Abstract
Alzheimer’s disease (AD) exerts a tremendous socio-economic burden worldwide. Although reduced cerebral blood flow is an early and persistent symptom that precedes the loss of cognitive function in AD, the underlying molecular and cellular mechanisms remain unclear. The present study investigated whether capillary endothelial inward rectifier potassium 2 (Kir2.1) expression is reduced in TgF344-AD (AD) rats and contributes to neurovascular uncoupling and cognitive deficits in AD. Three- to fourteen-month-old AD rats expressing mutant human APP and PS1 and age-matched wild-type (WT) F344 rats were studied. AD rats exhibited higher amyloid beta (Aβ) expression in the brain as early as 3 months of age and amyloid plaques by 4 months of age. Functional hyperemic responses induced by whisker stimulation were impaired at 4 months of age, which were exacerbated in 6-month- and 14-month-old AD rats. The expression of Kir2.1 protein was significantly lower in the brains of 6-month-old AD versus WT rats, and Kir2.1 coverage was lower in the cerebral microvasculature of AD than in WT rats. Aβ1–42 reduced the Kir2.1 expression in cultured capillary endothelial cells. Cerebral parenchymal arterioles with attached capillaries exhibited a reduced vasodilator in response to 10 mM K+ applied to capillaries, and constricted less following administration of a Kir2.1 channel blocker, compared to WT vessels. These results indicate that capillary endothelial Kir2.1 expression is reduced and contributes to impaired functional hyperemia in AD rats at early ages, perhaps secondary to elevated Aβ expression.
Keywords: Alzheimer’s disease, Brain hypoperfusion, Neurovascular coupling, Kir2.1, Cognitive impairment
Introduction
Alzheimer’s disease (AD) represents the most common form of dementia. Six and a half million Americans are living with AD or other dementias. The healthcare costs for AD and AD-related dementia (ADRD) exceeded $321 billion in 2022 [1]. The classic hallmarks of AD are amyloid beta (Aβ) accumulation and phosphorylated tau protein aggregation, resulting in the formation of extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs). In addition, AD is associated with cholinergic insufficiency, inflammation, and cerebrovascular dysfunction [2, 3]. Increasing evidence has implicated cerebral hypoperfusion as one of the earliest pathological findings in AD [3–7], and dynamic cerebral blood flow (CBF) regulation is reduced in AD patients and animal models [8–13].
Dynamic CBF regulation refers to functional hyperemia, which relies on neurovascular coupling (NVC) to redirect blood flow to the areas of activated neurons independent of blood pressure changes. The functional unit of NVC consists of neurons, astrocytic endfeet, capillary endothelial cells (ECs), pericytes, and smooth muscle cells (SMCs) [11]. When local neurons are activated, Ca2+ mobilization occurs in neurons and adjacent astrocytes, evoking an elevation of extracellular K+ concentration to about 10 mM [14, 15]. The elevated extracellular K+ activates inward rectifier potassium 2 (Kir2.1) channels on capillary ECs, producing a hyperpolarization current, which retrogradely propagates from capillary to arteriolar ECs via gap junctions and from EC to SMCs or alpha-smooth muscle actin (a-SMA)-positive pericytes via myoendothelial projections at upstream capillary branch points and parenchymal arterioles (PAs) [16]. In response to the hyperpolarizing signal, SMCs and a-SMA-positive pericytes relax, leading to an increase in blood flow to the activated region [17–21]. As such, nutrients are provided, and metabolic by-products are removed.
Cerebrovascular endothelial Kir2.1 channel dysfunction has been proposed as a potential contributor to neurovascular uncoupling in AD [22–24]. There is evidence that there is a diminished vasodilator response to the Kir2.1 channel activator and compromised NVC in AD transgenic rodent models [22, 23]. However, little is known about the time course of changes in capillary endothelial Kir2.1 expression or function, the initial sensor of neuronal activity [18, 25], in the development of AD. Recently, Mughal et al. [24] reported that capillary endothelial Kir2.1 channel–driven NVC is reduced in a middle-aged, 12–13-month-old 5xFAD mouse model of AD. However, the 5xFAD mouse model displays amyloid plaques as early as 2 months of age and memory dysfunction by 4–5 months of age. Thus, it remains uncertain whether capillary Kir2.1 channel dysfunction precedes the loss of NVC and cerebral hypoperfusion in the early stage of AD in this model.
To address this critical knowledge gap, the present study examined the hypothesis that reduced capillary endothelial Kir2.1 expression contributes to impaired functional hyperemic response in an early stage of AD using a TgF344-AD rat model of AD. This well-established AD model expresses human mutant APP and PS1 and recapitulates a broad range of AD-like pathologies.
Materials and methods
Animals
Experiments were performed on heterozygous TgF344-AD rats carrying mutant human APP KM670/671NL Swedish mutation and PS1 deltaE9 mutation driven by a mouse prion promoter on the Fischer 344 (F344) rat genetic background [26], and their wild-type (WT) F344 littermates. The animals were housed on a 12-h:12-h light/dark cycle with food and water provided ad libitum at the University of Mississippi Medical Center (UMMC). A total of 56 male WT and 56 male AD rats were studied. All procedures were approved by Institutional Animal Care and Use Committees of the UMMC and adhered to the NIH guidelines.
Previous studies have indicated that the TgF344-AD model first exhibits amyloid plaques and signs of memory dysfunction at 6 months of age. Significant tau tangles form, and neuronal loss are evident by 15 months of age [26]. In a recent study, we reported that these rats exhibit impaired myogenic responses of the middle cerebral artery and PAs and autoregulation of CBF at 4 months of age, 2 months prior to the development of cognitive dysfunction and the appearance of amyloid plaques [27]. The present study further evaluated the role of Aβ and cerebral vascular dysfunction in the early development of cognitive dysfunction in TgF344-AD rats. Functional hyperemic responses to whisker stimulation in vivo were studied on 3-month-, 4-month-, 6-month-, and 14-month-old rats. The effects of Aβ on the expression of Kir2.1 channels were studied in primary microvascular ECs isolated from 3-week-old F344 rats. Vascular reactivity to activation and blockage of the Kir2.1 channel was examined in perfused PAs with attached capillaries in 4-month-old rats based on results obtained from our functional hyperemia experiments.
Materials
The Kir2.1 inhibitor ML133 (#422689) and the Ca2+-activated intermediate-/small-conductance potassium (IK/SK) channel activator NS309 (#N8161) were purchased from Sigma-Aldrich. Stock solutions of ML133 (10 mM) and NS309 (1 mM) were prepared in dimethyl sulfoxide (DMSO; Invitrogen). Aβ1–42 (#AS24224) was obtained from AnaSpec. A stock solution of Aβ1–42 (0.1 mM) was prepared in 1.0% NH4OH. Test solutions were prepared by diluting the stock 1:1000 in physiological salt solution (PSS) or Endothelial Growth Medium (EGM). The final DMSO concentrations were less than 0.1%, which had no effect on cell viability or vascular responses.
Isolation and culture of microvascular endothelial cells
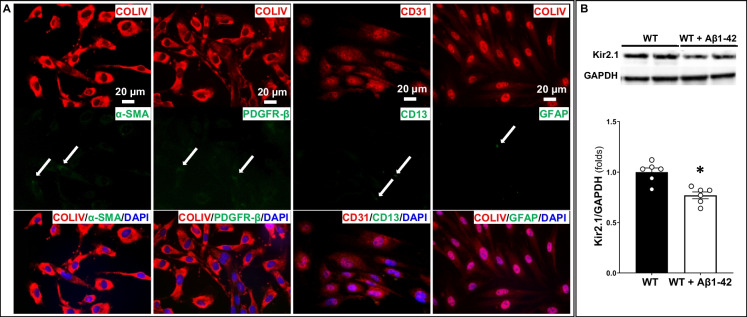
Rat brain microvascular ECs were isolated from 3-week-old WT animals as previously reported [28, 29]. The rats were euthanized with 4% isoflurane, and the brains were rapidly collected and placed in 5 mL sterile ice-cold PBS. After the removal of the brain stem, cerebellum, and meninges, the brain was homogenized in ice-cold serum-free Dulbecco’s modified Eagle’s medium (DMEM) with gentamicin (50 µg/mL). The homogenate was further dissociated in ice-cold DMEM supplemented with collagenase type II (1 mg/mL), DNase (15 µg/mL), and gentamicin (50 µg/mL), and incubated for 1.5 h at 37 °C on a shaker at 180 rpm. The digest was collected by centrifuging at 1000 g for 10 min at 4 °C and resuspended in 20% bovine serum albumin (BSA) in DMEM. The myelin layer was removed by centrifuging the tissue for 20 min. The pellets containing cells were then digested with collagenase/dispase (1 mg/mL, COLLDISP-RO Roche) and DNase (6.7 µg/mL) in DMEM for 1 h at 37 °C on a shaker at 180 rpm. Subsequently, cells were separated by centrifugation at 700 g on a 33% Percoll (GE17-0891–01, Sigma-Aldrich) gradient. The cells, visible as a cloud at the interphase, were collected and seeded onto a collagen type IV (0.1 mg/mL, C6745, Sigma-Aldrich) and fibronectin (0.1 mg/mL, F1141, Sigma-Aldrich)-coated 6-well plate. Freshly isolated cells were first maintained in EC medium containing 10% fetal bovine serum, #S11550; 1.5 ng/mL Fibroblast Growth Factor-Basic, #F0291, Sigma-Aldrich; 100 µg/mL heparin, #504015, USP; 50 µg/mL gentamicin, #G1397, Sigma-Aldrich; 4 µg/mL puromycin, #P9620, Sigma-Aldrich; and insulin (5 µg/mL), transferrin (5 µg/mL), and selenium (5 ng/mL) (ITS) solution (#cAP-26; ANGIO-PROTEOMIE, Boston, MA) at 37 °C with a humidified atmosphere of 5% CO2/95% air, for 2 days. Puromycin (4 µg/mL) was included in the media as a selection agent to achieve a high purity of ECs. From day 3, ECs were cultured with EGM (cAP-02; ANGIO-PROTEOMIE, Boston, MA). After the cells reached confluence (> 80%), they were dissociated with trypsin and seeded in 4-chamber slides for cell validation using immunocytochemistry. After 8 days in culture, the cell purity was more than 80% ECs (Fig. 6A).
Fig. 6.
The Kir2.1 expression was decreased in capillary endothelial cells (ECs) treated with Aβ1–42. A Validation of rat brain microvascular endothelial cells using immunocytochemistry. By puromycin selection, a high-purity microvascular endothelial cell is demonstrated by the positive expression of endothelial cell markers CD31 ( +) and COLIV ( +) and the lack of expression of the smooth muscle marker α-SMA ( −), the pericyte marker PDGFR-β ( −), and the astrocyte marker GFAP ( −). B Aβ1–42 treatment reduced Kir2.1 expression in capillary ECs. Mean values ± SEM are presented. Four to six rats were used. *P < 0.05 from the corresponding values in untreated capillary ECs. WT, wild-type; Kir2.1, inward rectifier potassium 2 channel
Immunostaining for Aβ expression
Brains were collected and fixed in a zinc-formalin solution overnight. Free-floating 35-µm brain sections were prepared using a Compresstome microtome (VF-310-0Z; Precisionary Instruments, Natick, MA). The sections were washed with PBS containing 0.1% Triton X-100 (PBST) 10 times for 5 min and were incubated with 0.1% trypsin at 37 °C for 2 h and sodium citrate buffer (10 mM, pH 6.0) at 80 °C for 3.5 h for antigen retrieval. After three washes with PBST, the sections were incubated with primary antibodies against Aβ peptide (A-beta 40/42) (MOAB-2, 1:500; M-1586; Biosensis Pty Ltd., Thebarton, South Australia) in PBST containing 10% BSA and 1% Triton X-100 at 4 °C overnight, followed by an Alexa Fluor 555–conjugated goat-anti-mouse secondary antibody (1:1000, Thermo Fisher Scientific) at room temperature for 1 h. After three washes with PBST, the sections were incubated with a 1:500 dilution of NeuroTrace Green Fluorescent Nissl Stain (#N21480, Invitrogen) that labels neurons and pericytes [30] at room temperature for 2 h. The stain was removed by washing the sections three times for 15 min using PBST, mounted on slides with anti-fading mounting medium (ClearVue Mountant XYL, Fisher Scientific), and coverslipped. Images were captured using a Nikon Eclipse 55i fluorescence (upright) microscope and a DS-FiL1 color camera (Nikon, Melville, NY) using a × 20 objective. Neurons stained green with NeuroTrace and with MOAB-positive (red) were counted, and the percentages of MOAB-positive neurons in the neocortex and hippocampus were calculated and compared.
Functional hyperemic responses
Whisker-stimulated functional hyperemic responses were assessed by laser-Doppler flowmetry as previously described [31–33]. Briefly, rats were anesthetized with ketamine (30 mg/kg, i.m.) and Inactin (50 mg/kg, i.p.). This anesthetic combination better preserves baseline arterial pressure and cerebral vascular responses [33–35]. The rat body temperature was maintained at 37 °C utilizing a servo-controlled heating pad. The trachea was cannulated and connected to a ventilator (SAR-830, CWE, Inc.) coupled to a CO2 analyzer to maintain the end-tidal CO2 levels at 35 mmHg. The femoral artery was cannulated to monitor mean blood pressure (MAP). The head was fixed in a stereotaxic frame, and the periosteum was removed to expose the skull. A thin cranial window was created over the somatosensory cortex (∼ − 2 mm posterior, 6 mm lateral relative to bregma) using a dental drill until the pial circulation was visible. Changes in CBF were monitored using a laser-Doppler flow meter (LDF, PF5010, Perimed, Inc.) and a fiber optic probe (91–00124; Perimed, Inc., Las Vegas, NV). The contralateral whiskers were stimulated for 1 min (~ 10 Hz), and the increases in CBF were recorded as the % change in the flow above baseline. The response was recorded three times over 5-min intervals.
Western blot
Western blots were used to detect the Kir2.1 expression levels as we previously described [33, 36, 37]. Proteins were either extracted from untreated and Aβ1–42-treated (0.1 µM, 48 h) WT ECs or the cerebral cortex of 6-month-old WT and AD rats. The tissues were homogenized in a radioimmunoprecipitation assay (RIPA) buffer (R0278, Sigma-Aldrich) containing protease and phosphatase inhibitors (PPIs; Thermo Fisher Scientific) and centrifuged at 4 °C at a speed of 11,000 g for 15 min. The supernatant was collected, and protein concentrations were measured using a Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). Aliquots of protein (30 µg) were mixed with 2 × Laemmli sample buffer and 2-mercaptoethanol (Bio-Rad) and heated at 95 °C for 5 min. The proteins were separated on a 4–20% sodium dodecyl sulfate–polyacrylamide gel and transferred to 0.2-µm nitrocellulose membranes using a Trans-Blot Turbo Transfer System (Bio-Rad). The membranes were blocked with 5% non-fat milk in Tris-buffered saline solution with 0.1% Tween-20 (TBST) at room temperature for 1 h. Subsequently, the blots were probed with primary antibodies against Kir2.1 (1:800; APC-159; Alomone Labs, Jerusalem, Israel) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2500; 2118S; Cell Signaling Technology, Danvers, MA) at 4 °C overnight. The membranes were washed with TBST and probed for 1 h with a goat anti-rabbit (1:10,000; ab6721, Abcam) secondary antibody. The protein bands were detected using SuperSignal™ West Dura Extended Duration Substrate (34075, Thermo Fisher Scientific). A ChemiDoc Imager System (Bio-Rad) was utilized to quantify the relative densities of each specific immunoreactive band.
Immunohistochemistry
Immunohistochemistry was used to detect the coverage of Kir2.1 channels on the cerebral microvasculature as previously described [37–39]. The rat brains were collected and fixed in 4% paraformaldehyde (PFA) overnight (4 °C), and then consecutively preserved in 10% and 30% sucrose at 4 °C overnight. One hemisphere was embedded with Tissue-Tek O.C.T. embedding medium (4583; Sakura Finetek USA, Torrance, CA) and frozen, and 50-µm coronal sections were prepared using a cryostat (CryoStar NX50, Thermo Fisher Scientific). Free-floating sections were kept in PBS and subject to antigen retrieval by incubating them with 0.1% trypsin solution at 37 °C for 2 h, followed by heating in sodium citrate buffer (10 mM, pH 6.0) at 80 °C for 3.5 h. After three washes in PBST, the sections were incubated with primary antibodies against collagen IV (Col IV) (1:200, MA1-22148, Thermo Fisher Scientific) and Kir2.1 (1:200; APC-159; Alomone Labs, Jerusalem, Israel) (in 10% BSA and 2% Triton X-100) for 72 h at 4 °C. The sections were washed three times with PBST and then probed with Alexa Fluor 488–labeled (anti-mouse) and Alexa Fluor 555–labeled (anti-rabbit) secondary antibodies (Thermo Fisher Scientific) (in 10% BSA and 2% Triton X-100) overnight at 4 °C. After washing with PBST, the sections were mounted on a slide. An antifading mounting medium was applied, and the slides were coverslipped. Images were acquired for comparing vascular density or Kir2.1 coverage using a Nikon C2 + laser scanning confocal Eclipse Ti2 inverted microscope using a × 20 objective.
Cerebrovascular reactivity responses
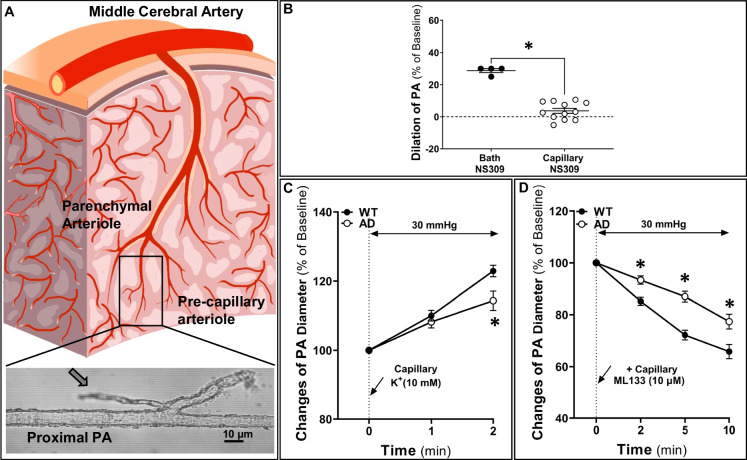
The rats were euthanized with isoflurane, followed by rapid decapitation. The brain was quickly collected and immersed in ice-cold, Ca-free PSS. A capillary-parenchymal arteriole (CaPA, about 15–30 µm) was microdissected (Fig. 7A), cannulated with glass pipettes, mounted in a pressure myograph chamber (Living System Instrumentation, Burlington, VT), and bathed at 37 °C using a PSS containing 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 18 NaHCO3, 5 HEPES, 1.18 NaH2PO4, and 10 glucose (in mM, pH 7.4) bubbled with 5% CO2 [33, 36, 38, 40, 41]. The arterioles were pressurized at 30 mmHg and were equilibrated for 40 min to develop myogenic tone [42, 43]. To test the viability of a vessel, 40 mM KCl [44] was applied to the bath, and a > 15% vasoconstriction was defined as an intact vessel. A glass micropipette with a 5-µm tip diameter was advanced next to the end of the capillary to deliver drugs locally. A PSS containing 10 mM KCl was directly applied to the ends of the capillary to mimic the physiological response associated with the local activation of neurons, and the change in diameter of upstream PA was followed for 3 min. Next, a PSS containing 10 µM ML133 [21], a Kir2.1 blocker, was injected near the end of the capillary 3 min after the initial stimulation with 10 mM KCl, and the changes in the diameter of the upstream PA were recorded.
Fig. 7.
The Kir2.1 activity was compromised in Alzheimer’s disease (AD) capillary ECs. A Schematic indication (box) of capillary-parenchymal arteriole (CaPA) with attached capillaries at the middle cerebral artery trajectory used for the ex vivo neurovascular coupling function studies. B Capillary focal administration was validated as 1 µM NS309 capillary treatment has no effect on upstream (proximal) arteriolar inner diameters (IDs). C Capillary stimulation with 10 mM K+ evoked a weaker upstream arteriolar dilation in AD vessels. D In the presence of 10 mM K+, blockage Kir2.1 channel with 10 µM ML133 triggered smaller vasoconstriction in the AD group, suggesting attenuated Kir2.1 channel activity in AD capillaries. Mean values ± SEM are presented. N = 5–9 rats per group. Two to three vessels per rat were studied, and 5 locations per vessel were measured. *P < 0.05 from the corresponding values in age-matched wild-type (WT) vessels. Kir2.1, inward rectifier potassium 2 channel
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, Inc., La Jolla, CA). Means values ± standard error are presented. A two-way ANOVA for repeated measures followed by the Holm-Sidak post hoc test was utilized to determine the significance of the differences between WT and AD rats in the functional hyperemic and cerebrovascular reactivity responses. An unpaired t test was used to compare the differences in Aβ and Kir2.1 expression between WT and AD rats. A P value of < 0.05 was considered to be statistically significant.
Results
Aβ expression
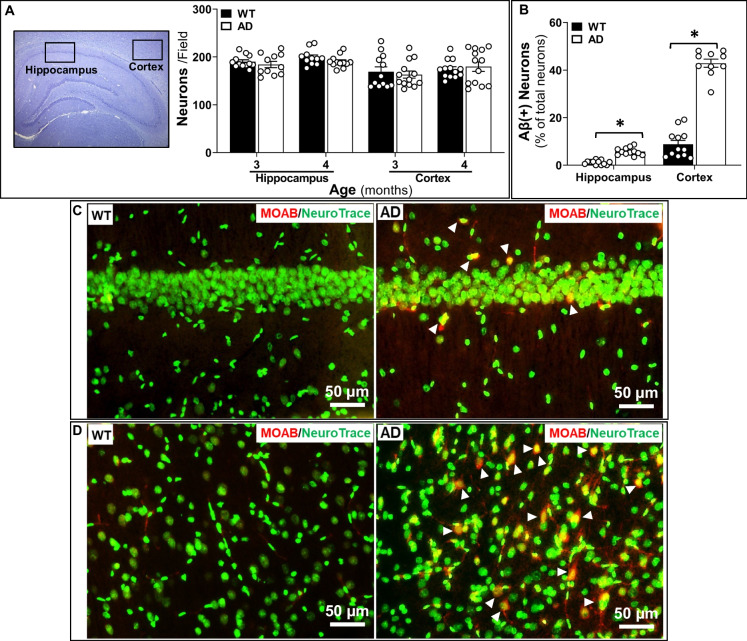
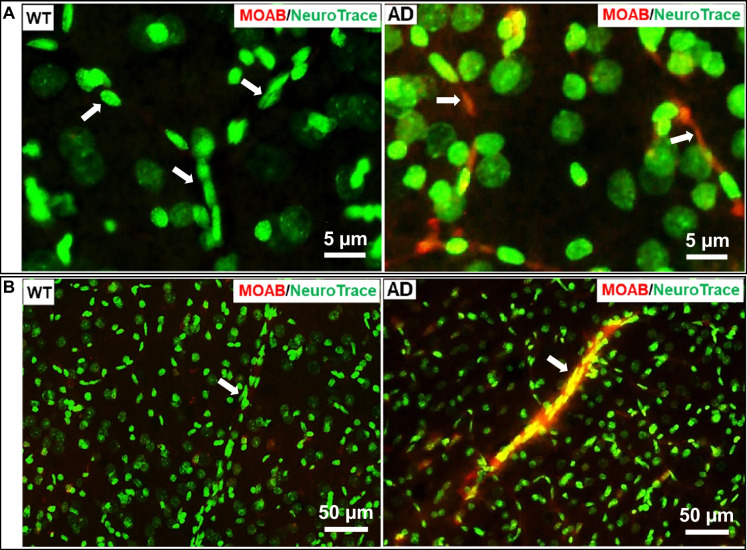
Aβ expression was compared between AD and WT rats at 3 months and 4 months of age using a MOAB antibody, which specifically detects Aβ but not amyloid precursor protein (APP) [45], and NeuroTrace 500/525, which labels neurons and longitudinal pericytes along microvasculature [30]. We confirmed that the numbers of neurons in the hippocampus and cortex were not significantly different in AD and WT rats at 3 months and 4 months of age (Fig. 1A). Three-month-old AD brains displayed higher percentages of Aβ-positive neurons than WT rats in both the hippocampus (WT vs. AD: 1.01 ± 0.07% vs. 5.76 ± 0.31%, P < 0.01) (Fig. 1B, C) and cortex layers 3–5 (WT vs. AD: 8.82 ± 0.48% vs. 42.90 ± 0.53%, P < 0.01) (Fig. 1B, D). We observed a high level of Aβ accumulation in the wall of some PAs (Fig. 2B) and in most capillaries (Fig. 2A) in comparison to WT rats in which there was very little staining in the cerebral microcirculation. Furthermore, we found that 4-month-old AD rats already exhibit a higher number of amyloid plaques in both the hippocampus and cortex compared to age-matched WT subjects (Fig. 3A, B). Taken together, these data demonstrate that the transgenic AD rats have a higher endogenous production of Aβ peptides, and these peptides accumulate in capillaries and arterioles at a much earlier age than previously thought.
Fig. 1.
Three-month-old Alzheimer’s disease (AD) rats manifest higher Aβ expression. A Neuron numbers are similar in the hippocampus and cortex of 3-month- and 4-month-old AD and wild-type (WT) rats. B Percentage of Aβ-positive neurons in the hippocampus and cortex is higher in 3-month-old AD rats than in age-matched WT animals. C Representative image to show Aβ-positive neurons (triangles) in the hippocampus of 3-month-old AD rats. D Aβ accumulated in neurons (triangles) in the cortex of 3-month-old AD rats. Mean values ± SEM are presented. N = 4 rats per group. Three to four fields of the hippocampus and three to four fields in layers 3–5 of the cortex per section were imaged and quantified. *P < 0.05 from the corresponding value in age-matched WT rats
Fig. 2.
Three-month-old Alzheimer’s disease (AD) rats manifest Aβ accumulation in the wall of parenchymal arterioles (PAs) and capillaries. A Aβ was avidly expressed in cerebral capillary endothelial cells (ECs) of AD rats. B Aβ accumulated (arrows) in the wall of PAs of AD rats. WT, wild-type
Fig. 3.
Four-month-old Alzheimer’s disease (AD) rats display amyloid plaques. A Representative image showing that 4-month-old AD rats display amyloid plaques (line arrows) at the cortex area. B The 4-month-old AD rats exhibited amyloid plaques in both the hippocampus and cortex. Mean values ± SEM are presented. N = 4 rats per group. Whole sections were quantified. *P < 0.05 from the corresponding value in age-matched WT rats
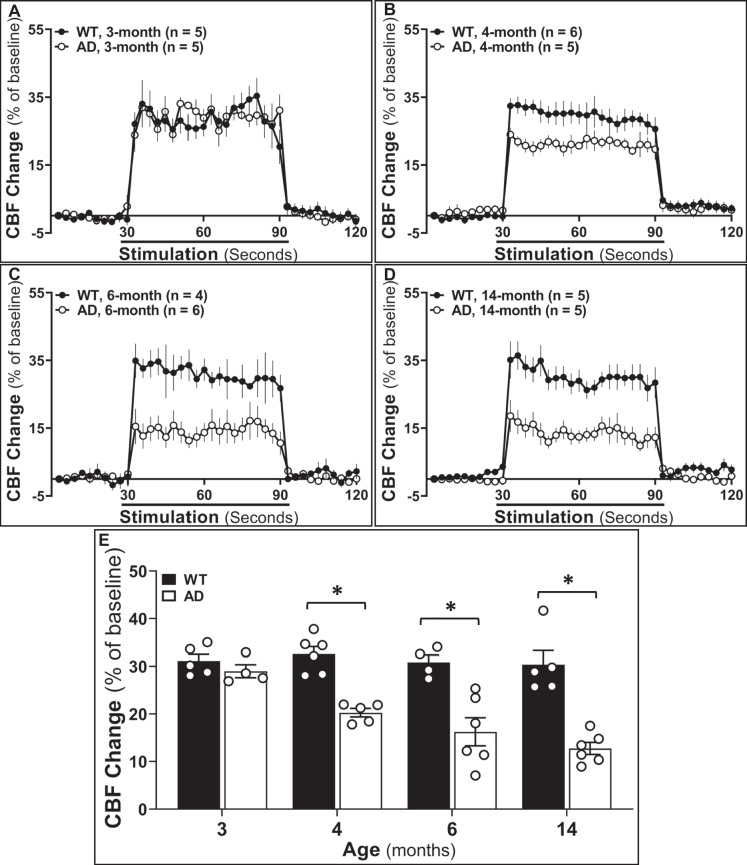
AD rats exhibit impaired functional hyperemia starting at 4 months of age
The functional hyperemic response redistributes blood flow to the areas of activated neurons independent of blood pressure changes. We compared the changes in CBF in the somatosensory cortex in response to whisker stimulation in AD and WT rats at 3-month, 4-month, 6-month, and 14-month age points (Fig. 4). As presented in Fig. 4A, the rise in CBF in response to whisker stimulation was similar in 3-month-old AD and WT rats. CBF increased by 28.99 ± 1.36% (AD) and 31.14 ± 1.37% (WT), respectively (Fig. 4E), suggesting functional hyperemic responses were intact in AD rats at this age. However, functional hyperemia was significantly lower in 4-month-old AD rats (20.27 ± 0.90%) than in age-matched controls (32.56 ± 1.57%) (Fig. 4B, E). Interestingly, the impaired functional hyperemia was evident 2 months prior to the earliest reports of memory dysfunction in this rat model of AD. We found a further age-dependent decline in whisker-stimulated evoked blood flow response in 6-month-old (16.23 ± 2.95%) and 14-month-old (13.04 ± 1.66%) AD rats (Fig. 4C–E), whereas the functional hyperemic response did not decrease with age in 6-month- and 14-month-old WT rats (6 months: 30.82 ± 1.52%; 14 months: 30.33 ± 2.52%) (Fig. 4C–E). These results suggest that impaired NVC in TgF344-AD rats occurs earlier than cognitive impairments and coincides with the first appearance of Aβ in the cerebral microcirculation and the formation of Aβ plaques.
Fig. 4.
Alzheimer’s disease (AD) rats exhibit impaired functional hyperemic responses starting from 4 months of age. A–D Comparison of whisker-stimulated evoked cerebral blood flow (CBF) responses in 3-month-, 4-month-, 6-month-, and 14-month-old wild-type (WT) and AD rats. E Increases in CBF during the 60-s stimulation of the whiskers in 3-month-, 4-month-, 6-month-, and 14-month-old WT and AD rats. Mean values ± SEM are presented. N = 4–6 rats per group. *P < 0.05 from the corresponding values in age-matched WT rats
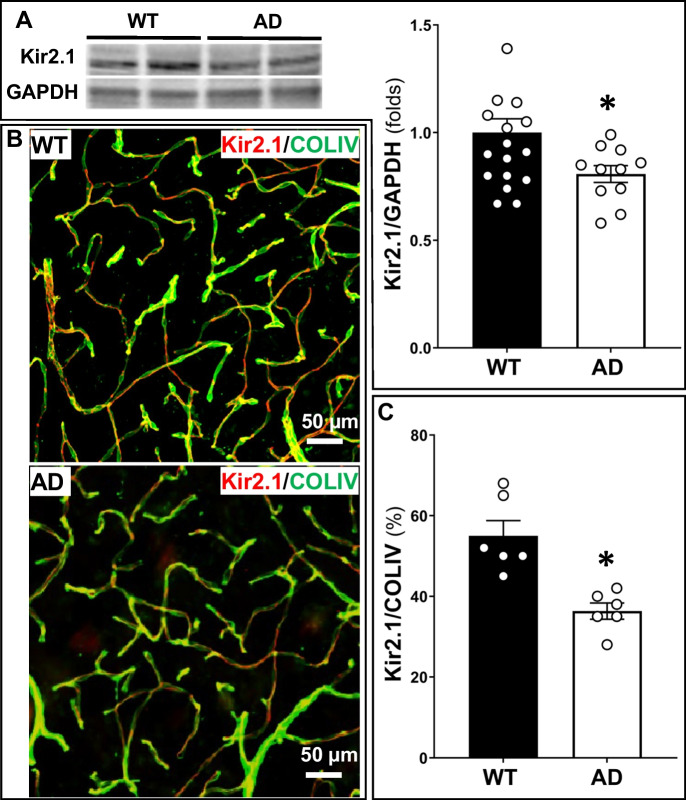
The Kir2.1 expression was decreased in the cerebral capillaries of AD rats and capillary ECs treated with Aβ1–42
The expression of Kir2.1 determined by western blot is reduced in the brain of 6-month-old AD rats compared to age-matched WT controls (Fig. 5A). We further confirmed that the expression of Kir2.1 on the cerebral capillaries expressed as the ratio of red (Kir2.1) to green (collagen IV) fluorescence intensity was lower in the brain of 6-month-old AD rats than in that of WT controls (Fig. 5B, C).
Fig. 5.
The Kir2.1 expression in the brain was decreased in Alzheimer’s disease (AD) rats. A Kir2.1 expression was lower in the brain of 6-month-old AD rats than in that of age-matched wild-type (WT) rats. B, C The density of Kir2.1 coverage on the cerebral microvasculature was lower in the brain of TgF344-AD WT rats. Mean values ± SEM are presented. N = 4–6 rats per group. *P < 0.05 from the corresponding values in age-matched wild-type rats and naïve capillary endothelial cells (ECs). *P < 0.05 from the corresponding value in age-matched WT rats. Kir2.1, inward rectifier potassium 2 channel
To explore one potential mechanism by which the expression of Kir2.1 on cerebral capillaries is reduced in AD, we treated WT capillary ECs with Aβ1–42 (0.1 µM) for 48 h. The Aβ1–42 treatment markedly reduced the Kir2.1 expression (0.77 ± 0.03 folds) compared to untreated ECs (Fig. 6B).
The Kir2.1 activity was compromised in AD capillary ECs
To assess the contribution of reduced Kir2.1 channel expression to impaired functional hyperemic responses in the AD rats, we freshly isolated CaPA with attached capillaries from 4-month-old AD and WT rats and measured the changes in inner diameters (IDs) in response to local superfusion of 10 mM KCl and after administration of ML133, a Kir2.1 blocker, to the ends of the capillary using a micropipette (Fig. 7). In the administration of NS309, an activator to Ca2+-activated IK/SK channels, 1 µM had no effect on the diameter of the PAs when applied downstream to the capillaries due to the lack of Ca2+-activated IK/SK channels on capillaries, but it dilated PAs by around 30% when added to the bath (Fig. 7B). The baseline IDs of the PAs were similar in the WT and AD groups (32.80 ± 0.92 µm and 26.99 ± 1.48 µm, respectively). After equilibration at 30 mmHg for 40 min, WT and AD PAs both developed myogenic tone and constricted to a similar level (WT vs. AD: 16.54 ± 0.53 µm vs. 15.65 ± 1.07 µm). The vasodilator response of the PAs to focal administration of 10 mM KCl to the end of the capillary for 30 s was significantly greater in WT than in AD vessels (AD vs. WT: 122.93 ± 1.67% vs. 114.34 ± 2.79%, P < 0.01) (Fig. 7C). To confirm that Kir2.1 channel activity mediated the response to KCl, we established a new baseline value 3 min after the initial application of KCl and then superfused the end of the capillary with the selective Kir2.1 blocker ML133 (10 µM) for 30 s. After 10 min, ML133 decreased the diameter of WT vessels to 65.82 ± 2.75% while the diameter of the AD PAs only fell to 77.36 ± 2.85% (P < 0.01) (Fig. 7D).
Discussion
AD is a devastating neurodegenerative disease. One in three seniors dies with AD or other dementias [1]. Most investigators have focused on the role of amyloid and tau in the pathogenesis of the disease. However, the high failure rate of late-stage therapies that reduce Aβ accumulation and/or hyperphosphorylated tau therapies [46] has renewed interest in strategies improving neurovascular function at the early stages of this disease. The transgenic rat model, TgF344-AD, recapitulates the complete repertoire of AD-like pathologies, including age-dependent Aβ accumulation that precedes tauopathy, gliosis, neuron loss in the cerebral cortex and hippocampus, and cognitive impairments [26, 27, 47–49], as well as cerebrovascular dysfunction [13, 27, 50–52]. However, the time course and mechanisms involved in the pathogenesis of cerebrovascular dysfunction, neurodegeneration, and loss of cognitive function remain to be determined. Previous studies have indicated that the earliest formation of amyloid plaques and loss of cognitive function occurs at 6 months of age [26]. Neurodegeneration has not been detected until the later stages of the disease at 9–15 months of age [26]. Impaired cerebral vascular response to hypercapnia has been reported at 9 months of age [50]. Recently, we reported that an impaired myogenic response of cerebral arteries and autoregulation of CBF is a very early event that was detected 2 months prior to the loss of cognitive function or the formation of amyloid plaques [27]. However, the role of Aβ in altering cerebral vascular function and the mechanisms by which changes in autoregulation of CBF might promote loss of cognitive function remain to be determined. In the present study, we found for the first time that TgF344-AD rats exhibit neurovascular uncoupling as early as 4 months of age, and this is associated with elevated expression of Aβ protein in cerebral capillaries and PAs. We further demonstrated that expression of the Kir2.1 channel is reduced in capillary ECs, contributing to the disruption of capillary-to-arteriole electrical signaling that is associated with compromised NVC in AD.
Aβ accumulation in AD
Aβ is produced by the enzymatic processing of APP [53–55], a transmembrane protein mainly expressed in neurons [53]. β-Secretase first cleaves APP into a soluble APP β and carboxyl-terminal fragment (CTF) β. The latter is then cleaved by γ-secretase into soluble and insoluble Aβ fragments. Presenilin 1 (PS1) or presenilin 2 (PS2) is associated with the catalytic core of γ-secretase. Mutations of APP and PS1 or PS2 have been confirmed to contribute to the overproduction of Aβ fragments, Aβ deposition, and amyloid plaques in AD patients and animal models [54]. TgF344-AD rats express 2.6 times more APP and 6.2 times more PS1 compared to normal littermates, resulting in a phenotype characterized by age-dependent increases in soluble and insoluble Aβ fragments, as well as progressive cognitive decline [26]. Most of the previous papers have reported that Aβ deposition can first be detected in walls of PAs at 7 months [56] of age and plaques detected between 9 and 15 months [13, 26, 50].
Using MOAB antibody, which labels β-sheet structure [56], we observed that 3-month-old AD rats manifest a higher expression of intraneuronal Aβ peptide. We observed that Aβ predominately accumulates in layer-5 pyramidal neurons. Less Aβ-positive neurons were found in the hippocampus. Aβ accumulated in the walls of PAs and was avidly expressed in cerebral capillary ECs of AD rats. This phenomenon is likely due to the overexpression of mutant APP and PS1 driven by a mouse prion promoter in this model [26]. Interestingly, we found that 4-month-old AD rats displayed amyloid plaques while age-matched WT littermates did not. Our results indicate that TgF344-AD rats develop amyloid plaques much earlier than reported previously [13, 26, 50]. The reason for this difference is that nearly all previous studies were done using older animals (9–15 months of age), and the plaques were detected with Congo red or thiazine rather than Aβ antibodies.
Neurovascular uncoupling in AD
Aβ1–42 is predominately localized in intracellular plaques, and Aβ1–40 is largely deposited at the cerebral arteries in association with cerebral amyloid angiopathy [57], which has been identified in up to 98.5% of AD patients postmortem [58]. There is no doubt that Aβ accumulation in vessels alters cerebral vascular function in models of AD. Notably, previous studies have shown that Aβ1–40 can directly alter cerebrovascular responses in vitro and functional hyperemic responses in vivo [9, 11, 23]. An emerging body of evidence also demonstrated that the CBF response to somatosensory neuronal activation is reduced within many mouse models of AD [8–13]. Interestingly, the overexpression of APP or Aβ does not attenuate the intensity of neuronal activity, reflected by increased glucose uptake in active areas [9]. Recent studies have also revealed that the capillary is the initial sensor of neuronal activity, and pial arteries and parenchymal arterioles are the effectors of flow changes during NVC [17]. Given that capillary dilatation is not required for the development of the function hyperemic response [59], much attention has been directed at the mechanisms for the impaired dilatory response in pial and parenchymal arterioles in AD. Nevertheless, very few studies have probed the role of the capillary in the development of NVC dysfunction early in the development of AD in young animals prior to the appearance of amyloid plaques and loss of cognitive function [24]. One of the major findings of the present study is that TgF-344 AD rats display compromised functional hyperemia in response to whisker stimulation as early as 4 months of age, a full 2 months earlier than the cognitive impairments. This was associated with reduced expression of Kir2.1 channel protein in the brain and expression of Kir2.1 channel protein on capillaries. These results are also consistent with the present finding that the vasodilatory response of pre-capillary PAs in response to focal administration of KCl to capillaries is impaired in 4-month-old AD rats.
The present data indicate that neurovascular uncoupling and impairments in learning and memory using an 8-arm swim test can be detected in TgF344-AD rats at the age of 4 months and 6 months, respectively. A recent study reported that the TgF344-AD model displayed impaired spatial reference memory in the Barnes maze at 4 months of age [60]. These results suggest that the onset of neurovascular uncoupling precedes or coincides with the initial development of cognitive impairment in this model of AD. Previous studies have reported that neurovascular dysfunction, including impaired functional hyperemic responses, almost universally accompanies the progressive loss of cognitive function during the later stages in AD patients and animal models [61–64]. Other researchers reported that the reduced nitric oxide production is associated with neurovascular uncoupling, which is likely to contribute to cognitive impairment with aging in normal mice [65, 66]. Moreover, even a short-term (3-week) pharmacological disruption of neurovascular coupling in normal mice using inhibitors of epoxygenase, cyclooxygenase, and nitric oxide synthase was correlated to impaired spatial and recognition memory and motor performance [67]. Taken together, impaired neurovascular coupling likely contributes to the loss of cognitive function in AD and aging.
Capillary endothelial Kir2.1 dysfunction is associated with compromised NVC in AD
Kir2.1 is found on vascular endothelial cells and SMCs [68]. Impaired Kir2.1 function on artery SMCs and ECs has been reported to contribute to compromised NVC in the late-stage of AD in mouse models [22–24, 69], which has been suggested to be related to Aβ-induced oxidative stress and inflammation [70]. PAs serve as the bottleneck of cerebral microcirculation [71]. Dilation of these arterioles is highly dependent on the activation of endothelial Kir2.1 and the Ca+-activated IK/SK channels [72] during dynamic CBF regulation.
The role of capillary EC Kir2.1 channels to functional hyperemia in vivo has been established in studies showing that injection of 10 mM KCl into the cerebral cortex near a capillary dilates upstream arterioles and that superfusion of 15 mM KCl over the cortical surface induces a robust increase in CBF that was attenuated by 100 µM barium to block the Kir2.1 channel in WT mice and was absent in EC-specific Kir2.1 KO mice [17]. Moreover, the essential role of this pathway to functional hyperemia was unequivocally confirmed by the findings that whisker-stimulated increases in CBF were blunted by 80% by bathing the cortical surface with barium and reduced by 50% in EC-specific Kir2.1 KO mice [17].
While reduced Kir2.1 on arteriolar endothelium has recently been accepted to cause impaired NVC in various mouse models of AD [24, 69], the role of Ca+-activated IK/SK channels on arteriolar endothelium has mixed reports [22, 69]. The primary sensor of the elevated extracellular K+ in response to neuronal activity is capillary endothelial Kir2.1 channel [18]. Previous studies have implied that reduced capillary endothelial Kir2.1 contributes to neurovascular uncoupling during the later stage of AD and that the restoration of Kir2.1 by exogenous administration of PIP2 improves reduced CBF [24]. Our study, for the first time, reveals that capillary endothelial Kir2.1 activity is impaired at the very early stage of AD in the TgF344-AD rat, possibly due to the detrimental effects of soluble Aβ peptides on the expression of the Kir2.1 channel in cerebral capillary ECs.
One limitation of the present study is that the functional hyperemic responses were measured in anesthetized animals, and anesthesia can impact the response. For example, isoflurane dose-dependently increases basal CBF and blunts subsequent functional hyperemia [73]. Therefore, we utilized ketamine/Inactin anesthesia which has minimal effects on baseline CBF and better preserves functional hyperemic responses [38]. Another limitation is that CBF was measured using laser Doppler flowmetry which measures RBC flux in a volume of 2 mm in diameter and 2–3 mm deep in the cortex, but it does not provide the spatial information available using laser speckle imaging or discern the contribution of individual penetrating arterioles and capillaries as measured using two-photon microscopy. Additionally, many cellular components (neurons, capillary ECs, pericytes, and SMCs) and astrocytic endfeet participate in NVC via modulating Ca2+ mobilization and subsequent K+ efflux into the extracellular space [11]. Thus, another limitation is that capillary endothelial function was studied in vitro using the perfused CaPA preparation [17], which excludes the modulatory influence of other vasodilatory mediators (epoxyeicosatrienoic acids, nitric oxide, and prostaglandins) that are released by surrounding neurons, pericytes, and glial cells in vivo.
Conclusions
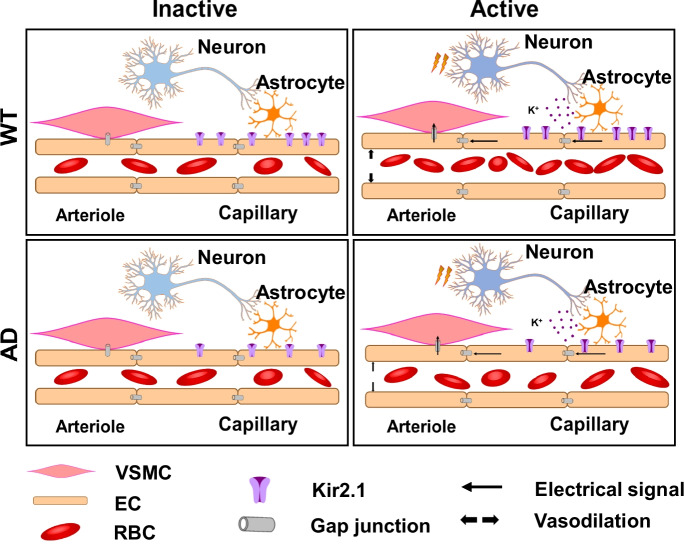
The present study supports the view that impaired dynamic CBF regulation precedes learning and memory disturbances in the well-established TgF344 rat model of AD. We found that reduced capillary endothelial Kir2.1 expression and function contribute to the compromised NVC in the earliest stage of AD (Fig. 8). These results suggest that novel therapeutic approaches to augment Kir2.1 channel expression and function in the cerebral circulation may be useful to restore functional hyperemic responses and cerebral perfusion and slow the loss of cognitive function in AD. However, specific Kir2.1 channel activators have yet to be discovered [74]. Moreover, Kir2.1 channels are expressed in cerebral vascular smooth muscle and other cell types throughout the body [68, 75], which raises the distinct possibility of detrimental side effects. Recent studies have revealed that the opening of the Kir2.1 channel requires the binding of PIP2 [21, 68, 74–76]. Exogenous application of PIP2 applied to capillaries dilates upstream parenchymal arterioles in vitro using the CaPA preparation [77]. Moreover, Mughal et al. [24] found that whisker-stimulated and KCl-induced cerebral hyperemic responses were markedly impaired in a mouse model of familial AD (5xFAD). As predicted, acute i.v. administration of a PIP2 acutely rescued the impaired capillary endothelial Kir2.1 function and neurovascular uncoupling in this model [24]. They further illustrate that restoration of electrical capillary signaling by exogenous supplementation of PIP2 or a precursor is a potential novel therapeutic approach for the treatment of cerebral vascular dysfunction in AD. However, further studies are needed to determine whether chronic administration of PIP2 analogs has a sustained effect on NVC and can improve cerebral hemodynamics and cognitive function in models of AD.
Fig. 8.
Proposed mechanisms for neurovascular uncoupling in TgF344-AD rats. As capillary endothelial Kir2.1 is the initial sensor of neuronal activities, reduced capillary Kir2.1 expression in Alzheimer’s disease (AD) is associated with impaired capillary-arteriole electric signal and results in compromised neurovascular coupling. WT, wild-type; VSMC, vascular smooth muscle cell; EC, endothelial cell; RBC, red blood cell; Kir2.1, inward rectifier potassium 2 channel
Author contribution
F. F., X. F., and R. J. R. conceived and designed the research project; X. F., J. B., P. R., and H. Z. performed the experiments; X. F., J. B., P. R., R. J. R., and F. F. analyzed the data; X. F., R. J. R., and F. F. interpreted the results of experiments; X. F., H. Z., and F. F. prepared the figures; X. F. and R. J. R. drafted, edited, and revised the manuscript; X. F., F. F., J. B., P. R., H. Z., R. J. R., and J. M. W. approved the final version of the manuscript.
Funding
This study was supported by grants R01 AG057842, RF1 AG079336, and HL138685 from the National Institutes of Health; 23PRE1018124 from the American Heart Association; and G20221001-3551 from Sigma Xi.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fan Fan, Email: FFan@augusta.edu.
Richard J. Roman, Email: rroman@umc.edu
References
- 1.Gaugler J, James B, Johnson T, Reimer J, Solis M, Weuve J. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 2.Dhapola R, Hota SS, Sarma P, Bhattacharyya A, Medhi B, Reddy DH. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology. 2021;29:1669–1681. doi: 10.1007/s10787-021-00889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7:11934. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korte N, Nortley R, Attwell D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020;140:793–810. doi: 10.1007/s00401-020-02215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracko O, Cruz Hernández JC, Park L, Nishimura N, Schaffer CB. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J Cereb Blood Flow Metab. 2021;41:1501–1516. doi: 10.1177/0271678x20982383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Zhang J, Roman RJ, Fan F. From 1901 to 2022, how far are we from truly understanding the pathogenesis of age-related dementia? GeroScience. 2022;44:1879–1883. doi: 10.1007/s11357-022-00591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang X, Crumpler RF, Thomas KN, Mazique JN, Roman RJ and Fan F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol Int. 2022. 10.1556/2060.2022.00020. [DOI] [PMC free article] [PubMed]
- 8.Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niwa K, Younkin L, Ebeling C, Turner SK, Westaway D, Younkin S, Ashe KH, Carlson GA, Iadecola C. Abeta 1–40-related reduction in functional hyperemia in mouse neocortex during somatosensory activation. Proc Natl Acad Sci U S A. 2000;97:9735–9740. doi: 10.1073/pnas.97.17.9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Tong XK, Hosseini Kahnouei M, Vallerand D, Hamel E, Girouard H. Impaired hippocampal neurovascular coupling in a mouse model of Alzheimer’s disease. Front Physiol. 2021;12:715446. doi: 10.3389/fphys.2021.715446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 12.Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. doi: 10.1523/jneurosci.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo IL, Lam WW, Oakden W, Hill ME, Koletar MM, Morrone CD, Stanisz GJ, McLaurin J, Stefanovic B. Early alterations in brain glucose metabolism and vascular function in a transgenic rat model of Alzheimer’s disease. Prog Neurobiol. 2022;217:102327. doi: 10.1016/j.pneurobio.2022.102327. [DOI] [PubMed] [Google Scholar]
- 14.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straub AC, Zeigler AC, Isakson BE. The myoendothelial junction: connections that deliver the message. Physiology (Bethesda) 2014;29:242–249. doi: 10.1152/physiol.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, Hill-Eubanks D, Nelson MT. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20:717–726. doi: 10.1038/nn.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22:183–196. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harraz OF, Longden TA, Dabertrand F, Hill-Eubanks D, Nelson MT. Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc Natl Acad Sci U S A. 2018;115:E3569–e3577. doi: 10.1073/pnas.1800201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moshkforoush A, Ashenagar B, Harraz OF, Dabertrand F, Longden TA, Nelson MT, Tsoukias NM. The capillary Kir channel as sensor and amplifier of neuronal signals: modeling insights on K+-mediated neurovascular communication. Proc Natl Acad Sci U S A. 2020;117:16626–16637. doi: 10.1073/pnas.2000151117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sackheim AM, Villalba N, Sancho M, Harraz OF, Bonev AD, D'Alessandro A, Nemkov T, Nelson MT and Freeman K. Traumatic brain injury impairs systemic vascular function through disruption of inward-rectifier potassium channels. Function (Oxf). 2021;2. 10.1093/function/zqab018. [DOI] [PMC free article] [PubMed]
- 22.Lacalle-Aurioles M, Trigiani LJ, Bourourou M, Lecrux C, Hamel E. Alzheimer’s disease and cerebrovascular pathology alter inward rectifier potassium (KIR 2.1) channels in endothelium of mouse cerebral arteries. Br J Pharmacol. 2022;179:2259–2274. doi: 10.1111/bph.15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor JL, Pritchard HAT, Walsh KR, Strangward P, White C, Hill-Eubanks D, Alakrawi M, Hennig GW, Allan SM, Nelson MT, Greenstein AS. Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2022;119:e2204581119. doi: 10.1073/pnas.2204581119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mughal A, Harraz OF, Gonzales AL, Hill-Eubanks D, Nelson MT. PIP2 improves cerebral blood flow in a mouse model of Alzheimer’s disease. Function (Oxf) 2021;2:zqab010. doi: 10.1093/function/zqab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 26.Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci. 2013;33:6245–6256. doi: 10.1523/JNEUROSCI.3672-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang X, Tang C, Zhang H, Border JJ, Liu Y, Shin SM, Yu H, Roman RJ, Fan F. Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer’s disease. Geroscience. 2023 doi: 10.1007/s11357-023-00773-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruck T, Bittner S, Epping L, Herrmann AM and Meuth SG. Isolation of primary murine brain microvascular endothelial cells. J Vis Exp. 2014:e52204. 10.3791/52204. [DOI] [PMC free article] [PubMed]
- 30.Damisah EC, Hill RA, Tong L, Murray KN, Grutzendler J. A fluoro-Nissl dye identifies pericytes as distinct vascular mural cells during in vivo brain imaging. Nat Neurosci. 2017;20:1023–1032. doi: 10.1038/nn.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Zhang H, Wu CY, Yu T, Fang X, Ryu JJ, Zheng B, Chen Z, Roman RJ, Fan F. 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility. Prostaglandins Other Lipid Mediat. 2021;154:106548. doi: 10.1016/j.prostaglandins.2021.106548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, Guo Y, Li M, Gao W, Fang X. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. GeroScience. 2020;42:1387–1410. doi: 10.1007/s11357-020-00233-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Zhang H, Liu Y, Li L, Guo Y, Jiao F, Fang X, Jefferson JR, Li M, Gao W, Gonzalez-Fernandez E, Maranon RO, Pabbidi MR, Liu R, Alexander BT, Roman RJ, Fan F. Sex differences in the structure and function of rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2020;318:H1219–H1232. doi: 10.1152/ajpheart.00722.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol Regul Integr Comp Physiol. 2015;308:R379–R390. doi: 10.1152/ajpregu.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan F, Geurts AM, Pabbidi MR, Smith SV, Harder DR, Jacob H, Roman RJ. Zinc-finger nuclease knockout of dual-specificity protein phosphatase-5 enhances the myogenic response and autoregulation of cerebral blood flow in FHH.1BN rats. PLoS One. 2014;9:e112878. doi: 10.1371/journal.pone.0112878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Wang S, Liu Y, Fan L, Booz GW, Roman RJ, Chen Z, Fan F. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience. 2020;42:547–561. doi: 10.1007/s11357-020-00179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Zhang H, Wang S, Guo Y, Fang X, Zheng B, Gao W, Yu H, Chen Z, Roman RJ, Fan F. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am J Physiol Heart Circ Physiol. 2021;320:H549–h562. doi: 10.1152/ajpheart.00726.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Lv W, Zhang H, Liu Y, Li L, Jefferson JR, Guo Y, Li M, Gao W, Fang X, Paul IA, Rajkowska G, Shaffery JP, Mosley TH, Hu X, Liu R, Wang Y, Yu H, Roman RJ, Fan F. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience. 2020;42:1387–1410. doi: 10.1007/s11357-020-00233-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Zhang H, Wu CYC, Yu T, Fang X, Ryu JJ, Zheng B, Chen Z. Roman RJ and Fan F. 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility. Prostaglandins Other Lipid Mediat. 2021;154:106548. doi: 10.1016/j.prostaglandins.2021.106548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang H, Zhang C, Liu Y, Gao W, Wang S, Fang X, Guo Y, Li M, Liu R, Roman RJ, Sun P, Fan F. Influence of dual-specificity protein phosphatase 5 on mechanical properties of rat cerebral and renal arterioles. Physiol Rep. 2020;8:e14345. doi: 10.14814/phy2.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Jiao F, Border JJ, Fang X, Crumpler RF, Liu Y, Zhang H, Jefferson J, Guo Y, Elliott PS, Thomas KN, Strong LB, Urvina AH, Zheng B, Rijal A, Smith SV, Yu H, Roman RJ, Fan F. Luseogliflozin, a sodium-glucose cotransporter-2 inhibitor, reverses cerebrovascular dysfunction and cognitive impairments in 18-mo-old diabetic animals. Am J Physiol Heart Circ Physiol. 2022;322:H246–H259. doi: 10.1152/ajpheart.00438.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J Cereb Blood Flow Metab. 2013;33:1486–1492. doi: 10.1038/jcbfm.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosehart AC, Johnson AC, Dabertrand F. Ex vivo pressurized hippocampal capillary-parenchymal arteriole preparation for functional study. J Vis Exp. 2019 doi: 10.3791/60676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golding EM, Steenberg ML, Johnson TD, Bryan RM., Jr The effects of potassium on the rat middle cerebral artery. Brain Res. 2000;880:159–166. doi: 10.1016/s0006-8993(00)02793-1. [DOI] [PubMed] [Google Scholar]
- 45.Youmans KL, Tai LM, Kanekiyo T, Stine WB, Jr, Michon SC, Nwabuisi-Heath E, Manelli AM, Fu Y, Riordan S, Eimer WA, Binder L, Bu G, Yu C, Hartley DM, LaDu MJ. Intraneuronal Aβ detection in 5xFAD mice by a new Aβ-specific antibody. Mol Neurodegener. 2012;7:8. doi: 10.1186/1750-1326-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer’s disease. J Biomed Sci. 2020;27:18. doi: 10.1186/s12929-019-0609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anckaerts C, Blockx I, Summer P, Michael J, Hamaide J, Kreutzer C, Boutin H, Couillard-Després S, Verhoye M, Van der Linden A. Early functional connectivity deficits and progressive microstructural alterations in the TgF344-AD rat model of Alzheimer’s disease: a longitudinal MRI study. Neurobiol Dis. 2019;124:93–107. doi: 10.1016/j.nbd.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM, Weinshenker D. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain. 2017;140:3023–3038. doi: 10.1093/brain/awx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernaud VE, Bulen HL, Peña VL, Koebele SV, Northup-Smith SN, Manzo AA, Valenzuela Sanchez M, Opachich Z, Ruhland AM, Bimonte-Nelson HA. Task-dependent learning and memory deficits in the TgF344-AD rat model of Alzheimer’s disease: three key timepoints through middle-age in females. Sci Rep. 2022;12:14596. doi: 10.1038/s41598-022-18415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joo IL, Lai AY, Bazzigaluppi P, Koletar MM, Dorr A, Brown ME, Thomason LA, Sled JG, McLaurin J, Stefanovic B. Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease. Sci Rep. 2017;7:46427. doi: 10.1038/srep46427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang X, Zhang H, Border JJ, Rivers PL, Strong LB, Cooper J, Crumpler RF, Thomas KN, Roman RJ and Fan F. Contribution of beta-amyloid accumulation to cerebral hypoperfusion in Alzheimer’s disease. FASEB J. 2022;36. 10.1096/fasebj.2022.36.S1.R2710.
- 52.Fang X, Zhang H, Wang S, Liu Y, Gao W, Roman RJ, Fan F. Abstract P076: cerebral vascular dysfunction precedes cognitive impairment in Alzheimer’s disease. Hypertension. 2020;76:AP076–AP076. doi: 10.1161/hyp.76.suppl_1.P076. [DOI] [Google Scholar]
- 53.Apátiga-Pérez R, Soto-Rojas LO, Campa-Córdoba BB, Luna-Viramontes NI, Cuevas E, Villanueva-Fierro I, Ontiveros-Torres MA, Bravo-Muñoz M, Flores-Rodríguez P, Garcés-Ramirez L, de la Cruz F, Montiel-Sosa JF, Pacheco-Herrero M, Luna-Muñoz J. Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab Brain Dis. 2022;37:39–50. doi: 10.1007/s11011-021-00814-4. [DOI] [PubMed] [Google Scholar]
- 54.Canobbio I, Abubaker AA, Visconte C, Torti M, Pula G. Role of amyloid peptides in vascular dysfunction and platelet dysregulation in Alzheimer’s disease. Front Cell Neurosci. 2015;9:65. doi: 10.3389/fncel.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stavljenic-Rukavina A. Molecular mechanisms in Alzheimer’s disease. eJIFCC. 2004;15:100–103. [PMC free article] [PubMed] [Google Scholar]
- 56.Bishay J, Beckett TL, Lai AY, Hill ME, McMahon D, McLaurin J. Venular amyloid accumulation in transgenic Fischer 344 Alzheimer’s disease rats. Sci Rep. 2022;12:15287. doi: 10.1038/s41598-022-19549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer’s disease - one peptide, two pathways. Nat Rev Neurol. 2020;16:30–42. doi: 10.1038/s41582-019-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Attems J, Lauda F, Jellinger KA. Unexpectedly low prevalence of intracerebral hemorrhages in sporadic cerebral amyloid angiopathy: an autopsy study. J Neurol. 2008;255:70–76. doi: 10.1007/s00415-008-0674-4. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fowler CF, Goerzen D, Devenyi GA, Madularu D, Chakravarty MM, Near J. Neurochemical and cognitive changes precede structural abnormalities in the TgF344-AD rat model. Brain Commun. 2022;4:fcac072. doi: 10.1093/braincomms/fcac072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Al-Shahi Salman R, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, González HM, Yuan C, Lockhart SN, Hughes TM, Chen CLH, Sachdev P, O'Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MMB, Román GC, Hamel E, Seshadri S, Gottesman RF, van Buchem MA, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM, Zlokovic BV. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15:158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Tang C, Liu Y, Border JJ, Roman RJ, Fan F. Impact of impaired cerebral blood flow autoregulation on cognitive impairment. Front Aging. 2022;3:1077302. doi: 10.3389/fragi.2022.1077302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarantini S, Nyúl-Tóth Á, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, Ungvari Z, Csiszar A. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience. 2021;43:2387–2394. doi: 10.1007/s11357-021-00405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harraz OF, Hill-Eubanks D, Nelson MT. PIP2: a critical regulator of vascular ion channels hiding in plain sight. Proc Natl Acad Sci U S A. 2020;117:20378–20389. doi: 10.1073/pnas.2006737117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hakim MA, Behringer EJ. Development of Alzheimer’s disease progressively alters sex-dependent KCa and sex-independent KIR channel function in cerebrovascular endothelium. J Alzheimers Dis. 2020;76:1423–1442. doi: 10.3233/jad-200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer’s dementia. Cell Mol Neurobiol. 2003;23:681–689. doi: 10.1023/A:1025092617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci U S A. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Behringer EJ, Hakim MA, Blackwell J and Pires PW. Endothelial KIR channel dysfunction in aged cerebral parenchymal arterioles. FASEB J. 2022;36. 10.1096/fasebj.2022.36.S1.R5757. [DOI] [PMC free article] [PubMed]
- 73.Okamoto H, Meng W, Ma J, Ayata C, Roman Richard J, Bosnjak Zeljko J, Kampine John P, Huang Paul L, Moskowitz Michael A, Hudetz AG. Isoflurane-induced cerebral hyperemia in neuronal nitric oxide synthase gene deficient mice. Anesthesiology. 1997;86:875–884. doi: 10.1097/00000542-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 74.van der Schoor L, van Hattum EJ, de Wilde SM, Harlianto NI, van Weert AJ, Bloothooft M and van der Heyden MAG. Towards the development of AgoKirs: new pharmacological activators to study K ir 2.x channel and target cardiac disease. Int J Mol Sci. 2020;21. 10.3390/ijms21165746. [DOI] [PMC free article] [PubMed]
- 75.Sancho M and Welsh DG. Chapter eight - KIR channels in the microvasculature: regulatory properties and the lipid-hemodynamic environment. In: Jackson WF, ed. Current topics in membranes. Academic Press; 2020;85: 227–259. [DOI] [PubMed]
- 76.Li J, Lü S, Liu Y, Pang C, Chen Y, Zhang S, Yu H, Long M, Zhang H, Logothetis DE, Zhan Y, An H. Identification of the Conformational transition pathway in PIP2 Opening Kir Channels. Sci Rep. 2015;5:11289. doi: 10.1038/srep11289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dabertrand F, Harraz OF, Koide M, Longden TA, Rosehart AC, Hill-Eubanks DC, Joutel A and Nelson MT. PIP2 corrects cerebral blood flow deficits in small vessel disease by rescuing capillary Kir2.1 activity. Proc Natl Acad Sci U S A. 2021;118. 10.1073/pnas.2025998118. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.