Abstract
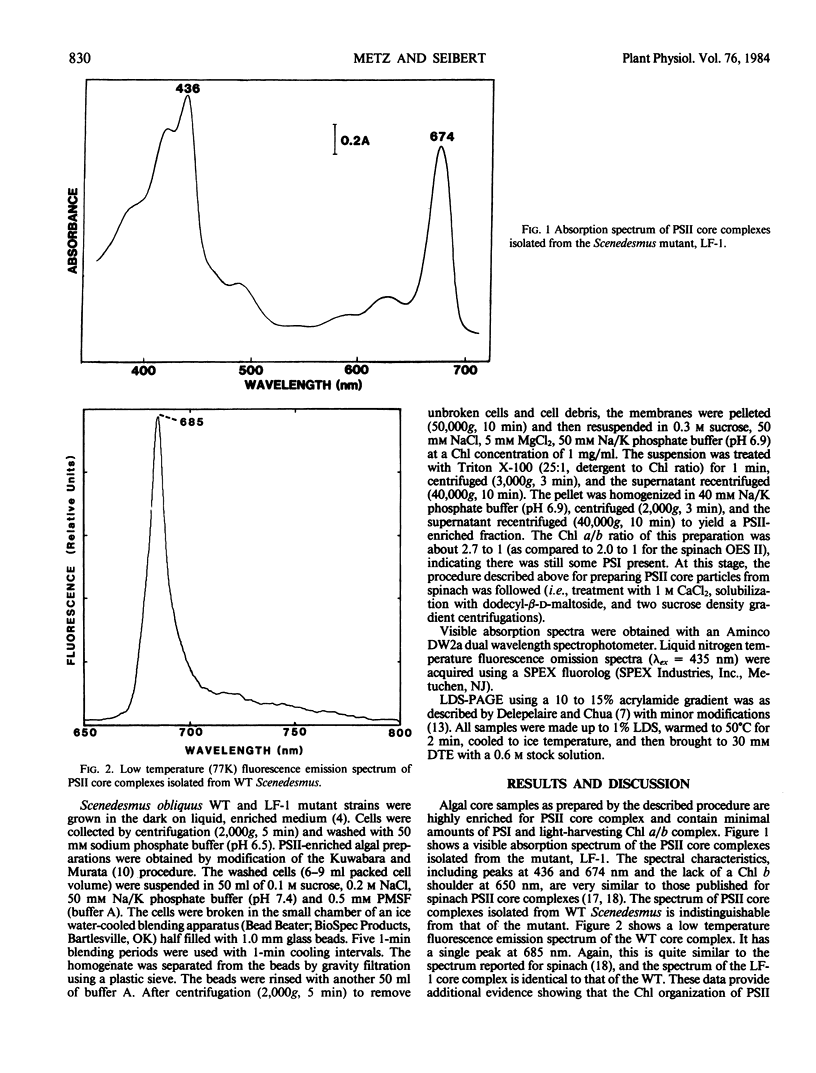
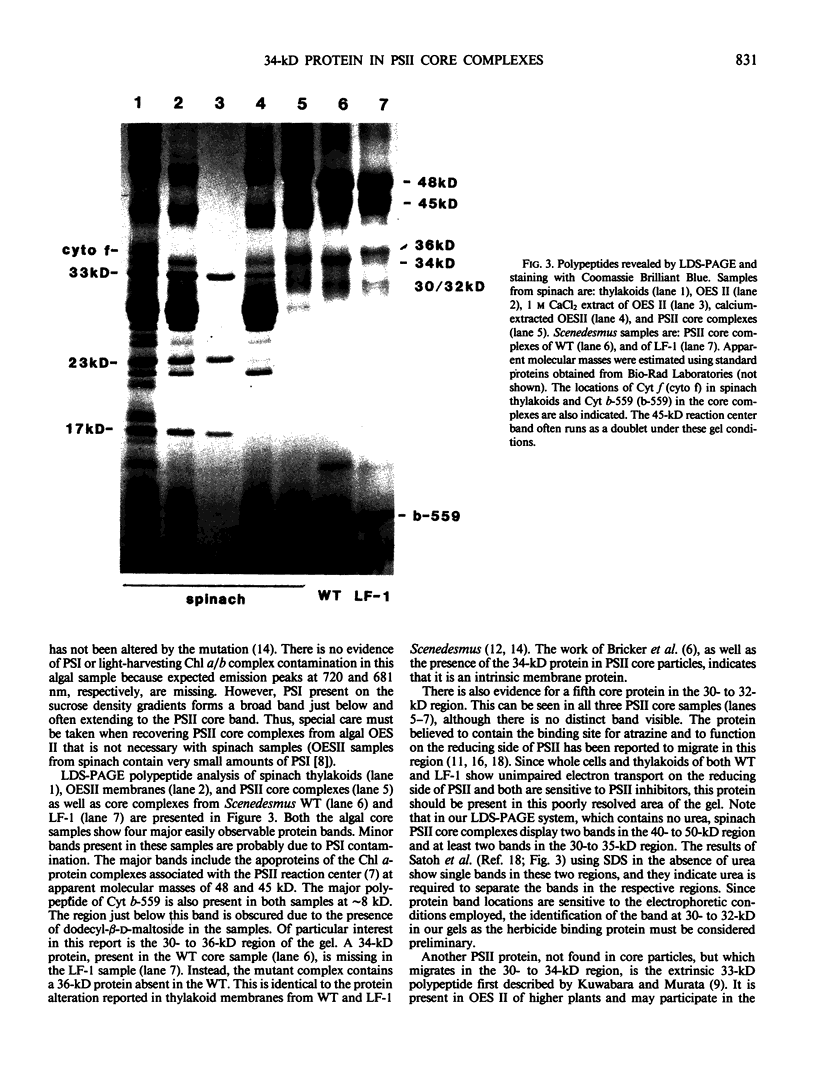
Photosystem II (PSII) reaction center core complexes have been isolated and characterized from wild type (WT) Scenedesmus obliquus and from its LF-1 mutant. LF-1 thylakoids are blocked on the oxidizing side of PSII and have a reduced Mn content. Visible absorption and low temperature fluorescence spectra of both core complexes are identical and resemble those reported for spinach (Satoh, Butler 1978 Plant Physiol 61: 373-379). Lithium dodecyl sulfate-polycrylamide gel electrophoresis reveals that a protein alteration, originally observed in thylakoid membranes (Metz, Wong, Bishop 1980 FEBS Lett 114: 61-66), is retained in the PSII core particles. That is, a 34-kilodalton (kD) polypeptide, present in the WT core complex, is missing in the mutant, and the core complex of the mutant contains a 36-kD protein not present in the WT. The 34-kD intrinsic protein is also observed in O2-evolving PSII preparations and PSII core complexes from spinach. It is distinct from the 33-kD extrinsic protein first reported by T. Kuwabara and N. Murata (1979 Biochim Biophys Acta 581: 228-236). We suggest that the 34-kD protein is a site of Mn binding in the PSII membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T., Murata N. Purification and characterization of 33 kilodalton protein of spinach chloroplasts. Biochim Biophys Acta. 1979 Dec 14;581(2):228–236. doi: 10.1016/0005-2795(79)90242-3. [DOI] [PubMed] [Google Scholar]
- Metz J., Bishop N. I. Identification of a chloroplast membrane polypeptide associated with the oxidizing side of photosystem II by the use of select low-fluorescent mutants of Scenedesmus. Biochem Biophys Res Commun. 1980 May 30;94(2):560–566. doi: 10.1016/0006-291x(80)91268-1. [DOI] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Butler W. L. Low temperature spectral properties of subchloroplast fractions purified from spinach. Plant Physiol. 1978 Mar;61(3):373–379. doi: 10.1104/pp.61.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd W. D., Kaplan S. Effect of heat and 2-mercaptoethanol on intracytoplasmic membrane polypeptides of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Aug;135(2):656–667. doi: 10.1128/jb.135.2.656-667.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Alt J., Herrmann R. G. Localization of the genes for the two chlorophyll a-conjugated polypeptides (mol. wt. 51 and 44 kd) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J. 1983;2(12):2229–2237. doi: 10.1002/j.1460-2075.1983.tb01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]