Abstract
Allergen immunotherapy (AIT) is a causative treatment for various allergic diseases such as allergic rhinitis, allergic asthma, and bee venom allergy that induces tolerance to offending allergens. The need for uniform practice guidelines in AIT is continuously growing because of the increasing discovery of potential candidates for AIT and evolving interest in new therapeutic approaches. This guideline is an updated version of the Korean Academy of Asthma Allergy and Clinical Immunology recommendations for AIT published in 2010. This updated guideline proposes an expert opinion by allergy, pediatrics, and otorhinolaryngology specialists with an extensive literature review. The guideline deals with basic knowledge and methodological aspects of AIT, including mechanisms, clinical efficacy, patient selection, allergens extract selection, schedule and doses, management of adverse reactions, efficacy measurements, and special consideration in pediatrics. The guidelines for sublingual immunotherapy will be covered in detail in a separate article.
Keywords: Allergens, immunotherapy, guideline
INTRODUCTION
Allergen immunotherapy (AIT) is a causative treatment of allergic diseases in which allergen extracts are regularly administered in a gradually escalated dose, leading to immune tolerance and consequent alleviation of allergic diseases.1,2,3 AIT has been used to treat allergic diseases for over 110 years and is the only treatment that can cure allergic diseases. It has been proven effective in asthma, allergic rhinitis, and bee venom allergy and has recently been expanded to atopic dermatitis and food allergy. In addition to traditional subcutaneous immunotherapy (SCIT), sublingual immunotherapy (SLIT) has been developed and widely used for the past 30 years.4
In 2010, the Korean Academy of Asthma Allergy and Clinical Immunology (KAAACI) Allergen Immunotherapy and Allergen working group published expert opinions on the principles and methods of AIT to help clinicians implement AIT.5 However, there has been an increasing need for updated AIT guidelines to reflect the changing clinical practices, especially in Korea.
This guideline updates the KAAACI recommendations for AIT published in 2010 and proposes an expert opinion by specialists in allergy, pediatrics, and otorhinolaryngology with an extensive literature review. The target audience of this guideline includes primary care physicians who manage SCIT or SLIT for patients with allergies. This guideline deals with basic knowledge and methodological aspects of AIT, including its mechanisms, clinical efficacy, patient selection, allergens extract selection in Korea, schedule and doses, management of adverse reactions, efficacy measurements, and special consideration in pediatrics. Each topic begins with a keynote summary with a level of evidence. The quality of evidence was rated according to the Grading of Recommendations Assessment, Development, and Evaluation approach.6
The guidelines outlined here do not cover SLIT, which will be reported in detail in a separate article. However, the practices employing other routes of allergen administration, including oral immunotherapy (OIT), intralymphatic immunotherapy (ILIT), and epicutaneous immunotherapy (EPIT), are beyond the scope of the present guideline.
MECHANISM
Summary statement
1. AIT induces immunological tolerance by upregulating CD4+ CD25+ regulatory T and B cells (High).
2. Successful AIT is associated with activating CD4+ T helper 1 (Th1) cells (High).
Given that the mechanism of AIT remains highly intricate and has not yet been fully elucidated, researchers are relentlessly endeavoring to explain the precise mechanisms and clinical effects. Immunotherapy can be categorized as SCIT, SLIT, OIT, ILIT, and EPIT, according to the route of allergen administration. Although SCIT has been studied extensively among these, the molecular mechanism of immunotherapy seems similar across different routes of administration.7
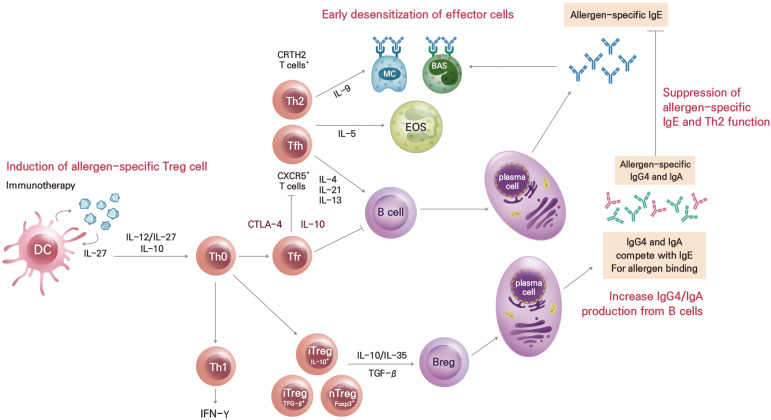
AIT reduces histamine secretion by mast cells and basophils, decreases the production of type 2 inflammatory mediators, such as interleukin (IL)-4, IL-5, IL-13, and IL-9, suppresses the proliferation of eosinophils, and decreases the number of eosinophils within inflamed tissues.8 AIT aims to rebalance the Th2 immune response towards a Th1 response, inducing immunological tolerance by upregulating CD4+CD25+ regulatory T and B cells and increasing allergen-specific immunoglobulin G4 (sIgG4) concentrations.9,10,11 Long-term AIT suppresses not only an early-phase response but also a late-phase response (Figure).12
Figure. Mechanisms of allergen immunotherapy.
BAS, basophil; Breg, regulatory B; DC, dendritic cell; EOS, eosinophil; Foxp3, forkhead box P3; IFN, interferon; Ig, immunoglobulin; IL, interleukin; iTreg, inducible regulatory T; MC, mast cell; nTreg, natural regulatory T; Tfh, follicular helper T; Tfr, follicular regulatory T; CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells; CXCR5, C-X-C motif chemokine receptor 5; CTLA4, cytotoxic T-lymphocyte-associated protein 4; TGF, transforming growth factor; Th, T helper.
Exposure to high doses of allergens activates regulatory dendritic cells, which induces the secretion of IL-12, IL-27, and IL-10 and downregulates CD86 expression.13 The released IL-12 and IL-27 stimulate the production of regulatory T cells, while IL-10 suppresses peripheral T cells, monocytes, and macrophages.14 IL-10 inhibits the production of IL-5 by Th2 cells, suppresses the number and function of eosinophils, reduces the number of mast cells, and prevents degranulation.15 Regulatory T cells secrete cytokines, such as IL-10, IL-35, and transforming growth factor (TGF)-β.14 IL-10 and TGF-β convert CD4+CD25− T cells to CD4+CD25+ T cells through the induction of forkhead box P3 (FOXP3).12 Furthermore, IL-10 and IL-35 induce regulatory B cells, which induce the production of allergen-specific IgG (sIgG), sIgG4 and allergen-specific IgA (sIgA) but inhibit that of specific allergen-specific IgE (sIgE).8 , 16 Because sIgG4 has the same epitope as sIgE antibodies, they competitively inhibit allergens from binding IgE antibodies, ultimately suppressing the activities of mast cells and basophils.12 Moreover, the elevation of sIgG4 levels is related to the dose of the allergen, sIgG4 concentration generally increases by 10–100 times through AIT.17 Furthermore, its activity as a blocking antibody to neutralize circulating sIgE is also important in developing tolerance.17 The production of sIgG4 blocking antibodies takes about 6–10 weeks with SCIT, and about 24 weeks with SLIT, with the IgG+ memory B cells retained until 1–3 years after treatment.18,19
AIT can induce type 2 innate lymphoid cells (ILC2s),20 which in turn produce IL-10, and the consequent elevation of IL-10+ ILC2s has been strongly associated with improvements in clinical symptoms.21 In addition, the CD25-expressing type 1 ILCs in the blood increases as a trained immune response during AIT. These pieces of evidence substantiate the importance of ILCs in AIT.21
CLINICAL EFFICACY
Summary statement
1. AIT is effective in treating bee venom allergy (High).
2. AIT is effective in treating allergic rhinitis (High).
3. AIT is effective in treating allergic asthma (High).
4. Further research is needed for the efficacy of AIT in oral allergy syndrome (OAS) (Low).
5. AIT against house dust mites (HDM) is effective for treating atopic dermatitis in patients sensitized to HDM (Moderate).
Bee venom allergy
SCIT using bee venom is effective in patients with a history of bee venom-induced anaphylaxis.22 Bee venom immunotherapy reduces the risk of a systemic reaction to bee venom by up to 98%. Therefore, SCIT is recommended for patients who develop a systemic reaction to bee venom and show increased sIgE levels.23 The first goal of bee venom SCIT is to prevent a life-threatening reaction. The second goal is to alleviate anxiety pertinent to insect stings to enhance the patient’s quality of life.24
Allergic rhinitis
Recent systematic reviews found that SCIT and SLIT are clinically effective in patients with allergic rhinitis by ameliorating their nasal and eye symptoms and reducing drug consumption.25,26 The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines recommend AIT for the treatment of moderate or severe intermittent or continuous allergic rhinitis that does not respond well to pharmacological treatment in both pediatric and adult patients.27 AIT has been confirmed to be effective in treating allergic rhinitis triggered by pollen, HDM, animal dander, and mold.28,29,30,31 AIT improves the quality of life of patients with allergic rhinitis, prevents its progression to asthma, and reduces sensitization to new allergens. Studies have shown that the clinical effects are retained even after the therapy is concluded with AIT for an adequate duration of at least 3 years.1,3
Allergic asthma
AIT improves asthma symptoms, drug consumption, and allergen-specific airway hypersensitivity in allergic asthma.32 There is a moderate or high level of evidence supporting the effectiveness of SCIT and SLIT in reducing asthma symptoms and drug requirements in allergic asthma.33,34 These therapies may lower potential adverse effects of corticosteroids by reducing the required dose of inhaled corticosteroids for asthma control.35,36 Even in pediatric patients with asthma, both SCIT and SLIT alleviate asthma symptoms and reduce drug requirements.37,38
Oral allergy syndrome
SCIT and SLIT for oak pollen extracts did not significantly improve OAS symptoms in patients with apple allergy.39,40,41 In contrast, a recent randomized controlled trial (RCT) investigating the efficacy of SLIT for birch pollen in patients with OAS reported a significant increase in Mal d1- specific IgG4 and improvement in OAS symptoms, suggesting AIT for birch pollen is beneficial in improving symptoms of OAS in some patients.42,43,44,45,46 However, further studies are needed for an in-depth understanding of the efficacy of AIT for OAS.
Atopic dermatitis
In studies on patients with atopic dermatitis sensitized to HDM, SCIT significantly decreased the SCORing Atopic Dermatitis (SCORAD) index and drug requirements.47 , 48 A meta-analysis on 1,957 adult and pediatric patients reported that SCIT or SLIT for HDM significantly improved the SCORAD score (odds ratio [OR], 1.53; 95% confidence interval [CI], 1.31–1.78) and quality of life (OR, 1.44; 95% CI, 1.03–2.01).49 Furthermore, a meta-analysis of 8 RCTs that compared AIT with a placebo showed that AIT effectively alleviated atopic dermatitis in patients sensitized to inhalant allergens (OR, 5.35; 95% CI, 1.61–17.77).50 However, significant heterogeneity in the types of antigens, dose, treatment duration, patient’s age, and disease severity among the included studies calls for caution when interpreting the therapeutic effects of AIT on atopic dermatitis.
Food allergy
Until now, no evidence shows the usefulness of SCIT and SLIT for food allergies other than OAS. In contrast, a meta-analysis of 22 clinical trials on OIT reported that AIT for milk, eggs, and peanuts produced substantial therapeutic effects (relative risk [RR], 0.14; 95% CI, 0.08–0.24).51 However, the meta-analysis regarding the effect of OIT after the discontinuation of AIT involving eggs and milk was performed based on only 4 studies. Moreover, this study could not find any long-term benefits of OIT (RR, 0.29; 95% CI, 0.08–1.13). In conclusion, OIT is recommended for children aged 4–5 years with continuous milk, egg, and peanut allergies, as it increases the threshold that triggers a clinical reaction.52
PATIENT SELECTION
Summary statement
1. AIT is indicated for patients with allergic rhinitis (with or without allergic asthma or conjunctivitis) who are sensitized to clinically relevant allergens and have moderate to severe symptoms despite appropriate pharmacological treatment (High).
2. Bee venom immunotherapy is indicated for patients with systemic reactions and specific IgE to bee venom (High).
3. Although the upper or lower age limit for AIT is not specific, immunotherapy is indicated in cooperative and compliant patients. In elderly patients with increased risks of adverse reactions due to underlying diseases, the cost-effectiveness of AIT should be considered (Very low).
General considerations
Table 1 summarizes the indications and contraindications for AIT. AIT should be performed only in cases strongly suggesting allergic rhinitis or rhinoconjunctivitis.53,54,55 Furthermore, it may be performed on patients with allergic asthma who frequently develop symptoms when exposed to the causative allergen despite continued pharmacological treatment.56 Identifying the causative allergen that triggers symptoms is essential for prescribing AIT. Therefore, evidence for sIgE sensitization (positive for skin prick test or serum sIgE) and symptoms upon exposure to the allergen is necessary.54 Although component-resolved diagnostics (CRD) may be conducive to identifying the causative allergen, evidence supporting its use is inadequate. Moreover, the clinical association with allergen sensitization in the target organ may be examined through nasal or conjunctival challenge tests. Still, the universal application of these tests in clinical practice is practically challenging, along with a lack of clear evidence for its utility and diagnostic criteria.3
Table 1. Indications and contraindications of allergen immunotherapy.
| Indications and contraindications | Details | ||
|---|---|---|---|
| Indications | |||
| 1. Allergic rhinitis | ✓ Patients with evidence of sIgE based on an allergen skin test or blood sIgE test and rhinitis symptoms induced by allergen exposure. | ||
| ✓ And satisfies one or more of the criteria below | |||
| (1) Limited effects on symptom relief by allergen avoidance or pharmacological therapy | |||
| (2) Intolerable to pharmacological therapy due to the adverse reactions | |||
| (3) Patients who wish to avoid long-term drug use | |||
| (4) Patients with allergic rhinitis to prevent the onset of asthma and reduce sensitization to new allergens | |||
| 2. Asthma | ✓ Patients with evidence of sIgE based on an allergen skin test or blood sIgE test and asthma symptoms induced by allergen exposure. | ||
| ✓ And satisfies one or more of the criteria below | |||
| (1) Limited effects on symptom relief by allergen avoidance or pharmacological therapy | |||
| (2) Intolerable to pharmacological therapy due to the adverse reactions | |||
| (3) Patients who wish to avoid long-term drug use | |||
| 3. Bee venom allergy | ✓ Adolescent and adult patients who had anaphylactic reactions (particularly hypotension or hypoxemia/wheezing) after insects (honeybee, wasp) sting and confirmed sensitization to specific venom by an allergen skin test or blood sIgE test. | ||
| ✓ Adolescent and adult patients (aged ≥ 16) who had systemic skin reactions other than anaphylaxis after insects (honeybee, wasp) sting and confirmed sensitization to specific venom by an allergen skin test or blood sIgE test (Mostly immunotherapy is not needed in adolescents or children aged < 16 years when patients had localized skin reaction) | |||
| Contraindications | |||
| 1. Absolute contraindications | ✓ Uncontrolled or severe asthma patients | ||
| ✓ Patients diagnosed with malignancies, refractory active autoimmune diseases | |||
| ✓ Immunotherapy cannot be initiated in pregnant women* | |||
| ✓ Eosinophilic esophagitis† | |||
| 2. Relative contraindications | ✓ Partly controlled asthma | ||
| ✓ Patients taking beta-blockers or ACEI | |||
| ✓ Severe cardiovascular disease (ex. coronary artery disease, severe arrhythmia, uncontrolled hypertension) | |||
| ✓ Completely resolved systemic autoimmune disease or localized immune disease | |||
| ✓ Severe psychological impairment or disease | |||
| ✓ Primary or secondary immunodeficiency | |||
| ✓ History of systemic reaction to allergen immunotherapy | |||
| ✓ Children aged under 5 | |||
| ✓ Low compliance | |||
sIgE, allergen-specific immunoglobulin E; ACEI, angiotensin-converting enzyme inhibitors.
*Immunotherapy can be continued if treatment is initiated before pregnancy; †Limited explanation for SLIT.
Allergic rhinitis
AIT is indicated for patients with moderate to severe symptomatic allergic rhinitis whose symptoms affect daily life or sleep despite pharmacological treatment. According to the ARIA classification, these patients are regarded to have moderate-to-severe allergic rhinitis, whose symptoms are either intermittent or persistent.27 Furthermore, AIT in pediatric or young patients with allergic rhinitis may be considered under the following conditions: the patient wants a cure for allergic rhinitis; the rhinitis symptoms are not controlled through pharmacological treatments; long-term treatment is difficult due to adverse drug reactions; or the patient wishes to avoid long-term drug use. In addition, AIT can be practiced to prevent the onset of asthma and reduce sensitization to new allergens.57 Products whose clinical efficacy on allergic rhinitis has been proven through well-designed clinical trials should be used for AIT.58,59 For patients who develop symptoms in response to indoor allergens, such as HDM and pet dander, avoidance strategies such as air purifiers with high-efficiency particulate air (HEPA) filters, impermeable bedding, and cleaning may be considered before initiating AIT. However, although avoidance strategies may reduce indoor allergen concentrations, they have limited effects on symptom relief.60,61,62,63,64,65,66,67
Bee venom immunotherapy
Bee venom immunotherapy is applied to individuals of all ages who have had an anaphylactic reaction after an insect sting and have sIgE for bee venom. Although bee venom skin test is known to be useful, with approximately 65% positivity in patients who have had an anaphylactic reaction to a bee sting, currently, it cannot be performed in Korea due to the unavailability of reagents. Instead, the causative bee can be identified through ImmunoCAP™ assays (Thermo Fisher Scientific/Phadia, Uppsala, Sweden). In the ImmunoCAP™ assays, it is common to observe positive results simultaneously for honeybee (Apidae) and the wasp (Vespidae) in cases of bee venom anaphylaxis, owing to cross-reactivity between the 2 types of venom. Then CRD testing may be useful for identifying the causative bee. In particular, Api m 1 is highly specific for honeybee venom, while Ves v 5 is highly specific for wasp venom without cross-reactivities.68 Although bee venom immunotherapy is not generally recommended for individuals with only localized skin reactions following bee stings, it has been reported to reduce the size and duration of local reactions. Therefore, bee venom immunotherapy may be considered in adolescents (aged 16 years and over) and adults who cannot avoid exposure and have a large local reaction (> 10 cm). AIT is not recommended for children (under age 16) with only local skin reactions. Bee venom immunotherapy aims to improve the quality of life for patients by reducing the risk of serious anaphylactic reactions and allowing them to engage in outdoor activities.24 Nevertheless, in Korea, bee venom immunotherapy products have not been approved by the Ministry of Food and Drug Safety (MFDS). The lack of a stable product supply is a pressing challenge for their implementation in practice as of the time of developing this guideline. We hope these issues will be resolved soon and that bee venom immunotherapy will become available to bee venom anaphylaxis patients in Korea.
Contraindications and special considerations
Physicians must also consider the absolute or relative contraindications, where the risk of AIT may outweigh the anticipated benefits (Table 1).57 Severe uncontrolled asthma, despite pharmacological treatments, is considered an absolute contraindication to AIT, as these patients are at a heightened risk for mortality in the event of anaphylaxis caused by AIT. No specific age limits have been recommended for initiating AIT; however, special caution should be exercised when considering children under 6 or older adults. In particular, age under 5 is a relative contraindication for SCIT, as they may have difficulty cooperating during injections. SCIT may be performed in children aged ≥ 5 years who are not resistant to injections.56 Nevertheless, some studies have investigated the efficacy and safety of SLIT in patients with allergic rhinitis aged ≥ 3 years, wherein most of the large-scale studies have been conducted in patients aged ≥ 5 years.25,69,70 Older adult patients often have comorbidities that may increase the risk of adverse events associated with AIT.
Special consideration is required for pregnant women and patients with malignancies, immunodeficiencies, or autoimmune diseases. While AIT cannot be initiated in pregnant women, it can be continued if treatment is initiated before pregnancy.71,72 Although some researchers have concerns regarding the potential negative impact of AIT on the immune system in patients with autoimmune diseases, immunodeficiency syndromes, or malignancies, no compelling evidence suggests the harmful effects of AIT.71 Nevertheless, specific contraindications for each product should be reviewed by examining the summary of the manufacturer’s instructions.
ALLERGEN SELECTION
Summary statement
1. Treatment effects of AIT are proven in target allergens, including pollens, HDM, Hymenoptera venom, furry pet animals, cockroaches, and mold (e.g., Alternaria, Cladosporium) (High).
2. Major allergens in Korea consist of HDM, pollens (birch, oak, mugwort, ragweed, Japanese hop, grass), and furry pet animals (Very low).
3. The selection of target allergens for AIT should be based on allergen exposure and subsequent symptoms and the results of skin prick test, sIgE levels or both (High).
4. The target allergen for AIT should be confined to a single or minimal number of allergens with clinical relevance (Very low).
5. Among pollens with cross-reactivity to each other, one pollen in the same genus or subfamily can be chosen as a target allergen for immunotherapy (Moderate).
6. The concentration of each constituent allergen must be equal to or larger than that proven to be therapeutically effective. If the target maintenance dose cannot be tolerated, a lower dose, referred to as the maintenance dose, may provide clinical benefits (High).
Allergen standardization
The greatest challenge to AIT is the high variability in the methods used to specify allergen content and potency across manufacturers. In terms of HDM allergen products for SCIT approved in Korea (50:50 mixture of Dermatophagoides farinae and Dermatophagoides pteronyssinus), Hollister-Stier (Spokane, WA, USA) specifies the potency in allergy units (AU)/mL as designated by the US Food and Drug Administration (FDA) based on standardized allergens. However, Allergopharma (Reinbek, Germany) uses its biological potency units of therapeutic units (TU)/mL. Furthermore, Allergy Therapeutics (Worthing, West Sussex, UK) does not specify the accurate allergen concentration or antigenic potency. Allergopharma and Allergy Therapeutics use allergoids, which are modified proteins obtained by exposing allergens to formaldehyde or glutaraldehyde to reduce their antigenicity that causes acute hypersensitivity reactions while maintaining immunogenicity, thereby inducing immune tolerance. However, such allergoid products have been reported to exhibit low immunogenicity and antigenicity in an in vitro study,73 and the protein modification makes it difficult to compare their antigenicity with other products. Moreover, even if allergens with the same potency are included, the therapeutic effect may be altered by the type of immunomodulatory adjuvant used (e.g., aluminum hydroxide, tyrosine). In a Korean study comparing the allergenic potency of the above 3 HDM immunotherapy products, the allergen concentration and antigenic potency were the highest in order of the product from Hollister-Stier, Allergopharma, and Allergy Therapeutics.74 According to the principle that the clinical effect of AIT is directly proportional to the dose of allergen administered, it can be assumed that a product with higher allergenic potency would produce better clinical effects. However, since different manufacturers use different raw materials for preparing allergen extracts, such as using only HDM bodies or extracting both the bodies and excreta of HDM, it is difficult to conclude the superiority among reagents.75 As an alternative, the dose of major component allergens in the specific allergen products may help predict the therapeutic effects of AIT if they can be identified and measured (Table 2).30,39,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93 However, some AIT products available in Korea do not disclose the content of allergens or component allergens or include allergens for which major component allergens have not been established. Therefore, verifying whether high-quality clinical trials have been conducted on the specific allergen and major component antigens is crucial. Based on this information, individual physicians must make their judgment when selecting the allergen product for each patient.
Table 2. Effective maintenance dose of major component allergen for allergen immunotherapy.
| Allergen | Author | Year | Major allergen | Effective dose | Suboptimal dose |
|---|---|---|---|---|---|
| Dermatophagoides | Ewan et al.76 | 1988 | Der p 1 | 11.9 mcg | |
| Haugaard et al.77 | 1993 | 7.0 mcg | 0.7 mcg | ||
| Olsen et al.78 | 1997 | 7.0 mcg | |||
| Short ragweed | Van Metre et al.79 | 1980 | Amb a 1 | 11 mcg | |
| Creticos et al.80 | 1985 | 6 mcg | |||
| Creticos et al.81 | 1989 | 12.4 mcg | 0.6 mcg | ||
| Furin et al.82 | 1991 | 24 mcg | 2 mcg | ||
| Grass | Varney et al.83 | 1991 | Phl p 5 | 18.6 mcg | |
| Dolz et al.84 | 1996 | Dac q 5 + Lol p 5 + Phl p 5 | 15 mcg | ||
| Walker et al.85 | 2001 | Phl p 5 | 20 mcg | ||
| Frew et al.75 | 2006 | Phl p 5 | 20 mcg | 2 mcg | |
| Birch | Bodtger et al.86 | 2002 | Bet v 1 | 12 mcg | |
| Khinchi et al.39 | 2004 | 3.28 mcg | |||
| Cat dander | Hedlin et al.87 | 1991 | Fel d 1 | 17.3 mcg | |
| Alvarez-Cuesta et al.30 | 1994 | 11.3 mcg | |||
| Varney et al.88 | 1997 | 15 mcg | |||
| Ewbank et al.89 | 2003 | 15 mcg | 3 mcg | ||
| Nanda et al.90 | 2004 | 15 mcg | 3 mcg | ||
| Dog dander | Lent et al.91 | 2006 | Can f 1 | 15 mcg | 3 mcg |
| Alternaria | Horst et al.92 | 1990 | Alt a 1 | 1.6 mcg | |
| Kuna et al.93 | 2011 | 8.0 mcg |
Allergen selection
Allergens, whose efficacy in AIT has been confirmed via double-blind placebo-controlled trials, include pollen (birch, ragweed, grass), HDM, bee venom, pet dander, and mold (Alternaria, Cladosporium). These trials were mostly conducted using a single allergen, except for grass pollen mixtures.2,3,23,94 Selecting the appropriate allergen is crucial to achieving the treatment effects of AIT. To this end, the causative allergen may be confirmed through a skin prick test or serum sIgE measurements. Serum sIgE testing consists of singleplex (ImmunoCAP™, Immulite®) and multiplex (e.g., MAST-CLA [Optigen®], AdvanSure™) testing, which provides quantitative IgE concentrations for a single antigen, and semi-quantitative IgE concentrations for hundreds of antigens, respectively. In addition, CRD can be performed to differentiate between polysensitization, cross-reactivity, and polyallergy. For example, if a positive reaction is seen in skin prick tests for all tree, grass, and weed pollen, this may be due to cross-reactivity caused by pan allergens, indicating the possibility of polysensitization. In such cases, polyallergy may be diagnosed if the sample tests positive for major component allergens such as Bet v 1 of birch pollen, Phl p 1 of timothy grass, Art v 1 of mugwort, and Amb a 1 of ragweed.95 However, there may be no symptoms even if the results of skin prick tests or serum sIgE measurements are positive. Hence, the following 3 conditions must be met to identify the causative allergen for AIT:
① A positive result in an allergy skin prick test or serum sIgE measurement should be confirmed.
② The patient should have frequent exposure to the sensitized allergen in their daily living.
③ Allergic symptoms should be induced upon exposure to the sensitized allergen.
In recent 2 decades, studies have shown that 10%–90% of patients with allergies are sensitized to 2 or more allergens, and 50%–80% of patients requiring AIT have been identified as having polysensitization.96,97,98,99,100 Studies conducted in Korea show that polysensitization increases with age, with an annual increase in the proportion of patients with polysensitization compared to those with monosensitization.101,102 Furthermore, a survey of allergists in Korea revealed that 87% of respondents performed AIT using 2 or more allergens.103 However, studies conducted in other countries have reported that 50% of patients with polysensitization could be asymptomatic, indicating the need to evaluate the correlation between patient symptoms and causative allergen rather than unquestionably accepting the results of polysensitization tests. The recommendations of European and American guidelines for selecting allergens for AIT are contrasting. The European guidelines recommend selecting a single or minimal number of allergens, while the American guidelines suggest including most allergens that test positive. Omitting allergens with clinical relevance in AIT can reduce the therapeutic efficacy. Conversely, including all allergens for which sIgE is detected without confirming clinical relevance can dilute the concentration of essential allergens and thus diminish the therapeutic efficacy. Although there is a debate on which approach is more effective, a single or a minimal number of allergens is generally recommended when selecting allergens for AIT.104,105
Important allergens in Korea
Various types of inhalant allergens have been reported as important triggers in Korea, including HDM, weed pollen (mugwort, ragweed, and Japanese hops), tree pollen (e.g., birch, oak), grass pollen, pet dander, and mold,106 and AIT may be performed on these allergens.107 HDM are the most commonly used allergen in AIT and have the most extensive documentation of clinical outcomes in Korea.103 However, as the number of patients sensitized to pollen has increased amid the increased incidence of allergic diseases in Korea due to climate and environmental changes, pollen is an important allergen in Korea, although AIT for some pollens, such as Japanese hop, is currently unavailable. In the country, tree pollen (e.g., birch, oak) is mainly observed from March to May, grass pollen from May to September, and weed pollen (mugwort, ragweed, and Japanese hops) from August to October.101 The pollen forecast website from the Korean Pollen Allergy Research Association (http://www.pollen.or.kr) provides information on the pollen aerobiology of Korea. When selecting pollen allergens for AIT, it is important to consider symptom variations according to the season and cross-reactivities across allergens. Recently, pet allergy has emerged as a significant issue in Korea, with a growing number of households with pets and reports demonstrating allergic symptoms in 20%–30% of adults exposed to pets. Skin prick tests and serum sIgE measurements have been reported to best predict allergic symptoms upon pet exposure.108,109 Molds can be sources of indoor and outdoor inhalant allergens, and 5%–20% of patients with allergies are reported to be sensitized to mold. However, mold allergens can easily be overlooked, as most patients test positive for other common indoor and outdoor allergens along with mold.110 Therefore, dog and cat dander as well as outdoor mold (Alternaria, Cladosporium) may be included in AIT in addition to HDM and pollen allergens. However, studies on the clinical efficacy of AIT for mold allergy are still rare in Korea compared to other countries. In addition, with the global expansion of activity space among the Korean people, there are cases in which individuals are sensitized to inhalant allergens that are not present in Korea but are important in other countries. AIT for these patients must be planned in consideration of their conditions.
In Korea, products from Allergy Therapeutics, Allergopharma, and Hollister-Stier have been approved for SCIT; however, the types of available allergens for each product vary. For SLIT, products from Lofarma (Milano, Italy), Stallergenes Greer (Antony, France), and ALK-Abelló (Hørsholm, Denmark) have been introduced, but all these products are only available for immunotherapy for HDM (Table 3).
Table 3. Available allergen extracts for allergen immunotherapy in Korea.
| Method | Manufacturer | Product name | Allergens | |
|---|---|---|---|---|
| SCIT | Allergy Therapeutics | Tyrosine S | HDMs | Dermatophagoides farinae |
| Dermatophagoides pteronyssinus | ||||
| Tree pollen | Alder pollen | |||
| Ash pollen | ||||
| Beech pollen | ||||
| Birch pollen | ||||
| Hazel pollen | ||||
| Oak pollen | ||||
| Weed pollen | Mugwort pollen | |||
| Ragweed pollen | ||||
| Grass pollen | Grass mixed* | |||
| Mold | Alternaria | |||
| Cladosporium | ||||
| Animal epithelia | Cat hair | |||
| Dog hair | ||||
| Horse hair | ||||
| Insect | Cockroach | |||
| Allergopharma | NOVO-HELISEN Depot | HDMs | Dermatophagoides farinae 50% + Dermatophagoides pteronyssinus 50% | |
| Animal epithelia | Cat fur | |||
| Dog hair | ||||
| Mold | Alternaria | |||
| ALLERGOVIT | Tree pollen | Birch pollen | ||
| Birch pollen 35% + Alder pollen 30% + Hazel pollen 35% | ||||
| Grass, Weed pollen | Grass mixed† 60%+Mugwort pollen 20%+Rye pollen 20% | |||
| Grass, Tree pollen | Grass mixed 60%+Birch pollen 20%+Rye pollen 20% | |||
| Grass pollen | Grass mixed 60%+Rye pollen 40% | |||
| Hollister-Stier | HDMs | Dermatophagoides farinae | ||
| Dermatophagoides pteronyssinus | ||||
| Tree pollen | Alder pollen | |||
| Beech pollen | ||||
| Birch pollen | ||||
| Oak pollen | ||||
| Tree mixed‡ | ||||
| Grass pollen | Grass mixed§ | |||
| Weed pollen | Ragweed pollen | |||
| Mugwort pollen | ||||
| Mold | Alternaria | |||
| Animal epithelia | Cat fur | |||
| Dog hair, Dog dander | ||||
| Insect | Cockroach | |||
| SLIT | Lofarma | Lais | HDMs | Dermatophagoides farinae 50%+Dermatophagoides pteronyssinus 50% |
| Stallergenes Greer | Staloral | |||
| Actair | ||||
| ALK-Abelló | Acarizax | |||
| Zhejiang Wolwo Bio-Pharma | Chanllergen | Dermatophagoides farina 100% | ||
SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy.
*Grass mixed included pollens of Bent, Brome, Cocksfoot, Dogstail, Oat grass, Fescue meadow, Foxtail meadow, Meadow grass, Rye grass, Timothy grass, Vernal, and Yorkshire fog; †Grass mixed included pollens of Kentucky bluegrass, Meadow fescue, Orchard grass, Rye grass, Timothy grass, and Velvet grass; ‡Tree mixed included pollens of White Ash, River/Red Birch, Black Walnut, Common Cottonwood, American Elm, Shagbark Hickory, Hard Maple, Red Oak, American Sycamore, and Black Willow; §Grass mixed included pollens of Kentucky Bluegrass, Orchard grass, Redtop, and Timothy grass.
Allergen extracts preparation
Prescription and preparation of AIT
After selecting the allergen for immunotherapy, a detailed prescription is necessary. The prescription must include patient information, the physician who is prescribing the extract, and information about the allergen contained in the extract.
Initial dose
The initial AIT dose is escalated incrementally according to the manufacturer’s instructions. For SCIT, some manufacturers provide more diluted initial doses for patients at higher risk of hypersensitivity reactions and recommend determining the initial dose through skin tests.
Maintenance dose
During AIT, extracts are prepared in wihch the concentration of each constituent allergen must be equal to or larger than that proven to be therapeutically effective. The appropriately planned dose is referred to as the target maintenance dose. If the dose cannot be tolerated, a lower dose may still provide clinical benefits, and this dose is referred to as the maintenance dose. In numerous studies, administering allergen extracts containing 1.6–24 μg of major allergens as maintenance doses has been proven to be effective.75,77,81,86,89,91,92,111,112
Allergen mixing
In some cases, various allergen extracts are mixed for AIT. When multiple antigens are mixed, the effective concentration of each allergen can be altered due to the dilution effect. The number of allergens that can be mixed may be limited depending on the product. Therefore, the prescribing physician should consider the following principles to include the minimum possible allergens.
① Mixing of cross-reacting extracts: In Korea, it is difficult to accurately distinguish cross-reactivity, polysensitization, and polyallergy due to the limited CRD. Considering these practical limitations, cross-reactivity between clinically important allergens should first be evaluated. In the case of tree pollen, it is common for many botanically related pollens to cross-react, so it is sufficient to choose one pollen in the genus or subfamily of pollens that cross-react from the phylogenetic classification (Table 4).113 For instance, as there is strong cross-antigenicity between birch and alder pollens, birch pollen is generally chosen. Among grass pollens, however, Bermuda grass does not have cross-antigenicity with other grass pollens.114 Among weed pollens, there is no cross-antigenicity between mugwort and ragweed pollens.113 On the contrary, there is strong cross-antigenicity between mugwort and plants in the Asteraceae, so it is sufficient to include only mugwort pollen.113 Japanese hop pollen has no cross-antigenicity with mugwort and ragweed pollen; however, it is unavailable in Korea. Among HDM allergens, the major allergens of D. pteronyssinus and D. farinae show strong cross-antigenicity. Therefore, D. pteronyssinus and D. farinae are generally included in the mixture for AIT in a 1:1 ratio, and the appropriate dose is calculated by summing the doses for the 2 allergens.
② Mixing with proteolytic enzymes: Mold and cockroach extracts contain proteolytic enzymes that can damage the antigenicity when mixed with other allergens.111,115,116 Therefore, cockroach and mold extracts should be prepared in separate vials from other allergens.
Table 4. Phylogenetic classification of major pollens in Korea.
| Allergen | Family | Genus | Common name |
|---|---|---|---|
| Tree pollen | Betulaceae | Alnus | Alder |
| Betula | Birch | ||
| Corylus | Hazel | ||
| Fagaceae | Quercus | Oak | |
| Fagus | Beech | ||
| Taxodiaceae | Cryptomeria | Japanese cedar | |
| Weed pollen | Asteraceae/Compositae | Ambrosia | Ragweed |
| Artemisia | Mugwort | ||
| Canabaceae | Humulus | Japanese hop | |
| Grass pollen | Pooideae | Lolium | Rye grass |
| Dactylis | Orchard grass | ||
| Phleum | Timothy grass | ||
| Poa | Kentucky blue grass | ||
| Chloridoideae | Cynodon | Bermuda grass |
Allergen extract handling
Storage
Allergen extracts should be stored at 4°C to reduce the rate of potency loss.117 The higher the dilution (1:10 vol/vol or greater), the more fragile the allergens are to temperature; they lose their potency more quickly than concentrated extracts, resulting in a shorter shelf life.117,118 The expiration date of AIT extracts is determined by considering various factors such as storage temperature, presence of stabilizers and preservatives, allergen concentrations, presence of proteolytic enzymes, and the volume of the storage vial. The Hollister–Stier allergen solution, one of the products for immunotherapy, contains 0.03% human serum albumin to preserve allergenic potency and 0.4% phenol to inhibit bacterial growth. This product is currently available in Korea and is recommended to be mixed with other allergens by healthcare professionals. The raw allergen material is supplied in 50% glycerin, which is diluted during the allergen preparation.
Dilution of maintenance concentrate
The maintenance concentrate is sequentially diluted to prepare the build-up regimen for AIT.
Labeling of dilution concentration
Unified and standardized labeling of diluted extract from maintenance concentrate can reduce the risk of erroneous administration of inadequate allergen concentrates.
Personalization of AIT glass vials
Medication errors can occur during AIT, such as injecting the wrong allergen belonging to another patient or with inappropriate concentration. Therefore, the patient’s name, in addition to the registration number, date of birth, or both, should be specified on individual maintenance concentrate and diluted extract vials to reduce the risk of medication errors. Mixing allergens within a syringe is not recommended because of the potential for cross-contamination of extracts.
SCHEDULES AND DOSES
Summary statement
1. If AIT is maintained at 4–8 weeks intervals for more than 3–5 years, improvement in symptoms and disease progression will be kept for a long time after the scheduled immunotherapy (High).
2. Rush immunotherapy can reach maintenance doses more quickly than conventional immunotherapy and does not increase the risk of systemic reactions compared to conventional immunotherapy, given adequate premedication (High).
3. Premedication with H1-antihistamines may reduce the frequency of systemic adverse reactions during the build-up phase of AIT (High).
4. In the event of a serious systemic adverse reaction, the schedule and doses should be appropriately adjusted (Very low).
5. Patients should be followed up at least once every 3 months while receiving AIT (Very low).
General considerations
1) AIT should be performed by an allergist or experienced physician trained for AIT procedures in an institution or clinic with proper clinical personnel, materials, emergency healthcare settings, and enough facilities to manage adverse reactions to AIT.
2) Prior to beginning AIT, a patient must be informed about the procedure, duration, anticipated effects, and adverse effects of AIT, and such information should be specified in an informed consent form. This information should also be documented in the patient’s record.
3) Before administering the AIT procedure, it is necessary to correctly assess the symptoms (e.g., allergy symptoms or infection signs such as fever), presence of adverse reactions to the previous AIT, and time elapsed since the last AIT.119
4) Generally, a 26–27-gauge, 1-mL syringe with a 0.5-inch-long needle is used for subcutaneous injection. An injection should be administered on the external side of a central third of the upper arm after cleaning the injection site with an alcohol swab, and the skin should be lifted during the administration to facilitate subcutaneous injection. Intramuscular and intradermal injections are not recommended, and intravenous injection is prohibited.
5) Healthcare professionals should monitor the patient for adverse reactions for at least 30 minutes after the injection. The patient is returned home if no adverse reactions occur.
Build-up phase
Starting doses
Starting doses of allergen AIT vary across products, but generally the recommended starting dose is 1:1,000–1:10,000 dilution of the target maintenance dose. Although the maintenance dose could be reached more quickly with a higher starting dose, the risk for a systemic adverse reaction is also elevated.112 Therefore, the starting dose is determined considering the patient’s medical history and the existence of symptoms. If the patient is sensitized to seasonal allergens, such as pollens, initiating a new AIT regimen during a season where the symptoms are exacerbated is generally not recommended. AIT should be started for perennial allergens, such as HDM, while symptoms are not severe.
Injection intervals during build-up phases
AIT build-up methods can be categorized as conventional, cluster, rush, and ultra-rush (Supplementary Table S1). Conventional AIT involves increasing the dose at 1–2-week intervals and usually reaches the maintenance dose after 2–3 months. Cluster AIT involves administering 2–3 doses per day, which can shorten the time required to reach the maintenance dose. Rush and ultra-rush AIT involve continuous incremental AIT doses to reach the maintenance dose in 1–3 days. Hence, rapid symptom relief may be expected, and the risk for a systemic adverse reaction is not elevated with appropriate premedication.120,121,122,123,124
Premedication
Antihistamines can be administered 2 hours before the injection to reduce the risk of local and systemic adverse reactions.125 Previous studies on premedication for AIT showed excellent preventive effects of adverse reactions with premedication using single H1-antihistamine or combinations of H1-antihistamines, H2-blockers, and oral glucocorticosteroids.126 The effects of these 2 methods in reducing adverse reactions did not differ.127 In cases where adverse reactions were observed despite premedication with H1- antihistamines, combined oral glucocorticosteroids can be considered. In cases of rush or ultra-rush AIT injecting a high amount of allergens per day, premedications with combined oral glucocorticosteroids and H1-antihistamines may be needed.
Adverse reactions during the build-up phase
In recent studies, cluster AIT has shown faster symptom improvement without increasing the risk of systemic reactions than conventional AIT; however, rush and ultra-rush immunotherapies showed relatively higher risk and severity of adverse reactions.128 As previously mentioned, adverse reactions may occur during the build-up phase in the rush and ultra-rush AIT despite premedication with H1-antihistamines. In such cases, premedications with combined systemic glucocorticosteroids and H1-antihistamines may be considered.129,130 Furthermore, the allergen content and potency differ across products even though the same allergen is used for AIT. Therefore, close monitoring for adverse reactions is required when starting AIT with a relatively higher allergen content and more potent antigenicity product.131
Dose adjustment in build-up phases
No systematic review or meta-analysis on dose adjustment after missing a scheduled AIT dose has been reported. However, the risk for adverse reactions increases with longer injection intervals during the build-up phase. The dose can be continuously escalated if the missed injection can be given within one week of the scheduled visit. The last dose can be maintained if delayed by 8–13 days. If delayed by 14–21 days, the dose can be reduced by 25% of the last dose. If delayed by 22–28 days, the dose can be reduced by 50% of the last dose.71
Maintenance phase
Injection intervals during the maintenance phase
In general, the maintenance phase of AIT is performed in 4–8-week intervals. Caution must be taken not to exceed the maximum dose specified in the manufacturer’s instructions. Maintaining AIT for at least 3–5 years is recommended for long-term symptom control and improvement of disease progression.1
Dose adjustment in maintenance phases
As with the build-up phase, no systematic review or meta-analysis on dose adjustment after missing a scheduled visit during the maintenance phase has been reported. If the scheduled AIT dose is missed and more than 4 weeks have elapsed from the date, the dose should be reduced by half or more from the last administered dose (or the dose from the previous visit). Generally, the longer the time elapsed from the scheduled visit, the lower the dose should be recommended for the next AIT.59 If no systemic adverse reaction occurs with the reduced dose, the dose can be gradually increased to the previous maintenance dose. AIT can sometimes exacerbate symptoms, and in such cases, it is recommended to maintain or reduce the previous dose.132
Follow-up
Follow-up
Patients should be followed up at least once every 3 months during the AIT for the following purposes:
- To assess the effectiveness
- To educate on safety and monitoring of adverse reactions
- To educate patients on improving their compliance
- To assess the need for adjustments in the AIT dosing schedule or allergen content
Archive of documents and records
The composition of the allergen extracts, details of the AIT procedure and informed consent forms, the doses and dosing schedule, and other relevant information must be documented and stored.
Adjustment of AIT dosage
The dosage of AIT should be adjusted under the following circumstances:
- Exchange for a new vial of freshly produced allergen extract
- Development of adverse reactions after previous AIT
- Patient’s missing a previously scheduled injection
- AIT performed during the pollen season in a patient sensitized to pollen
Facility
A clinic room with an emergency setting for immediate treatment of adverse reactions, such as anaphylaxis, is preferred for AIT. Caution should also be taken in patients at high risk of systemic reactions (e.g., taking beta-blockers or those with uncontrolled asthma).
Duration
The duration of AIT should be determined based on the potential benefits and risks of continuing or discontinuing treatment. The recommended duration of AIT is at least 3 years, and while some patients may remain in remission after treatment, some may relapse.
Patient transfer
When a patient is transferred to another hospital during AIT, the treating physician should decide whether to continue the current AIT regimen or change to a new one. It is important to provide detailed records of the patient's AIT schedule, types of allergens included, the manufacturer of the products, patient’s history of systemic adverse reactions (e.g., type and treatment), and patient compliance.
The risk for a systemic reaction is not elevated if the existing schedule is maintained, but even minor changes in the extract components can increase the risk of systemic reactions. Therefore, when changing the AIT prescription, appropriate sIgE testing and adjustment of the AIT schedule should be considered.
ADVERSE REACTIONS
Summary statement
1. Local adverse reactions following AIT injections are not predictive of subsequent systemic reactions. However, the risk of systemic reactions may be high in patients with frequent and large local adverse reactions (Low).
2. The risk of severe systemic adverse reactions after appropriately administered AIT is low, but fatal reactions may occur (Low).
3. Because most systemic adverse reactions following AIT occur within 30 min of injection, patients should be observed in the office for at least 30 minutes after injection (Low).
4. AIT should not be given if the likelihood of recovery from systemic adverse events is low (such as beta-blockers and angiotensin-converting enzyme inhibitors [ACEI]) or the therapeutic agents for systemic adverse events are contraindicated (Low).
Local adverse reactions
Local adverse reactions to AIT are very common (an incidence rate of about 26%–86%), and the development of systemic reactions based on the onset of local reactions is difficult to predict.133 However, it has been reported that the incidence of local reactions is 4-fold higher in patients with a systemic reaction, suggesting that the risk of a systemic reaction may be high among patients who frequently develop large local reactions.134 The scheduled build-up dose can be maintained in local reactions less than 10 cm. If the patient complains of symptoms such as itching and pain, H1-antihistamines can be given. However, there is a risk of a systemic reaction if the dose is escalated as scheduled in cases of much larger local reactions causing wheals or erythema with diameters more than 10 cm. Hence, in these cases, reducing the dose by half or more from the last administered dose or to the previous dose and premedications with H1-antihistamines and oral glucocorticoids are recommended.
Systemic adverse reactions
While the reported incidence of systemic reactions is less than 1% in patients who underwent the conventional AIT, the incidence was as high as 36% in some studies that administered rush immunotherapy.135,136 According to a study conducted in the United States, the incidence of serious systemic reactions involving severe respiratory failure, hypotension, and emergency use of epinephrine injections was 5.4 events per 1,000,000 injections,132 and the mortality was about 1 person per 2,500,000 injections.137 Therefore, while severe systemic reactions to AIT are not common, there is a risk of fatal systemic reactions.
Approximately 70%–90% of systemic reactions occurred within 30 minutes of injection.132,135,137 However, one study reported that 38% of cases occurred between 30 minutes to 6 hours after injection. In contrast, another study reported that 8% of cases occurred 2 hours after the injection, calling for caution even after 30 minutes.138,139 Therefore, a minimum 30-minute hospital stay is mandatory for patients on AIT. In addition, longer waiting times and carrying an epinephrine auto-injector are desirable for the high-risk group of systemic reactions. For patients who have developed a severe systemic reaction, the risk-benefit must be weighed, and AIT continuation should be reconsidered and decided carefully.112
Risk factors for a systemic adverse reaction
Risk factors for severe systemic reactions include uncontrolled asthma, AIT performed in seasons of symptom exacerbation, errors in injection dosage, previous history of systemic reactions, use of beta-blockers, strong positive reactions to the allergen on skin tests, and administration of newly prepared allergen extracts (new vials).132,137 For asthmatics, appropriate symptom control is needed before starting AIT, while partly or uncontrolled asthma is contraindicated.132,137
Risk factors other than strong positive reaction to an allergen in skin tests and injection dosage errors can be reduced by assessing the patient before administering the injection. Pre-injection assessment should include asthma symptoms, exacerbation of allergy symptoms, concomitant use of beta-blockers or ACEI, recent changes in health status including pregnancy, and previous adverse reactions to AIT. Measuring maximum expiratory flow to assess lung function can also be helpful for patients with asthma. Meanwhile, AIT is contraindicated for patients with uncontrolled serious systemic diseases (Table 1).112 Treatment modalities other than AIT must also be considered for patients with diseases that undermine their ability to recover from a serious systemic adverse reaction, such as severe pulmonary functional impairment, uncontrolled asthma, unstable angina, recent onset of myocardial infarction, severe arrhythmia, and uncontrolled hypertension.112
Anaphylaxis
The greatest risk of AIT is anaphylaxis. In rare cases, anaphylaxis can be fatal despite appropriate treatment. Therefore, AIT should be performed under the supervision and backup of clinical experts, including physicians and healthcare professionals trained for emergency care for anaphylaxis. The administering healthcare professional should closely monitor the patient’s symptoms and be able to recognize and treat anaphylaxis upon onset promptly. The World Allergy Organization (WAO) classified anaphylaxis that may occur after AIT into 5 stages based on symptoms.140 Healthcare professionals should be familiar with the pharmacological action, adverse reactions, and administration of epinephrine and understand possible reasons for the lack of response to treatment.112,141,142 AIT should not be done in patients with conditions where epinephrine, the first-line treatment for anaphylaxis, is contraindicated. Supplementary Table S2 lists equipment for the treatment of anaphylaxis and measures to reduce the risk of anaphylaxis.112 As previously mentioned, beta-blockers warrant caution, as they reduce the effects of epinephrine, and ACEI may induce more severe symptoms of anaphylaxis, as they may disrupt the compensatory mechanism of the renin-angiotensin system.1
MEASUREMENTS OF CLINICAL EFFICACY
Summary statement
1. Clinical parameters reflecting symptoms and medication requirements are primary tools for measuring AIT efficacy. Changes in lung function test (forced expiratory volume for 1 second [FEV1] or peak expiratory flow rate [PEFR]) in asthma patients, visual analog scale (VAS), and Combined Symptoms and rescue Medication Scores (CSMS) in rhinitis patients may be useful (High).
2. Measuring serum sIgE, sIgG4 levels or skin prick tests may be considered for efficacy measurement during the AIT (Very low).
Symptoms and medication requirements
One classic index used to assess subjective symptoms of a disease is the VAS presented in the ARIA guidelines.143 VAS was significantly correlated with disease-specific quality of life scores (Rhinoconjunctivitis Quality of Life Questionnaire, RQLQ), as well as changes in medication requirements,144 and is used in RCTs for developing AIT agents in children and adults.75,93,145,146 In addition, the US FDA and the European Medicines Agency (EMA) recommend the use of a CSMS, which scores daily rhinoconjunctivitis symptoms based on 4 items for rhinitis, 2 items for conjunctivitis, and 3 types of medications.147 However, studies analyzing the applicability of these scoring systems as parameters for assessing the success of AIT in actual patient care outside clinical trials are limited. Therefore, assessments primarily rely on patient reports and subjective evaluations of symptom improvement; however, quality of life surveys such as Short Form (SF)-12, SF-36, and disease-specific quality of life measurements such as RQLQ can also be helpful.
Blood test
Serum sIgE has been shown to transiently increase during the early stages of AIT and then decrease; however, it is not correlated with the prognosis or clinical effects of AIT. The variation in total IgE (tIgE) levels in response to AIT differs between studies.148 While retrospective studies have reported that the sIgE/tIgE ratio after SCIT or SLIT for HDM is associated with the improvement of symptoms in rhinitis and/or asthma,149,150 prospective randomized trials have not demonstrated such an association. Studies have shown that serum sIgG4 increases with SCIT and is associated with a decrease in sensitivity in nasal provocation tests,151 and that the sIgG4/sIgE ratio is associated with a decrease in skin reaction during SCIT.152,153 However, these biomarkers currently have limitations in determining the effectiveness of AIT due to a lack of optimal measurement methodologies. Therefore, further research is necessary to understand the role of the sIgE/tIgE ratio in AIT efficacy.
Skin tests and provocation tests
Skin prick, intradermal, nasal and conjunctival provocation, nonspecific bronchial provocation, and pulmonary function tests can be used to assess the therapeutic efficacy of AIT.147,148 Although skin tests have been employed to indicate immunotherapeutic efficacy in some clinical studies aimed at developing therapeutic agents,154,155,156 they cannot accurately reflect allergen exposure in natural settings. In particular, intradermal testing has been demonstrated not to be correlated with the degree of symptom improvement and, therefore, cannot be used to measure immunotherapeutic efficacy in clinical practice.147,148 Nasal and conjunctival provocation tests have not been standardized in terms of their administration and interpretation. Therefore, these provocation tests are limited in replacing clinical parameters, such as changes in symptoms and medication requirements, to assess the therapeutic efficacy of AIT.
AIT IN CHILDREN
Summary statement
1. AIT in children can prevent new allergen sensitization and reduce the occurrence of future allergic diseases, such as asthma in rhinitis patients (High).
2. As with adults, AIT in children is most effective. SCIT or SLIT is recommended for allergic rhinitis, asthma, and bee venom allergy. Allergens mainly used for AIT are HDM, pollen, bee venom, and pet dander, which are similar to those of adults (High).
3. For safety reasons, pediatric AIT is recommended for those aged 5 years or older. If AIT is performed in children younger than this, SLIT may be considered because injection therapy may be difficult (High).
4. Since both SCIT and SLIT are equally effective in children, the choice depends on the preference, cost, and compliance of the patient or caregiver (High).
In children, it should be considered that an allergic march may progress if effective treatment for allergic diseases is not provided. Pediatric AIT can be considered a fundamental treatment for the underlying mechanisms of allergic diseases, as it induces immune tolerance to weaken hypersensitivity to antigens, even if a child develops an allergic disease due to environmental influences.
Most previous studies have reported that AIT is highly effective for children.107,157 Recent studies on the effects of AIT in children can be summarized as follows. First, it helps control symptoms in children with asthma and allergic rhinitis158,159 and improves the clinical severity of atopic dermatitis.50,160 Secondly, it reduces airway hypersensitivity57 and the progression to asthma in children with allergic rhinitis.158,159 Thirdly, it can prevent sensitization to new allergens in patients who have undergone AIT.158,159 As with adults, AIT is generally recommended for children with allergic rhinitis, asthma, and bee venom allergy, mainly those who are sensitized to HDM, pollen, or bee venom allergens. According to European guidelines, AIT can be considered even in cases with mild allergic rhinitis symptoms if the goal is to attain long-term therapeutic effects or prevent asthma.57 In children with well-controlled allergic asthma induced by HDM, SCIT or SLIT is recommended as an adjunctive therapy to routine asthma treatment to reduce symptoms and drug consumption.2 Furthermore, bee venom AIT may be performed on children aged 5 years and over who clearly show sensitization to bee venom and exhibit a systemic reaction.23 However, evidence for the efficacy of AIT to prevent asthma in preschool-age children is lacking.57,161
Currently, the efficacy and safety of AIT targeting some inhalant allergens limited to some types of pollen and HDM have been substantiated. However, there is limited evidence for AIT in children for allergens such as pet dander, mold, and cockroaches.
For safety reasons, the minimum age for AIT has been established as 5 years based on expert opinions. Most large clinical trials involving children have focused on those aged 5 years and above, including school-age children and adolescents.162,163 The evidence for the effectiveness of AIT in preschool-aged children is still very limited. Children under the age of 5 years are rarely included in RCTs due to concerns about potential safety and the ability to diagnose allergic rhinitis in this age group accurately. It may be difficult to recognize improvement or exacerbation of symptoms in young children, in addition to the challenges of communicating symptoms of adverse reactions. Particularly, they are highly likely to face difficulties due to repeated injections associated with SCIT. On the contrary, SLIT is relatively safe in this age group, even though it causes similar symptoms and frequency of adverse reactions to those in older children.164,165,166,167 Moreover, compliance of such patients may be low, and in one study, nearly half of the patients discontinued treatment within the first few months.168
The safety profile of SCIT and SLIT in children has been thoroughly evaluated in most clinical trials.169,170 Common adverse effects seen in SCIT are local reactions at the injection site, such as erythema, itching, or swelling. Systemic reactions (e.g., asthma, systemic hives, and anaphylaxis) have been reported in approximately 2% of all patients who underwent SCIT. In addition, very rare but fatal or near-fatal systemic reactions have also been reported.132 SLIT mainly causes less severe, localized reactions limited to the oral mucosa; nevertheless, abnormal reactions such as asthma, hives, and abdominal pain have also been reported. In addition, the risk of eosinophilic esophagitis, which is considered a contraindication for SLIT, has been reported.3,171
CONCLUSION
AIT effectively reduces symptoms of allergic asthma and rhinitis and potentially modifies the underlying course of the disease. This guideline provides expert opinions based on an extensive literature review instead of evidence-based recommendations by systematic review and meta-analysis. Clinical indications and contraindications for AIT are provided in Table 1. Major pollen allergens in Korea are summarized in Table 4. Although this guideline suggests the optimal dosing schedule, an individualized approach and modifications are recommended considering the situation for each patient and clinic. Systemic adverse reactions may occur during AIT; therefore, AIT should be performed at the clinic with first aid kits and trained health care providers (Supplementary Table S2).
AIT is still not widely used for various reasons, including a lack of awareness, long duration, and the reimbursement policy. In addition, unmet needs should be addressed, such as developing biomarkers for clinical efficacy, the availability of clinically important allergens such as Japanese hop, the cost-effectiveness of AIT in Korea, and the development of new allergoids with higher immunogenicity and lower allergenicity.
This guideline should be updated regularly as new evidence emerges. Furthermore, Korean AIT guidelines providing evidence-based recommendations by systematic review and meta-analysis will be helpful.
ACKNOWLEDGMENTS
This guideline for allergen immunotherapy was developed with the support of the Korean Academy of Asthma Allergy and Clinical Immunology (KAAACI). The authors would like to thank all members of the Allergen Immunotherapy and Allergen working group at the KAAACI. This study was supported by a grant from the KAAACI in 2022.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Examples of protocol for allergen immunotherapy
Recommended equipment and medications to treat anaphylaxis
References
- 1.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556–568. doi: 10.1016/j.jaci.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 2.Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI Guidelines on Allergen Immunotherapy: house dust mite-driven allergic asthma. Allergy. 2019;74:855–873. doi: 10.1111/all.13749. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI Guidelines on Allergen Immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765–798. doi: 10.1111/all.13317. [DOI] [PubMed] [Google Scholar]
- 4.Greenhawt M, Oppenheimer J, Nelson M, Nelson H, Lockey R, Lieberman P, et al. Sublingual immunotherapy: a focused allergen immunotherapy practice parameter update. Ann Allergy Asthma Immunol. 2017;118:276–282.e2. doi: 10.1016/j.anai.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Hur GY, Kim TB, Kim ST, Han MY, Nahm DH, Lee YW, et al. Allergy immunotherapy. Korean J Asthma Allergy Clin Immunol. 2010;30:153–183. [Google Scholar]
- 6.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Bohle B, Kinaciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen-specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol. 2007;120:707–713. doi: 10.1016/j.jaci.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Shamji MH, Sharif H, Layhadi JA, Zhu R, Kishore U, Renz H. Diverse immune mechanisms of allergen immunotherapy for allergic rhinitis with and without asthma. J Allergy Clin Immunol. 2022;149:791–801. doi: 10.1016/j.jaci.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Gardner LM, Thien FC, Douglass JA, Rolland JM, O’Hehir RE. Induction of T ‘regulatory’ cells by standardized house dust mite immunotherapy: an increase in CD4+ CD25+ interleukin-10+ T cells expressing peripheral tissue trafficking markers. Clin Exp Allergy. 2004;34:1209–1219. doi: 10.1111/j.1365-2222.2004.02009.x. [DOI] [PubMed] [Google Scholar]
- 10.Varona R, Ramos T, Escribese MM, Jimeno L, Galán A, Würtzen PA, et al. Persistent regulatory T-cell response 2 years after 3 years of grass tablet SLIT: links to reduced eosinophil counts, sIgE levels, and clinical benefit. Allergy. 2019;74:349–360. doi: 10.1111/all.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavón-Romero GF, Parra-Vargas MI, Ramírez-Jiménez F, Melgoza-Ruiz E, Serrano-Pérez NH, Teran LM. Allergen Immunotherapy: Current and Future Trends. Cells. 2022;11:11. doi: 10.3390/cells11020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127:18–27. doi: 10.1016/j.jaci.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Zimmer A, Bouley J, Le Mignon M, Pliquet E, Horiot S, Turfkruyer M, et al. A regulatory dendritic cell signature correlates with the clinical efficacy of allergen-specific sublingual immunotherapy. J Allergy Clin Immunol. 2012;129:1020–1030. doi: 10.1016/j.jaci.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485–1498. doi: 10.1016/j.jaci.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Schandené L, Alonso-Vega C, Willems F, Gérard C, Delvaux A, Velu T, et al. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368–4374. [PubMed] [Google Scholar]
- 16.van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol. 2016;138:654–665. doi: 10.1016/j.jaci.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J Allergy Clin Immunol. 2003;112:915–922. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 18.Heeringa JJ, McKenzie CI, Varese N, Hew M, Bakx AT, Aui PM, et al. Induction of IgG2 and IgG4 B-cell memory following sublingual immunotherapy for ryegrass pollen allergy. Allergy. 2020;75:1121–1132. doi: 10.1111/all.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couroux P, Ipsen H, Stage BS, Damkjaer JT, Steffensen MA, Salapatek AM, et al. A birch sublingual allergy immunotherapy tablet reduces rhinoconjunctivitis symptoms when exposed to birch and oak and induces IgG4 to allergens from all trees in the birch homologous group. Allergy. 2019;74:361–369. doi: 10.1111/all.13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitthamsiri W, Pradubpongsa P, Sangasapaviliya A, Boonpiyathad T. Decreased CRTH2 expression and response to allergen re-stimulation on innate lymphoid cells in patients with allergen-specific immunotherapy. Allergy Asthma Immunol Res. 2018;10:662–674. doi: 10.4168/aair.2018.10.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eljaszewicz A, Ruchti F, Radzikowska U, Globinska A, Boonpiyathad T, Gschwend A, et al. Trained immunity and tolerance in innate lymphoid cells, monocytes, and dendritic cells during allergen-specific immunotherapy. J Allergy Clin Immunol. 2021;147:1865–1877. doi: 10.1016/j.jaci.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 22.James C, Bernstein DI. Allergen immunotherapy: an updated review of safety. Curr Opin Allergy Clin Immunol. 2017;17:55–59. doi: 10.1097/ACI.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sturm GJ, Varga EM, Roberts G, Mosbech H, Bilò MB, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: hymenoptera venom allergy. Allergy. 2018;73:744–764. doi: 10.1111/all.13262. [DOI] [PubMed] [Google Scholar]
- 24.Golden DB, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, et al. Stinging insect hypersensitivity: a practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118:28–54. doi: 10.1016/j.anai.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 25.Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. doi: 10.1186/1939-4551-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malling HJ, Bousquet J. Subcutaneous immunotherapy for allergic rhinoconjunctivitis, allergic asthma, and prevention of allergic diseases. Clin Allergy Immunol. 2008;21:343–358. [PubMed] [Google Scholar]
- 27.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466–476. doi: 10.1016/j.jaci.2010.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Ariano R, Kroon AM, Augeri G, Canonica GW, Passalacqua G. Long-term treatment with allergoid immunotherapy with Parietaria. Clinical and immunologic effects in a randomized, controlled trial. Allergy. 1999;54:313–319. doi: 10.1034/j.1398-9995.1999.00900.x. [DOI] [PubMed] [Google Scholar]
- 29.Malling HJ, Stahl Skov P. Diagnosis and immunotherapy of mould allergy. VIII. Qualitative and quantitative estimation of IgE in Cladosporium immunotherapy. Allergy. 1988;43:228–238. [PubMed] [Google Scholar]
- 30.Alvarez-Cuesta E, Cuesta-Herranz J, Puyana-Ruiz J, Cuesta-Herranz C, Blanco-Quirós A. Monoclonal antibody-standardized cat extract immunotherapy: risk-benefit effects from a double-blind placebo study. J Allergy Clin Immunol. 1994;93:556–566. doi: 10.1016/s0091-6749(94)70067-2. [DOI] [PubMed] [Google Scholar]
- 31.Aas K. Hyposensitization in house dust allergy asthma. A double-blind controlled study with evaluation of the effect on bronchial sensitivity to house dust. Acta Paediatr Scand. 1971;60:264–268. doi: 10.1111/j.1651-2227.1971.tb06655.x. [DOI] [PubMed] [Google Scholar]
- 32.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186. doi: 10.1002/14651858.CD001186.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Erekosima N, Suarez-Cuervo C, Ramanathan M, Kim JM, Chelladurai Y, Segal JB, et al. Effectiveness of subcutaneous immunotherapy for allergic rhinoconjunctivitis and asthma: a systematic review. Laryngoscope. 2014;124:616–627. doi: 10.1002/lary.24295. [DOI] [PubMed] [Google Scholar]
- 34.Lin SY, Erekosima N, Kim JM, Ramanathan M, Suarez-Cuervo C, Chelladurai Y, et al. Sublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic review. JAMA. 2013;309:1278–1288. doi: 10.1001/jama.2013.2049. [DOI] [PubMed] [Google Scholar]
- 35.Mosbech H, Deckelmann R, de Blay F, Pastorello EA, Trebas-Pietras E, Andres LP, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568–575.e7. doi: 10.1016/j.jaci.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 36.de Blay F, Kuna P, Prieto L, Ginko T, Seitzberg D, Riis B, et al. SQ HDM SLIT-tablet (ALK) in treatment of asthma--post hoc results from a randomised trial. Respir Med. 2014;108:1430–1437. doi: 10.1016/j.rmed.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Passalacqua G, Guerra L, Compalati E, Canonica GW. The safety of allergen specific sublingual immunotherapy. Curr Drug Saf. 2007;2:117–123. doi: 10.2174/157488607780598340. [DOI] [PubMed] [Google Scholar]
- 38.Penagos M, Passalacqua G, Compalati E, Baena-Cagnani CE, Orozco S, Pedroza A, et al. Metaanalysis of the efficacy of sublingual immunotherapy in the treatment of allergic asthma in pediatric patients, 3 to 18 years of age. Chest. 2008;133:599–609. doi: 10.1378/chest.06-1425. [DOI] [PubMed] [Google Scholar]
- 39.Khinchi MS, Poulsen LK, Carat F, André C, Hansen AB, Malling HJ. Clinical efficacy of sublingual and subcutaneous birch pollen allergen-specific immunotherapy: a randomized, placebo-controlled, double-blind, double-dummy study. Allergy. 2004;59:45–53. doi: 10.1046/j.1398-9995.2003.00387.x. [DOI] [PubMed] [Google Scholar]
- 40.Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. 2004;48:441–448. doi: 10.1002/mnfr.200400037. [DOI] [PubMed] [Google Scholar]
- 41.Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J Allergy Clin Immunol. 2007;119:937–943. doi: 10.1016/j.jaci.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–25.e43. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014;69:1008–1025. doi: 10.1111/all.12429. [DOI] [PubMed] [Google Scholar]
- 44.Skypala IJ, Hunter H, Krishna MT, Rey-Garcia H, Till SJ, du Toit G, et al. BSACI guideline for the diagnosis and management of pollen food syndrome in the UK. Clin Exp Allergy. 2022;52:1018–1034. doi: 10.1111/cea.14208. [DOI] [PubMed] [Google Scholar]
- 45.Biedermann T, Kuna P, Panzner P, Valovirta E, Andersson M, de Blay F, et al. The SQ tree SLIT-tablet is highly effective and well tolerated: results from a randomized, double-blind, placebo-controlled phase III trial. J Allergy Clin Immunol. 2019;143:1058–1066.e6. doi: 10.1016/j.jaci.2018.12.1001. [DOI] [PubMed] [Google Scholar]
- 46.Till SJ, Stage BS, Skypala I, Biedermann T. Potential treatment effect of the SQ tree SLIT-tablet on pollen food syndrome caused by apple. Allergy. 2020;75:2059–2061. doi: 10.1111/all.14242. [DOI] [PubMed] [Google Scholar]
- 47.Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006;61:202–205. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 48.Nahm DH, Lee ES, Park HJ, Kim HA, Choi GS, Jeon SY. Treatment of atopic dermatitis with a combination of allergen-specific immunotherapy and a histamine-immunoglobulin complex. Int Arch Allergy Immunol. 2008;146:235–240. doi: 10.1159/000115892. [DOI] [PubMed] [Google Scholar]
- 49.Yepes-Nuñez JJ, Guyatt GH, Gómez-Escobar LG, Pérez-Herrera LC, Chu AW, Ceccaci R, et al. Allergen immunotherapy for atopic dermatitis: systematic review and meta-analysis of benefits and harms. J Allergy Clin Immunol. 2023;151:147–158. doi: 10.1016/j.jaci.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 50.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132:110–117. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 51.Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. 2017;72:1133–1147. doi: 10.1111/all.13124. [DOI] [PubMed] [Google Scholar]
- 52.Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. 2018;73:799–815. doi: 10.1111/all.13319. [DOI] [PubMed] [Google Scholar]
- 53.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 54.Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631. doi: 10.1111/all.13201. [DOI] [PubMed] [Google Scholar]
- 55.Bousquet J, Lockey R, Malling HJ, Alvarez-Cuesta E, Canonica GW, Chapman MD, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Ann Allergy Asthma Immunol. 1998;81:401–405. doi: 10.1016/s1081-1206(10)63136-5. [DOI] [PubMed] [Google Scholar]
- 56.Moote W, Kim H, Ellis AK. Allergen-specific immunotherapy. Allergy Asthma Clin Immunol. 2018;14:53. doi: 10.1186/s13223-018-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halken S, Larenas-Linnemann D, Roberts G, Calderón MA, Angier E, Pfaar O, et al. EAACI Guidelines on Allergen Immunotherapy: prevention of allergy. Pediatr Allergy Immunol. 2017;28:728–745. doi: 10.1111/pai.12807. [DOI] [PubMed] [Google Scholar]
- 58.Bachert C, Larché M, Bonini S, Canonica GW, Kündig T, Larenas-Linnemann D, et al. Allergen immunotherapy on the way to product-based evaluation-a WAO statement. World Allergy Organ J. 2015;8:29. doi: 10.1186/s40413-015-0078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, Eng P, et al. Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD) Allergo J Int. 2014;23:282–319. doi: 10.1007/s40629-014-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reisman RE, Mauriello PM, Davis GB, Georgitis JW, DeMasi JM. A double-blind study of the effectiveness of a high-efficiency particulate air (HEPA) filter in the treatment of patients with perennial allergic rhinitis and asthma. J Allergy Clin Immunol. 1990;85:1050–1057. doi: 10.1016/0091-6749(90)90050-e. [DOI] [PubMed] [Google Scholar]
- 61.Terreehorst I, Hak E, Oosting AJ, Tempels-Pavlica Z, de Monchy JG, Bruijnzeel-Koomen CA, et al. Evaluation of impermeable covers for bedding in patients with allergic rhinitis. N Engl J Med. 2003;349:237–246. doi: 10.1056/NEJMoa023171. [DOI] [PubMed] [Google Scholar]
- 62.Jeon YH, Lee YJ, Sohn MH, Lee HR. Effects of vacuuming mattresses on allergic rhinitis symptoms in children. Allergy Asthma Immunol Res. 2019;11:655–663. doi: 10.4168/aair.2019.11.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park KH, Sim DW, Lee SC, Moon S, Choe E, Shin H, et al. Effects of air purifiers on patients with allergic rhinitis: a multicenter, randomized, double-blind, and placebo-controlled study. Yonsei Med J. 2020;61:689–697. doi: 10.3349/ymj.2020.61.8.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stillerman A, Nachtsheim C, Li W, Albrecht M, Waldman J. Efficacy of a novel air filtration pillow for avoidance of perennial allergens in symptomatic adults. Ann Allergy Asthma Immunol. 2010;104:440–449. doi: 10.1016/j.anai.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 65.Wood RA, Johnson EF, Van Natta ML, Chen PH, Eggleston PA. A placebo-controlled trial of a HEPA air cleaner in the treatment of cat allergy. Am J Respir Crit Care Med. 1998;158:115–120. doi: 10.1164/ajrccm.158.1.9712110. [DOI] [PubMed] [Google Scholar]
- 66.Björnsdottir US, Jakobinudottir S, Runarsdottir V, Juliusson S. The effect of reducing levels of cat allergen (Fel d 1) on clinical symptoms in patients with cat allergy. Ann Allergy Asthma Immunol. 2003;91:189–194. doi: 10.1016/s1081-1206(10)62176-x. [DOI] [PubMed] [Google Scholar]
- 67.Gherasim A, Jacob A, Schoettel F, Domis N, de Blay F. Efficacy of air cleaners in asthmatics allergic to cat in ALYATEC® environmental exposure chamber. Clin Exp Allergy. 2020;50:160–169. doi: 10.1111/cea.13511. [DOI] [PubMed] [Google Scholar]
- 68.Müller UR, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5. Allergy. 2009;64:543–548. doi: 10.1111/j.1398-9995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 69.Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: World Allergy Organization Position Paper 2009. Allergy. 2009;64(Suppl 91):1–59. doi: 10.1111/j.1398-9995.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 70.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 71.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 72.Oykhman P, Kim HL, Ellis AK. Allergen immunotherapy in pregnancy. Allergy Asthma Clin Immunol. 2015;11:31. doi: 10.1186/s13223-015-0096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Henmar H, Lund G, Lund L, Petersen A, Würtzen PA. Allergenicity, immunogenicity and dose-relationship of three intact allergen vaccines and four allergoid vaccines for subcutaneous grass pollen immunotherapy. Clin Exp Immunol. 2008;153:316–323. doi: 10.1111/j.1365-2249.2008.03710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HS, Chae BW. Allergen standarization of Dermatophagoides farinae extracts. Korean J Asthma Allergy Clin Immnol. 1996;16:7. [Google Scholar]
- 75.Frew AJ, Powell RJ, Corrigan CJ, Durham SR UK Immunotherapy Study Group. Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment-resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319–325. doi: 10.1016/j.jaci.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Ewan PW, Alexander MM, Snape C, Ind PW, Agrell B, Dreborg S. Effective hyposensitization in allergic rhinitis using a potent partially purified extract of house dust mite. Clin Allergy. 1988;18:501–508. doi: 10.1111/j.1365-2222.1988.tb02900.x. [DOI] [PubMed] [Google Scholar]
- 77.Haugaard L, Dahl R, Jacobsen L. A controlled dose-response study of immunotherapy with standardized, partially purified extract of house dust mite: clinical efficacy and side effects. J Allergy Clin Immunol. 1993;91:709–722. doi: 10.1016/0091-6749(93)90190-q. [DOI] [PubMed] [Google Scholar]
- 78.Olsen OT, Larsen KR, Jacobsan L, Svendsen UG. A 1-year, placebo-controlled, double-blind house-dust-mite immunotherapy study in asthmatic adults. Allergy. 1997;52:853–859. doi: 10.1111/j.1398-9995.1997.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 79.Van Metre TE, Jr, Adkinson NF, Jr, Amodio FJ, Lichtenstein LM, Mardiney MR, Jr, Norman PS, et al. A comparative study of the effectiveness of the Rinkel method and the current standard method of immunotherapy for ragweed pollen hay fever. J Allergy Clin Immunol. 1980;66:500–513. doi: 10.1016/0091-6749(80)90012-3. [DOI] [PubMed] [Google Scholar]
- 80.Creticos PS, Adkinson NF, Jr, Kagey-Sobotka A, Proud D, Meier HL, Naclerio RM, et al. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985;76:2247–2253. doi: 10.1172/JCI112233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Creticos PS, Marsh DG, Proud D, Kagey-Sobotka A, Adkinson NF, Jr, Friedhoff L, et al. Responses to ragweed-pollen nasal challenge before and after immunotherapy. J Allergy Clin Immunol. 1989;84:197–205. doi: 10.1016/0091-6749(89)90325-4. [DOI] [PubMed] [Google Scholar]
- 82.Furin MJ, Norman PS, Creticos PS, Proud D, Kagey-Sobotka A, Lichtenstein LM, et al. Immunotherapy decreases antigen-induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991;88:27–32. doi: 10.1016/0091-6749(91)90297-2. [DOI] [PubMed] [Google Scholar]
- 83.Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265–269. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dolz I, Martínez-Cócera C, Bartolomé JM, Cimarra M. A double-blind, placebo-controlled study of immunotherapy with grass-pollen extract Alutard SQ during a 3-year period with initial rush immunotherapy. Allergy. 1996;51:489–500. doi: 10.1111/j.1398-9995.1996.tb04655.x. [DOI] [PubMed] [Google Scholar]
- 85.Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol. 2001;107:87–93. doi: 10.1067/mai.2001.112027. [DOI] [PubMed] [Google Scholar]
- 86.Bødtger U, Poulsen LK, Jacobi HH, Malling HJ. The safety and efficacy of subcutaneous birch pollen immunotherapy - a one-year, randomised, double-blind, placebo-controlled study. Allergy. 2002;57:297–305. doi: 10.1034/j.1398-9995.2002.1o3532.x. [DOI] [PubMed] [Google Scholar]
- 87.Hedlin G, Graff-Lonnevig V, Heilborn H, Lilja G, Norrlind K, Pegelow K, et al. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955–964. doi: 10.1016/0091-6749(91)90417-m. [DOI] [PubMed] [Google Scholar]
- 88.Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy. 1997;27:860–867. [PubMed] [Google Scholar]
- 89.Ewbank PA, Murray J, Sanders K, Curran-Everett D, Dreskin S, Nelson HS. A double-blind, placebo-controlled immunotherapy dose-response study with standardized cat extract. J Allergy Clin Immunol. 2003;111:155–161. doi: 10.1067/mai.2003.41. [DOI] [PubMed] [Google Scholar]
- 90.Nanda A, O’connor M, Anand M, Dreskin SC, Zhang L, Hines B, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339–1344. doi: 10.1016/j.jaci.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 91.Lent AM, Harbeck R, Strand M, Sills M, Schmidt K, Efaw B, et al. Immunologic response to administration of standardized dog allergen extract at differing doses. J Allergy Clin Immunol. 2006;118:1249–1256. doi: 10.1016/j.jaci.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 92.Horst M, Hejjaoui A, Horst V, Michel FB, Bousquet J. Double-blind, placebo-controlled rush immunotherapy with a standardized Alternaria extract. J Allergy Clin Immunol. 1990;85:460–472. doi: 10.1016/0091-6749(90)90156-x. [DOI] [PubMed] [Google Scholar]
- 93.Kuna P, Kaczmarek J, Kupczyk M. Efficacy and safety of immunotherapy for allergies to Alternaria alternata in children. J Allergy Clin Immunol. 2011;127:502–508.e1-6. doi: 10.1016/j.jaci.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 94.Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2003;(4):CD001186. doi: 10.1002/14651858.CD001186. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann-Sommergruber K, Hilger C, Santos AF, de las Vecillas L, Dramburg S, editors. Molecular allergology user’s guide 2.0. Zurich (Swiss): The European Academy of Allergy and Clinical Immunology (EAACI); 2022. [Google Scholar]
- 96.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 97.Bousquet PJ, Castelli C, Daures JP, Heinrich J, Hooper R, Sunyer J, et al. Assessment of allergen sensitization in a general population-based survey (European Community Respiratory Health Survey I) Ann Epidemiol. 2010;20:797–803. doi: 10.1016/j.annepidem.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 98.Calderón MA, Cox L, Casale TB, Moingeon P, Demoly P. Multiple-allergen and single-allergen immunotherapy strategies in polysensitized patients: looking at the published evidence. J Allergy Clin Immunol. 2012;129:929–934. doi: 10.1016/j.jaci.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 99.Ciprandi G, Alesina R, Ariano R, Aurnia P, Borrelli P, Cadario G, et al. Characteristics of patients with allergic polysensitization: the POLISMAIL study. Eur Ann Allergy Clin Immunol. 2008;40:77–83. [PubMed] [Google Scholar]
- 100.Demoly P, Passalacqua G, Pfaar O, Sastre J, Wahn U. Management of the polyallergic patient with allergy immunotherapy: a practice-based approach. Allergy Asthma Clin Immunol. 2016;12:2. doi: 10.1186/s13223-015-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim YJ, Lee MY, Yang AR, Sol IS, Kwak JH, Jung HL, et al. Trends of sensitization to inhalant allergens in Korean children over the last 10 years. Yonsei Med J. 2020;61:797–804. doi: 10.3349/ymj.2020.61.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim HY, Shin YH, Han MY. Determinants of sensitization to allergen in infants and young children. Korean J Pediatr. 2014;57:205–210. doi: 10.3345/kjp.2014.57.5.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hur GY, Kim TB, Han MY, Nahm DH, Park JW Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI) A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013;5:277–282. doi: 10.4168/aair.2013.5.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burbach GJ, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, Bonini S, et al. GA2LEN skin test study II: clinical relevance of inhalant allergen sensitizations in Europe. Allergy. 2009;64:1507–1515. doi: 10.1111/j.1398-9995.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 105.Mahler V, Esch RE, Kleine-Tebbe J, Lavery WJ, Plunkett G, Vieths S, et al. Understanding differences in allergen immunotherapy products and practices in North America and Europe. J Allergy Clin Immunol. 2019;143:813–828. doi: 10.1016/j.jaci.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 106.Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, et al. Sensitization rates for inhalant allergens in Korea: a multicenter study. Korean J Asthma Allergy Clin Immnol. 2003;23:483–493. [Google Scholar]
- 107.Adkinson NF, Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, et al. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med. 1997;336:324–331. doi: 10.1056/NEJM199701303360502. [DOI] [PubMed] [Google Scholar]
- 108.Yang MS, Lee SP, Kwon YJ, Lee SM. Dog and cat allergies and allergen avoidance measures in Korean adult pet owners who participated in a pet exhibition. Allergy Asthma Immunol Res. 2018;10:155–164. doi: 10.4168/aair.2018.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kang SY, Yang MS, Park SY, Kim JH, Won HK, Kwon OY, et al. The role of allergen-specific IgE in predicting allergic symptoms on dog and cat exposure among Korean pet exhibition participants. World Allergy Organ J. 2020;13:100488. doi: 10.1016/j.waojou.2020.100488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015;7:205–220. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kordash TR, Amend MJ, Williamson SL, Jones JK, Plunkett GA. Effect of mixing allergenic extracts containing Helminthosporium, D. farinae, and cockroach with perennial ryegrass. Ann Allergy. 1993;71:240–246. [PubMed] [Google Scholar]
- 112.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Allergen immunotherapy: a practice parameter second update. J Allergy Clin Immunol. 2007;120:S25–S85. doi: 10.1016/j.jaci.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 113.Lee YW, Choi SY, Lee EK, Sohn JH, Park JW, Hong CS. Cross-allergenicity of pollens from the Compositae family: Artemisia vulgaris, Dendranthema grandiflorum, and Taraxacum officinale . Ann Allergy Asthma Immunol. 2007;99:526–533. doi: 10.1016/S1081-1206(10)60382-1. [DOI] [PubMed] [Google Scholar]
- 114.Leavengood DC, Renard RL, Martin BG, Nelson HS. Cross allergenicity among grasses determined by tissue threshold changes. J Allergy Clin Immunol. 1985;76:789–794. doi: 10.1016/0091-6749(85)90749-3. [DOI] [PubMed] [Google Scholar]
- 115.Nelson HS, Iklé D, Buchmeier A. Studies of allergen extract stability: the effects of dilution and mixing. J Allergy Clin Immunol. 1996;98:382–388. doi: 10.1016/s0091-6749(96)70162-8. [DOI] [PubMed] [Google Scholar]
- 116.Grier TJ, LeFevre DM, Duncan EA, Esch RE. Stability of standardized grass, dust mite, cat, and short ragweed allergens after mixing with mold or cockroach extracts. Ann Allergy Asthma Immunol. 2007;99:151–160. doi: 10.1016/S1081-1206(10)60639-4. [DOI] [PubMed] [Google Scholar]
- 117.Anderson MC, Baer H. Antigenic and allergenic changes during storage of a pollen extract. J Allergy Clin Immunol. 1982;69:3–10. doi: 10.1016/0091-6749(82)90080-x. [DOI] [PubMed] [Google Scholar]
- 118.Sridhara S, Singh BP, Arora N, Verma J, Gangal SV. A study on antigenic and allergenic changes during storage in three different biological extracts. Asian Pac J Allergy Immunol. 1992;10:33–38. [PubMed] [Google Scholar]
- 119.Alvarez-Cuesta E, Bousquet J, Canonica GW, Durham SR, Malling HJ, Valovirta E, et al. Standards for practical allergen-specific immunotherapy. Allergy. 2006;61(Suppl 82):1–20. doi: 10.1111/j.1398-9995.2006.01219_1.x. [DOI] [PubMed] [Google Scholar]
- 120.Tabar AI, Muro MD, García BE, Alvarez MJ, Acero S, Rico P, et al. Dermatophagoides pteronyssinus cluster immunotherapy. A controlled trial of safety and clinical efficacy. J Investig Allergol Clin Immunol. 1999;9:155–164. [PubMed] [Google Scholar]
- 121.Casanovas M, Martín R, Jiménez C, Caballero R, Fernández-Caldas E. Safety of an ultra-rush immunotherapy build-up schedule with therapeutic vaccines containing depigmented and polymerized allergen extracts. Int Arch Allergy Immunol. 2006;139:153–158. doi: 10.1159/000090392. [DOI] [PubMed] [Google Scholar]
- 122.Harvey SM, Laurie S, Hilton K, Khan DA. Safety of rush immunotherapy to multiple aeroallergens in an adult population. Ann Allergy Asthma Immunol. 2004;92:414–419. doi: 10.1016/S1081-1206(10)61776-0. [DOI] [PubMed] [Google Scholar]
- 123.Kim ME, Kim JE, Sung JM, Lee JW, Choi GS, Nahm DH. Safety of accelerated schedules of subcutaneous allergen immunotherapy with house dust mite extract in patients with atopic dermatitis. J Korean Med Sci. 2011;26:1159–1164. doi: 10.3346/jkms.2011.26.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee SH, Kim ME, Shin YS, Ye YM, Park HS, Nahm DH. Safety of ultra-rush schedule of subcutaneous allergen immunotherapy with house dust mite extract conducted in an outpatient clinic in patients with atopic dermatitis and allergic rhinitis. Allergy Asthma Immunol Res. 2019;11:846–855. doi: 10.4168/aair.2019.11.6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nielsen L, Johnsen CR, Mosbech H, Poulsen LK, Malling HJ. Antihistamine premedication in specific cluster immunotherapy: a double-blind, placebo-controlled study. J Allergy Clin Immunol. 1996;97:1207–1213. doi: 10.1016/s0091-6749(96)70186-0. [DOI] [PubMed] [Google Scholar]
- 126.Wang L, Wang C, Lou H, Zhang L. Antihistamine premedication improves safety and efficacy of allergen immunotherapy. Ann Allergy Asthma Immunol. 2021;127:363–371.e1. doi: 10.1016/j.anai.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 127.Brockow K, Kiehn M, Riethmüller C, Vieluf D, Berger J, Ring J. Efficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trial. J Allergy Clin Immunol. 1997;100:458–463. doi: 10.1016/s0091-6749(97)70135-0. [DOI] [PubMed] [Google Scholar]
- 128.Tabar AI, Echechipía S, García BE, Olaguibel JM, Lizaso MT, Gómez B, et al. Double-blind comparative study of cluster and conventional immunotherapy schedules with Dermatophagoides pteronyssinus . J Allergy Clin Immunol. 2005;116:109–118. doi: 10.1016/j.jaci.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 129.Temiño VM, Wu P, Konig J, Fahrenholz JM. Safety of multiple aeroallergen rush immunotherapy using a modified schedule. Allergy Asthma Proc. 2013;34:255–260. doi: 10.2500/aap.2013.34.3651. [DOI] [PubMed] [Google Scholar]
- 130.Rieker-Schwienbacher J, Nell MJ, Diamant Z, van Ree R, Distler A, Boot JD, et al. Open-label parallel dose tolerability study of three subcutaneous immunotherapy regimens in house dust mite allergic patients. Clin Transl Allergy. 2013;3:16. doi: 10.1186/2045-7022-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim JT, Kim H, Kim SH, Kim DJ, Shin Y, Kim JD, et al. Comparison of allergenic properties among commercially available house dust mite allergen extracts in Korea. Yonsei Med J. 2021;62:86–90. doi: 10.3349/ymj.2021.62.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amin HS, Liss GM, Bernstein DI. Evaluation of near-fatal reactions to allergen immunotherapy injections. J Allergy Clin Immunol. 2006;117:169–175. doi: 10.1016/j.jaci.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 133.Tankersley MS, Butler KK, Butler WK, Goetz DW. Local reactions during allergen immunotherapy do not require dose adjustment. J Allergy Clin Immunol. 2000;106:840–843. doi: 10.1067/mai.2000.110468. [DOI] [PubMed] [Google Scholar]
- 134.Roy SR, Sigmon JR, Olivier J, Moffitt JE, Brown DA, Marshall GD. Increased frequency of large local reactions among systemic reactors during subcutaneous allergen immunotherapy. Ann Allergy Asthma Immunol. 2007;99:82–86. doi: 10.1016/S1081-1206(10)60626-6. [DOI] [PubMed] [Google Scholar]
- 135.Malling HJ. Minimising the risks of allergen-specific injection immunotherapy. Drug Saf. 2000;23:323–332. doi: 10.2165/00002018-200023040-00005. [DOI] [PubMed] [Google Scholar]
- 136.Lockey RF, Nicoara-Kasti GL, Theodoropoulos DS, Bukantz SC. Systemic reactions and fatalities associated with allergen immunotherapy. Ann Allergy Asthma Immunol. 2001;87:47–55. doi: 10.1016/s1081-1206(10)62195-3. [DOI] [PubMed] [Google Scholar]
- 137.Bernstein DI, Wanner M, Borish L, Liss GM Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J Allergy Clin Immunol. 2004;113:1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 138.Lang DM, Alpern MB, Visintainer PF, Smith ST. Elevated risk of anaphylactoid reaction from radiographic contrast media is associated with both beta-blocker exposure and cardiovascular disorders. Arch Intern Med. 1993;153:2033–2040. [PubMed] [Google Scholar]
- 139.Müller UR, Haeberli G. Use of beta-blockers during immunotherapy for Hymenoptera venom allergy. J Allergy Clin Immunol. 2005;115:606–610. doi: 10.1016/j.jaci.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 140.Cox L, Larenas-Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569–574. 574.e1–567. doi: 10.1016/j.jaci.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 141.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 142.Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis: an updated practice parameter. J Allergy Clin Immunol. 2005;115:S483–S523. doi: 10.1016/j.jaci.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 143.Bousquet J, Schünemann HJ, Togias A, Bachert C, Erhola M, Hellings PW, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145:70–80.e3. doi: 10.1016/j.jaci.2019.06.049. [DOI] [PubMed] [Google Scholar]
- 144.Bousquet PJ, Combescure C, Klossek JM, Daurès JP, Bousquet J. Change in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol. 2009;123:1349–1354. doi: 10.1016/j.jaci.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 145.Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A Study Group. Efficacy and safety of preseasonal-specific immunotherapy with an aluminium-adsorbed six-grass pollen allergoid. Allergy. 2005;60:801–807. doi: 10.1111/j.1398-9995.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 146.Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J Allergy Clin Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 147.Pfaar O, Demoly P, Gerth van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854–867. doi: 10.1111/all.12383. [DOI] [PubMed] [Google Scholar]
- 148.Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156–1173. doi: 10.1111/all.13138. [DOI] [PubMed] [Google Scholar]
- 149.Di Lorenzo G, Mansueto P, Pacor ML, Rizzo M, Castello F, Martinelli N, et al. Evaluation of serum s-IgE/total IgE ratio in predicting clinical response to allergen-specific immunotherapy. J Allergy Clin Immunol. 2009;123:1103–1110. 1110.e1–1104. doi: 10.1016/j.jaci.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 150.Li Q, Li M, Yue W, Zhou J, Li R, Lin J, et al. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014;164:210–217. doi: 10.1159/000365630. [DOI] [PubMed] [Google Scholar]
- 151.Reisinger J, Horak F, Pauli G, van Hage M, Cromwell O, König F, et al. Allergen-specific nasal IgG antibodies induced by vaccination with genetically modified allergens are associated with reduced nasal allergen sensitivity. J Allergy Clin Immunol. 2005;116:347–354. doi: 10.1016/j.jaci.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 152.Lima MT, Wilson D, Pitkin L, Roberts A, Nouri-Aria K, Jacobson M, et al. Grass pollen sublingual immunotherapy for seasonal rhinoconjunctivitis: a randomized controlled trial. Clin Exp Allergy. 2002;32:507–514. doi: 10.1046/j.0954-7894.2002.01327.x. [DOI] [PubMed] [Google Scholar]
- 153.Bousquet J, Calvayrac P, Guérin B, Hejjaoui A, Dhivert H, Hewitt B, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. I. In vivo and in vitro parameters after a short course of treatment. J Allergy Clin Immunol. 1985;76:734–744. doi: 10.1016/0091-6749(85)90680-3. [DOI] [PubMed] [Google Scholar]
- 154.Francis JN, James LK, Paraskevopoulos G, Wong C, Calderon MA, Durham SR, et al. Grass pollen immunotherapy: IL-10 induction and suppression of late responses precedes IgG4 inhibitory antibody activity. J Allergy Clin Immunol. 2008;121:1120–1125.e2. doi: 10.1016/j.jaci.2008.01.072. [DOI] [PubMed] [Google Scholar]
- 155.Des Roches A, Paradis L, Knani J, Hejjaoui A, Dhivert H, Chanez P, et al. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. V. Duration of the efficacy of immunotherapy after its cessation. Allergy. 1996;51:430–433. doi: 10.1111/j.1398-9995.1996.tb04643.x. [DOI] [PubMed] [Google Scholar]
- 156.Bousquet J, Hejjaoui A, Soussana M, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. IV. Comparison of the safety and efficacy of two dosages of a high-molecular-weight allergoid. J Allergy Clin Immunol. 1990;85:490–497. doi: 10.1016/0091-6749(90)90160-6. [DOI] [PubMed] [Google Scholar]
- 157.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 158.Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI Allergen Immunotherapy User’s Guide. Pediatr Allergy Immunol. 2020;31(Suppl 25):1–101. doi: 10.1111/pai.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pfaar O, Bousquet J, Durham SR, Kleine-Tebbe J, Larché M, Roberts G, et al. One hundred and ten years of allergen immunotherapy: a journey from empiric observation to evidence. Allergy. 2022;77:454–468. doi: 10.1111/all.15023. [DOI] [PubMed] [Google Scholar]
- 160.Yu N, Luo H, Liang D, Lu N. Sublingual immunotherapy in mite-sensitized patients with atopic dermatitis: a randomized controlled study. Postepy Dermatol Alergol. 2021;38:69–74. doi: 10.5114/ada.2021.104281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kristiansen M, Dhami S, Netuveli G, Halken S, Muraro A, Roberts G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2017;28:18–29. doi: 10.1111/pai.12661. [DOI] [PubMed] [Google Scholar]
- 162.Wahn U, Tabar A, Kuna P, Halken S, Montagut A, de Beaumont O, et al. Efficacy and safety of 5-grass-pollen sublingual immunotherapy tablets in pediatric allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2009;123:160–166.e3. doi: 10.1016/j.jaci.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 163.Bufe A, Eberle P, Franke-Beckmann E, Funck J, Kimmig M, Klimek L, et al. Safety and efficacy in children of an SQ-standardized grass allergen tablet for sublingual immunotherapy. J Allergy Clin Immunol. 2009;123:167–173.e7. doi: 10.1016/j.jaci.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 164.Rienzo VD, Minelli M, Musarra A, Sambugaro R, Pecora S, Canonica WG, et al. Post-marketing survey on the safety of sublingual immunotherapy in children below the age of 5 years. Clin Exp Allergy. 2005;35:560–564. doi: 10.1111/j.1365-2222.2005.02219.x. [DOI] [PubMed] [Google Scholar]
- 165.Fiocchi A, Pajno G, La Grutta S, Pezzuto F, Incorvaia C, Sensi L, et al. Safety of sublingual-swallow immunotherapy in children aged 3 to 7 years. Ann Allergy Asthma Immunol. 2005;95:254–258. doi: 10.1016/S1081-1206(10)61222-7. [DOI] [PubMed] [Google Scholar]
- 166.Agostinis F, Tellarini L, Canonica GW, Falagiani P, Passalacqua G. Safety of sublingual immunotherapy with a monomeric allergoid in very young children. Allergy. 2005;60:133. doi: 10.1111/j.1398-9995.2004.00616.x. [DOI] [PubMed] [Google Scholar]
- 167.Shao J, Cui YX, Zheng YF, Peng HF, Zheng ZL, Chen JY, et al. Efficacy and safety of sublingual immunotherapy in children aged 3-13 years with allergic rhinitis. Am J Rhinol Allergy. 2014;28:131–139. doi: 10.2500/ajra.2014.28.4006. [DOI] [PubMed] [Google Scholar]
- 168.Pajno GB, Caminiti L, Crisafulli G, Barberi S, Landi M, Aversa T, et al. Adherence to sublingual immunotherapy in preschool children. Pediatr Allergy Immunol. 2012;23:688–689. doi: 10.1111/j.1399-3038.2012.01317.x. [DOI] [PubMed] [Google Scholar]
- 169.Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. 2017;43:13. doi: 10.1186/s13052-016-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Calderon MA, Gerth van Wijk R, Eichler I, Matricardi PM, Varga EM, Kopp MV, et al. Perspectives on allergen-specific immunotherapy in childhood: an EAACI position statement. Pediatr Allergy Immunol. 2012;23:300–306. doi: 10.1111/j.1399-3038.2012.01313.x. [DOI] [PubMed] [Google Scholar]
- 171.Arasi S, Passalacqua G, Caminiti L, Crisafulli G, Fiamingo C, Pajno GB. Efficacy and safety of sublingual immunotherapy in children. Expert Rev Clin Immunol. 2016;12:49–56. doi: 10.1586/1744666X.2016.1102058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of protocol for allergen immunotherapy
Recommended equipment and medications to treat anaphylaxis