather than on estradiol levels (statements 14 and 15) and to use 18 growing follicles as the threshold (statement 16) for favoring a GnRHa trigger. Tailoring according to other patient characteristics and desires can and should be done on an individual basis. While in the HERA consensus, 15 follicles ≥ 14 mm, was used to characterize a hyper-response, the Delphi panel chose to use the number of follicles ≥ 10 mm to guide the choice of trigger, acknowledging the importance of smaller follicles in the occurrence of OHSS and, at the same time, enabling flexibility in the choice of trigger.
OHSS prevention and the use of a dopaminergic agent
It is hypothesized that dopamine agonists given around the time of triggering or oocyte collection reduce the rate of OHSS by reducing VEGF production and VEGF receptor 2-dependent vascular permeability [8, 44]. A meta-analysis on the role of cabergoline in the prevention of OHSS and a Cochrane review on interventions to reduce OHSS found cabergoline to be useful [45–47]. The ASRM guidelines too state that “there is good evidence that dopamine agonists starting at the time of hCG trigger for several days reduces the incidence of OHSS” [8]. However, the above meta-analysis and guidelines are based on studies that used an hCG trigger for final oocyte maturation. The role of dopamine agonists after the GnRHa trigger is less studied. While the mechanism of OHSS reduction should not be different, the cost-effectiveness of such an intervention with the lower rates of OHSS associated with GnRHa trigger deserves further study. In a retrospective study comparing no treatment vs. dopamine agonist alone vs. dopamine agonist and luteal phase GnRH antagonist in patients at risk for OHSS after GnRHa trigger, dopamine agonist reduced patient discomfort and bloating. However, the rates of severe OHSS were comparable between the groups [48]. The Delphi panel agrees that after the GnRHa trigger, there is no need for the addition of a dopamine agonist (statement 17), which is in line with the ESHRE guidelines which state that the addition of cabergoline as a preventive measure for OHSS is not recommended when GnRHa is used for triggering acknowledging the lack of evidence regarding its cost-effectiveness [16].
Dopamine agonist treatment can be initiated with an ovulation trigger or on the day of oocyte pickup, potentially reducing its efficacy in OHSS prevention. In an RCT comparing dopamine agonist administration on the day of the hCG trigger to the administration on the day of oocyte pickup (OPU), no difference was found in oocyte maturation rates, fertilization rates, implantation rates, or CPR [49]. Severe OHSS rates were also comparable between the groups; however, the study was underpowered to draw a conclusion in this regard. A retrospective study comparing dopamine antagonist administration beginning on the day of the GnRHa trigger (in a GnRH antagonist cycle) to the administration on the day of OPU found lower rates of mild and moderate OHSS in the former group; however, neither group developed severe OHSS as expected in GnRH antagonist cycles using GnRHa trigger. The studies assessing dopamine agonists for OHSS prevention differ in their length of treatment and in the time of dopamine agonist initiation. To the best of our knowledge, no study comparing different durations of dopamine agonist administration was done; however, most studies did so for 7–8 days. The Delphi panel recommends administering dopamine agonists on the day of the hCG trigger for 7 days (statements 18 and 19). It is worth emphasizing that dopamine agonist treatment is less effective in preventing late, pregnancy associated, OHSS. Further, prospective trials should ideally be done to better clarify the length of treatment needed and the optimal time to initiate treatment. With that in mind, considering the lower incidence of OHSS with the increased use of GnRH antagonist cycles in anticipated hyper-responders, it is unlikely that studies of sufficient power would be conducted.
A freeze-all strategy prevents late OHSS [8, 50, 51] and is thus recommended by both the ASRM and the ESHRE guidelines. The use of other interventions depends on the trigger being used. When the trigger is a GnRHa, the risk of severe OHSS is almost negligible, and the Delphi panel believes that no other intervention is warranted (statement 21). On the other hand, as discussed above, when the trigger used includes hCG, a dopamine agonist reduces the risk of early OHSS. Aspirin, when initiated from the start of OS, has been found to reduce the incidence of OHSS [52, 53]; however, the effectiveness of aspirin when administered from the time of trigger has not been studied and thus cannot be recommended. GnRH antagonists have been shown to decrease the secretion of VEGF from human granulosa luteinized cells [54] and as such reinitiating GnRH antagonist in the luteal phase has been studied as an intervention to reduce the rates of OHSS. In a case report, Lainas et al. first described successful outpatient management of early severe OHSS with the reinitiation of GnRH antagonist [55]. In a larger prospective observational study including 40 women, Lainas et al. described how early severe OHSS can be managed with the use of luteal GnRH antagonist [56]. Similar findings were reported in a retrospective study on the matter [57]. Data on the role of reinitiating GnRH antagonists in patients triggered with GnRHa is limited. In a retrospective study, Shrem et al. found that the rates of mild and moderate OHSS were lower in high-risk women triggered with GnRHa who received GnRH antagonists on top of cabergoline for OHSS prevention [48]. With that in mind, no patient in the cohort suffered from severe OHSS questioning the cost-effectiveness of this intervention. To date, evidence regarding the use of luteal phase GnRH antagonists is limited to observational studies and, as such, is not recommended by the Delphi panel (statement 20).
OHSS is a risk factor for thromboembolism, and as such, guidelines recommend thromboprophylaxis in cases of severe OHSS [8, 58]. Since not all cases of OHSS are severe, the Delphi panel believes that thromboprophylaxis should be tailored per case severity and concomitant risk factors such as pregnancy, age, and existing thrombophilia.
A supplemental bolus of hCG at ovum pick up, or later, can “rescue” the corpus luteum and thus preserve implantation and ongoing pregnancy rates, which are reduced with GnRHa trigger only [59–62]. In a prospective randomized trial comparing hCG to GnRHa trigger with 1500 IU supplement of hCG on the day of ovum pick up, no statistical difference in delivery rates was found between the groups, and no cases of OHSS occurred in the GnRHa trigger group [61]. Of note, the average number of oocytes collected in each group was 9. Another prospective RCT on 118 patients at risk for OHSS (15–25 oocytes retrieved) found similar reproductive outcomes between hCG trigger versus GnRHa trigger with supplemental hCG, and no cases of OHSS in the latter group [63]. On the other hand, a RCT on 212 patients with an excessive response to ovarian stimulation (defined as ≥ 18 follicles ≥ 11 mm) triggered with GnRHa found that while pregnancy rates after fresh embryo transfer with intensified luteal support using low-dose hCG were comparable to pregnancy rates following the freeze-all strategy, moderate-severe OHSS occurred more frequently in women who attempted fresh transfer [64]. Two retrospective studies also described severe OHSS as a complication of low-dose hCG support albeit at low rates 0.72–1.4% [65, 66], and another retrospective study described 26% rate of severe OHSS with the hCG luteal support [67]. Lastly, a study of low-dose hCG luteal support (three boluses of hCG of 250, 500, or 1000 IU) observed 4.2% cases of moderate OHSS and 3.6% cases of severe OHSS [59]. Of note, 85.7% of the severe OHSS cases were late OHSS related to pregnancy. This highlights the risk of a fresh transfer, being related not only to the luteal support with hCG rescue but also to hCG from the pregnancy itself and the ensuing late OHSS. This, coupled with data described above, of a decrease in LBR when more than 15–20 oocytes are collected (which the trials on luteal rescue may have been underpowered to detect), and the high success rates of a freeze-all policy in hyper-responders [14] support the Delphi consensus of not adding hCG and attempt a fresh transfer in women perceived to be at high risk for OHSS prior to trigger (statement 23).
FET protocols in a hyper-responder
The optimal endometrial preparation protocol for FET is under debate [68]. A Cochrane review on the matter stated that there is insufficient evidence for the use of any specific preparation protocol for FET [69]. A recent multicenter retrospective cohort study found a lower LBR and a higher pregnancy loss rate in programmed endometrial preparation cycles as compared to natural or stimulated cycles [70]. Two meta-analyses on the matter also found higher rates of LBR in natural or stimulated cycles compared to programmed endometrial preparation [71, 72]. When addressing obstetrical and neonatal outcomes, a meta-analysis found programmed endometrial preparation to be associated with increased rates of large for gestational age neonates and macrosomia [73], and a register-based cohort study found higher rates of hypertensive disorders of pregnancy, postpartum hemorrhage, and cesarian section in programmed endometrial preparation cycles [74]. Finally, a meta-analysis on the subject also found a higher risk for hypertensive disorders of pregnancy, preeclampsia, post-partum hemorrhage, and cesarian section in programmed cycles [75]. Data specific to hyper-responders is limited, and, at this time, the Delphi panel does not believe that the hyper-response itself necessitates a specific adjustment of the FET protocol (statement 24).
The theoretical basis for the need for a washout period between a stimulation cycle and a FET cycle stems from the notion that the negative effects supraphysiological estradiol levels may have caused on endometrial receptivity continues in the immediately following cycle. Indeed, an online survey on the matter showed that most ART clinics worldwide defer the frozen embryo transfer in lieu of immediate transfer in the following menstrual cycle [76]. However, recent trials challenge the assumption of a lower LBR following transfer in the immediately following cycle both when analyzing a FET following a fresh failed embryo transfer [77–79] and when analyzing freeze-all cycles [80–86]. A meta-analysis of retrospective studies comparing immediate to delayed embryo transfer found improved LBR (aOR 1.2 CI 1.01–1.44) and improved CPR (aOR 1.22 CI 1.07–1.39) in favor of immediate FET [87]. In a subgroup analysis of FET after freeze-all cycles, the CPR was in favor of immediate transfer (aOR 1.27 CI 1.02–1.59) whereas the LBR did not differ significantly (aOR 1.15 CI 0.92–1.44). Of note, freeze-all cycles had several indications and were not limited to hyper-response and OHSS prevention only. Recently, two prospective RCTs found higher ongoing pregnancy rates and LBR following embryo transfer in the immediately following cycle [88, 89]; however, these trials were not limited to freeze-all cycles and consisted of programmed cycles. When studying obstetrical and neonatal outcomes, immediate embryo transfer seems comparable to deferred transfer [82, 90].
Considering the data above suggesting at least comparable outcomes and the possible added emotional stress related to postponing the frozen embryo transfer, the Delphi panel supports FET in the immediately following cycle (statement 25). With that in mind, further prospective RCT comparing immediate to delayed FET, following freeze all for the indication of a hyper-response, are needed to better consult women belonging to this specific population.
OHSS management
OHSS is classified into 4 stages based on clinical and laboratory features [8]. Data on the admission threshold for OHSS management is sparse and likely related to outpatient resources and capabilities which vary geographically. The Delphi panel recommends patient admission following the diagnosis of severe OHSS (statement 26) as recommended by other guidelines [91]. Although clinically apparent ascites classifies OHSS as severe, there is no consensus among the Delphi panelists whether this clinical feature alone requires patient admission/continued inpatient care. Indeed, outpatient paracentesis and culdocentesis is a viable option [8], and the lack of a Delphi consensus on this matter is too likely related to geographic variation in outpatient management capabilities. Moreso, other factors such as hydration status, renal function, and ability to tolerate oral intake are likely more important in the decision regarding inpatient and outpatient management; however these were not addressed by the Delphi panel and are likely also influenced by the varying outpatient management capabilities.
Our study is not without limitations. While we aimed to form a diverse and geographically representative panel, the lack of representation from countries such as China and India and the whole African continent is a limitation in our study design. The panel focus was on stimulation protocols for IVF with the aim of conception. The answers might differ when treating a hyper-responder interested in oocyte cryopreservation. An important aspect of treatment is patients’ comfort, financial and emotional costs, and compliance. As such, including patient representatives in at least part of the questions could have added helpful information to the treating physician when choosing a stimulation protocol and its goals. Lastly, when attempting to reach a consensus that, on the one hand, would include the expertise of a diverse panel and, on the other hand, could serve as a guideline, some arbitrary cutoff values (such as 80 kg as the cutoff for higher gonadotropin doses or 18 or more as the AFC that would put a patient at risk of a hyper-response) are needed. Similarly, and with the aim of simplifying the consensus, the statements could not discuss all possible distributions of follicle size and number or the contribution of different estradiol levels on decision-making. Notwithstanding the above-mentioned limitations, our study provides for the first-time guidelines regarding the management of a hyper-responder patient taking into consideration the more frequent use of the GnRH antagonist protocol with agonist trigger and freeze all in such patients and outcomes other than OHSS. Hopefully, with further research and experience, these guidelines could be fine-tuned in the future.
In conclusion, this Delphi consensus utilized a systematic compilation of experts’ opinions to generate a guideline for the management of a hyper-responder patient. It is worth emphasizing that the Delphi process is a form of expert opinion not meant to replace evidence-based medicine but rather to provide a guideline where such evidence is lacking. We believe that these guidelines can and should be used in the design of future studies on the matter.
Supplementary information
(PDF 226 kb)
(DOCX 14 kb)
Acknowledgements
Hera was the goddess of women, marriage, family, and childbirth in ancient Greek mythology and, as such, was felt to make a fitting title for a woman with hyper-response.
Data availability
Authors agree to make data and materials supporting the results or analyses presented in their paper available upon reasonable request.
Declarations
Competing interests
All declarations of interest are outside the submitted work. Peter Humaidan reports reception of lecture honoraria from Merck, Gedeon Richter, and IBSA. Antonio La Marca reports reception of consulting fees from Merck, Organon, Ferring, Gedeon Richter, Theramex, Beckman Coulter, and Roche. Samuel Santos-Ribeiro reports reception of research funding from Roche Diagnostics, Organon, and Theramex; reception of consulting fees from Organon, MSD, and Ferring; honoraria for lectures from Ferring, Besins, MSD/Organon, Theramex, and Gedeon Richter; reception of equipment materials or other services from Roche Diagnostics and Ferring; and is deputy of the SQART SIG in ESHRE. Alessandro Conforti reports reception of grants from the University of Naples Federico II; reception honoraria from Medea, Event Planet, and Merck. Baris Ata reports reception of consulting fees from Merck GmBH–Turkey; reception of payment or honoraria from Abbott, Merck GmBH, and Ferring; reception of support for attending meetings or travel from IBSA; is president of the Turkish Society of Reproductive Medicine; and is an executive committee member of ESHRE. Juan Garcia Velasco reports reception of payment or honoraria for lectures or educational events from Merck, Ferring, MSD, Organon, Theramex, and Gedeon Richter. George Lainas reports reception of payment or honoraria for lectures or educational events from Merck and Ferring, payment for expert testimony from Merck, and support for attending meetings from ESHRE. He also participated in data safety monitoring or advisory board of Merck. Filippo Maria Ubaldi is the scientific director of GeneraLife and minority shareholder of the company. He is also the president of SIFES-MR (the Italian Society of Fertility, Sterility and Reproductive Medicine) and a member of the scientific board of Medea. In the last 3 years, F.M. Ubaldi has received honoraria or consultation fees from Merck, MSD, Ferring, Gedeon Richter, Organon, and IBSA. Sesh Sunkara reports reception of payment or honoraria for lectures from Merck Ferring and MSD. Raoul Orvieto reports reception of consulting fees from Merck and Ferring and payment or honoraria for lectures from Merck and Ferring. Nikolaos Polyzos reports reception of grants or contracts from Merck Serono, IBSA, Organon, Ferring, Roche, Theramex, Besins Healthcare, and Gedeon Richter and reception of consulting fees from Merck Serono, IBSA, Organon, Ferring, Besins Healthcare, and Gedeon Richter. Hakan Yarali reports unrestricted grants from Merck, honoraria for lectures from Merck and IBSA, and support for attending meetings from Merck, IBSA, and Ferring. Human Fatemi reports receiving research grants from Merck Serono and Organon; consulting fees from Ferring Global; speaker honoraria from Organon, Merck Serono, and Ferring; and participation in data safety monitoring or advisory board for Ferring. Sandro Esteves reports unrestricted research grants from Merck KGaA; reception of consulting fees from Merck, MeD.E.A, and event planet; reception of honoraria for lectures from Merck, MeD.E.A, and event planet; has a patent on the ART calculator, is an unpaid advisory board member for Nature Reviews and for Urology, Is the Head, Department of Education and Research, Brazilian Society of Urology (São Paulo section; unpaid), and is the Co-chair, Male Infertility Special Interest Group, WHO Infertility Guidelines (unpaid). Craig LaTasha is a site investigator for Ferring Pharmaceuticals. He was an advisory board member for Ferring Pharmaceuticals. Ariel Weissman, Christophe Blockeel, Christos Venetis, Seang Lin Tan, Michael Dahan, Bulent Urman, RJ Norman, Richard Paulson, and Ido Feferkorn report no conflicts of interest.
Footnotes
The original online version of this article was revised: In this article the author’s name "LaTasha B. Craig" was incorrectly written as "C. LaTasha".
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/11/2023
A Correction to this paper has been published: 10.1007/s10815-023-03003-7
References
- 1.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod [Internet]. 2011;26:1768–1774. doi: 10.1093/humrep/der106. [DOI] [PubMed] [Google Scholar]
- 2.Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril [Internet]. 2018;110:661–670.e1. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Hum Reprod [Internet]. 2019;34:491–505. doi: 10.1093/humrep/dey388. [DOI] [PubMed] [Google Scholar]
- 4.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update [Internet]. 2019;25:2–14. doi: 10.1093/humupd/dmy033. [DOI] [PubMed] [Google Scholar]
- 5.Boynukalin FK, Turgut NE, Gultomruk M, Ecemis S, Yarkiner Z, Findikli N, et al. Impact of elective frozen vs. fresh embryo transfer strategies on cumulative live birth: Do deleterious effects still exist in normal & hyper responders? PLoS One. 2020;15 [cited 2022 Mar 17]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/32589634/ [DOI] [PMC free article] [PubMed]
- 6.Ioannidou PG, Bosdou JK, Lainas GT, Lainas TG, Grimbizis GF, Kolibianakis EM. How frequent is severe ovarian hyperstimulation syndrome after GnRH agonist triggering in high-risk women? A systematic review and meta-analysis. Reprod Biomed Online [Internet]. 2021;42:635–650. doi: 10.1016/j.rbmo.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Orvieto R. Can we eliminate severe ovarian hyperstimulation syndrome? Hum Reprod [Internet]. 2005;20:320–322. doi: 10.1093/humrep/deh613. [DOI] [PubMed] [Google Scholar]
- 8.Committee of the American Society for Reproductive Medicine P. Pfeifer S, Butts MSCES, Dumesic D, Fossum G, Gracia MSCEC, et al. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril [Internet]. 2016;106:1634–1647. doi: 10.1016/j.fertnstert.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Feferkorn I, Ata B, Esteves SC, La Marca A, Paulson R, Blockeel C, et al. The HERA (Hyper-response Risk Assessment) Delphi consensus definition of hyper-responders for in-vitro fertilization. J Assist Reprod Genet. 2023; [cited 2023 Mar 21]; Available from: https://pubmed.ncbi.nlm.nih.gov/36933094/ [DOI] [PMC free article] [PubMed]
- 10.Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. 2014;101:967–973. doi: 10.1016/j.fertnstert.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 11.Polyzos NP, Drakopoulos P, Parra J, Pellicer A, Santos-Ribeiro S, Tournaye H, et al. Cumulative live birth rates according to the number of oocytes retrieved after the first ovarian stimulation for in vitro fertilization/intracytoplasmic sperm injection: a multicenter multinational analysis including ∼15,000 women. Fertil Steril. 2018;110:661–670.e1. doi: 10.1016/j.fertnstert.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson Å, Källen K, Thurin-Kjellberg A, Bergh C. The number of oocytes retrieved during IVF: a balance between efficacy and safety. Hum Reprod [Internet]. 2018;33:58–64. doi: 10.1093/humrep/dex334. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfers in high responders. Fertil Steril [Internet]. 2011;96:516–518. doi: 10.1016/j.fertnstert.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 14.Chen Z-J, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. New Engl J Med [Internet]. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 15.Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. Risk of severe ovarian hyperstimulation syndrome in GnRH antagonist versus GnRH agonist protocol: RCT including 1050 first IVF/ICSI cycles. Hum Reprod [Internet]. 2016;31:1253–1264. doi: 10.1093/humrep/dew051. [DOI] [PubMed] [Google Scholar]
- 16.Ovarian Stimulation TEGGO, Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open [Internet]. 2020;2020:1–13. [Google Scholar]
- 17.Oudshoorn SC, van Tilborg TC, Eijkemans MJC, Oosterhuis GJE, Friederich J, van Hooff MHA, et al. Individualized versus standard FSH dosing in women starting IVF/ICSI: an RCT. Part 2: the predicted hyper responder. Hum Reprod [Internet]. 2017;32:2506–2514. doi: 10.1093/humrep/dex319. [DOI] [PubMed] [Google Scholar]
- 18.Lensen SF, Wilkinson J, Leijdekkers JA, la Marca A, Mol BWJ, Marjoribanks J, et al. Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev. 2018;2 [cited 2022 Jun 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/29388198/ [DOI] [PMC free article] [PubMed]
- 19.Friis Petersen J, Løkkegaard E, Andersen LF, Torp K, Egeberg A, Hedegaard L, et al. A randomized controlled trial of AMH-based individualized FSH dosing in a GnRH antagonist protocol for IVF. Hum Reprod Open [Internet]. 2019;2019 [cited 2022 Jun 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/30895268/ [DOI] [PMC free article] [PubMed]
- 20.Mannaerts BMJL, Rombout F, Out HJ, Bennink HC. Clinical profiling of recombinant follicle stimulating hormone (rFSH; Puregon): relationship between serum FSH and efficacy. Hum Reprod Update [Internet]. 1996;2:153–161. doi: 10.1093/humupd/2.2.153. [DOI] [PubMed] [Google Scholar]
- 21.Rose TH, Röshammar D, Erichsen L, Grundemar L, Ottesen JT. Population pharmacokinetic modelling of FE 999049, a recombinant human follicle-stimulating hormone, in healthy women after single ascending doses. Drugs R D [Internet]. 2016;16:173–180. doi: 10.1007/s40268-016-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose TH, Röshammar D, Erichsen L, Grundemar L, Ottesen JT. Characterisation of population pharmacokinetics and endogenous follicle-stimulating hormone (FSH) levels after multiple dosing of a recombinant human FSH (FE 999049) in healthy women. Drugs RD [Internet]. 2016;16:165–172. doi: 10.1007/s40268-016-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledger WL, Fauser BCJM, Devroey P, Zandvliet AS, BMJL M. Corifollitropin alfa doses based on body weight: clinical overview of drug exposure and ovarian response. Reprod Biomed Online [Internet]. 2011;23:150–159. doi: 10.1016/j.rbmo.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Leijdekkers JA, van Tilborg TC, Torrance HL, Oudshoorn SC, Brinkhuis EA, Koks CAM, et al. Do female age and body weight modify the effect of individualized FSH dosing in IVF/ICSI treatment? A secondary analysis of the OPTIMIST trial. Acta Obstet Gynecol Scand [Internet]. 2019;98:1332–1340. doi: 10.1111/aogs.13664. [DOI] [PubMed] [Google Scholar]
- 25.Lainas GT, Lainas TG, Sfontouris IA, Venetis CA, Bosdou JK, Chatzimeletiou A, et al. Association between body mass index and oocyte maturation in patients triggered with GnRH agonist who are at high risk for severe ovarian hyperstimulation syndrome: an observational cohort study. Reprod Biomed Online [Internet]. 2020;40:168–175. doi: 10.1016/j.rbmo.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Aboulghar MA, Mansour RT, Serour GI, Rhodes CA, Amin YM. Reduction of human menopausal gonadotropin dose before coasting prevents severe ovarian hyperstimulation syndrome with minimal cycle cancellation. J Assist Reprod Genet [Internet]. 2000;17:298. doi: 10.1023/A:1009470602525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatemi H, Bilger W, Denis D, Griesinger G, la Marca A, Longobardi S, et al. Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: a systematic review covering 10 years (2007-2017). Reprod Biol Endocrinol [Internet]. 2021;19 [cited 2022 Aug 26] Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33975610/ [DOI] [PMC free article] [PubMed]
- 28.Mahony MC, Hayward B, Mottla GL, Richter KS, Beall S, Ball GD, et al. Recombinant human follicle-stimulating hormone alfa dose adjustment in US clinical practice: an observational, retrospective analysis of a real-world electronic medical records database. Front Endocrinol (Lausanne) [Internet]. 2021;12 [cited 2022 Aug 26]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/34956077/ [DOI] [PMC free article] [PubMed]
- 29.D’Angelo A, Brown J, Amso NN. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev [Internet]. 2011; [cited 2022 Aug 26]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/21678336/
- 30.D’Angelo A, Amso NN, Hassan R. Coasting (withholding gonadotrophins) for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev [Internet]. 2017;5 [cited 2022 Aug 26]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/28535578/ [DOI] [PMC free article] [PubMed]
- 31.Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25:1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 32.Tso LO, Costello MF, Albuquerque LET, Andriolo RB, Macedo CR. Metformin treatment before and during IVF or ICSI in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2020;12 [cited 2022 Jun 23] Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33347618/ [DOI] [PMC free article] [PubMed]
- 33.Wu Y, Tu M, Huang Y, Liu Y, Zhang D. Association of metformin with pregnancy outcomes in women with polycystic ovarian syndrome undergoing in vitro fertilization: a systematic review and meta-analysis. JAMA Netw Open [Internet]. 2020;3:e2011995. doi: 10.1001/jamanetworkopen.2020.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siristatidis CS, Basios G, Pergialiotis V, Vogiatzi P. Aspirin for in vitro fertilisation. Cochrane Database Syst Rev. 2016;11 [cited 2022 Aug 26]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/27807847/ [DOI] [PMC free article] [PubMed]
- 35.Orvieto R. Prediction of ovarian hyperstimulation syndrome. Challenging the estradiol mythos. Human Reproduction [Internet]. 2003;18:665–667. doi: 10.1093/humrep/deg166. [DOI] [PubMed] [Google Scholar]
- 36.Orvieto R. Controlled ovarian hyperstimulation - an inflammatory state. J Soc Gynecol Investig. 2004;11:424–426. doi: 10.1016/j.jsgi.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Orvieto R, Ben-Rafael Z. Ovarian hyperstimulation syndrome: a new insight into an old enigma. J Soc Gynecol Investig [Internet]. 1998;5:110–113. doi: 10.1016/S1071-5576(97)00113-5. [DOI] [PubMed] [Google Scholar]
- 38.Ata B, Tulandi T. Pathophysiology of ovarian hyperstimulation syndrome and strategies for its prevention and treatment. Expert Rev Obstet Gynecol. 2014;4:299–311. doi: 10.1586/eog.09.10. [DOI] [Google Scholar]
- 39.Levy T, Orvieto R, Homburg R, Peleg D, Dekel A, Ben-Rafael Z. Severe ovarian hyperstimulation syndrome despite low plasma oestrogen concentrations in a hypogonadotrophic, hypogonadal patient. Human Reproduction [Internet]. 1996;11:1177–1179. doi: 10.1093/oxfordjournals.humrep.a019350. [DOI] [PubMed] [Google Scholar]
- 40.Papanikolaou EG, Pozzobon C, Kolibianakis EM, Camus M, Tournaye H, Fatemi HM, et al. Incidence and prediction of ovarian hyperstimulation syndrome in women undergoing gonadotropin-releasing hormone antagonist in vitro fertilization cycles. Fertil Steril. 2006;85:112–120. doi: 10.1016/j.fertnstert.2005.07.1292. [DOI] [PubMed] [Google Scholar]
- 41.Tarlatzi TB, Venetis CA, Devreker F, Englert Y, Delbaere A. What is the best predictor of severe ovarian hyperstimulation syndrome in IVF? A cohort study. J Assist Reprod Genet [Internet]. 2017;34:1341. doi: 10.1007/s10815-017-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahnberg A, Enskog A, Brännström M, Lundin K, Bergh C. Prediction of ovarian hyperstimulation syndrome in women undergoing in vitro fertilization. Acta Obstet Gynecol Scand [Internet]. 2009;88:1373–1381. doi: 10.3109/00016340903287482. [DOI] [PubMed] [Google Scholar]
- 43.Griesinger G, Verweij PJM, Gates D, Devroey P, Gordon K, Stegmann BJ, et al. Prediction of ovarian hyperstimulation syndrome in patients treated with Corifollitropin alfa or rFSH in a GnRH antagonist protocol. PLoS One [Internet]. 2016;11:e0149615. doi: 10.1371/journal.pone.0149615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, Novella-Maestre E, Alonso-Muriel I, Sanchez-Criado J, et al. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology [Internet]. 2006;147:5400–5411. doi: 10.1210/en.2006-0657. [DOI] [PubMed] [Google Scholar]
- 45.Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1 [cited 2022 Apr 30]. Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/28111738/ [DOI] [PMC free article] [PubMed]
- 46.Leitao VMS, Moroni RM, Seko LMD, Nastri CO, Martins WP. Cabergoline for the prevention of ovarian hyperstimulation syndrome: systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2014;101:664–675.e7. doi: 10.1016/j.fertnstert.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Tang H, Mourad SM, Wang A, Zhai S di, Hart RJ. Dopamine agonists for preventing ovarian hyperstimulation syndrome. Cochrane Database Syst Rev [Internet]. 2021 2021 [cited 2022 Aug 20]. Available from: /pmc/articles/PMC8092425/. [DOI] [PMC free article] [PubMed]
- 48.Shrem G, Steiner N, Balayla J, Volodarsky-Perel A, Tannus S, Son WY, et al. Use of cabergoline and post-collection GnRH antagonist administration for prevention of ovarian hyperstimulation syndrome. Reprod Biomed Online. 2019;39:433–438. doi: 10.1016/j.rbmo.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 49.Seow KM, Lin YH, Bai CH, Chen HJ, Hsieh BC, Huang LW, et al. Clinical outcome according to timing of cabergoline initiation for prevention of OHSS: a randomized controlled trial. Reprod Biomed Online. 2013;26:562–568. doi: 10.1016/j.rbmo.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 50.The ESHRE Guideline Group on Ovarian Stimulation. Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI†. Hum Reprod Open [Internet]. 2020;2020:1–13. doi: 10.1093/hropen/hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2021;2 [cited 2022 Aug 20]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33539543/ [DOI] [PMC free article] [PubMed]
- 52.Revelli A, Dolfin E, Gennarelli G, Lantieri T, Massobrio M, Holte JG, et al. Low-dose acetylsalicylic acid plus prednisolone as an adjuvant treatment in IVF: a prospective, randomized study. Fertil Steril. 2008;90:1685–1691. doi: 10.1016/j.fertnstert.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 53.Várnagy Á, Bódis J, Mánfai Z, Wilhelm F, Busznyák C, Koppán M. Low-dose aspirin therapy to prevent ovarian hyperstimulation syndrome. Fertil Steril. 2010;93:2281–2284. doi: 10.1016/j.fertnstert.2009.01.085. [DOI] [PubMed] [Google Scholar]
- 54.Asimakopoulos B, Nikolettos N, Nehls B, Diedrich K, Al-Hasani S, Metzen E. Gonadotropin-releasing hormone antagonists do not influence the secretion of steroid hormones but affect the secretion of vascular endothelial growth factor from human granulosa luteinized cell cultures. Fertil Steril [Internet]. 2006;86:636–641. doi: 10.1016/j.fertnstert.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 55.Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Kolibianakis EM. Management of severe early ovarian hyperstimulation syndrome by re-initiation of GnRH antagonist. Reprod Biomed Online [Internet]. 2007;15:408–412. doi: 10.1016/S1472-6483(10)60366-5. [DOI] [PubMed] [Google Scholar]
- 56.Lainas GT, Kolibianakis EM, Sfontouris IA, Zorzovilis IZ, Petsas GK, Tarlatzi TB, et al. Outpatient management of severe early OHSS by administration of GnRH antagonist in the luteal phase: an observational cohort study. Reprod Biol Endocrinol. 2012;10 [cited 2022 Aug 23]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/22938051/ [DOI] [PMC free article] [PubMed]
- 57.Mills G, Dahan MH. Gonadotropin releasing hormone (GnRH) antagonist administration to decrease the risk of ovarian hyperstimulation syndrome in GNRH agonist cycles triggered with human chorionic gonadotropin. Arch Gynecol Obstet [Internet]. 2022; [cited 2022 Aug 31]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/35932297/ [DOI] [PubMed]
- 58.VTE, thrombophilia, antithrombotic therapy, and pregnancy - antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest [Internet]. 2012;141:e691S–736S. [cited 2022 Aug 23]; Available from: http://journal.chestnet.org/article/S0012369212601366/fulltext [DOI] [PMC free article] [PubMed]
- 59.Castillo JC, Dolz M, Bienvenido E, Abad L, Casan EM, Bonilla-Musoles F. Cycles triggered with GnRH agonist: exploring low-dose HCG for luteal support. Reprod Biomed Online [Internet]. 2010;20:175–181. doi: 10.1016/j.rbmo.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Humaidan P. Luteal phase rescue in high-risk OHSS patients by GnRHa triggering in combination with low-dose HCG: a pilot study. Reprod Biomed Online [Internet]. 2009;18:630–634. doi: 10.1016/S1472-6483(10)60006-5. [DOI] [PubMed] [Google Scholar]
- 61.Humaidan P, Ejdrup Bredkjær H, Westergaard LG, Yding Andersen C. 1,500 IU human chorionic gonadotropin administered at oocyte retrieval rescues the luteal phase when gonadotropin-releasing hormone agonist is used for ovulation induction: a prospective, randomized, controlled study. Fertil Steril [Internet]. 2010;93:847–854. doi: 10.1016/j.fertnstert.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 62.Humaidan P, Bungum L, Bungum M, Andersen CY. Rescue of corpus luteum function with peri-ovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reprod Biomed Online [Internet]. 2006;13:173–178. doi: 10.1016/S1472-6483(10)60612-8. [DOI] [PubMed] [Google Scholar]
- 63.Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod [Internet]. 2013;28:2511–2521. doi: 10.1093/humrep/det249. [DOI] [PubMed] [Google Scholar]
- 64.Santos-Ribeiro S, Mackens S, Popovic-Todorovic B, Racca A, Polyzos NP, van Landuyt L, et al. The freeze-all strategy versus agonist triggering with low-dose hCG for luteal phase support in IVF/ICSI for high responders: a randomized controlled trial. Hum Reprod [Internet]. 2020;35:2808–2818. doi: 10.1093/humrep/deaa226. [DOI] [PubMed] [Google Scholar]
- 65.Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi-centre studies in IVF patients. Hum Reprod [Internet]. 2013;28:2511–2521. doi: 10.1093/humrep/det249. [DOI] [PubMed] [Google Scholar]
- 66.Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Tournaye H, Humaidan P, et al. Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high-risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Hum Reprod [Internet]. 2013;28:2529–2536. doi: 10.1093/humrep/det304. [DOI] [PubMed] [Google Scholar]
- 67.Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod [Internet]. 2013;28:2522–2528. doi: 10.1093/humrep/det124. [DOI] [PubMed] [Google Scholar]
- 68.Orvieto R, Kirshenbaum M, Gleicher N. Is embryo cryopreservation causing macrosomia-and what else? Front Endocrinol (Lausanne) [Internet]. 2020;11 [cited 2023 Mar 16]; Available from: https://pubmed.ncbi.nlm.nih.gov/32047479/ [DOI] [PMC free article] [PubMed]
- 69.Glujovsky D, Pesce R, Sueldo C, Quinteiro Retamar AM, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev. 2020;10 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/33112418/ [DOI] [PMC free article] [PubMed]
- 70.Vinsonneau L, Labrosse J, Porcu-Buisson G, Chevalier N, Galey J, Ahdad N, et al. Impact of endometrial preparation on early pregnancy loss and live birth rate after frozen embryo transfer: a large multicenter cohort study (14 421 frozen cycles). Hum Reprod Open [Internet]. 2022;2022 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/35274060/ [DOI] [PMC free article] [PubMed]
- 71.Gan J, Rozen G, Polyakov A. Treatment outcomes of blastocysts thaw cycles, comparing the presence and absence of a corpus luteum: a systematic review and meta-analysis. BMJ Open [Internet]. 2022;12:e051489. doi: 10.1136/bmjopen-2021-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu H, Zhou P, Lin X, Wang S, Zhang S. Endometrial preparation for frozen-thawed embryo transfer cycles: a systematic review and network meta-analysis. J Assist Reprod Genet. 2021;38:1913–1926. doi: 10.1007/s10815-021-02125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosalik K, Carson S, Pilgrim J, Luizzi J, Levy G, Heitmann R, et al. Effects of different frozen embryo transfer regimens on abnormalities of fetal weight: a systematic review and meta-analysis. Hum Reprod Update [Internet]. 2021;28:1–14. doi: 10.1093/humupd/dmab037. [DOI] [PubMed] [Google Scholar]
- 74.Asserhøj LL, Spangmose AL, Aaris Henningsen AK, Clausen TD, Ziebe S, Jensen RB, et al. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril [Internet]. 2021;115:947–956. doi: 10.1016/j.fertnstert.2020.10.039. [DOI] [PubMed] [Google Scholar]
- 75.Busnelli A, Schirripa I, Fedele F, Bulfoni A, Levi-Setti PE. Obstetric and perinatal outcomes following programmed compared to natural frozen-thawed embryo transfer cycles: a systematic review and meta-analysis. Hum Reprod [Internet]. 2022;37 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/35553678/ [DOI] [PubMed]
- 76.RESULTS - Frozen-Thawed Embryo Transfer - IVF-Worldwide [Internet]. [cited 2022 Aug 24]. Available from: https://ivf-worldwide.com/survey/frozen-thawed-embryo-transfer/results-frozen-thawed-embryo-transfer.html.
- 77.Huang J, Lu X, Xie Q, Lin J, Cai R, Kuang Y. Timing of frozen-thawed embryo transfer after controlled ovarian stimulation in a non-elective freeze-all policy. Ann Transl Med [Internet]. 2019;7:752–752. doi: 10.21037/atm.2019.11.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Santos-Ribeiro S, Siffain J, Polyzos NP, van de Vijver A, van Landuyt L, Stoop D, et al. To delay or not to delay a frozen embryo transfer after a failed fresh embryo transfer attempt? Fertil Steril [Internet]. 2016;105:1202–1207.e1. doi: 10.1016/j.fertnstert.2015.12.140. [DOI] [PubMed] [Google Scholar]
- 79.Horowitz E, Mizrachi Y, Farhi J, Shalev A, Raziel A, Weissman A. Modified natural-cycle cryopreserved embryo transfer: is a washout period needed after a failed fresh cycle? Reprod Biomed Online [Internet]. 2019;39:439–445. doi: 10.1016/j.rbmo.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Santos-Ribeiro S, Polyzos NP, Lan VTN, Siffain J, Mackens S, van Landuyt L, et al. The effect of an immediate frozen embryo transfer following a freeze-all protocol: a retrospective analysis from two centres. Hum Reprod [Internet]. 2016;31:2541–2548. doi: 10.1093/humrep/dew194. [DOI] [PubMed] [Google Scholar]
- 81.Lattes K, Checa MA, Vassena R, Brassesco M, Vernaeve V. There is no evidence that the time from egg retrieval to embryo transfer affects live birth rates in a freeze-all strategy. Hum Reprod [Internet]. 2017;32:368–374. doi: 10.1093/humrep/dew306. [DOI] [PubMed] [Google Scholar]
- 82.Bourdon M, Santulli P, Maignien C, Pocate-Cheriet K, Alwohaibi A, Marcellin L, et al. The interval between oocyte retrieval and frozen-thawed blastocyst transfer does not affect the live birth rate and obstetrical outcomes. PLoS One. 2018;13 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/30339700/ [DOI] [PMC free article] [PubMed]
- 83.Ozgur K, Bulut H, Berkkanoglu M, Humaidan P, Coetzee K. Frozen embryo transfer can be performed in the cycle immediately following the freeze-all cycle. J Assist Reprod Genet [Internet]. 2018;35:135–142. doi: 10.1007/s10815-017-1048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higgins C, Healey M, Jatkar S, Vollenhoven B. Interval between IVF stimulation cycle and frozen embryo transfer: is there a benefit to a delay between cycles? Aust N Z J Obstet Gynaecol [Internet]. 2018;58:217–221. doi: 10.1111/ajo.12696. [DOI] [PubMed] [Google Scholar]
- 85.He Y, Zheng H, Du H, Liu J, Li L, Liu H, et al. Delayed frozen embryo transfer failed to improve live birth rate and neonatal outcomes in patients requiring whole embryo freezing. Reprod Biol Endocrinol [Internet]. 2020;18 [DOI] [PMC free article] [PubMed]
- 86.Yildiz S, Turkgeldi E, Kalafat E, Keles I, Gokyer D, Ata B. Do live birth rate and obstetric outcomes vary between immediate and delayed embryo transfers following freeze-all cycles? J Gynecol Obstet Hum Reprod. 2021;50 [cited 2023 Mar 16]; Available from: https://pubmed.ncbi.nlm.nih.gov/34506996/ [DOI] [PubMed]
- 87.Bergenheim SJ, Saupstad M, Pistoljevic N, Andersen AN, Forman JL, Løssl K, et al. Immediate versus postponed frozen embryo transfer after IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update [Internet]. 2021;27:623–642. doi: 10.1093/humupd/dmab002. [DOI] [PubMed] [Google Scholar]
- 88.Song JY, Dong FY, Li L, Zhang XX, Wang AJ, Zhang Y, et al. Immediate versus delayed frozen embryo transfer in women following a failed IVF-ET attempt: a multicenter randomized controlled trial. Reprod Biol Endocrinol. 2021;19 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/34461950/ [DOI] [PMC free article] [PubMed]
- 89.Li H, Sun X, Yang J, Li L, Zhang W, Lu X, et al. Immediate versus delayed frozen embryo transfer in patients following a stimulated IVF cycle: a randomised controlled trial. Hum Reprod [Internet]. 2021;36:1832–1840. doi: 10.1093/humrep/deab071. [DOI] [PubMed] [Google Scholar]
- 90.Zuo N, Gao Y, Zhang N, Li D, Wang X. Effects of immediate versus delayed frozen embryo transfer in high responder patients undergoing freeze-all cycles. BMC Pregnancy Childbirth [Internet]. 2021;21 [cited 2022 Aug 24]; Available from: https://pubmed-ncbi-nlm-nih-gov.proxy3.library.mcgill.ca/34182954/ [DOI] [PMC free article] [PubMed]
- 91.Timmons D, Montrief T, Koyfman A, Long B. Ovarian hyperstimulation syndrome: a review for emergency clinicians. Am J Emerg Med. 2019;37:1577–1584. doi: 10.1016/j.ajem.2019.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 226 kb)
(DOCX 14 kb)
Data Availability Statement
Authors agree to make data and materials supporting the results or analyses presented in their paper available upon reasonable request.