Abstract
Adhesive molecular bonds between blood cells are essential for thrombosis and hemostasis as they provide means for platelet adhesion, aggregation, and signaling in flowing blood. According to the nowadays conventional definition, a “catch” bond is a type of non-covalent bio-molecular bridge, whose dissociation lifetime counter-intuitively increases with applied tensile force. Following recent experimental findings, such receptor-ligand protein bonds are vital to the blood cells involved in the prevention of bleeding (hemostatic response) and infection (immunity). In this review, we examine the up-to-date experimental discoveries and theoretical insights about catch bonds between the blood cells, their biomechanical principles at the molecular level, and their role in platelet thrombosis and hemostasis.
Keywords: Cell adhesion, Catch bonds, Blood platelets, Hemostasis
Introduction
Adhesion in physics is the ability of dissimilar solid and/or liquid surfaces to stick to each other. Generally speaking, this is a complex interfacial phenomenon that may involve several molecular mechanisms and distinct inter-molecular forces, including (but not limited to) van der Waals forces, electrostatics, elasticity, viscous drag, capillary and wetting effects, molecular binding, diffusion, chemical reactions, etc. Adhesion is ubiquitous in nature and has great importance for the integrity, signaling, and functioning of biological systems on levels from individual cells and tissues, to organisms and their colonies.
At the cellular and the tissue levels of organization, biological adhesion mainly relies on molecular bridging between cell adhesion molecules (CAMs) and their ligands. This is a vast group of macro-molecules that reside on a cell’s surface (usually represented by trans-membrane proteins) and are involved in the binding of cells to other cells, surfaces, or extracellular matrix (ECM).
The specificity and selectivity of this binding arising from the complexity of proteins and their spacial organization is the most noticeable distinguishing feature of biological adhesion. It means not only that some ligands are preferred among others (that is also true for the “non-living” systems, as the strength of adhesion generally depends on the physical and chemical properties of interacting surfaces). Moreover, the kinetics of bridging between CAM and its ligand depends on their mutual orientation and, more importantly, on the conformation of these proteins. Given that proteins are prone to thermal motion, the binding between CAMs is usually described as a stochastic process, which complicates its understanding and mathematical description. Other factors that influence the protein-protein interactions involved in biological adhesion should be taken into account, including instantaneous mechanical forces on the proteins, molecular crowding, solvent quality, salt concentration, acidity of the solution, etc.
It is known that biological adhesion may serve as a source of self-organization of the living matter in tissues (Tsai et al. 2022). Moreover, it may be a cell’s method to receive information about its surroundings, mechanical properties of a substrate, and hydrodynamic conditions in fluid media (Martino et al. 2018). Besides that, several protective functions of an organism rely on cell adhesion, e.g., immunity (Springer 1990) and hemostasis (Ruggeri and Mendolicchio 2007).
Apart from their stochastic formation and dissociation kinetics, one of the most intriguing properties of receptor-ligand protein bonds is their biomechanical behavior under external load. Naturally, it may be assumed that the bridging molecules would slip apart under tensile mechanical force (i.e., reduce the lifetime of the complex as the force increases) (Thomas et al. 2008). Such behavior, indeed, was experimentally observed (Bell 1978), and the molecular interactions responsible for this received the name “slip” bonds. However, there are numerous examples of counter-intuitive opposite behavior when the lifetime increases with tension (Beste and Hammer 2008; Rakshit et al. 2012; Sauer et al. 2016) (Fig. 1A). These types of molecular interactions are typical of those cells that need to sustain strong external forces usually arising from hydrodynamic drag, i.e., there is a need to recruit those cells to the vessel walls from the flowing fluid (Fig. 1B). The “catch” bonds provide a way for cells to stabilize their attachments exactly when they need it most (Thomas et al. 2008), e.g., to recruit blood platelets at the site of a vascular injury or to attract the leukocytes to inflamed or infected tissues. Besides that, several bacteria (e.g., E.coli ) are known to demonstrate the catching adhesive mechanisms that allow them to form persistent and wash-resistant biofilms on different surfaces (Tchesnokova et al. 2008; Thomas 2008; Thomas et al. 2006, 2004). The physical mechanisms of catch bonds rely on complex allosteric transitions in proteins under external mechanical force (Evans and Ritchie 1997; Thomas et al. 2006). In realistic systems, any molecular bond would eventually be overpowered by high enough force. The catch bonds only exist within a certain range of low-to-intermediate forces, therefore, the catch bonds are always followed by the slip bonds, hence termed “catch-slip bonds”. More examples can be found in the literature indicating a prevalence of hybrid “catch-slip” or other variations, such as “flex” bonds, and their high physiological relevance for the blood circulation system (Kim et al. 2010).
Fig. 1.
A A scheme of a force-clamp experiment and the typical bond lifetime dependencies on the force. B Manifestation of the catch- and slip-bonds in white blood cell rolling experiments. Schematic plots for slip, ideal catch, and realistic catch-slip bonds are indicated by dash-dotted (green), dashed (red), and solid (blue) lines respectively
In this review, we address known molecular mechanisms that govern the biomechanical behavior of CAMs found in blood platelets, leukocytes, and other blood cells, describe their peculiarities found in experiments, address some issues in their theoretical understanding and discuss their impact on the hemostatic response. We also review some mechanisms of sustainable cell adhesion that involve physical effects in addition to protein-ligand bridging and conclude by expressing our point of view about future directions of studies in the field.
History of “slip” and “catch” adhesive bonds
Despite the diversity of the CAM family, the “physics of bond breaking” is generally common in terms of thermodynamics and mechanics. The pioneering theoretical study of G.I. Bell (Bell 1978) describes the bond breakage event as a thermally-activated process that could be described by Arrhenius equation, so that the characteristic lifetime of a non-loaded bond depends exponentially on the fraction of the potential energy barrier (i.e., the “activation energy”) and the “thermal” energy kT:
| 1 |
where is the natural frequency of attempts to escape from the bounded state. Moreover, Bell considered a molecular bond under mechanical load (a stretching force of a magnitude F) and suggested the following expression for the loaded bond lifetime based on a physical (mechanical work) argument:
| 2 |
or equivalently for the dissociation rate constant :
| 3 |
where is the dissociation rate of a non-loaded bond and is a phenomenological parameter (with a dimension of length) that defines the bond’s compliance. The latter expression is usually referred to as the Bell’s law for a molecular bond dissociation rate: the stronger the force, the faster the bond breaks. Note that this law is described by a monotonous analytical function of the applied force F.
As for the bond formation rate , it was assumed to be a diffusive process dependent on the distance between reactive centers of the proteins (CAMs), their diffusion constants, and mechanical elasticity. A compliant spring allows scanning a larger volume of space during the diffusion-driven “search” for the ligand. Therefore, only the dissociation event is meant to be force-dependent and thus mechanically sensitive.
Furthermore, Bell’s theory was extended to account for multivalent ligands (Bell 1979), and, more importantly, reduced the complexity and diversity of biological adhesion to a mathematical formalism that relies on ordinary differential equations of the form:
| 4 |
where , , and are respectively the numbers of the present molecular bonds at time t (or sometimes bonds per unit surface area of adhering cells), available (non-bound) receptors, and available ligands.
A bit later (Dembo et al. 1988; Dembo 1994) modeled the rolling motion of white blood cells in the shear flow of a viscous fluid and proposed to relate the forward reaction rate , and the reverse reaction rate, , to the separation distance between the cell’s microvillus tip and the adhesive surface. This approach introduced a more elaborated mechanistic mathematical description into the field and draw a lot of attention of mathematicians and physicists to the problem of biological adhesion of blood cells in flow. Dembo et al. (1988) proposed to account for the changes in the mechanical stiffness of CAMs in the bound state and near the transition to the broken state, manifesting the essential non-linearity and the non-Hookean nature of the energy landscape for a ligand-receptor pair.
Noticeably, the possibility of catch molecular bonds was first theoretically proposed in this work in 1988 (ahead of direct experimental observations) from an analogy between the tension-induced conformational transitions in CAMs and the mechanics of a Chinese finger trap toy. Although a slightly different formulation was used, generally, they assumed that the increment in the exponent of Eq. 3 (i.e., the parameter ) may have a negative sign. If the bond becomes stiffer near the transition (bond breakage) then its lifetime counter-intuitively increases with the external force. It was thought that the possibility of catch and slip behavior of CAMs depends on the structure and mechanics of these proteins: pulling with a small force should first tighten a bond and extend its lifetime, and then higher forces should deform the proteins and lower a principal energy barrier to speed up the bond rupture.
This formalism allowed for computational modeling of the adhering cell dynamics under external mechanical load, e.g., hydrodynamic forces (Hammer and Lauffenburger 1987). In combination with real-time microscopy experiments Bell’s theory became a powerful tool for biophysical analysis and understanding of blood cells rolling in a flowing fluid (Fig. 1B). A number of studies confirmed that Bell-like slip-kinetics for various cells and different adhesion substrates are observed at least at low and moderate wall shear rates. For instance, early experimental studies of leukocyte rolling under venous flow over inflamed vascular endothelium (Lawrence et al. 1990; Lawrence and Springer 1991) revealed that binding of neutrophil integrins (LFA-1, Mac-1, CD11/CD18) and selectins (LECAM-1) to endothelial adhesion molecules (ICAM-1 and CD62 respectively) could be well-described by the slip-bonds since the number of adherent cells monotonously decreased with the wall shear stress (WSS) in fluid. These studies focused on physiological wall shear stresses typical for venules (1–10 dyn/cm2). Furthermore, Hammer and Apte (1992) analyzed the receptor-mediated adhesion of white blood cells to ligand-coated surfaces in viscous shear flow by using the approach from Dembo et al. (1988) and determined that the experiments could be described by the slip-bonds that are “exceedingly close to the ideal bond limit,” i.e., is almost independent of the tension force.
A bit later Finger et al. (1996) studied leukocyte adhesion to different substrates in a wide range of the wall shear stress from 0.1 to 10 dyn/cm2. It was experimentally discovered that the adhesion between L-selectin on leukocytes and the peripheral node addressin protein (PNAd) depends non-monotonously on the hydrodynamic shear and actually demonstrates a threshold behavior in the low-shear regime: the shear stress above 0.4 dyn/cm2 was required to observe the leukocyte rolling in vitro, and for 0.41.0 dyn/cm2 the number of tethered and rolling cells was surprisingly growing with the shear stress. However, it was not the case when the white blood cells were to roll in vitro over the surfaces covered with E-selectin, P-selectin, or Vascular cell adhesion molecule 1 (VCAM-1) even at the lowest shear stresses tested. It was then proposed that the catch-bonds are not merely a theoretical abstraction, but an actual mechanism that prevents inappropriate blood cell aggregation in low shear.
Following that discovery, Doggett et al. (2002) experimentally revealed a similar “selectin-like” bond kinetics for the tethering of blood platelets GPIb receptor to a surface with immobilized von Willebrand factor proteins (VWF). It was concluded that the wild-type proteins demonstrate the protective no-binding behavior in the low shear, unlike their mutant variants (Doggett et al. 2002; Yago et al. 2008), suggesting that the adhesive properties required for the healthy hemostasis depend on the structures of both the receptor and the ligand, and are very sensitive even to point mutations in these proteins (Tischer et al. 2014). The tension-activated catch bonds were found to be typical for adhesins FimH in bacteria (Alonso-Caballero et al. 2018; Languin-Cattoën et al. 2023; Sauer et al. 2016; Tchesnokova et al. 2008; Thomas et al. 2002, 2004), actin, and myosin complexes in muscles (Guo and Guilford 2006; Holmes et al. 2004), cadherins that maintain tissue integrity (Manibog et al. 2014), and fibrin fibers self-assembled during blood clotting (Litvinov et al. 2018), i.e., the systems that need to increase their mechanical stability under external load. In these cases, it can be assumed that tension-dependent bond kinetics plays a regulatory function and may participate in mechanotransduction (Ju et al. 2016; Morikis et al. 2017).
The molecular reasons for the catch-bond behavior remained unclear until the early 2000-s when more sensitive ( 1 pN) biophysical techniques, such as atomic force microscopy (AFM) (Arya et al. 2002), biomembrane force probe (Evans et al. 2004), optical tweezers (Kim et al. 2010) and other force-spectroscopy methods, allowed for studies of the ligand-receptor adhesion in a wide range of stretching forces and loading rates. These setups assume that the adhering cells (and even individual molecular bonds (Neuman and Nagy 2008)) can be pulled apart in a more controllable way providing a sensitive technique for relating the bond’s lifetime to the pulling force and loading rate that is widely used nowadays (Yang et al. 2020).
Using a sensitive force probe to test the leukocyte adhesion bond between the P-selectin and the P-selectin glycoprotein ligand 1 (PSGL-1), several teams discovered the catch bond response for this pair of proteins (Evans et al. 2004; Marshall et al. 2003), as well as for bonds between L-selectin and PSGL-1 (McEver and Zhu 2010; Sarangapani et al. 2004). Evans et al. (2004) also found that it behaves as a mechanochemical switch, as the loading rate selects between two possible dissociation pathways. Their “two-pathway model” is one of the first explanations of the phenomenological catch bond behavior that takes into account the structural complexity and mechanical properties of CAMs. It assumed the existence of one bonded state and several competing ways to reach the free (unbound) state. The escape from the bonded state to the free state is done by overcoming potential energy barriers and can take place along two independent (“orthogonal”) generalized reaction coordinates so that the “energy landscape” of the protein aggregate is more sensitive to the force in one of these directions. In other words, the potential barrier height in the direction of coordinate 1 lowers faster with the increase of the pulling force than in the direction of coordinate 2, thus favoring pathway 1 to pathway 2. Under the term “reaction coordinate” this theory implies not just the increase of the distance between the reaction (adhesion) centers in the proteins, but also includes the deformations of either the receptor, the ligand, or both. It can be shown that the kinetic rate of such bond dissociation is Evans et al. (2004); Pereverzev et al. (2005); Thomas et al. (2008):
| 5 |
where may have different signs. The expression on the right-hand side of Eq. 5 reaches minimum if
| 6 |
manifesting a critical force for switching between the catch (low force) and the slip (high force) behavior. At the same time, this model allows for the case when two quantitatively different slip paths (with both ) coexist and thus slow down the bond breakage kinetics at high forces (Kim et al. 2010). It needs to be mentioned that the unbinding events under the stationary and the transient applied force are dynamically different for such molecular bonds, so the bond strength should, in general, depend on the pulling rate (Evans and Ritchie 1997).
An alternative “allosteric model” (Thomas et al. 2006) assumes that there exist two different bond states, separated by a small energy barrier, each having an independent escape (bond rupture) pathway and a transition path between bonded states 1 and 2. It was shown that the two-pathway may be considered as a limiting case of the allosteric model (Pereverzev et al. 2011). At present, the allosteric theory is more widespread due to its mechanistic idea, which opens the prospect of relating protein structure and conformational transitions to observed bond kinetics. Both two-pathway and allosteric models imply a manifestation of multiple degrees of freedom of the ligand-receptor complex, which are related to either rotations or deformations of the adhering proteins. However, the existence of multiple bound states is essential, but not sufficient for the emergence of the catch bond regime (Barsegov and Thirumalai 2005, 2006). The theoretical concepts are illustrated in Fig. 2.
Fig. 2.
A A scheme illustrating the physical concept of the two-pathways model for the catch bond behavior. B The energy landscape of the protein complex: several reaction coordinates may be involved in the bond dissociation process. C A scheme, illustrating the allosteric model. D The alterations of the bond’s energy landscape caused by the external force lead to a preferential transition into a state with a greater activation barrier for dissociation
An important aspect of biomolecular adhesion was also indicated: the catch-slip kinetics do not necessarily impart to cells an adhesive behavior exhibiting a shear threshold (Beste and Hammer 2008), which may be important for regulating blood cell aggregation in vivo. It has been also indicated in several cases that point mutations may have a drastic effect on bond rupture kinetics and thus alter the motion and adhesion of blood cells under flow (Coburn et al. 2011; Yago et al. 2008). Besides that, mechanochemical aspects may complicate our understanding of these phenomena, as several shear-dependent molecular effects may take place at the same time, e.g., autoinhibition by blocking of the binding sites (Aponte-Santamaria et al. 2015; Zhao et al. 2022), forced protein unfolding (Ju et al. 2016, 2015), cell membrane tethering (Jackson 2007; McEver and Zhu 2010), etc.
With known protein structures obtained from the X-ray crystallography, it is possible to relate the structural mechanics of CAMs to their functions and binding kinetics by utilizing the advantages of steered molecular dynamics (SMD) simulations (Ju et al. 2015; Zhang et al. 2020). Several protein shapes and bond geometries favoring the catch-slip bonds have been classified and characterized. The conceptual models and the arising bond rupture kinetics are well-illustrated in a substantial review (Thomas et al. 2008). Remarkable recent theoretical studies (Barkan and Bruinsma 2023; Makarov 2016; Zhuravlev et al. 2016) propose a generalized phenomenological approach to reveal the mechanical determinants required for catch and slip bonds. It has been emphasized that such mechano-sensitive behavior is a natural consequence of the multidimensionality of the free energy landscape for a protein-protein bond (Makarov 2016; Zhuravlev et al. 2016). Here, we focus on particular catch-like molecular interactions found in blood cells, in particular blood platelets, describe their peculiarities, and discuss their importance for hemostasis and thrombosis.
Platelets
The hemostasis under strong hemodynamic streaming (i.e., in arteries, arterioles, and venules) relies on adhesion and aggregation of blood platelets over the vascular injury to provide reinforcement for further clotting and fibrin polymerization (Celi et al. 2002; Savage and Ruggeri 2007). The interactions between the platelet adhesion receptors—mainly glycoproteins GPIb and GPIIb/IIIa—and their protein ligands—von Willebrand factor (VWF) and fibrinogen (Fg) respectively exhibit conformational transitions that result in non-monotonous relations between bond lifetime and the pulling force (Butera et al. 2018; Coburn et al. 2011; Yago et al. 2008). The picture of platelet recruitment, rolling, and adhesion under different shear conditions is quite complex. In this section, we address the known molecular mechanisms that lead to catch-like bond kinetics in platelets.
Platelet GPIb receptor and A1 domain of von Willebrand factor
An important role in the initial stages of hemostasis in vessels with high shear stress is played by the von Willebrand factor protein. This protein is able to bind to collagen and platelet membrane receptors and thus slow down cells in the area of damage to the blood vessel. The von Willebrand factor monomer consists of a sequence of domains that perform different functions (Fig. 3A). Domain A1 contains the binding site for the platelet membrane receptor GPIb and implements the primary adhesion of cells to collagen. In a healthy vessel, collagen fibers are localized in subendothelial structures, but if the integrity of the walls is violated, they are exposed and interact with the components of the hemostasis system. The D′-D3 domain contains the binding site for the blood coagulation factor FVIII, while the A3 domain contains the binding site for collagen. The proteolytic regulation of the length of VWF multimers by ADAMTS13 metalloproteinase is carried out through the A2 domain, and the A2 domain is able to block the A1 domain and thereby prevent binding to GPIb (Springer 2014).
Fig. 3.
A Domain structure of the von Willebrand factor multimer. VWF monomers dimerize through the formation of disulfide bonds between C-terminal domains (CTCK). In the Golgi apparatus, VWF dimers assemble into multimers, forming disulfide bonds between the N-terminal domains of D′-D3. B Organization of the GPIb-IX complex. Proteins GPI and GPIX form the stalk. The GPIb subunit binds via a macroglycopeptide and a mechanosensing domain (MSD) to the transmembrane portion of the complex. Von Willebrand factor binds to GPIb and, under the action of blood flow shear forces, unfolds the MSD, which initiates a change in the conformation of GPI and GPIX and transmission of an intracellular signal for platelet activation. C, D Molecular mechanism of GPIb (red) and A1VWF (blue) bond strengthening under high pulling force. Rectangles indicate the main sections of GPIb, forming a connection with A1VWF. When pinning the A1VWF end linkers and applying force to GPIb (similar to the process of breaking the bond under physiological conditions), the protein complex first rotates around the pinned ends. Thus the bond between molecules is maintained for a longer time, which is typical for catch-bonds. Initially, the position of the protein complex is shown in grey. The wild-type 1SQ0 structure was taken from the PDB archive. E A1VWF mutations described in the literature, characteristic of von Willebrand disease type 2B (blue spheres) and 2 M (red spheres), as well as the Y1271C/C1272R double mutation (cyan spheres), leading to an increase in VWF affinity for GPIb
Von Willebrand factor monomers are synthesized by endothelial cells and megakaryocytes. The molecular weight of one subunit is about 260 kD. VWF monomers dimerize in the endoplasmic reticulum through the formation of disulfide bonds between C-terminal domains (CTCK). Linear multimers are formed in the Golgi apparatus and consist of a variable number of subunits linked by disulfide bonds between the N-terminal D′-D3 domains. VWF dimers can be coiled for compact storage in Golgi apparatuses and Waibel-Palade bodies. Von Willebrand factor is also found in platelet granules and is secreted when cells are activated.
The initial contact between circulating platelets and the VWF is through the binding of the A1 domain of the VWF to the N-terminal domain of the glycoprotein Ib receptor. GPIb is part of the GPIb-IX protein complex localized on the platelet membrane. The receptor complex consists of four different transmembrane proteins and includes the polypeptides GPIb, GPIb, GPIX and a macroglycopeptide region (Fig. 3B). The von Willebrand factor A1 domain is a ligand for the extracellular domain of GPIb. GPIb contains a leucine-rich repeat domain (LRRD) that interacts with the VWF A1 domain. It is known from the literature that the mechanosensitive domain (MSD) is located in the near-membrane region of GPIb-IX (Hansen et al. 2018). When von Willebrand factor binds to GPIb and forces in the bloodstream reach a threshold, MSD unfolds. This process results in a change in the conformation of GPIb and GPIX and initiates intracellular signal transduction.
Experimental evidence
Under conditions of arterial blood flow, special adhesion proteins embedded in the platelet membrane play an important role in hemostasis. Platelet aggregation is a two-stage process (Maxwell et al. 2007). The first stage consists of the formation of weak adhesive bonds of platelets with each other and with subendothelial collagen using membrane receptors glycoprotein (GP) Ib and integrins (GPIIb/IIIa). In the second stage, the aggregate is stabilized by changing the shape of platelets caused by ADP. This leads to the formation of stronger bonds in the emerging hemostatic plug. There are various mechanisms of platelet aggregation, the use of which depends on the rate of shear in the bloodstream. Under conditions of relatively low shear (less than 1000 s-1), aggregation is triggered by soluble agonists, which cause a change in the shape of platelets and increase the affinity of the platelet integrin receptor. Activated integrin can interact with liquid-phase fibrinogen, a dimeric molecule that physically binds adjacent activated platelets. Under conditions of a higher shear (from 1000 to 10000 ), additional receptors, ligands, and membrane bonds are involved in the processes initiating aggregation. This mechanism involves adhesive interactions of the integrin between platelets via ADP-independent activation. Under conditions of higher shear (greater than 10,000 ), which are observed in stenotic arteries, the interaction of von Willebrand factor with platelet receptor glycoprotein Ib plays an important role. In contrast to aggregation at physiological shear rates, activation of integrin or platelets is not required in this case, and aggregation is provided solely by VWF: GPIb adhesive bonds. GPIb is part of the GPIb-IX-V protein complex localized on the platelet membrane.
“Catch-slip” kinetics is characteristic of the interaction between the A1 domain of VWF and GPIb. This behavior helps the platelets resist the shear forces of the blood flow by remaining attached to the blood vessel wall or other platelets. The manifestation of catch bonds in these molecules was captured in a number of experimental works, including videomicroscopy in a flow chamber (Coburn et al. 2011; Yago et al. 2008), studies using a biomembrane force probe (Ju et al. 2013).
In addition, in the experimental work Ref. (Kim et al. 2010) it was shown that the GPIb: A1VWF complex exists in two different states with different dissociation kinetics. One state is observed at low force values, and the other appears at higher values of tensile force and contributes to bond stabilization. Such “flex-bond” kinetics is important for activating the formation of a platelet aggregate with an increase in hydrodynamic forces. The origin of the catch-bond mechanism can be explained by the mechanochemical properties of the proteins.
Several studies suggest that apart from adhesion the platelet GPIb-IX-V receptor serves as a mechanical signal transmitter in both inside-out and outside-in directions (Feghhi et al. 2016; Ju et al. 2016). Ref. Feghhi et al. (2016) suggests that mechanical tension propagating from the actin filament network of platelet cytoskeleton may be transferred to catch bonds formed between GPIb-IX-V and VWF to maintain their adhesion at a wound site even when external forces are absent. The authors used arrays of flexible nanoposts to measure platelet contractility on VWF with inhibited integrins. This study shows that mechanical inside-out signals can be transmitted not only through integrins, as previously thought, but also through the GPIb-IX-V complex. The mechanism nonetheless relies on the force-dependent dissociation kinetics of the GPIb: A1VWF complex regardless of the nature of the tension force. Remarkably, it can explain why type 2B von Willebrand disease platelets bind rapidly to VWF via slip bonds, but fail to maintain their adhesion for a long time enough to prevent bleeding.
Outside-in mechanical signals could be induced by the unfolding of the leucine-rich repeat domain (LRRD) of GPIb and the juxtamembrane mechanosensing domain (MSD) of the GPIb-IX-V receptor complex. In the experimental work Ref. (Ju et al. 2016), by using a micropipette driven by a piezo actuator it was shown that LRRD unfolding enhances the calcium signal, and MSD unfolding affects the type of this signal. When both LRRD and MSD unfold, a signal featured by an initial latent phase followed by a sharp calcium spike is observed, otherwise, calcium concentration only fluctuates around the baseline. By unfolding the LRRD, the lifetime of the VWF: GPIb bond is increased, which allows the macroglycopeptide located in the GPIb-IX-V complex to transfer force to the MSD and induce intracellular signal transduction. This process is likely mechanical rather than chemical. Therefore, it was suggested that LRRD unfolding can prolong the bond lifetime via the catch-bond mechanism (Ju et al. 2015), and thus GPIb can discriminate ligands based on the bond lifetime and regulate further platelet response (Ju et al. 2016).
Molecular dynamics insights on the mechanism of catch-slip transitions in GPIb: VWF bonds
Molecular dynamics methods make it possible to study in more detail, at the atomic level, the process of interaction between GPIb and VWF. Steered molecular dynamics (SMD) simulations of VWF pinning and force applied to GPIb showed that bond breaking occurs in several steps (Ju et al. 2015). First, the GPIb forms a bond with the VWF in the area of the -switch and -finger (Fig. 3C, D). Under the action of force, the protein complex rotates counterclockwise and the GPIb -switch disaggregates, with the connection in the -finger area. Due to this rotation, the bond between molecules is maintained for a longer time, which is typical for catch-bonds. Then, at high force values, the LRRD GPIb unfolds, and at the final stage, the connection between the A1 VWF and the GPIb -finger is broken. Salt bridges play an important role in maintaining the connection between proteins. They do not allow molecules to slide relative to each other and ensure the rotation of the protein complex (Interlandi and Thomas 2010).
From Ref. (Interlandi and Thomas 2010) it is known that there is also a way of separating proteins through thermal fluctuations by destabilizing the -switch structure. In this case, the protein complex turns in the opposite direction than under the action of force. In addition, bond breaking through thermal fluctuations has been shown to be inhibited by force.
Theoretical aspects
The mechanism for the catch-slip transitions in GPIb: A1VWF seems to rely on several principles (Interlandi and Thomas 2010; Ju et al. 2016, 2015; Kim et al. 2010; Yago et al. 2008), schematically illustrated in Fig. 4. To begin, the thermally activated partial unfolding of the -switch on GPIb leads to the slip bond-like detachment of these proteins. Apparently, this pathway works under low or zero tension and manifests as a slip bond. Next, under intermediate and high tension forces, the rotation of the A1VWF globule around the cysteine lock leads to tangential orientation (with respect to the pulling direction) of the -switch binding -strand. It has been established that the N- and C-terminal linkers of the A1 domain are responsible for protection against this rotation as they form salt bridges with adjacent amino acid residues at the A1VWF surface (Ju et al. 2013; Zhao et al. 2022). Now the hydrogen bonds between the -switch and the A1 domain have to break all at once in order to release the bond. This is energetically demanding and less probable than the unzipping of the partially unfolded -switch in the initial orientation of the complex. Besides that, the -finger at the N-terminal side of the GPIb chain gets involved and forms salt bridges with the turned-up surface of the A1VWF and manifests the catch scenario (Yago et al. 2008). Further dynamics depend on the local tension distribution over the GPIb. If the LRRD stays intact, the sliding and rebinding along the direction of the pulling force helps to maintain the bond (Yago et al. 2008). Otherwise, If the local tension in the LRRD part of GPIb exceeds the critical value, then a local unfolding takes place with the N-terminal part of GPIb still being attached to the A1VWF (Ju et al. 2016, 2015). This mechanism dissipates the mechanical energy and prolongs the bond. It has also been shown that this LRRD-unfolding mechanism is relevant for the mechano-chemical signal transduction through the membrane (Ju et al. 2016; Zhang et al. 2015) that leads to intermediate integrin activation (Chen et al. 2019). Therefore, at least two sub-pathways resembling the catch bond behavior have been proposed in the literature. Kim et al. (2010) measured the kinetic rates of transition between the bond states and the off-rates from both states by using the optical tweezers technique. They identified such A1VWF: GPIb bond as a 2-state “flex-bond” introducing a new term for the case, when one conformation manifests in a low force regime and a switch to another conformation is induced by a higher force. Each pathway was well fit to an exponential Bell-like off-rate dependence on force, therefore suggesting that the complex A1VWF: GPIb dissociates as a slip bond from either of its states, so the two-state model could be applied.
Fig. 4.
A cartoon representing known mechanisms responsible for the catch bond behavior of GPIb: A1VWF complex under mechanical tension
Mutations
The literature describes such mutations of the A1 domain of VWF as R1306Q, I1309V R1341Q, H1268D, P1337L, V1316M, I1372S, Y1271C/C1272R, R1450E leading to von Willebrand disease type 2B, as well as E1359K, G1324S, G1324A, S1285F, I1425F, A1437T, R1308L, R1374H, F1369I, characteristic of von Willebrand disease type 2M (Fig. 3E) (Blenner et al. 2014; Tischer et al. 2014, 2016; Yago et al. 2008). The clinical manifestations of these mutations are represented by a wide spectrum from insufficient interaction of VWF with platelets to severe forms of thrombocytopenia; however, the reasons for the change in the kinetics of VWF interaction with platelets remain poorly understood.
Mutations R1306Q and R1450E, characteristic of von Willebrand disease type 2B, exhibit slip kinetics of interaction: at low values of strength, the lifetime of the bond has a high value, which then decreases with increasing force (Yago et al. 2008). Due to the long lifetime of the VWF and GPIb bonds at low load, the complexes of molecules in the bound state are removed from the body, which leads to thrombocytopenia.
It is known from Ref. Blenner et al. (2014) that the Y1271C/C1272R mutation increases the affinity for GPIb by 10 times compared to the wild-type (WT), although this mutation represents a displacement of the disulfide bond by only one amino acid. A similar effect was also found with a combination of R1306Q and I1309V mutations. Single substitutions R1306Q and I1309V increased the affinity for GPIb by several times. It was also observed that with these substitutions, GPIb is less curved, and its leucine repeats are closer to A1 VWF. It is possible that additional bonds can be formed in this region, which keep the proteins in a bonded state.
There are hypotheses suggesting that type 2B mutations can affect the conformation of the VWF A1 domain or the entire multimer, increasing its affinity for platelets. Mutations of type 2M, in turn, can also change the global folding of the A1 domain and lead to loss of function.
It was shown in Ref. Tischer et al. (2014) that mutations R1306Q, I1309V, G1324S, R1308L, and I1372S change the thermodynamic stability of A1 VWF against partial unfolding, but globally the globule does not change its conformation. Mutations A1437T and R1341Q have fewer secondary structures and retain part of the tertiary structure. Also, mutations F1369I, E1359K, I1425F, S1285F, R1374H, H1268D, P1337L, and V1316M have a reduced content of the tertiary structure. Changes in the mechanical properties of the A1 globule were also described in Ref. Tischer et al. (2016), where the G1324A and G1324S mutations characteristic of von Willebrand disease type 2 M were considered. The A1 VWF globule is less flexible with such substitutions, which is associated with the steric consequences of adding a side chain and the formation of an additional hydrogen bond. Thus, mutations can affect the mechanical properties of the A1 globule and lead to a change in the interaction with GPIb. The low stiffness of the globule can lead to misfolding of the A1 domain, which leads to an increase or loss of function. At the same time, the high rigidity of the A1 domain may prevent strong attachment to GPIb.
Thus, mutations located on the surface of the A1 domain near the site of contact with GPIb (especially in the -finger and -switch region) can affect adhesion directly through the formation or loss of molecular bonds, for example, salt bridges or hydrogen bonds. At the same time, the effect of mutations located in the center of the globule is more difficult to explain. Most likely, such mutations change the rigidity of the globule: less rigid A1 globules can retain the bond with GPIb for a longer time than rigid WT globules due to deformations. Mutations localized in the region of terminal linkers of the A1 domain of VWF can facilitate the rotation of the globule in the direction of blood flow forces, which is the reason for the manifestation of catch kinetics.
Platelet integrins (GPIIb/IIIa)
Platelets contain three types of integrins that mediate platelet adhesion to collagen, fibronectin and laminin, and two types of integrins, among which the majority belongs to the platelet receptor glycoprotein (GP) IIb/IIIa, formed by integrins and . This receptor plays an important role in platelet adhesion and aggregation (Savage and Ruggeri 2007). Mutations of either of its subunits result in the bleeding disorder Glanzmann thrombasthenia. At the same time, many anti-platelet drugs aim at inhibition of ligand binding to , and this strategy is effective in the prevention and treatment of arterial thrombosis (Coller 1997).
The integrins are transmembrane proteins consisting of 2 chains (subunits and ) that can change their conformation from resting to active after a biochemical stimulus (inside-out signaling) (Fig. 5). X-ray crystallography (Zhu et al. 2008), nuclear magnetic resonance (NMR) (Metcalf et al. 2010), and electron microscopy (Cormier et al. 2018; Weisel et al. 1992) have revealed a complex domain structure rearrangement as the integrins switch between an inactive (resting) and an active (ligand-binding) conformations. The active (open) form of on stimulated platelets can bind fibrinogen, von Willebrand factor, and fibronectin, mediating platelet aggregation and thus having major importance for platelet hemostasis and thrombosis (Huang et al. 2019). They are also known to be responsible for outside-in signal transduction in activated and aggregated platelets (Chen and Ju 2019); therefore, adhesive properties are also important for the regulation of thrombosis and hemostasis. Together with platelet receptors GPIb, the integrins form an important mechanosensing axis (Zhang and Cheng 2019).
Fig. 5.

Inactive (bent) conformation of the glycoprotein (GP) IIb/IIIa, formed by integrins (blue) and (red), according to PDB 3FCS: A, B the isosurface (QuickSurf) representations, rotated around the vertical axis; C the ribbon representation; and D its sketch, indicating the bent “legs” and the contacting “heads” of two integrin chains. The figure was rendered in VMD software (Humphrey et al. 1996), and the secondary structures were determined with the built-in STRIDE software (Frishman and Argos 1995)
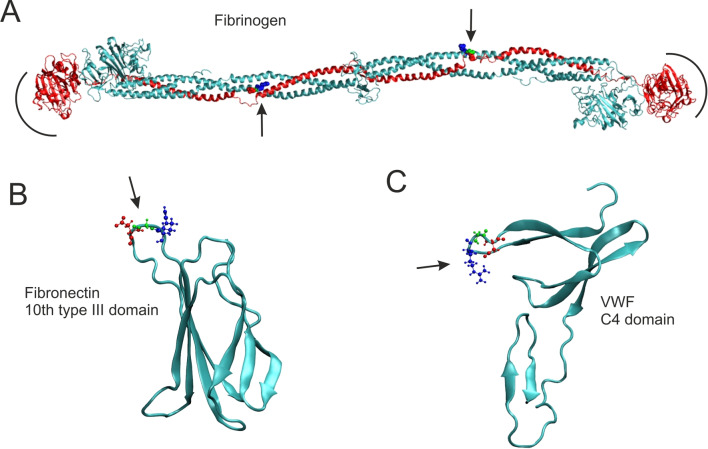
The integrin specifically binds to the peptide sequences: arginine-glycine-aspartic acid (Arg-Gly-Asp or RGD in the one letter code) and Leu-Gly-Gly-Ala-Lys-Gln-Ala-Gly-Asp-Val (LGGAKQAGDV) (Bennett et al. 1988). Both are present in fibrinogen—a fibril-forming glycoprotein that circulates in blood of all vertebrates and plays a major role in blood clotting (Fig. 6A). Both fibronectin and von Willebrand factor contain the RGD loop protruding from the protein globule and thus can form bonds with this receptor as well Bachman et al. (2015); Weisel et al. (1992) (Fig. 6B, C).
Fig. 6.
Relevant ligands to integrin . A Fibrinogen (PDB 3GHG). The semi-circles indicate locations of the primary binding sites at the globular ends of the -chains, yet the actual binding motif is not resolved in this structure. B Fibronectin 10th type III domain (PDB 1TTG). C von Willebrand factor C4 domain (PDB 6FWN). Arrows indicate the RGD-loop serving as the integrin binding site. The structures were rendered in VMD software (Humphrey et al. 1996), and the secondary structures were determined with the built-in STRIDE software (Frishman and Argos 1995)
The character of bonds between the integrin and its primary ligands—fibrinogen (Fg) and fibronectin (FN)—has been debatable for a period of time. Litvinov et al. (2011) used an optical-trap-based force clamp technique to investigate the dissociation of bonds formed by the integrin and its ligand (Fg) with a pulling force ranging from 5 pN to 50 pN. They studied purified integrins adsorbed on silica spheres and activated by Mn2+ ions. It was found that in these conditions the interactions behave as classical slip bonds (Litvinov et al. 2011). It was also found that the integrin : Fg complex may be characterized by at least two states that differ in their zero force on-rates and off-rates (Litvinov et al. 2012).
Recent studies show that the integrin bond kinetics is governed by the conformation of this receptor (Chen et al. 2019). Unlike previous works, Chen et al. (2019) used whole platelets (not purified integrins), so that the interacting membrane proteins were in more or less natural conditions. A platelet was held in a force clamp against a bead covered with a specific ligand (Fg or FN). They also stimulated platelets biochemically (by different soluble agonists) and mechanically (via loading the GPIb: VWF bonds with an auxiliary probe). It was found that bond lifetimes depend on the activation state of the platelet. Moreover, there were indicated 3 states of platelet integrins activation manifesting in different dissociation kinetics: (i) resting, (ii) intermediate, and (iii) highly active (Fig. 7A). In the resting state the two subunits of the integrin are in bent-closed conformation, and its binding to fibronectin and fibrinogen demonstrated a slip and a weak catch behavior respectively (with comparatively low lifetimes less than 5 s). The “switchblade”-like unbending (Bennett 2017) happens in response to a mechanical stimulus (Chen et al. 2019) manifesting an intermediate integrin activation. Once upregulated to this intermediate state with extended and closed subunits, the integrin binding to Fg is a typical catch bond with the maximal lifetime corresponding to the pulling force of 20 pN. Notably, this force value correlates with the characteristic force for VWF activation (Fu et al. 2017) and platelet GPIb-VWF binding (Ju et al. 2015; Zhang et al. 2015). The catch behavior becomes even stronger when the integrin is further activated to the completely extended state via biochemical stimulation by adenosine diphosphate (ADP): the peak lifetime force shifts to 30 pN and the lifetime itself reaches 20 s for both ligands (Fg and FN) (Chen et al. 2019). The typical plots illustrating bond lifetime-force dependencies are sketched in Fig. 7B. A scissor-like separation of the and -subunit stalks is the mechanical origin for this strong activation (Bennett 2017; Dai et al. 2015). Presumably, in the highly active state the molecular catch bond mechanism of : Fg and : FN bonds are similar; however, further molecular dynamics studies are required. The intermediate state was reported only in cases of biomechanical stimulation of the platelets (Chen et al. 2019), therefore, indicating a possible mechanosensitive regulation pathway for platelet aggregation that is closely related to the slip-to-catch bond transition in integrins . Besides, different conformations of the integrin are reported to have different molecular stiffness (Chen et al. 2019), which may have an impact on the bond lifetime and give a hint about the mechanism of catch bond in this case: stiffer receptor demonstrates longer bond lifetimes and favors the “catch” dynamics. In addition, a dynamic allosteric effect in Calf-1 domain of integrins has been revealed by molecular dynamic simulations (Goguet et al. 2017) and once again highlighted the role of mechanical flexibility in ligand-receptor adhesion According to these simulations, the mutations associated with impaired integrin functions (and thus platelet-associated bleeding) cause long-range structural effects: the mutated structure barely showed any modifications at the mutation sites, while distant conformational changes were the case.
Fig. 7.

The conformation-dependent character of the molecular bonds between the integrin (GPIIa/IIIa) and fibrinogen. A The conformations of the extracellular domains of the integrin (from left to right): a bent-closed inactive, an extended-closed intermediate, and a highly active extended-open, according to Chen et al. (2019). B A schematic representation of experimental (Chen et al. 2019) dependencies of the bond lifetime on the tension force for three respective conformations of the integrin. As the activation progresses, the catch regime extends to greater forces and the overall lifetime increases
The most probable location of the bonds : Fg was observed in Ref. Weisel et al. (1992) by means of electron microscopy. It was found that the activated integrins attach to the C-terminal globular part of the fibrinogen -chain, and not to the RGD-loop in the -chain. The binding site for fibrinogen on the headpiece is located at the interface of a “specificity-determining” loop in the head of subunit and a “cap” composed of four loops (“blades”) of the so-called -propeller domain of the subunit (Xiao et al. 2004). The fibrinogen -chain is thus squeezed between these integrin headpieces, resembling a pair of pliers. The mechanics of this bond is quite different from the GPIb: A1VWF complex.
Another known ligand to the platelet integrins is the VWF, specifically, its domain C4 of the C-terminal stem (Chen et al. 2022; Chen and Ju 2019; Springer 2014; König et al. 2019; Weisel et al. 1992; Xu et al. 2019). Opening of force-bearing disulfide bonds in the VWF C4 domain leads to pronounced conformational changes that considerably affect the accessibility of the integrin-binding motif (Kutzki et al. 2023). In other words, the hydrodynamics-induced stretching of VWF impairs integrin-mediated platelet binding by unfolding the RGD motif. This study indicates the importance of the local loop shape of the RGD for binding the integrins . It also indicates possible new feedback between mechanics and molecular interactions in the VWF-dependent shear-induced platelet aggregation. C-Terminal missense mutations in VWF cause reduced binding to integrin and manifests in a variant of von Willebrand disease (König et al. 2019). However, it is unknown yet whether these adhesive interactions are catch-slip or purely slip bonds.
Overall, the catch bond mechanism was demonstrated to be conformation-dependent for integrins (also known as GPIIb/IIIa platelet receptors). The activation increases the maximal bond lifetime, changes the bond regime from slip to catch, and, importantly, stretches the “catch” regime to a wider range of the tension forces - up to 30 pN (Chen et al. 2019; Chen and Ju 2019). Such an effect reinforces the platelet aggregation under arterial flow and supports the development of platelet thrombi even under pathological shear stresses in stenosed arteries. Structural studies and molecular dynamics provide a picture of several local conformational rearrangements in the integrin head that regulate affinity for ligand, as well as global structural transitions in the integrin leg domains that transmit the mechano-chemical signals (Xiao et al. 2004) (Fig. 8). We may thus conclude that the adhesivity and signal transduction in these classes of CAMs are closely connected and rely on the mechanochemistry of the receptor proteins. Like the GPIb receptor with its mechanosensitive domain, the integrins are not just passive sticky molecules, rather they provide a cell with remote allosteric control of the surrounding environment (Hynes 2002).
Fig. 8.
Complex long-range allostery in integrins presumably controls its adhesive catch-slip bond properties and participates in signal transmission. A Several conformational changes in the unbent extracellular integrin domains. The cartoon is inspired by the structural data of Xiao et al. (2004). B, C Local rearrangement of the binding site of integrin, according to the crystallography data from Zhu et al. (2008): the bent upper leg part of the -subunit (red) PDB 3FCS (B) and the straightened one PDB 3FCU (C) in contact with the -propeller domain of the -subunit (blue). The local conformational transition in the integrin “head” domains makes the ligand binding pocket between them narrower and the heads are stiffer due to elongation of the -sheets in the -subunit (the “blades” of the “propeller” are more structured in panel (C). The panels B and C were rendered in VMD software (Humphrey et al. 1996), and the secondary structures were determined with the built-in STRIDE software (Frishman and Argos 1995)
Although exact molecular mechanics of :Fg-mediated bonds require further studies, it is now clear that its ability to establish high-affinity catch bonds is triggered in response to either biomechanical or biochemical stimulation of platelets (Chen et al. 2019; Ju et al. 2016; Packham and Rand 2011; Zhang and Cheng 2019) and that thrombosis in arteries and arterioles relies on strength and lifetime on these bonds (Fullard 2004; Maxwell et al. 2007; Kaneva et al. 2021; Masalceva et al. 2022; Stalker et al. 2013). Novel antithrombotic approaches could be aimed at downregulation of the catch bond behavior of activated integrins at the large forces by altering the mechanical properties of particular domains (Rosetti et al. 2015) or by the usage of conformation-specific inhibitors (Schwarz et al. 2006).
Other integrins: common structural aspects of catch bonds
It is instructive to inspect the catch bonds formed by other receptors of the integrin family. Atomic force microscopy experiments showed that ion-stimulated integrins bind to fibronectin in the catch-bond manner (Kong et al. 2009). This receptor expressed on leukocytes and platelets plays an important role in supporting platelet thrombus growth on fibrillar fibronectin (present in atherosclerotic plaque), yet it appears to be dispensable for hemostasis (Janus-Bell et al. 2021). Other family integrins can form catch bonds with their ligands (Choi et al. 2013) indicating a possible structural importance of -subunit for catch-bond behavior. Integrins can bind FN in different modes (Boettiger et al. 2001), even being in bent (inactive) conformation, as suggested by the crystal structures PDB 4MMX, 4MMZ (Agthoven et al. 2014). Although it is not exactly known whether these are slip or catch bonds, one may assume by the analogy with : FN bonds (Chen et al. 2019) that this bond is a slip bond in the bent state and a catch one in the unbent state.
The major cell-binding site for many integrins is the three amino acid sequence RGD on the 10th type III repeat of FN (Agthoven et al. 2014; Chi-Rosso et al. 1997; Leahy et al. 1996). Therefore, one may assume that the relatively small RGD motif may still be enough to form the catch bonds with the integrins, while the tension-dependent unfolding of the RGD loop may abolish the binding, just like in VWF-C4 domain (Xu et al. 2019). These studies demonstrate that it may be a common feature of particular integrins to form catch bonds (in the activated state), making them prime candidates for mechanosensing molecules, while others may form only slip bonds.
The ability to change its conformation and thus adhesive properties in response to biochemical signaling makes the understanding difficult, but it underlines the importance of catch-slip transitions in the regulation of thrombosis and hemostasis. The structural basis for allostery in integrin ectodomains seems to be quite common throughout this family of CAMs (Xiao et al. 2004). For instance, steered molecular dynamics simulations of the leukocyte integrin : ICAM-1 catch bonds suggested the three-state model and provided a structural mechanism for catch bond in that case, including domain swinging and integrin extension (Zhu and Chen 2012). Several intermediate conformations of the “upper legs” domains define the mechanical properties of the receptor (Fig. 8A), as well as the local conformation of the integrin’s main binding site (Fig. 8B, C). The conformation of the “legs” also governs the directions of the tension force transmission during cell adhesion (Rosetti et al. 2015). The distance between the “lower legs” seems to be the driving geometrical factor (Cormier et al. 2018; Xiao et al. 2004; Zhu and Chen 2012). It is controlled by the intracellular parts of this protein (Dong et al. 2012; Metcalf et al. 2010; Rosetti et al. 2015; Zhu and Chen 2012) provided by their connections to the cytoskeleton and action of talin and kindlin inside a cell (Bennett 2017; Huang et al. 2019). The catch-bond properties may be tuned and controlled due to arising local allosteric changes in the head domains, making the integrin “head” connection narrower and stiffer, as the “leg” parts are being separated.
The extracellular integrin domains are thought to serve as a bidirectional mechanical signal transmitter (Hynes 2002). In order to communicate over unusually large distances through the extended and flexible lower leg of the -subunit, large separations between the cytoplasmic domains would be required (Dong et al. 2012; Kim et al. 2003; Springer and Dustin 2012). The comparison of structural data from several studies suggests that such common molecular machinery may be characteristic of the whole integrin family (Chen et al. 2010; Rosetti et al. 2015; Xiao et al. 2004; Zhu and Chen 2012). Bidirectional trans-membrane integrin signaling is thus mechanical in its nature and is likely to be common for all integrin-expressing cells (Hynes 2002). Despite recent progress in understanding of the complex biomechanics of the integrin family receptors, the impact of structural peculiarities of different integrins (including mutated variants) on their ability to form the catch bonds needs to be studied further.
P-selectin on activated platelets
The selectins represent another family of CAMs, which consists of 3 known members: platelet selectin (or, shortly, P-selectin), leukocyte selectin (L-selectin), and endothelial selectin (E-selectin). All of them are single-chain transmembrane glycoproteins that share a similar cassette structure, yet differ in their length due to a variable number of consensus repeat domains (Kansas 1992; Ley 2003). The largest one is the P-selectin found in platelet -granules and also in Weibel-Palade bodies of vascular endothelial cells (McEver 2015). After platelet activation, P-selectin is translocated from intracellular granules to the external membrane to assist the integrins in the stabilization of platelet aggregates. This receptor has been experimentally observed on strongly activated platelets in aggregates or thrombi both in vivo (Stalker et al. 2013) and in vitro (Muthard and Diamond 2013). Expression of P-selectin on the platelet surface correlates strongly with the mean platelet aggregate size (Merten and Thiagarajan 2000). During an inflammatory response, P-selectin is also expressed on endothelial cells (together with E-selectin) (Scalia et al. 1999). The most studied ligand is P-selectin glycoprotein ligand-1 (PSGL-1), abundantly found at the tips of leukocyte microvilli (Huo and Xia 2009), expressed on inflamed endothelial cells (Costa Martins et al. 2007) and activated platelets (Frenette et al. 2000). PSGL-1 can bind to all three members of the family, yet binds with the highest affinity to P-selectin (Li et al. 1996; Wilkins et al. 1995).
Platelets and leukocytes co-localize in the areas of vascular injury, at sites of vascular inflammation and thrombosis (André 2004), the platelets roll on inflamed endothelium under shear flow (Frenette et al. 1995) and induce leukocyte rolling in vivo (Dole et al. 2005; Konstantopoulos et al. 1998). It has been found in vitro that platelets adhered to endothelial cell-bound ultra-large von Willebrand factor strings expressed P-selectin suggesting their rapid activation (Bernardo et al. 2005). Leukocytes are recruited on activated platelets due to P-selectin: PSGL-1 binding (Ludwig et al. 2004). VWF-bound activated platelets are a suitable matrix for leukocyte tethering and rolling under high arterial wall shear stress (2–4 Pa), since platelets express P-selectin at higher density compared to endothelial cells Yeo et al. 1994). These bonds thus contribute to both inflammatory and hemostatic responses, being an important link between the two protective systems of an organism. Since the P-selectin: PSGL-1 binding has an effect on thrombosis and thrombo-inflammation, it is instructive to inspect the mechano-chemical properties of these bonds.
The catch-slip character of P-selectin: PSGL-1 bond has been established experimentally during the very early studies of the catch bond phenomenon (Evans et al. 2004; Marshall et al. 2003; Xiao et al. 2004). Experimental observations of Xiao et al. (2004) suggest that platelet-neutrophil adhesion in suspension exhibits the shear-threshold phenomenon at low shear stress (less than 0.05 Pa) due to a force-dependent regulation of P-selectin: PSGL-1 binding. Marshall et al. (2003) measured P-selectin: PSGL-1 bond lifetime versus tension force using both AFM and a flow chamber neutrophil rolling technique. They found that the transition from catch to slip regime takes place at 20–40 pN and the peak measured lifetime is about 1.01.2 s. As wall shear stress was increased, the number of rolling neutrophil cells in their flow chamber experiments first increased and then decreased in accordance with the expected catch-slip bond dynamics. We see that the catch-bond transition takes place in approximately the same force range, as for the integrins : Fg and GPIb: A1VWF bonds. However, due to a bigger radius of neutrophils (compared to platelets) the wall shear stress, at which this transition occurs, is quite low (0.020.04 Pa)—at least it is much lower than physiological arterial wall shear stress (2–4 Pa). According to this, one may assume that in arteries and arterioles the P-selectin: PSGL-1 bonds that involve leukocytes should work in the high-force slip regime, while the catch regime may be important in venules and slow flow recirculation zones. However, as a platelet has a 10-times smaller size than a typical leukocyte and the drag force scales as
| 7 |
one may expect that for the blood platelets, which express P-selectin on their membranes and adhere to the inflamed vessel walls, the catch regime of P-selectin: PSGL-1 bonds may be relevant in physiological hemodynamic conditions. It may be one of the mechanical properties participating in the regulation and signaling of both immune and hemostatic responses. White blood cell rolling occurs primarily in the low-shear venous environment, due to the weak strength of P-selectin: PSGL-1 bonds (McEver 2001). Leukocyte rolling on ultra-large VWF strings decorated with platelets is significantly slower than their rolling on endothelial cells, which delineates a new mechanism of leukocyte participation in thrombotic and thrombo-inflammatory events in arterial conditions and micro-vasculature (Bernardo et al. 2005).
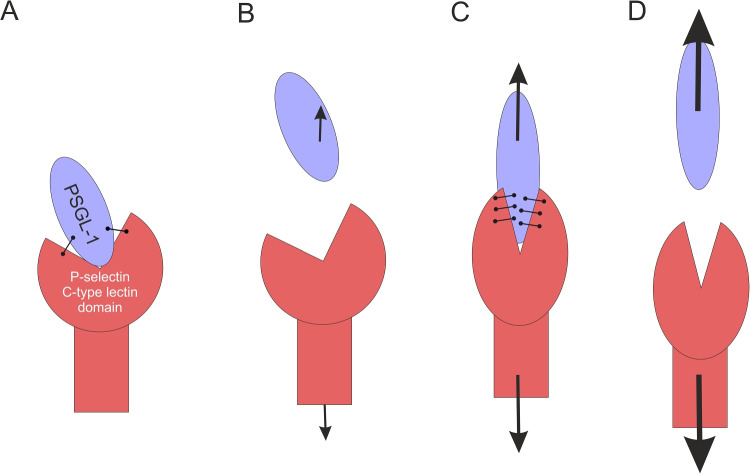
Based on the structural information, a model has been proposed to explain the catch-slip bond behavior of the P-selectin: PSGL-1 complex in vivo (Beste and Hammer 2008; Konstantopoulos et al. 2003). A two-state model of P-selectin was proposed based on the data from structural studies (Somers et al. 2000) that suggest the existence of “bent” and “extended” conformations of the lectin headpiece relative to the EGF-like domain of P-selectin (Fig. 9). In addition to this global transition, smaller structural changes are evident in the PSGL-1-binding site (Konstantopoulos et al. 2003; Somers et al. 2000). At low forces, the receptor assumes the bent conformation with a lower number of hydrogen bonds at the P-selectin: PSGL-1 binding interface (Fig. 10A, B). At intermediate levels of force, due to mechanical tension, the complex favors the extended conformation(Beste and Hammer 2008), which is assumed to exhibit greater resistance to failure (Fig. 10C). This could be due to a favorable orientation of the binding interface and a conformational change in the binding pocket (Konstantopoulos et al. 2003). Structural studies suggest that a cooperative effect takes place in the C-type lectin domain. Bending alters the environment around Ala28 and triggers the movement of a loop Asn83-Asp89 towards the binding site, thus increasing the area of contact with the ligand (Phan et al. 2006; Waldron and Springer 2009). This leads to the catch behavior under appropriate forces arising from the bending-dependent modulations of the binding strength (Barkan and Bruinsma 2023). Further force increase leads to the breakage of the high-affinity complex (Fig. 10D). A progressive exposure of buried residues that initiate new attractive interactions (hydrogen bonds or salt bridges) at the receptor-ligand interface could be a key to the catch bond mechanism in this case. Hypothetically, mechanical forces could also elongate the PSGL-1 molecule and expose its determinants for P-selectin recognition. The steered molecular dynamics studies in the future could provide a better understanding of the dynamical catch-slip transitions and their biophysical mechanisms in selectin receptors.
Fig. 9.
A, B Crystal structure of P-selectin lectin and EGF domains (red) in complex with PSGL-1 peptide (blue) and a polysaccharide (green), rendered from PDB 1G1S. C Ligand-free P-selectin in bent conformation, rendered from PDB 1G1Q. Notice that in the stretched conformation (A, B) the loop (residues Asn83-Asp89) leans to the ligand binding site, unlike in the bent one (C) (Phan et al. 2006; Waldron and Springer 2009). The figure was rendered in VMD software (Humphrey et al. 1996), and the secondary structures were determined with the built-in STRIDE software (Frishman and Argos 1995)
Fig. 10.
A cartoon of a hypothetical mechanism of catch bond in P-selectin: PSGL-1 complex. The complex initially formed from relaxed structures of both ligand and receptor A contains a certain number of stabilizing hydrogen bonds in the adhesion interface. Under a low force B the complex dissociates through a slip pathway without any significant deformation of CAMs. However, if the deformations caused by an intermediate force C lead to an increased site of molecular contacts between the P-selectin C-type lectin domain and PSGL-1, hence catch bond. Extremely high force though can overcome even this bond D leading again to slip behavior. The catch behavior arises from a combined (cooperative) effect of bending and binding site plasticity of the C-type lectin domain. This scheme is based on the ideas of Beste and Hammer (2008); Konstantopoulos et al. (2003); Somers et al. (2000); Phan et al. (2006); Waldron and Springer (2009); Barkan and Bruinsma (2023)
Vascular endothelium
Vascular endothelial cells play a central role in triggering of thrombotic events in cases of inflammation, damage or infection while preventing coagulation and platelet adhesion in normal physiological conditions. Apart from the already mentioned secretion of Weibel-Palade bodies (with VWF and P-selectin) and the exposure of the PSGL-1 to bind activated platelets and leukocytes, they also express E-selectins—receptors similar in structure to P-selectins.
E-selectin, like its family members L- and P-selectin, is capable of forming catch bonds with its main ligand PSGL-1 (Snook and Guilford 2010) with a characteristic catch-to-slip transition at a force 30 pN. E-selectin that is shorter in length compared to P-selectin (Kappelmayer and Nagy 2017). E-selectin: PSGL-1 binding has been shown to be crucial for leukocyte tethering under flow. Experiments (Morikis et al. 2017) have also shown that endothelial E-selectins form catch bonds with L-selectin on neutrophils, thus mediating rolling and transmitting signals into the neutrophils to trigger the activation of high-affinity integrins necessary for shear-resistant adhesion and transendothelial migration. However, this seems to be true only for humans, not mice (Zöllner et al. 1997). The transition from catch to slip regime takes place at the force of 20–25 pN, and the tether lifetime is reported to be up to several seconds. An unusual triphasic slip-catch-slip force dependence of E-selectin: sLex (Sialyl Lewis X) polysaccharide ligand dissociation has been detected in optical trap experiments (Wayman et al. 2010), and the physiological role of this phenomenon is yet to be understood.
An alternative way of establishing bonds between the endothelium and the platelets is via PECAM-1 (platelet/endothelial cell adhesion molecule 1) has been reported in literature (Woodfin et al. 2007). However, there is a lack of information in the literature, on whether it forms catch bonds or if its adhesive functions are shear-dependent.
Another class of CAMs that is important for vascular integrity is cadherins (“calcium-dependent adhesion molecules”) (Maître and Heisenberg 2013). Cadherins mediate robust cell-cell adhesion by binding in multiple conformations. One of these adhesive states called an X-dimer, forms catch bonds that strengthen and become longer lived in the presence of mechanical force (Duong and Vestweber 2020; Manibog et al. 2014; Rakshit et al. 2012). In contrast, another form—strand-swap dimers—forms slip-only bonds (Rakshit et al. 2012). The steered molecular dynamics once again showed that catch bond formation is mechanically regulated by the flexibility of the ectodomain (Manibog et al. 2014): the tensile force bends the cadherin extracellular region so that they form long-lived hydrogen bonds that lock the X-dimers into a tight contact. However, at low concentrations of Ca2+ ions the structure of cadherin becomes more flexible and fluctuating, thus fewer amount of hydrogen bonds are formed, and catch bond formation is eliminated.
Additional remarks about leukocytes
The leukocytes have played an immense role in the experimental study and theoretical conceptualization of cell-cell adhesion and, in particular, historically lead to the very idea of catch and slip bonds. We have already mentioned that selectins and integrins that present on monocytes and neutrophils demonstrate catch bond kinetics manifesting in shear-threshold recruitment to a substrate in venous (low wall shear stress) hemodynamic conditions (Ley 2003).
L-selectin (known also as LECAM-1/CD62) is present on the leukocyte membranes, yet it is a weak ligand to the PSGL-1 and is the shortest receptor in its family (Ley 2003). Hammer and Apte (1992) analyzed the receptor-mediated adhesion of white blood cells to ligand-coated surfaces in viscous shear flow in order to identify the receptor-ligand pairs that are responsible for observed cell’s dynamics. By comparing the model with the experiments (Andrian et al. 1991; Lawrence et al. 1990; Lawrence and Springer 1991; Ley et al. 1991), they showed that L-selecting binding to endothelium promotes the initial rolling and adhesion of neutrophils, while the leukocyte integrin (LFA-1) binding to the Inter-Cellular Adhesion Molecule-1 (ICAM-1, also CD54) strengthens the adhesion, providing arrest and extravasation. The L-selectin: PSGL-1 complexes are also reported to behave under mechanical tension as the catch-slip bonds for neutrophils and microspheres rolling in vitro at the wall shear stress of 0.01–1 Pa (Yago et al. 2004). It was concluded that L-selectin is responsible for signaling, as the initial attachment of neutrophils to endothelium and their rolling is a prerequisite for subsequent adhesion strengthening through integrins LFA-1 and ICAM-1 that also exhibits the catch behavior (Chen et al. 2010), and the mechanisms are presumably common to platelet integrins (Rosetti et al. 2015). The role of catch-slip L-selectin: PSGL-1 binding in signaling requires further studies with a focus on the L-selectin cytoplasmic tail and its hypothetical mechanical response from the adhesion bonds and arising tension.
Shear-enhanced adhesion beyond catch bonds: alternative mechanisms to stick stronger
As we see, in many cases, those biomolecules that mediate cell adhesion in the blood flow form catch bonds. It serves to provide a reliable binding to resist the drag force and cooperates with cellular mechanotransduction. However, there are physical mechanisms that also contribute to the shear-enhanced adhesion, apart from the allostery-induced bond enforcement. For instance, tethering due to membrane protrusions is observed for leukocytes (Chen et al. 2016; Cugno et al. 2021), platelets (Jackson 2007), as well as bacteria (Omidvar et al. 2021). Such protrusions elongate the distance between the binding point and the cell center and could be much longer than the maximal size of a protein receptor. Alternatively, the protein ligand may elongate itself, as it happens to the wall-attached VWF multimers in the blood flow (Fu et al. 2017; Kushchenko and Belyaev 2020). There is a mechanical argument behind this phenomenon: elongated ligand-receptor bonds provide more stable adhesion in a shear flow near the wall as they reorient the tension force in the direction parallel to the flow counterbalancing the drag more effectively (Belyaev 2018b). Besides, a softer cell may form a wider interface with the substrate thus providing a greater number of bonds to maintain adhesion. For instance, platelets after activation become soft and spread providing a greater contact surface (Margolis et al. 1980; Lee et al. 2012). In addition, a shear-sensitive ligand may become sticky under higher shear due to local conformational transitions and deactivate itself under low shear, like a multimeric VWF is capable of self-inhibition in low shear stress (Aponte-Santamaria et al. 2015; Belyaev and Kushchenko 2023; Fu et al. 2017; Liu et al. 2022; Posch et al. 2016; Schneider et al. 2007; Springer 2014; Vergauwe et al. 2014). Such multi-level shear-induced activation processes provide a fine-tuning of platelet/leukocyte adhesion and aggregation during the hemostatic response in vivo and also regulate its functional activity to prevent undesirable thrombosis.
Future perspectives
Despite current progress in understanding the biophysics of adhesive molecular bonds between blood cells, several aspects of this problem still require attention.
Mechanical signal transduction by catch-slip bonds. Recent works (Hansen et al. 2018; Huang et al. 2019; Ju et al. 2016; Morikis et al. 2017) shed light on cell’s ways to convert external mechanical stimulus into internal electrochemical activity in platelets and leukocytes, but we are far from the complete picture. How the signal passes through the membrane and in what form the information/energy is transferred to the cytosol proteins can be in the scope of further studies.
Cheap and rapid information processing unit at the entry point. A non-linear catch-slip bond seems to be a molecular allostery-based machine (Lorimer et al. 2018; Tverdislov et al. 2015) designed to analyze whether a platelet/leukocyte needs to adhere at a certain point of the vasculature or not, and whether it needs to get activated. This approach could be applied to controlled drug delivery and other bio-engineering applications.
Platelet aggregates rely on catch-slip bonds formed by their receptors GPIb and GPIIb/IIIa. The understanding of thrombus rheology in relation to the cell adhesion peculiarities would be appreciated for systemic models of thrombosis and hemostasis (Belyaev et al. 2018a).
Relations between the structure of a receptor and its adhesive and mechanical properties are still far from complete understanding. It is not clear if all possible variants of the catch bond-favoring allostery are described up to date; what effects could be caused by mutations in CAMs and to which extent these mutations are dangerous; if there are some common rational principles to design the adhesive molecules (Tverdislov and Malyshko 2020). With modern experimental techniques combined to novel theoretical (Barkan and Bruinsma 2023) and computational approaches, such as steered molecular dynamics (Languin-Cattoën et al. 2023), coarse-grained protein modeling (Languin-Cattoën et al. 2021), protein structure prediction (Jumper et al. 2021), and computational peptide design (Wang et al. 2023) it might be possible to find new medications against platelet-related thrombotic disorders.
Conclusion
The majority of blood cell adhesion receptors exhibit a catch bond behavior in physiologically relevant hemodynamic conditions. The key to this phenomenon is the flexibility of the ligand binding domain of CAMs The mechanical in turn relies on structural peculiarities and physico-chemical properties of these molecules. In platelets, both main adhesion receptors (GPIb and GPIIb/IIIa) form the catch-slip bonds with their specific ligands. This phenomenon has a crucial meaning for the regulation of hemostasis in vivo. Catch bonds serve not only for the strengthening of cell adhesion and tissue integrity: they also play a noticeable role in mechanosensing and signal transduction, as well as in the regulation of hemostasis.
Acknowledgements
The authors acknowledge funding by the Russian Science Foundation (RSF), grant No. 22-21-00221 (https://rscf.ru/en/project/22-21-00221/).
Author contribution
I.V.F. wrote the section “Platelet GPIb receptor and A1 domain of von Willebrand factor” and prepared the figures for it. A.V.B. wrote other sections, conceptualized the review, and outlined the paper. All authors reviewed and finalized the manuscript.
Funding
This study was funded by the Russian Science Foundation (RSF), grant No. 22-21-00221 (https://rscf.ru/en/project/22-21-00221/).
Data availability
All protein structures presented in the paper are publicly available from Protein Data Bank (https://www.rcsb.org/).
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agthoven JFV, Xiong J-P, Alonso JL, Rui X, Adai BD, Goodman SL, Arnaout MA. Structural basis for pure antagonism of integrin v3 by a high-affinity form of fibronectin. Nat Struct Mol Biol. 2014;21(4):383–388. doi: 10.1038/nsmb.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caballero A, Schönfelder J, Poly S, Corsetti F, Sancho DD, Artacho E, Perez-Jimenez R (2018) Mechanical architecture and folding of e. coli type 1 pilus domains. Nat Commun 9(1). 10.1038/s41467-018-05107-6 [DOI] [PMC free article] [PubMed]
- André P. P-selectin in haemostasis. Br J Haematol. 2004;126(3):298–306. doi: 10.1111/j.1365-2141.2004.05032.x. [DOI] [PubMed] [Google Scholar]
- Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci. 1991;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte-Santamaria C, Huck V, Posch S, Bronowska AK, Grässle S, Brehm MA, Obser T, Schneppenheim R, Hinterdorfer P, Schneider SW, Baldauf C, Gräter F. Force-sensitive autoinhibition of the von Willebrand factor is mediated by interdomain interactions. Biophys J. 2015;108:2312–2321. doi: 10.1016/j.bpj.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya M, Anvari B, Romo GM, Cruz MA, Dong J-F, McIntire LV, Moake JL, López J. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99(11):3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- Bachman H, Nicosia J, Dysart M, Barker TH. Utilizing fibronectin integrin-binding specificity to control cellular responses. Adv Wound Care. 2015;4(8):501–511. doi: 10.1089/wound.2014.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan CO, Bruinsma RF (2023) Catch-slip bonding, pathway switching, and singularities in the flow of molecular deformation. Phys Rev Res 5(2). 10.1103/physrevresearch.5.023161
- Barsegov V, Thirumalai D (2005) Dynamics of unbinding of cell adhesion molecules: transition from catch to slip bonds. Proc Nat Acad Sci USA 102(6):1835–1839. 10.1073/pnas.0406938102 [DOI] [PMC free article] [PubMed]
- Barsegov V, Thirumalai D. Dynamic competition between catch and slip bonds in selectins bound to ligands. J Phys Chem B. 2006;110(51):26403–26412. doi: 10.1021/jp0653306. [DOI] [PubMed] [Google Scholar]
- Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- Bell GI. A theoretical model for adhesion between cells mediated by multivalent ligands. Cell Biophys. 1979;1(2):133–147. doi: 10.1007/bf02781347. [DOI] [PubMed] [Google Scholar]
- Belyaev AV, Dunster JL, Gibbins JM, Panteleev MA, Volpert V. Modelling thrombosis in silico: frontiers, challenges, unresolved problems and milestones. Phys Life Rev. 2018;26–27:57–95. doi: 10.1016/j.plrev.2018.02.005. [DOI] [PubMed] [Google Scholar]
- Belyaev AV. Long ligands reinforce biological adhesion under shear flow. Phys Rev E. 2018;97:042407. doi: 10.1103/PhysRevE.97.042407. [DOI] [PubMed] [Google Scholar]
- Belyaev AV, Kushchenko YK. Biomechanical activation of blood platelets via adhesion to von willebrand factor studied with mesoscopic simulations. Biomech Model Mechanobiol. 2023;22(3):785–808. doi: 10.1007/s10237-022-01681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JS (2017) IIb3 (GPIIb/IIIa) structure and function. In: Platelets in Thrombotic and Non-Thrombotic Disorders, Springer, pp 99–112. 10.1007/978-3-319-47462-5_8
- Bennett JS, Shattil SJ, Power JW, Gartner TK (1988) Interaction of fibrinogen with its platelet receptor differential effects of alpha and gamma chain fibrinogen peptides on the glycoprotein iib-iiia complex. J Biol Chem 263(26):12948–12953. 10.1016/S0021-9258(18)37654-3 [PubMed]
- Bernardo A, Ball C, Nolasco L, Choi H, Moake JL, Dong JF. Platelets adhered to endothelial cell-bound ultra-large von willebrand factor strings support leukocyte tethering and rolling under high shear stress. J Thromb Haemost. 2005;3(3):562–570. doi: 10.1111/j.1538-7836.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- Beste MT, Hammer DA. Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc Natl Acad Sci. 2008;105(52):20716–20721. doi: 10.1073/pnas.0808213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenner MA, Dong X, Springer TA. Structural basis of regulation of von willebrand factor binding to glycoprotein ib. J Biol Chem. 2014;289(9):5565–5579. doi: 10.1074/jbc.m113.511220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D, Lynch L, Blystone S, Huber F. Distinct ligand-binding modes for integrin v3-mediated adhesion to fibronectinversus vitronectin. J Biol Chem. 2001;276(34):31684–31690. doi: 10.1074/jbc.m103997200. [DOI] [PubMed] [Google Scholar]
- Butera D, Passam F, Ju L, Cook KM, Woon H, Aponte-Santamaría C, Gardiner E, Davis AK, Murphy DA, Bronowska A, Luken BM, Baldauf C, Jackson S, Andrews R, Gräter F, Hogg PJ (2018) Autoregulation of von Willebrand factor function by a disulfide bond switch. Sci Adv 4(2). 10.1126/sciadv.aaq1477 [DOI] [PMC free article] [PubMed]
- Celi A, Merrill-Skoloff G, Gross P, Falati S, Sim DS, Flaumenhaft R, Furie BC, Furie B (2002) Thrombus formation: direct real-time observation and digital analysis of thrombus assembly in a living mouse by confocal and widefield intravital microscope. J Thromb Haemost 1:60–68. 10.1046/j.1538-7836.2003.t01-1-00033.x [DOI] [PubMed]
- Chen Y, Ju LA. Biomechanical thrombosis: the dark side of force and dawn of mechano-medicine. Stroke Vasc Neurol. 2019;5(2):185–197. doi: 10.1136/svn-2019-000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Lou J, Zhu C. Forcing switch from short- to intermediate- and long-lived states of the a domain generates LFA-1/ICAM-1 catch bonds. J Biol Chem. 2010;285(46):35967–35978. doi: 10.1074/jbc.m110.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lu L, Shao J-Y. Endothelial surface protrusion by a point force. Biophys J. 2016;110(5):1150–1157. doi: 10.1016/j.bpj.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Ju LA, Zhou F, Liao J, Xue L, Su QP, Jin D, Yuan Y, Lu H, Jackson SP, Zhu C. An integrin IIb3 intermediate affinity state mediates biomechanical platelet aggregation. Nat Mater. 2019;18(7):760–769. doi: 10.1038/s41563-019-0323-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P-c, Kutzki F, Mojzisch A, Simon B, Xu E-R, Aponte-Santamaría C, Horny K, Jeffries C, Schneppenheim R, Wilmanns M, Brehm MA, Gräter F, Hennig J. Structure and dynamics of the von willebrand factor c6 domain. J Struct Biol. 2022;214(4):107923. doi: 10.1016/j.jsb.2022.107923. [DOI] [PubMed] [Google Scholar]
- Chi-Rosso G, Gotwals PJ, Yang J, Ling L, Jiang K, Chao B, Baker DP, Burkly LC, Fawell SE, Koteliansky VE. Fibronectin type III repeats mediate RGD-independent adhesion and signaling through activated 1 integrins. J Biol Chem. 1997;272(50):31447–31452. doi: 10.1074/jbc.272.50.31447. [DOI] [PubMed] [Google Scholar]
- Choi YI, Duke-Cohan JS, Chen W, Liu B, Rossy J, Tabarin T, Ju L, Gui J, Gaus K, Zhu C, Reinherz EL. Dynamic control of 1 integrin adhesion by the plexinD1-sema3e axis. Proc Natl Acad Scie. 2013;111(1):379–384. doi: 10.1073/pnas.1314209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn LA, Damaraju VS, Dozic S, Eskin SG, Cruz MA, McIntire LV. GPIb-vWF rolling under shear stress shows differences between type 2b and 2m von willebrand disease. Biophys J. 2011;100(2):304–312. doi: 10.1016/j.bpj.2010.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller BS. Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J Clin Investig. 1997;99(7):1467–1471. doi: 10.1172/jci119307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier A, Campbell MG, Ito S, Wu S, Lou J, Marks J, Baron JL, Nishimura SL, Cheng Y. Cryo-EM structure of the v8 integrin reveals a mechanism for stabilizing integrin extension. Nat Struct Mol Biol. 2018;25(8):698–704. doi: 10.1038/s41594-018-0093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Martins P, Garcia-Vallejo J-J, Thienen JV, Fernandez-Borja M, Gils JM, Beckers C, Horrevoets AJ, Hordijk PL, Zwaginga J-J. Pselectin glycoprotein ligand-1 is expressed on endothelial cells and mediates monocyte adhesion to activated endothelium. Arterioscler Thromb Vasc Biol. 2007;27(5):1023–1029. doi: 10.1161/atvbaha.107.140442. [DOI] [PubMed] [Google Scholar]
- Cugno A, Marki A, Ley K. Biomechanics of neutrophil tethers. Life. 2021;11(6):515. doi: 10.3390/life11060515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A, Ye F, Taylor DW, Hu G, Ginsberg MH, Taylor KA. The structure of a full-length membrane-embedded integrin bound to a physiological ligand. J Biol Chem. 2015;290(45):27168–27175. doi: 10.1074/jbc.m115.682377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembo M. On peeling an adherent cell from a surface. Lect Math Life Sci. 1994;24:51–77. [Google Scholar]
- Dembo M, Torney DC, Saxman K, Hammer DA (1988) The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proc R Soc Lond B 234:55–83. 10.1098/rspb.1988.0038 [DOI] [PubMed]
- Doggett TA, Girdhar G, Lawshé A, Schmidtke DW, Laurenzi IJ, Diamond SL, Diacovo TG. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb-vWF tether bond. Biophys J. 2002;83(1):194–205. doi: 10.1016/s0006-3495(02)75161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce weibel-palade-body secretion and leukocyte rolling in vivo: role of p-selectin. Blood. 2005;106(7):2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Mi L-Z, Zhu J, Wang W, Hu P, Luo B-H, Springer TA. integrin crystal structures and their functional implications. Biochemistry. 2012;51(44):8814–8828. doi: 10.1021/bi300734n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong CN, Vestweber D (2020) Mechanisms ensuring endothelial junction integrity beyond VE-cadherin. Front Physiol 11. 10.3389/fphys.2020.00519 [DOI] [PMC free article] [PubMed]
- Evans E, Ritchie K. Dynamic strength of molecular adhesion bonds. Biophys J. 1997;72:1541–1555. doi: 10.1016/S0006-3495(97)78802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a p-selectin adhesion bond. Proc Natl Acad Sci. 2004;101(31):11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghhi S, Munday AD, Tooley WW, Rajsekar S, Fura AM, Kulman JD, Ló JA, Sniadecki NJ. Glycoprotein ib-IX-v complex transmits cytoskeletal forces that enhance platelet adhesion. Biophys J. 2016;111(3):601–608. doi: 10.1016/j.bpj.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EB, Purl KD, Alon R, Lawrence MB, Andrian UH, Springer TA. Adhesion through l-selectin requires a threshold hydrodynamic shear. Nature. 1996;379(6562):266–269. doi: 10.1038/379266a0. [DOI] [PubMed] [Google Scholar]
- Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial p-selectin. Proc Natl Acad Sci. 1995;92(16):7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Denis CV, Weiss L, Jurk K, Subbarao S, Kehrel B, Hartwig JH, Vestweber D, Wagner DD. P-selectin glycoprotein ligand 1 (psgl-1) is expressed on platelets and can mediate platelet-endothelial interactions in vivo. J Exp Med. 2000;191(8):1413–1422. doi: 10.1084/jem.191.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman D, Argos P. Knowledge-based protein secondary structure assignment. Proteins Structure Function Genetics. 1995;23(4):566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- Fu H, Jiang Y, Yang D, Scheiflinger F, Wong WP, Springer TA. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat Commun. 2017;8:324. doi: 10.1038/s41467-017-00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullard J. The role of the platelet glycoprotein IIb / IIIa in thrombosis and haemostasis. Curr Pharm Des. 2004;10(14):1567–1576. doi: 10.2174/1381612043384682. [DOI] [PubMed] [Google Scholar]
- Goguet M, Narwani TJ, Petermann R, Jallu V, Brevern AG (2017) In silico analysis of glanzmann variants of calf-1 domain of IIb3 integrin revealed dynamic allosteric effect. Sci Rep 7(1). 10.1038/s41598-017-08408-w [DOI] [PMC free article] [PubMed]
- Guo B, Guilford WH. Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction. Proc Natl Acad Sci. 2006;103(26):9844–9849. doi: 10.1073/pnas.0601255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer DA, Apte SM (1992) Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J 63:35–57. 10.1016/S0006-3495(92)81577-1 [DOI] [PMC free article] [PubMed]
- Hammer DA, Lauffenburger DA (1987) A dynamical model for receptor-mediated cell adhesion to surfaces. Biophys J 52:475–487. 10.1016/S0006-3495(87)83236-8 [DOI] [PMC free article] [PubMed]
- Hansen CE, Qiu Y, McCarty OJT, Lam WA. Platelet mechanotransduction. Annu Rev Biomed Eng. 2018;20(1):253–275. doi: 10.1146/annurev-bioeng-062117-121215. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Trentham DR, Simmons R, Holmes KC, Schröder RR, Sweeney HL, Houdusse A. The structure of the rigor complex and its implications for the power stroke. Philos Trans R Soc Lond B Biol Sci. 2004;359(1452):1819–1828. doi: 10.1098/rstb.2004.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li X, Shi X, Zhu M, Wang J, Huang S, Huang X, Wang H, Li L, Deng H, Zhou Y, Mao J, Long Z, Ma Z, Ye W, Pan J, Xi X, Jin J (2019) Platelet integrin IIb3: signal transduction, regulation, and its therapeutic targeting. J Hematol Oncol 12(1). 10.1186/s13045-019-0709-6 [DOI] [PMC free article] [PubMed]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Huo Y, Xia L. P-selectin glycoprotein ligand-1 plays a crucial role in the selective recruitment of leukocytes into the atherosclerotic arterial wall. Trends Cardiovasc. Med. 2009;19(4):140–145. doi: 10.1016/j.tcm.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Interlandi G, Thomas W. The catch bond mechanism between von willebrand factor and platelet surface receptors investigated by molecular dynamics simulations. Proteins Structure Function Bioinformatics. 2010 doi: 10.1002/prot.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- Janus-Bell E, Yakusheva A, Scandola C, Receveur N, Ahmed UM, Mouriaux C, Bourdon C, Loubière C, Eckly A, Senis YA, Panteleev MA, Gachet C, Mangin PH. Characterization of the role of integrin 51 in platelet function, hemostasis, and experimental thrombosis. Thromb Haemost. 2021;122(05):767–776. doi: 10.1055/a-1659-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, Dong J-f, Cruz MA, Zhu C. The n-terminal flanking region of the a1 domain regulates the force-dependent binding of von willebrand factor to platelet glycoprotein ib. J Biol Chem. 2013;288(45):32289–32301. doi: 10.1074/jbc.m113.504001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, Lou J, Chen Y, Li Z, Zhu C. Force-induced unfolding of leucine-rich repeats of glycoprotein ib strengthens ligand interaction. Biophys J. 2015;109(9):1781–1784. doi: 10.1016/j.bpj.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju L, Chen Y, Xue L, Du X, Zhu C (2016) Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife 5. 10.7554/elife.15447 [DOI] [PMC free article] [PubMed]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, Bridgland A, Meyer C, Kohl SAA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneva VN, Dunster JL, Volpert V, Ataullahanov F, Panteleev MA, Nechipurenko DY. Modeling thrombus shell: linking adhesion receptor properties and macroscopic dynamics. Biophys J. 2021;120(2):334–351. doi: 10.1016/j.bpj.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas GS. Structure and function of l-selectin. APMIS. 1992;100(1–6):287–293. doi: 10.1111/j.1699-0463.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Kappelmayer J, Nagy B. The interaction of selectins and PSGL-1 as a key component in thrombus formation and cancer progression. Biomed Res Int. 2017;2017:1–18. doi: 10.1155/2017/6138145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301(5640):1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Kim J, Zhang C-Z, Zhang X, Springer TA (2010) A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 466:992–995. 10.1038/nature09295 [DOI] [PMC free article] [PubMed]
- Kong F, García AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185(7):1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König G, Obser T, Marggraf O, Schneppenheim S, Budde U, Schneppenheim R, Brehm MA. Alteration in GPIIb/IIIa binding of VWD-associated von willebrand factor variants with c-terminal missense mutations. Thromb Haemost. 2019;119(07):1102–1111. doi: 10.1055/s-0039-1687878. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev. 1998;33(1–2):141–164. doi: 10.1016/s0169-409x(98)00024-6. [DOI] [PubMed] [Google Scholar]
- Konstantopoulos K, Hanley WD, Wirtz D. Receptor-ligand binding: ‘catch’ bonds finally caught. Curr Biol. 2003;13(15):611–613. doi: 10.1016/s0960-9822(03)00529-3. [DOI] [PubMed] [Google Scholar]
- Kushchenko YK, Belyaev AV. Effects of hydrophobicity, tethering and size on flow-induced activation of von Willebrand factor multimers. J Theor Biol. 2020;485:110050. doi: 10.1016/j.jtbi.2019.110050. [DOI] [PubMed] [Google Scholar]
- Kutzki F, Butera D, Lay AJ, Maag D, Chiu J, Woon H-G, Kubař T, Elstner M, Aponte-Santamaría C, Hogg PJ, Gräter F. Disulfide bond reduction and exchange in c4 domain of von willebrand factor undermines platelet binding. J Thromb Haemost. 2023 doi: 10.1016/j.jtha.2023.03.039. [DOI] [PubMed] [Google Scholar]
- Languin-Cattoën O, Laborie E, Yurkova DO, Melchionna S, Derreumaux P, Belyaev AV, Sterpone F. Exposure of von Willebrand factor cleavage site in a1a2a3-fragment under extreme hydrodynamic shear. Polymers. 2021;13(22):3912. doi: 10.3390/polym13223912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languin-Cattoën O, Sterpone F, Stirnemann G. Binding site plasticity regulation of the FimH catch-bond mechanism. Biophys J. 2023 doi: 10.1016/j.bpj.2023.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Smith C, Eskin S, McIntire L. Effect of venous shear stress on CD18-mediated neutrophil adhesion to cultured endothelium. Blood. 1990;75(1):227–237. doi: 10.1182/blood.v75.1.227.227. [DOI] [PubMed] [Google Scholar]
- Leahy DJ, Aukhil I, Erickson HP. 2.0 a crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84(1):155–164. doi: 10.1016/s0092-86740081002-8. [DOI] [PubMed] [Google Scholar]
- Lee D, Fong KP, King MR, Brass LF, Hammer DA. Differential dynamics of platelet contact and spreading. Biophys J. 2012;102(3):472–482. doi: 10.1016/j.bpj.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9(6):263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Ley K, Gaehtgens P, Fennie C, Singer M, Lasky L, Rosen S. Lectinlike cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991;77(12):2553–2555. doi: 10.1182/blood.v77.12.2553.2553. [DOI] [PubMed] [Google Scholar]
- Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Post-translational modifications of recombinant p-selectin glycoprotein ligand-1 required for binding to p- and e-selectin. J Biol Chem. 1996;271(6):3255–3264. doi: 10.1074/jbc.271.6.3255. [DOI] [PubMed] [Google Scholar]
- Litvinov RI, Barsegov V, Schissler AJ, Fisher AR, Bennett JS, Weisel JW, Shuman H. Dissociation of bimolecular IIb3-fibrinogen complex under a constant tensile force. Biophys J. 2011;100(1):165–173. doi: 10.1016/j.bpj.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Mekler A, Shuman H, Bennett JS, Barsegov V, Weisel JW. Resolving two-dimensional kinetics of the integrin IIb3-fibrinogen interactions using binding-unbinding correlation spectroscopy. J Biol Chem. 2012;287(42):35275–35285. doi: 10.1074/jbc.m112.404848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov RI, Kononova O, Zhmurov A, Marx KA, Barsegov V, Thirumalai D, Weisel JW. Regulatory element in fibrin triggers tension-activated transition from catch to slip bonds. Proc Natl Acad Sci. 2018;115(34):8575–8580. doi: 10.1073/pnas.1802576115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZL, Bresette C, Aidun CK, Ku DN. SIPA in 10 milliseconds: VWF tentacles agglomerate and capture platelets under high shear. Blood Adv. 2022;6(8):2453–2465. doi: 10.1182/bloodadvances.2021005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer GH, Horovitz A, McLeish T. Allostery and molecular machines. Phil Trans R Soc B Biol Sci. 2018;373(1749):20170173. doi: 10.1098/rstb.2017.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig RJ, Schultz JE, Boehncke W-H, Podda M, Tandi C, Krombach F, Baatz H, Kaufmann R, Andrian UH, Zollner TM. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Investig Dermatol. 2004;122(3):830–836. doi: 10.1111/j.0022-202x.2004.22318.x. [DOI] [PubMed] [Google Scholar]
- Maître J-L, Heisenberg C-P. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23(14):626–633. doi: 10.1016/j.cub.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov DE (2016) Perspective: mechanochemistry of biological and synthetic molecules. J Chem Phys 144(3). 10.1063/1.4939791 [DOI] [PubMed]
- Manibog K, Li H, Rakshit S, Sivasankar S (2014) Resolving the molecular mechanism of cadherin catch bond formation. Nat Commun 5(1). 10.1038/ncomms4941 [DOI] [PubMed]
- Margolis LB, Tikhonov AN, Vasilieva EY. Platelet adhesion to fluid and solid phospholipid membranes. Cell. 1980;19(1):189–195. doi: 10.1016/0092-86748090400-6. [DOI] [PubMed] [Google Scholar]
- Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C (2003) Direct observation of catch bonds involving cell-adhesion molecules. Nature 423(6936):190–193. 10.1038/nature01605 [DOI] [PubMed]
- Martino F, Perestrelo AR, Vinarský V, Pagliari S, Forte G (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9. 10.3389/fphys.2018.00824 [DOI] [PMC free article] [PubMed]
- Masalceva AA, Kaneva VN, Panteleev MA, Ataullakhanov F, Volpert V, Afanasyev I, Nechipurenko DY. Analysis of microvascular thrombus mechanobiology with a novel particle-based model. J Biomech. 2022;130:110801. doi: 10.1016/j.jbiomech.2021.110801. [DOI] [PubMed] [Google Scholar]
- Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109:566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- McEver R. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86(09):746–756. doi: 10.1055/s-0037-1616128. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107(3):331–339. doi: 10.1093/cvr/cvv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol. 2010;26(1):363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102(16):1931–1936. doi: 10.1161/01.cir.102.16.1931. [DOI] [PubMed] [Google Scholar]
- Metcalf DG, Moore DT, Wu Y, Kielec JM, Molnar K, Valentine KG, Wand AJ, Bennett JS, DeGrado WF. NMR analysis of the IIb3 cytoplasmic interaction suggests a mechanism for integrin regulation. Proc Natl Acad Sci. 2010;107(52):22481–22486. doi: 10.1073/pnas.1015545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikis VA, Chase S, Wun T, Chaikof EL, Magnani JL, Simon SI. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood. 2017;130(19):2101–2110. doi: 10.1182/blood-2017-05-783027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip. 2013;13(10):1883. doi: 10.1039/c3lc41332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Methods. 2008;5(6):491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omidvar R, Ayala YA, Brandel A, Hasenclever L, Helmstädter M, Rohrbach A, Römer W, Madl J. Quantification of nanoscale forces in lectinmediated bacterial attachment and uptake into giant liposomes. Nanoscale. 2021;13(7):4016–4028. doi: 10.1039/d0nr07726g. [DOI] [PubMed] [Google Scholar]
- Packham MA, Rand ML. Historical perspective on ADP-induced platelet activation. Purinergic Signal. 2011;7(3):283–292. doi: 10.1007/s11302-011-9227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereverzev YV, Prezhdo OV, Forero M, Sokurenko EV, Thomas WE (2005) The two-pathway model for the catch-slip transition in biological adhesion. Biophys J 89:1446–1454. 10.1529/biophysj.105.062158 [DOI] [PMC free article] [PubMed]
- Pereverzev YV, Prezhdo E, Sokurenko EV. The two-pathway model of the biological catch-bond as a limit of the allosteric model. Biophys J. 2011;101(8):2026–2036. doi: 10.1016/j.bpj.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan UT, Waldron TT, Springer TA. Remodeling of the lectin-EGF-like domain interface in p- and l-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat Immunol. 2006;7(8):883–889. doi: 10.1038/ni1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch S, Aponte-Santamaria C, Schwarzl R, Karner A, Radtke M, Gräter F, Obser T, König G, Brehm MA, Gruber HJ, Netz RR, Baldauf C, Schneppenheim R, Tampe R, Hinterdorfer P. Mutual a domain interactions in the force sensing protein von Willebrand factor. J Struct Biol. 2016;197(1):57–64. doi: 10.1016/j.jsb.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Rakshit S, Zhang Y, Manibog K, Shafraz O, Sivasankar S. Ideal, catch, and slip bonds in cadherin adhesion. Proc Natl Acad Sci. 2012;109(46):18815–18820. doi: 10.1073/pnas.1208349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosetti F, Chen Y, Sen M, Thayer E, Azcutia V, Herter JM, Luscinskas FW, Cullere X, Zhu C, Mayadas TN. A lupus-associated mac-1 variant has defects in integrin allostery and interaction with ligands under force. Cell Rep. 2015;10(10):1655–1664. doi: 10.1016/j.celrep.2015.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100(12):1673–1685. doi: 10.1161/01.res.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, Rosen SD, McEver RP, Zhu C. Low force decelerates l-selectin dissociation from p-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 2004;279(3):2291–2298. doi: 10.1074/jbc.m310396200. [DOI] [PubMed] [Google Scholar]
- Sauer MM, Jakob RP, Eras J, Baday S, Eriş D, Navarra G, Bernèche S, Ernst B, Maier T, Glockshuber R (2016) Catch-bond mechanism of the bacterial adhesin FimH. Nat Commun 7(1). 10.1038/ncomms10738 [DOI] [PMC free article] [PubMed]
- Savage B, Ruggeri ZM. Platelet thrombus formation in flowing blood. In: Michelson AD, editor. Platelets. 2. Burlington: Elsevier; 2007. pp. 359–376. [Google Scholar]
- Scalia R, Armstead VE, Minchenko AG, Lefer AM. Essential role of pselectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J Exp Med. 1999;189(6):931–938. doi: 10.1084/jem.189.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Nat Acad Sci. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Meade G, Stoll P, Ylanne J, Bassler N, Chen YC, Hagemeyer CE, Ahrens I, Moran N, Kenny D, Fitzgerald D, Bode C, Peter K. Conformation-specific blockade of the integrin GPIIb/IIIa. Circ Res. 2006;99(1):25–33. doi: 10.1161/01.res.0000232317.84122.0c. [DOI] [PubMed] [Google Scholar]
- Snook JH, Guilford WH. The effects of load on e-selectin bond rupture and bond formation. Cel Mol Bioeng. 2010;3(2):128–138. doi: 10.1007/s12195-010-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of p- and e-selectin bound to SLeX and PSGL-1. Cell. 2000;103(3):467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Springer TA. Von Willebrand factor, Jedi knight of the bloodstream. Blood. 2014;124(9):1412–1425. doi: 10.1182/blood-2014-05-378638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Curr Opin Cell Biol. 2012;24(1):107–115. doi: 10.1016/j.ceb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121:1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokova V, Aprikian P, Yakovenko O, LaRock C, Kidd B, Vogel V, Thomas W, Sokurenko E. Integrin-like allosteric properties of the catch bond-forming FimH adhesin of escherichia coli. Journal of Biol Chem. 2008;283(12):7823–7833. doi: 10.1074/jbc.m707804200. [DOI] [PubMed] [Google Scholar]
- Thomas W (2008) Catch bonds in adhesion. Annu Rev Biomed Eng 10:39–57. 10.1146/annurev.bioeng.10.061807.160427 [DOI] [PubMed]
- Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV (2002) Bacterial adhesion to target cells enhanced by shear force. Cell 109(7):913–923. 10.1016/S0092-8674(02)00796-1 [DOI] [PubMed]
- Thomas WE, Nilsson LM, Forero M, Sokurenko EV, Vogel V. Sheardependent ‘stick-and-roll’ adhesion of type 1 fimbriated escherichia coli. Mol Microbiol. 2004;53(5):1545–1557. doi: 10.1111/j.1365-2958.2004.04226.x. [DOI] [PubMed] [Google Scholar]
- Thomas W, Forero M, Yakovenko O, Nilsson L, Vicini P, Sokurenko E, Vogel V (2006) Catch-bond model derived from allostery explains force-activated bacterial adhesion. Biophys J 90:753–764. 10.1529/biophysj.105.066548 [DOI] [PMC free article] [PubMed]
- Thomas WE, Vogel V, Sokurenko E. Biophysics of catch bonds. Annu Rev Biophys. 2008;37(1):399–416. doi: 10.1146/annurev.biophys.37.032807.125804. [DOI] [PubMed] [Google Scholar]
- Tischer A, Madde P, Moon-Tasson L, Auton M. Misfolding of vWF to pathologically disordered conformations impacts the severity of von willebrand disease. Biophys J. 2014;107(5):1185–1195. doi: 10.1016/j.bpj.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer A, Campbell JC, Machha VR, Moon-Tasson L, Benson LM, Sankaran B, Kim C, Auton M. Mutational constraints on local unfolding inhibit the rheological adaptation of von willebrand factor. J Biol Chem. 2016;291(8):3848–3859. doi: 10.1074/jbc.m115.703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TY-C, Garner RM, Megason SG. Adhesion-based self-organization in tissue patterning. Annu Rev Cell Dev Biol. 2022;38(1):349–374. doi: 10.1146/annurev-cellbio-120420-100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tverdislov VA, Malyshko EV. Chiral dualism as an instrument of hierarchical structure formation in molecular biology. Symmetry. 2020;12(4):587. doi: 10.3390/sym12040587. [DOI] [Google Scholar]
- Tverdislov VA, Malyshko EV, Ilchenko SA. From autowave mechanisms of self-assembly to molecular machines. Bull Russ Acad Sci Phys. 2015;79(12):1516–1520. doi: 10.3103/s1062873815120230. [DOI] [Google Scholar]
- Vergauwe RMA, Uji-i H, De Ceunynck K, Vermant J, Vanhoorelbeke K, Hofkens J. Shear-stress-induced conformational changes of von Willebrand factor in a water-glycerol mixture observed with single molecule microscopy. J Phys Chem B. 2014;118(21):5660–5669. doi: 10.1021/jp5022664. [DOI] [PubMed] [Google Scholar]
- Waldron TT, Springer TA. Transmission of allostery through the lectin domain in selectin-mediated cell adhesion. Proc Natl Acad Sci. 2009;106(1):85–90. doi: 10.1073/pnas.0810620105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Stebe KJ, Fuente-Nunez C, Radhakrishnan R. Computational design of peptides for biomaterials applications. ACS Appl Bio Mater. 2023 doi: 10.1021/acsabm.2c01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman AM, Chen W, McEver RP, Zhu C. Triphasic force dependence of e-selectin/ligand dissociation governs cell rolling under flow. Biophys J. 2010;99(4):1166–1174. doi: 10.1016/j.bpj.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992;267(23):16637–1664. doi: 10.1016/s0021-9258(18)42050-9. [DOI] [PubMed] [Google Scholar]
- Wilkins PP, Moore KL, McEver RP, Cummings RD. Tyrosine sulfation of p-selectin glycoprotein ligand-1 is required for high affinity binding to pselectin. J Biol Chem. 1995;270(39):22677–22680. doi: 10.1074/jbc.270.39.22677. [DOI] [PubMed] [Google Scholar]
- Woodfin A, Voisin M-B, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514–2523. doi: 10.1161/atvbaha.107.151456. [DOI] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang J-H, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432(7013):59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu E-R, Bülow S, Chen P-C, Lenting PJ, Kolsek K, Aponte-Santamaria C, Simon B, Foot J, Obser T, Schneppenheim R, Gräter F, Denis CV, Wilmanns M, Hennig J. Structure and dynamics of the platelet integrin-binding c4 domain of von willebrand factor. Blood. 2019;133(4):366–376. doi: 10.1182/blood-2018-04-843615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T, Wu J, Wey CD, Klopocki AG, Zhu C, McEver RP. Catch bonds govern adhesion through l-selectin at threshold shear. J Cell Biol. 2004;166(6):913–923. doi: 10.1083/jcb.200403144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, Lopez JA, Cruz MA, Dong J-F, McIntire LV, McEver RP, Zhu C (2008) Platelet glycoprotein Ib forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest 118:3195–3207. 10.1172/JCI35754 [DOI] [PMC free article] [PubMed]
- Yang B, Liu Z, Liu H, Nash MA (2020) Next generation methods for single-molecule force spectroscopy on polyproteins and receptor-ligand complexes. Front Mol Biosci 7. 10.3389/fmolb.2020.00085 [DOI] [PMC free article] [PubMed]
- Yeo E, Sheppard J, Feuerstein I. Role of p-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model) Blood. 1994;83(9):2498–2507. doi: 10.1182/blood.v83.9.2498.2498. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Cheng X. Platelet mechanosensing axis revealed. Nat Mater. 2019;18(7):661–662. doi: 10.1038/s41563-019-0393-5. [DOI] [PubMed] [Google Scholar]
- Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein ib-IX complex. Blood. 2015;125(3):562–569. doi: 10.1182/blood-2014-07-589507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lin Z, Fang Y, Wu J. Prediction of catch-slip bond transition of kindlin2/3 integrin via steered molecular dynamics simulation. J Chem Inf Model. 2020;60(10):5132–5141. doi: 10.1021/acs.jcim.0c00837. [DOI] [PubMed] [Google Scholar]
- Zhao YC, Wang H, Wang Y, Lou J, Ju LA (2022) The n-terminal autoinhibitory module of the a1 domain in von willebrand factor stabilizes the mechanosensor catch bond. RSC Chem Biol 3(6):707–720. 10.1039/d2cb00010e [DOI] [PMC free article] [PubMed]
- Zhu J, Luo B-H, Xiao T, Zhang C, Nishida N, Springer TA (2008) Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell 32(6):849–861. 10.1016/j.molcel.2008.11.018 [DOI] [PMC free article] [PubMed]
- Zhu C, Chen W (2012) Catch bonds of integrin/ligand interactions. In: Singlemolecule Studies of Proteins, Springer, ??? pp 77–96. 10.1007/978-1-4614-4921-8_3
- Zhuravlev PI, Hinczewski M, Chakrabarti S, Marqusee S, Thirumalai D (2016) Force-dependent switch in protein unfolding pathways and transition-state movements. Proc Natl Acad Sci 113(6). 10.1073/pnas.1515730113 [DOI] [PMC free article] [PubMed]
- Zöllner O, Lenter MC, Blanks JE, Borges E, Steegmaier M, Zerwes H-G, Vestweber D, et al. L-selectin from human, but not from mouse neutrophils binds directly to e-selectin. J Cell Biol. 1997;136(3):707–716. doi: 10.1083/jcb.136.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All protein structures presented in the paper are publicly available from Protein Data Bank (https://www.rcsb.org/).
Not applicable.