Abstract
Background
Immunotherapy is significantly revolutionizing cancer treatment and demonstrating promising efficacy in gastric cancer (GC) patients. However, only a subset of patients could derive benefits from targeted monoclonal antibody therapy against programmed death receptor 1 (PD-1). This study aims to identify suitable serum cytokines and blood cell ratios as predictive biomarkers to aid in the selection of GC patients likely to benefit from PD-1 inhibitors.
Materials and methods
This retrospective study included 41 GC patients who received PD-1 inhibitors combined with chemotherapy, 36 GC patients treated solely with chemotherapy, and 33 healthy controls. The study assessed the levels of seven cytokines: interleukin-2 (IL-2), IL-4, IL-6, IL-10, IL-17A, tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and various inflammatory markers, including the neutrophil-to-lymphocyte ratio (NLR), total lymphocyte count (TLC), platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR). Measurements were obtained using the inpatient system. Univariate and multivariate Cox regression analyses were performed to evaluate the predictive significance of these hematologic parameters for clinical outcomes.
Results
Levels of IL-6, IL-10, TNF-α, NLR, and PLR were significantly elevated in GC patients compared to healthy controls, while TLC and LMR were higher in the control group. Among the 41 patients receiving PD-1 inhibitors and chemotherapy, baseline IL-2 was associated with OS and PFS. Additionally, IL-6 and IL-17A correlated with OS, while NLR was linked to PFS (all P<0.05). These factors were identified as independent prognostic indicators in both univariate and multivariate analyses. Furthermore, almost all cytokine levels increased following the initiation of PD-1 inhibitor treatment.
Conclusions
The introduction of PD-1 inhibitors alongside chemotherapy in GC impacts serum cytokine levels. IL-2, IL-6, IL-17A, and NLR exhibit potential as reliable circulating predictive biomarkers for identifying patients who may benefit from PD-1 inhibitors combined with chemotherapy.
Keywords: gastric cancer, immune-checkpoint inhibitors, cytokines, predictive biomarker, blood cell ratio, overall survival, progression-free survival
Introduction
Gastric cancer is a significant global health concern, ranking fifth in terms of incidence and fourth in mortality worldwide (1). Certain regions, such as Eastern Asia, Eastern Europe, and South America, have particularly high rates of gastric cancer cases. In mainland China, a considerable number of patients are diagnosed at an advanced stage due to low screening rates and subtle clinical symptoms, resulting in missed opportunities for surgery and poorer prognoses (2). Fortunately, the development of immunotherapy for gastric cancer has shown promising results, changing traditional treatment approaches.
The immune checkpoint is a vital element of the immune system, consisting of receptors found on the surface of immune cells that can either positively or negatively regulate immune responses. For example, PD-1, located on the surface of T cells, functions as a natural brake to control the excessive activity of cytotoxic T effector cells when it binds to its ligand PD-L1. PD-L1 is commonly found in both normal tissues and tumor cells, and their interactions help limit immune-mediated tissue damage and support tumor cells in evading the immune system (3). Immunotherapy using Immune Checkpoint Inhibitors (ICIs) has emerged as a promising approach in the treatment of various cancers. ICIs target the PD-1/PD-L1 pathway to boost the reactivity of anti-tumor T cells. Notably, several PD-1 inhibitors (Nivolumab, Pembrolizumab, Sintilimab, Camrelizumab, Tislelizumab) and PD-L1 inhibitors (Atezolizumab, Avelumab, Durvalumab) have received approval for cancer therapy and have demonstrated effectiveness in an expanding range of malignancies, including gastroesophageal, melanoma, and lung cancers (4). However, despite these significant advancements, a considerable proportion of gastric cancer patients receiving ICIs do not derive therapeutic benefits (5). Numerous clinical studies have been conducted to identify biomarkers that can predict which gastric cancer patients are likely to respond well to ICIs therapy. Some potential biomarkers include PD-L1 expression, tumor mutational burden (TMB), microsatellite instability/mismatch repair (MSI/MMR) status, Epstein-Barr virus (EBV) infection, circulating tumor DNA (ctDNA), and gut microbiota. However, their practical application in day-to-day clinical practice still requires further confirmation (6).
Recent research has been rapidly uncovering the mechanisms linking infection, innate immunity, inflammation, and cancer (7). Cytokines, produced by activated immune cells, play a crucial role in this linkage. Pro-inflammatory cytokines such as IL-1β, IL-8, IL-12, TNF-α, IFN-γ, and anti-inflammatory cytokines like IL-4 and IL-10 have dual functions, activating anti-tumorigenic actions of T cells while also participating in tumor malignant transformation, growth, invasion, and metastasis (8). Cytokines can activate anti-tumorigenic actions of T cells and also contribute to tumor growth, invasion, and metastasis (9). Moreover, systemic inflammatory response (SIR) indicators, such as NLR, PLR, LMR, and TLC, have been reported to be associated with the prognosis of certain cancers (10). Changes in cytokine expression levels and cell composition in the tumor microenvironment (TME) can potentially influence the efficacy of ICIs in various malignancies (11). Therefore, multiplex cytokine and blood cell analysis could yield valuable prognostic assessments in patients.
This study aims to examine the association between baseline and post-treatment peripheral cytokines and blood cells in GC patients who received PD-1 inhibitors combined with chemotherapy. The goal is to identify clinically significant predictive factors for the efficacy of immunotherapy in patients with gastric cancer.
Materials and methods
Patient characteristics
December 2022. Among them, 41 GC patients received PD-1 inhibitors in combination with chemotherapy (Cohort 1), while 36 GC patients underwent chemotherapy alone (Cohort 2). The 33 healthy controls exhibited good health without any indications of tumors, viral infections, diabetes, connective tissue diseases, or liver/kidney impairments. Inclusion criteria for the 77 patients included: 1) histopathological confirmation of gastric cancer at stage II-IV according to the American Joint Committee on Cancer (AJCC); 2) receiving PD-1 inhibitors combined with chemotherapy (Cohort 1) or chemotherapy alone (Cohort 2) for a minimum of 3 cycles; 3) regular tumor assessments every 2 treatment courses using imaging evaluations, with Overall Survival (OS) and Progression-Free Survival (PFS) times recorded based on imaging results and follow-up phone calls; 4) blood samples collected for cytokine and blood cell analysis when tumor progression or response was observed; 5) physical condition scored according to the Eastern Cooperative Oncology Group guidelines (ECOG) ranging from 0 to 3 (12), and no dysfunction in vital organs detected. This study adhered to the principles outlined in the World Medical Association’s Declaration of Helsinki and received approval from the Medical Ethical Committee of our hospital. Since only anonymous data were used for this retrospective study, the ethics committee waived the requirement for informed consent.
Treatment
In Cohort 2, 36 GC patients were treated: 19 received first-line Sox (Oxaliplatin, Teggio) chemotherapy, 15 received first-line Sox combined with albumin-bound paclitaxel, and 2 received first-line Xelox (oxaliplatin and capecitabine). The median number of cycles for the first-line chemotherapy was 5, with a range from 3 to 10, and no subsequent PD-1 inhibitor treatment was administered. In Cohort 1, 41 patients were included: 14 received PD-1 inhibitors as part of the first-line therapy, while 27 received PD-1 inhibitors during subsequent-line therapy. The PD-1 inhibitors used were Sintilimab, Camrelizumab, and Tislelizumab, combined with chemotherapy over a 21-day cycle. The chemotherapy regimen was consistent with the description above. The median number of chemoimmunotherapy cycles was 5, with a range from 3 to 14.
Analysis of survival
Tumor assessments were performed after every two treatment courses using various imaging techniques such as CT, ultrasound, MRI, or PET-CT. The evaluation was conducted following the Response Evaluation Criteria of Solid Tumors 1.1 (RECIST1.1) criteria (13). PFS was calculated from the initiation of anti-tumor therapy to the date of disease progression. On the other hand, OS was measured from the date of the first treatment dose until death from any cause.
Blood sample collection and measurements
Plasma samples were collected from the patients before the first treatment and at the time of disease remission or progression. These samples were then centrifuged at 1000 g for 10 min at 4°C. After centrifugation, the supernatant (serum) was immediately extracted and analyzed on the spot or divided into aliquots and stored frozen at −80°C. Cytokine levels were assessed using the Human Cytokine 12 Plex Kit (Beijing ACRO Biosystems, catalog number: CRS- A002/A017/B001/B003/B005/B008) at the clinical laboratory department of our hospital. The panel of measured cytokines included IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, and IL-17A. Beyond that, blood routine examination was achieved by flow cytometry, NLR, PLR, and LMR were then calculated as the total neutrophil counts divided by the lymphocyte counts, platelet counts divided by the TLCs, and the TLCs divided by the total monocyte counts, respectively.
Cytokine cut-off value calculation
To assess the correlation between baseline blood parameters and survival, we categorized the baseline blood parameters into high-level and low-level groups. This categorization was based on either the median value or the optimal cut-off value ( Tables S1-2 ). To ascertain the most suitable cut-off value for the studied indicators, we employed the web-based software X-tile ( Table S3-4 ).
Statistical analyses
Patient characteristics underwent analysis using descriptive statistical methods. Continuous variables were summarized using medians and quartiles, and comparisons were conducted using the Mann-Whitney U test and the Kruskal-Wallis test. Categorical variables were presented as numbers (%) and analyzed using the chi-squared test and Fisher’s exact test. For the evaluation of independent prognostic factors, both univariate and multivariate analyses were performed. Hazard ratios (HRs) and 95% confidence intervals (CIs) were reported. In the multivariable model, only elements with a p-value of <0.1 from the univariate analysis were incorporated. The significance threshold for multivariate analyses was set at P < 0.05. OS and PFS were illustrated using the Kaplan-Meier method, and the log-rank test was employed to compare the survival curves. All statistical analyses were executed using SPSS version 26.0 software, and the figures were generated using GraphPad Prism version 8.0.
Results
Patients’ characteristics and survival outcomes
Table 1 presents the clinical characteristics and pre-treatment blood parameters of the 77 patients diagnosed with GC. Cohort 1 consisted of a higher percentage of patients in stages III-IV of the TNM classification (82.93% vs. 58.33%) and more patients who had not undergone gastric surgery (56.10% vs. 22.22%) compared to Cohort 2. The level of IL-6 was found to be higher in Cohort 1 than in Cohort 2 (12.06 vs. 4.85). Patients in Cohort 1, who received chemoimmunotherapy, experienced a significantly better PFS of 10.67 months compared to 8.1 months in Cohort 2 (p = 0.003). Additionally, Cohort 1 also showed an improved OS of 15.7 months compared to 10.83 months in Cohort 2 (p = 0.021). No statistically significant differences were observed between Cohort 1 and Cohort 2 regarding age, sex, ECOG score, presence of other chronic diseases (diabetes, hypertension, cardiopathy), history of smoking, and family history of cancer (p > 0.05).
Table 1.
Characteristics of patients at baseline.
| Clinical characteristics | GC patients (n=77) n (%) |
Cohort 1 (n=41) n (%) |
Cohort 2 (n=36) n (%) |
P | |
|---|---|---|---|---|---|
| Gender | male | 53 (68.831%) | 26 (63.415%) | 27 (75.000%) | 0.273 |
| female | 24 (31.169%) | 15 (36.585%) | 9 (25.000%) | ||
| Age | <60 | 41 (53.247%) | 24 (58.537%) | 17 (47.222%) | 0.321 |
| ≥60 | 36 (46.753%) | 17 (41.463%) | 19 (52.778%) | ||
| ECOG score | ≤2 | 63 (81.818%) | 34 (82.927%) | 29 (80.556%) | 0.788 |
| >2 | 14 (18.182%) | 7 (17.073%) | 7 (19.444%) | ||
| TNM stage | II | 22 (28.571%) | 7 (17.073%) | 15 (41.667%) | 0.017 |
| III-IV | 55 (71.429%) | 34 (82.927%) | 21 (58.333%) | ||
| Surgery history | Yes | 46 (59.740%) | 18 (43.902%) | 28 (77.778%) | 0.002 |
| No | 31 (40.260%) | 23 (56.098%) | 8 (22.222%) | ||
| Smoked | Yes | 27 (35.065%) | 15 (36.585%) | 12 (33.333%) | 0.765 |
| No | 50 (64.935%) | 26 (63.415%) | 24 (66.667%) | ||
| family cancer history | Yes | 14 (18.182%) | 6 (14.634%) | 8 (22.222%) | 0.389 |
| No | 63 (81.818%) | 35 (85.366%) | 28 (77.778%) | ||
| other chronic disease | Yes | 20 (25.974%) | 10 (24.390%) | 10 (27.778%) | 0.735 |
| No | 57 (74.026%) | 31 (75.610%) | 26 (72.222%) | ||
| mPFS (month) | median | 8.87 | 10.67 | 8.1 | 0.003 |
| mOS (month) | median | 14.83 | 15.7 | 10.83 | 0.021 |
| IL-2 | median[Q1, Q3] | 1.740[1.210,2.590] | 1.960[1.420,2.600] | 1.490[1.110,2.260] | 0.213 |
| IL-4 | median[Q1, Q3] | 1.990[0.910,3.310] | 2.370[1.020,3.310] | 1.930[0.800,3.080] | 0.444 |
| IL-6 | median[Q1, Q3] | 6.100[3.970,14.190] | 12.060[5.020,18.060] | 4.850[2.980,8.280] | <0.001 |
| IL-10 | median[Q1, Q3] | 2.690[1.870,3.790] | 2.860[1.870,3.840] | 2.650[1.890,3.650] | 0.748 |
| TNF-α | median[Q1, Q3] | 1.940[1.300,2.700] | 1.870[1.470,2.550] | 2.230[1.230,2.910] | 0.537 |
| IFN-γ | median[Q1, Q3] | 2.060[1.460,2.590] | 2.110[1.500,2.560] | 1.940[1.460,2.620] | 0.736 |
| IL-17A | median[Q1, Q3] | 5.680[2.900,9.260] | 5.680[2.340,9.890] | 5.720[3.190,8.910] | 0.779 |
| TLC | median[Q1, Q3] | 1.320[1.040,1.700] | 1.310[1.000,1.790] | 1.370[1.200,1.690] | 0.721 |
| NLR | median[Q1, Q3] | 2.338[1.571,3.444] | 2.600[1.692,4.500] | 2.338[1.571,2.628] | 0.234 |
| PLR | median[Q1, Q3] | 149.231[109.924,209.375] | 149.231[109.924,205.833] | 149.693[114.557,223.171] | 0.732 |
| LMR | median[Q1, Q3] | 3.478[2.370,4.238] | 3.462[2.167,4.238] | 3.714[2.726,3.953] | 0.713 |
Eastern Cooperative Oncology Group Performance Status (ECOG PS). P < 0.05 was considered statistically significant and shown in bold type.
Comparison of baseline blood parameters between GC patients and healthy individuals
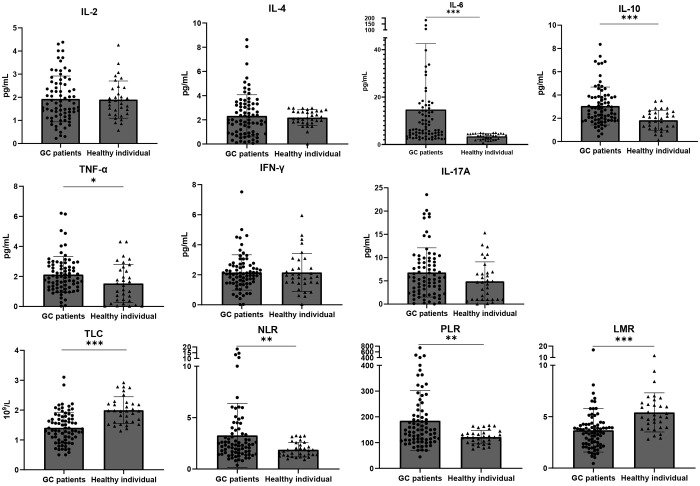
To clarify the significance of cytokines in GC diagnosis, we included 33 healthy participants. As displayed in Table S5 , there was no marked difference in age and gender distribution between the healthy controls and GC patients (p > 0.05), making subsequent results comparable. Figure 1 reveals that, aside from IL-2 and IL-4, levels of all other cytokines were elevated in GC patients compared to healthy individuals. Specifically, the differences in IL-6, IL-10, and TNF-αwere statistically significant (p<0.0001, p<0.0001, p = 0.021, respectively). It is worth highlighting that every blood cell component ratio studied exhibited statistical differences between the two cohorts. In healthy individuals, both TLC and LMR were higher (p<0.0001, p<0.0001), while GC patients had elevated NLR and PLR (p = 0.0085, p = 0.0034) ( Table S6 ).
Figure 1.
Baseline IL-6, IL-10, TNF-α, NLR and PLR are higher, TLC and LMR are lower in GC patients than in healthy individuals. Dot plots show the difference of baseline blood parameters between healthy volunteers (n = 33) and GC patients (n = 77). The top of the grey box shows the median value. All cytokines except for IL-2 and IL-4 were higher in GC patients, but only in the case of IL-6 (p<0.0001), IL-10 (p<0.0001) and TNFα (p = 0.021) these differences were statistically significant. TLC (p<0.0001) and LMR (p<0.0001) were higher in healthy individuals while NLR(p=0.0085 )and PLR (p=0.0034)were higher in GC patients. Error bars show the interquartile range. *p < 0.05, **p < 0.01, and ***p < 0.001.
Correlation between blood indexes and clinical features in GC patients
As illustrated in Table S7 , females exhibited notably higher baseline levels of IL-6 and PLR, while their NLR was significantly lower (p = 0.032, p = 0.046, p = 0.003, respectively). Elevated IL-6 levels were also observed in patients without a family history of cancer (p = 0.012) and in those aged above 60 years (p = 0.048). Patients who underwent gastric surgery had significantly increased levels of IFN-γ and NLR (p = 0.009, p = 0.017). Patients with an ECOG score of ≤2 had a notably raised TLC (p = 0.048). Conversely, LMR was distinctly lower in patients diagnosed with primary diseases such as hypertension, diabetes, and stroke (p = 0.028). There were no statistically significant differences in cytokine levels based on clinical stages or smoking histories (p > 0.05).
Associations between blood indexes and survival outcomes
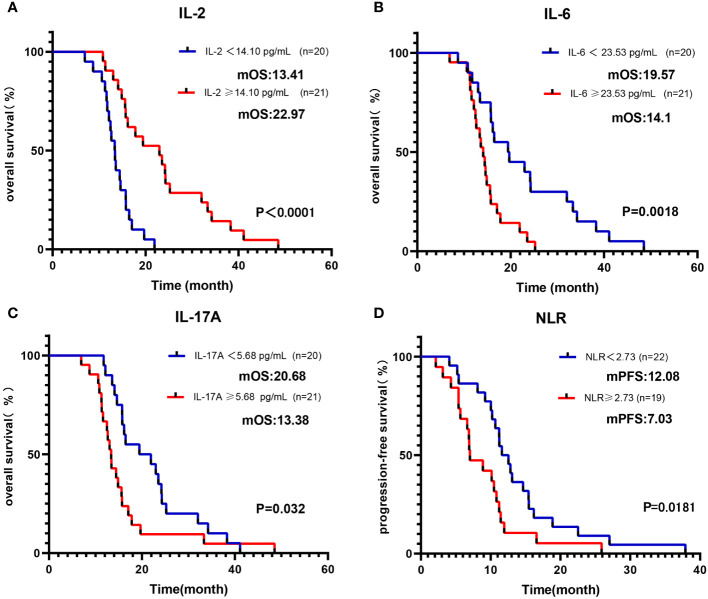
Initially, we categorized baseline blood parameters into a high-level group and a low-level group based on the median value. As outlined in Table 2 , univariate analysis revealed a significant association between OS and several factors, including IL-2, IL-6, IFN-γ, IL-17A, NLR, and ECOG (all p < 0.05). To account for other potential influences on survival outcomes, a multivariable Cox regression analysis was conducted. It confirmed that the IL-2-high group had an improved OS, whereas the IL-6-high and IL-17A-high groups exhibited reduced OS (all p < 0.05) ( Figures 2A–C ). Regarding PFS, the univariate analysis indicated significant associations with IL-2, IL-4, IL-6, IL-10, IFN-γ, and NLR (all p < 0.1). Subsequent multivariate analysis confirmed that the NLR-high group had a reduced PFS (p <0.01) ( Figure 2D ). In a similar manner, we conducted an analysis of the prognostic impact of blood parameters (categorized by the median) after the initial 2 treatment cycles. The multivariable regression analysis revealed that the IL-6-low group exhibited an enhanced OS and PFS, whereas the IL-2-high groups showed increased OS. Conversely, the IL-17A-high group demonstrated a diminished PFS (all with p < 0.05) ( Table S8 ).
Table 2.
Univariate and Multivariate analysis for PFS and OS of Cohort 1 baseline blood parameters grouped by median.
| OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | univariate analysis | multivariate analysis | univariate analysis | multivariate analysis | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| IL-2 | 0.217 (0.098-0.478) | 0.000 | 0.382 (0.165-0.888) | 0.025 | 0.432 (0.223-0.838) | 0.013 | 0.702 (0.294-1.674) | 0.425 |
| IL-4 | 0.745 (0.396-1.401) | 0.361 | - | - | 0.547 (0.287-1.041) | 0.066 | 0.73 (0.333-1.599) | 0.431 |
| IL-6 | 2.944 (1.453-5.965) | 0.003 | 3.018 (1.367-6.666) | 0.006 | 2.212 (1.145-4.273) | 0.018 | 1.882 (0.866-4.089) | 0.110 |
| IL-10 | 0.74 (0.393-1.396) | 0.353 | - | - | 0.516 (0.265-1.002) | 0.051 | 0.668 (0.311-1.437) | 0.302 |
| TNF-α | 0.88 (0.46-1.683) | 0.699 | - | - | 0.919 (0.49-1.722) | 0.791 | - | - |
| IFN-γ | 0.355 (0.174-0.725) | 0.004 | 0.553 (0.251-1.218) | 0.142 | 0.567 (0.296-1.084) | 0.086 | 0.79 (0.372-1.677) | 0.540 |
| IL-17A | 1.978 (1.049-3.729) | 0.035 | 2.143 (1.077-4.265) | 0.030 | 1.382 (0.739-2.587) | 0.311 | - | - |
| TLC | 0.956 (0.501-1.824) | 0.891 | - | - | 1.454 (0.727-2.908) | 0.289 | - | - |
| NLR | 2.162 (1.137-4.111) | 0.019 | 2.022 (0.981-4.166) | 0.056 | 2.38 (1.241-4.563) | 0.009 | 2.886 (1.418-5.876) | 0.003 |
| PLR | 1.052 (0.559-1.981) | 0.876 | - | - | 0.946 (0.508-1.763) | 0.862 | - | - |
| LMR | 1.156 (0.607-2.203) | 0.660 | - | - | 0.874 (0.467-1.635) | 0.673 | - | - |
| gender | 0.921 (0.479-1.768) | 0.804 | - | - | 0.875 (0.459-1.667) | 0.685 | - | - |
| age | 0.74 (0.39-1.403) | 0.357 | - | - | 0.968 (0.504-1.86) | 0.922 | - | - |
| ECOG (>2) | 10.172 (3.453-29.966) | 0.000 | 7.481 (2.19-25.548) | 0.001 | 1.659 (0.723-3.81) | 0.233 | - | - |
| TNM stage (III-IV) | 1.267 (0.526-3.055) | 0.598 | - | - | 0.953 (0.391-2.322) | 0.915 | - | - |
| surgery history | 1.33 (0.699-2.529) | 0.385 | - | - | 0.957 (0.512-1.79) | 0.891 | - | - |
| other chronic basic diseases | 1.001 (0.484-2.069) | 0.999 | - | - | 1.072 (0.521-2.208) | 0.850 | - | - |
| smoked | 0.903 (0.47-1.734) | 0.758 | - | - | 0.97 (0.508-1.852) | 0.927 | - | - |
| family cancer history | 1.373 (0.563-3.349) | 0.486 | - | - | 0.765 (0.319-1.833) | 0.548 | - | - |
Baseline blood parameters were grouped by the median. HR, hazard ratios; CI, confidence interval. basic disease (diabetes, hypertension, cardiopathy). Elements with a p-value of <0.1 in the univariate analysis and with a p-value of <0.05 in the multivariate analysis were in bold type.
Figure 2.
Kaplan-Meier curve of OS/PFS of Cohort 1 patients, grouped by median of baseline blood parameters. Kaplan-Meier OS curves according to baseline median of (A) IL-2; (B) IL-6; (C) IL-17A. Kaplan-Meier PFS curves according to baseline median of (D) NLR.
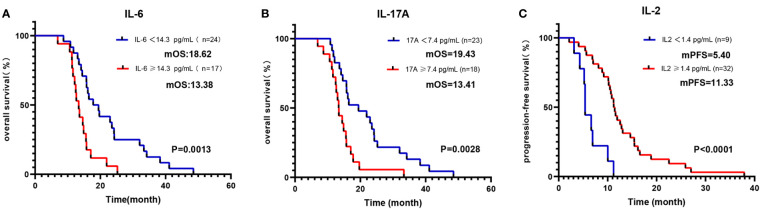
As detailed in Table 3 , the blood parameters of Cohort 1 were divided into high-level and low-level groups based on a cut-off value. Univariate analysis identified significant links between OS and parameters such as IL-2, IL-6, TNF-α, IFN-γ, IL-17A, NLR, and ECOG (all p <0.05). Further multivariate analysis verified that the IL-6-high and IL-17A-high groups had diminished OS (all p < 0.05) ( Figures 3A, B ). Similarly, univariate analysis revealed a significant relationship between PFS and variables like IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, NLR, and LMR (all p < 0.1). Upon multivariate assessment, the IL-2-high group was found to have a superior PFS (all p < 0.05) ( Figure 3C ). In a parallel manner, the Cox regression analysis of blood parameters (categorized by cut-off value) after the initial 2 treatment cycles indicated that the IL-2-high and IL-6-low groups experienced enhanced overall survival (OS) and progression-free survival (PFS), while the IL-17A-high groups displayed decreased PFS (all p < 0.01) ( Table S9 ).
Table 3.
Univariate and Multivariate analysis for PFS and OS of Cohort 1 baseline blood parameters grouped by cut-off value.
| OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | univariate analysis | multivariate analysis | univariate analysis | multivariate analysis | ||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| IL-2 | 0.215 (0.097-0.474) | 0.000 | 0.735 (0.267-2.026) | 0.552 | 0.195 (0.083-0.456) | 0.000 | 0.354 (0.127-0.983) | 0.046 |
| IL-4 | 0.713 (0.378-1.346) | 0.297 | – | – | 0.47 (0.243-0.909) | 0.025 | 0.62 (0.287-1.34) | 0.224 |
| IL-6 | 2.969 (1.489-5.92) | 0.002 | 3.092 (1.204-7.943) | 0.019 | 1.815 (0.923-3.569) | 0.084 | 2.114 (0.935-4.78) | 0.072 |
| IL-10 | 0.65 (0.337-1.254) | 0.199 | – | – | 0.423 (0.209-0.859) | 0.017 | 0.511 (0.226-1.156) | 0.107 |
| TNF-α | 0.278 (0.111-0.701) | 0.007 | 0.697 (0.189-2.573) | 0.588 | 0.45 (0.183-1.107) | 0.082 | 1.828 (0.53-6.301) | 0.339 |
| IFN-γ | 0.355 (0.174-0.725) | 0.004 | 0.475 (0.179-1.262) | 0.135 | 0.45 (0.213-0.949) | 0.036 | 0.414 (0.17-1.006) | 0.051 |
| IL-17A | 2.704 (1.378-5.306) | 0.004 | 2.715 (1.156-6.375) | 0.022 | 1.382 (0.739-2.587) | 0.311 | – | – |
| TLC | 0.703 (0.332-1.491) | 0.359 | – | – | 1.218 (0.63-2.354) | 0.558 | – | – |
| NLR | 2.967 (1.301-6.766) | 0.010 | 2.036 (0.758-5.463) | 0.158 | 2.22 (1.149-4.29) | 0.018 | 1.645 (0.653-4.141) | 0.291 |
| PLR | 0.775 (0.374-1.606) | 0.493 | – | – | 0.716 (0.352-1.46) | 0.359 | – | – |
| LMR | 0.508 (0.213-1.211) | 0.127 | – | – | 0.54 (0.274-1.065) | 0.075 | 0.575 (0.207-1.594) | 0.287 |
| gender | 0.921 (0.479-1.768) | 0.804 | – | – | 0.875 (0.459-1.667) | 0.685 | – | – |
| age | 0.74 (0.39-1.403) | 0.357 | – | – | 0.968 (0.504-1.86) | 0.922 | – | – |
| ECOG (>2) | 10.172 (3.453-29.966) | 0.000 | 7.546 (2.281-24.966) | 0.001 | 1.659 (0.723-3.81) | 0.233 | – | – |
|
TNM stage
(III-IV) |
1.267 (0.526-3.055) | 0.598 | – | – | 0.953 (0.391-2.322) | 0.915 | – | – |
| surgery history | 1.33 (0.699-2.529) | 0.385 | – | – | 0.957 (0.512-1.79) | 0.891 | – | – |
| other chronic basic disease | 1.001 (0.484-2.069) | 0.999 | – | – | 1.072 (0.521-2.208) | 0.850 | – | – |
| smoked | 0.903 (0.47-1.734) | 0.758 | – | – | 0.97 (0.508-1.852) | 0.927 | – | – |
| family cancer history | 1.373 (0.563-3.349) | 0.486 | – | – | 0.765 (0.319-1.833) | 0.548 | – | – |
Baseline blood parameters were grouped by cut-off value. Elements with a p-value of <0.1 in the univariate analysis and with a p-value of <0.05 in the multivariate analysis were in bold type.
Figure 3.
Kaplan-Meier curve of OS/PFS of Cohort 1 patients, grouped by cut-off values of baseline blood parameters. Kaplan-Meier OS curves according to baseline cut-off values of (A) IL-6; (B) IL-17A. Kaplan-Meier PFS curves according to baseline cut-off values of (C) IL-2.
Interestingly, these relationships between blood parameters and clinical outcomes were exclusive to Cohort 1. When focusing on Cohort 2, where patients underwent only chemotherapy, these associations were not evident ( Tables S10-11 ). This implies that baseline serum IL-2, IL-6, IL-17A, and NLR can independently forecast the efficacy of PD-1 inhibitors in GC patients.
Dynamic changes of cytokines once treatment was initiated in each cohort
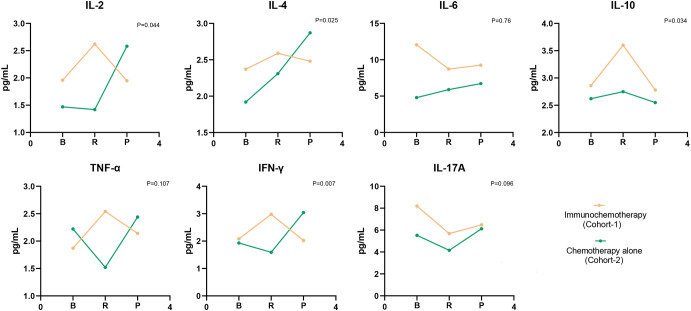
As depicted in Figure 4 , there was a general elevation from baseline to the moment the tumor exhibited its first complete response (CR) or partial response (PR) in all cytokines, with the exceptions being IL-6 and IL-17A in Cohort 1. Notably, the levels of IL-2, IL-4, IL-10, and IFN-γ were statistically significantly increases (p = 0.044, p = 0.025, p = 0.034, p = 0.007, respectively). In contrast, Cohort 2 displayed a decline in IL-2, TNF-α, IFN-γ, and IL-17A from baseline to response. While IL-4, IL-6, and IL-10 demonstrated a rise from baseline to tumor response, none of these changes reached statistical significance. We calculated the percentage variations in cytokine levels from baseline to response to determine if these quantitative shifts during treatment correlated with survival outcomes. As illustrated in Figure 5 , individuals in Cohort 1, where IL-2 levels increased by over 20% from baseline to response, showed a considerably improved OS (16.32 m vs. 13.03 m; p = 0.0154). This trend in IL-2 variation was also observed in Cohort 2 patients, but it did not maintain statistical significance (13.58 m vs. 12.49 m, p = 0.6537). We additionally computed the percentage variations in cytokine levels from baseline to the timepoint following 2 treatment cycles, but we did not observe any consistent trend.
Figure 4.
Evolution of cytokine levels in patients of two Cohorts. Values corresponded to the median of cytokine titers, and p values were obtained taking into account the difference of cytokine levels in the baseline and response period. B, baseline; R, response, include first complete response (CR) and partial response (PR); P, progression.
Figure 5.
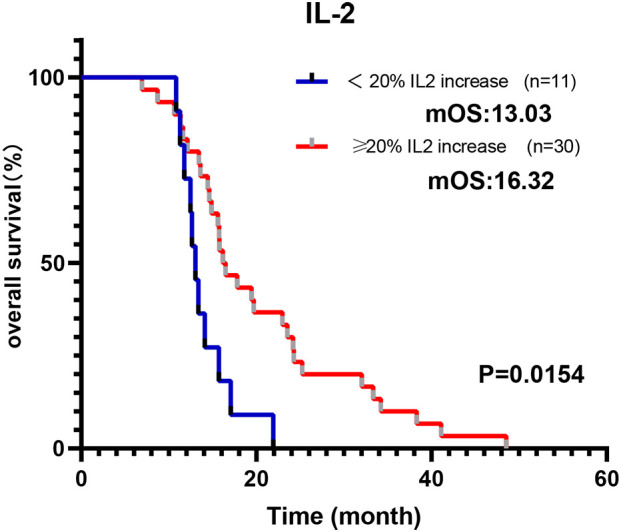
Modulation of IL-2 during immunochemotherapy treatment predicts a better prognosis. Cohort 1 patients whose IL-2 increased more than 20% from baseline to response indicate a longer OS.
Discussion
The detection of cytokines and blood cell parameters is preferable to other biomarkers due to their widespread use and minimally invasive sampling technique. In this study, we had access to two groups of patients treated either with immunochemotherapy or solely with chemotherapy. This allowed us to assess the biological impacts of incorporating Immune ICIs. Only associations that were notably significant in Cohort 1, distinct from those observed in Cohort 2 (chemotherapy-only group), were considered indicative of the effects related to ICIs. We employed statistical analysis to determine whether baseline levels and variations in cytokines and blood cell parameters could predict the efficacy of immunotherapy across different treatment outcomes. Initially, we compared baseline cytokine levels of GC patients against those found in healthy subjects. One significant observation was the universally elevated cytokine levels in GC patients, with the exceptions being IL-2 and IL-4. Notably, levels of IL-6, IL-10, and TNF-α were markedly increased in the GC patient group. As depicted in Figure 1 , there were no significant differences in the levels of IL-2, IL-4, INF-γ, and IL-17A between GC patients and healthy controls. IL-2 is primarily secreted by T cells (14), and our study observed a decrease in the total lymphocyte count among gastric cancer patients. Consistent with our findings, Mohammad et al. (15), reported no significant differences in IL-2 levels between gastric cancer patients and healthy controls. Furthermore, another study noted that patients with gastric cancer stage III or IV exhibited elevated levels of IL-2, while there was no distinction in the serum levels of IL-2 between patients with gastric cancer stage I or II and healthy controls (16), which aligns with our findings in Table S7 . Increased IL-4 levels have been frequently observed in various types of cancers. However, the evidence regarding the pro- or antitumoral role of IL-4 is conflicting, and this function is closely linked to IL-4 levels and its interaction with other immunological modulators (17). IFN-γ, produced by numerous immune cell subsets (including T cells, natural killer cells, B cells, and others), possesses both pro-tumor and anti-tumor activities (18). Nitu et al. reported that no significant differences existed in the concentration of IFN-γ between patients and healthy controls (19), which is consistent with our findings. Norma et al. also identified that circulating levels of IL-6 and IL-10 were discernibly higher in GC patients compared to a healthy control group (20), aligning with our results. Numerous studies indicate the pivotal role of IL-6 in a variety of malignancies (21–23). Elevated serum IL-6 concentrations have been documented in several solid tumors, including those of the lung, breast, pancreas, and stomach (24). The STAT3 pathway, when activated by IL-6, up-regulates the expression of cyclins and down-regulates the expression of the cyclin-dependent kinase (Cdk) inhibitor p21. This mechanism consequently promotes tumor cell cycle progression, leading to metastasis and tumor cell proliferation (25). Additionally, IL-6 has been reported to prevent cellular senescence by increasing telomerase activity, thereby promoting tumor growth (26). Studies indicate that IL-10 primarily inhibits the differentiation and antigen-presenting properties of DCs (dendritic cells) during the early stages of immune response (27). As a result, IL-10 significantly suppresses the production of IL-2 from antigen-presenting cells. In the absence of Th1-associated cytokines (like IL-2), the T-cell-mediated response is inevitable (28). While TNF-α, a pro-inflammatory cytokine, has been linked to promoting tumor metastasis and correlated with advanced cancer stages (29–31), its presence in cancers has also been associated with immune suppression. Animal model research further supports TNF-α’s role in promoting tumor growth and malignancy (32–35). Conversely, there are reports suggesting the benefits of the potent pro-inflammatory cytokine (TNF-α) in cancer treatments, especially given its recognition as a major factor in the anti-tumor activities of Coley’s toxins (36). In this study, both NLR and PLR were statistically elevated in GC patients compared to healthy controls. The neutrophil-to-lymphocyte ratio in peripheral blood reflects the balance between systemic inflammation and immunity. Consistent with our results, Mishra et al. discovered that the NLR is higher in cancer patients and its elevated level is linked to a worse.
In SIR studies, elevated NLR levels after ICI treatment have been linked to reduced survival rates in advanced esophagus cancer and lung cancer (10, 37). Consistent with these findings, our patients with a pre-treatment NLR above the median demonstrated a notably worse PFS. This negative correlation may be indicative of the interplay between intense inflammation and compromised immune function (38). While some studies suggest that a higher PLR corresponds to a worse prognosis in lung cancer patients (38), there is a dearth of research examining whether PLR, TLC, and LMR values differ between cancer patients and healthy individuals.
Cytokines represent a broad category of intercellular signaling proteins that play a pivotal role in almost every aspect of human immunology. However, the interaction of cytokine signaling activities is highly complex due to the redundancy and pleiotropy exhibited by cytokines. Moreover, there exists an intricate network of “cytokine cascades,” wherein the expression of a specific cytokine gene is invariably influenced by other cytokines (39). Cytokines are subject to regulation through various mechanisms. For instance, the anti-inflammatory cytokine IL-10 can suppress the expression of TNF-α and IFN-γ, a process referred to as feedback inhibition (40). IL-4, on the other hand, can suppress the production of IFN-γ by T cells, a phenomenon known as antagonism (41), IL-2, conversely, can enhance the production of IFN-γ (42), and IL-17A can synergistically stimulate TNF-α-induced IL-8 production (43).
To explore the prognostic and predictive role of cytokines, we examined the baseline and variations in cytokine levels and assessed their influence on patient outcomes across both cohorts. Cohort 2 had a higher number of patients in the early stages, and more had undergone radical surgery, which is traditionally considered a positive indicator for survival. However, the better OS in Cohort 1 implies that immunotherapy plays a more pivotal role in enhancing survival. A comparative analysis of the two cohorts allowed us to discern the specific effects associated with ICIs. Based on our findings, IL-2 can be perceived as a predictor of favorable response to ICIs. Higher baseline levels of IL-2 correlated with a significantly extended PFS and OS in Cohort 1, a distinction not observed in Cohort 2. IL-2 is a cytokine important in T-cell proliferation and promoting immune responses, as well as in increasing the activity of natural killer cells (44). Garrelds et al. identified that mice deficient in IL-2 are more prone to gastrointestinal inflammation, resembling human ulcerative colitis (45). Ren et al. documented that combining IL-2 with anti-PD-1 helps overcome tumor resistance to ICIs in mice by reactivating intratumoral CD8+ T cells rather than CD4+ Treg cells (46). Similarly, Ewan A et al. reported a two-year remission resulting from combined anti-PD-1 and intralesional IL-2 therapy in two patients with locoregional metastatic melanoma. This impressive response was partly due to an altered tumor microenvironment, including increased PD-L1 expression and CD8 T cell infiltration (47). Moreover, as shown in Figure 5 , patients of Cohort-1 whose IL-2 increased more than 20% from baseline as a response, had a longer OS, which conforms to our preceding view.
IL-6 seems to be a predictor of resistance to ICIs, as patients with higher levels of this factor were found to have significantly worse OS. These observations perfectly agree with the study by Yu et al., who reported that increased circulating levels of IL-6 are associated with poor outcomes in liver cancer patients who received therapy with PD-1 inhibitors (48). IL-6 is a pro-inflammatory cytokine that may contribute to tumor progression by stimulating angiogenesis, invasion, and metastasis (8, 49). In some studies, increased IL-6 serum levels were reported to be associated with metastasis and poor prognosis in prostate, ovarian, and gastrointestinal cancers (21, 50, 51). Tsukamoto et al. indicated that increased IL-6 levels could indicate decreased efficacy of PD-1 blockade in patients with melanoma, and IL-6 blockade augments PD-L1 expression on tumor cells (52). Consistently, a study using IL-6-deficient mice bearing a murine colon cancer cell line found that the lack of IL-6 enhances the induction of effector T cells and inhibits tumorigenesis. Additionally, PD-L1 expression levels on tumor cells were significantly increased in the IL-6-deficient mice compared with wild-type mice (53). These findings strongly indicate the negative immune role of IL-6, especially in patients receiving ICIs.
IL-17A is a prominent member of the IL-17 family of pro-inflammatory cytokines. Prior research has reported its upregulation in the serum and tumors of GC patients. Kang et al. suggested that IL-17A promotes gastric carcinogenesis by regulating the IL-17RC/NF-κB/NOX1 pathway (54). However, it is worth noting that Karl et al. (55) found decreased IL-17A levels in esophageal adenocarcinoma patients when compared to healthy controls. In our study, we observed a less pronounced elevation of IL-17A in GC patients in comparison to healthy controls (as shown in Figure 1 ). Furthermore, our study revealed that GC patients with lower levels of IL-17A experienced improved OS, as demonstrated in Figures 2C , 3B . Interestingly, IL-17A exhibited a noticeable decline from baseline to the point of maximum tumor remission. Accumulating evidence indicates that IL-17A activity may contribute to resistance to anti-tumor immunity and play a role in therapeutic failure. It is reported that the IL-17A signaling pathway can enhance the immunosuppressive activity of regulatory T cells (Tregs), leading to tumor growth and development (56). Liu et al. revealed that IL-17A increases PD-L1 expression through the p65/NRF1/miR-15b-5p axis, thereby promoting resistance to anti-PD-1 therapy. Blocking IL-17A improved the efficacy of anti-PD-1 treatment in murine models of MSS CRC (57). Another clinical analysis suggested that the activation of IL-17A signaling is associated with the failure of anti-PD-1 therapy in patients with colorectal cancer (58).
Prior research has shown that tumor cells release cytokines, vascular endothelial growth factors, and chemokines, which attract neutrophils into tumors. These neutrophils facilitate vascular invasion and contribute to the metastatic potential of tumor cells (59). Neutrophils also participate in creating an immunosuppressive microenvironment by releasing myeloperoxidase and arginase-1, and upregulating PD-L1. This, in turn, reduces the number of tumor-infiltrating lymphocytes (TIL) and leads to decreased effectiveness of immunotherapy (60). The correlation between peripheral blood NLR and clinical outcomes may be explained by the association between tumor-infiltrating lymphocytes and neutrophils, which results in reduced anti-tumor T-cell responses (61, 62).
As depicted in Figure 4 , we observed changes in cytokine levels after treatment in both Cohorts. Cancer cells are the primary sources of cytokines, so successful treatment can lead to reductions in specific cytokines, as observed for IL-2, TNF-α, IFN-γ, and IL-17A in Cohort 2. However, patients treated with chemotherapy alone exhibited stabilization or an increase in levels of IL-4, IL-6, and IL-10 cytokines, which may suggest that the crucial cell compartments contributing to the presence of these cytokines might not be affected by chemotherapy, such as M2 macrophages in the tumor microenvironment (63, 64). Furthermore, the addition of ICIs increased concentrations of cytokines after treatment globally and appeared to counteract the effect of chemotherapy, which typically decreases cytokine levels. It is believed that cytokine levels reflect the immunosuppressive state to some extent, where a high level of cytokines indicates that the body is more sensitive to PD-1 antibodies (65). This finding is consistent with our observation that GC patients in Cohort 1 with more than 20% variation in IL-2 from baseline to the point of maximum remission had better OS.
Conclusion
In conclusion, ongoing studies are actively investigating the predictive role of peripheral blood indicators in the effectiveness and prognosis of immunotherapy. However, comprehensive data on the use of Immune Checkpoint Inhibitors (ICIs) in advanced gastric cancer patients, both domestically and internationally, are still limited. Therefore, further prospective validation is required. To sum up, serum cytokines have varying significance in assessing the response of gastric cancer (GC) patients to anti-PD-1 therapy. Baseline levels of IL-2, IL-6, IL-17A, and Neutrophil-to-Lymphocyte Ratio (NLR), as well as changes in IL-2 levels over time, may serve as convenient predictive biomarkers for identifying GC patients who are likely to benefit from the addition of anti-PD-1 monoclonal antibodies to chemotherapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research and Clinical Trial Ethics Committee of the First Affiliated Hospital of Zhengzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The study was retrospective, with anonymous patient information obtained through the hospital record system, and all patients were deceased.
Author contributions
YH: Data curation, Formal Analysis, Writing – original draft. XL: Validation, Visualization, Writing – original draft. YY: Investigation, Software, Writing – review & editing. HS: Conceptualization, Writing – review & editing. SW: Investigation, Writing – review & editing. MG: Funding acquisition, Methodology, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Henan Province young and middle-aged health science and technology innovation leading talent training program (grant no. YXKC2022011); Beijing Science and Technology Innovation Medical Development Foundation (grant no. KC2021-JX-0186-48); Beijing Xisike Clinical Oncology Research Foundation (grant no. Y-HR2019-0303).
Abbreviations
PD-1, programmed death-1; GC, gastric cancer; IL-2, interleukin-2; TNF-α, tumor necrosis factor-alpha; IFN-γ, interferon-gamma; NLR, neutrophil-to-lymphocyte ratio; TLC, total lymphocyte count; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; OS, overall survival; PFS, progression-free survival; PD-L1, programmed cell death-ligand 1; ICIs, immune checkpoint inhibitors; TMB, tumor mutational burden; MSI/MMR, microsatellite instability/mismatch repair; EBV, Epstein Barr virus; ctDNA, circulating tumor DNA; SIR, systemic inflammatory response; TME, tumor microenvironment; HR, hazard ratios; CI, confidence intervals; CR, complete response; PR, partial response; CAFs, cancer-associated fibroblasts; DCs, dendritic cells; TIL, tumor-infiltrating lymphocytes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1274431/full#supplementary-material
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Zaanan A, Bouché O, Benhaim L, Buecher B, Chapelle N, Dubreuil O, et al. Gastric cancer: french intergroup clinical practice guidelines for diagnosis, treatments and follow-up (Snfge, ffcd, gercor, unicancer, sfcd, sfed, sfro). Dig Liver Dis (2018) 50(8):768–79. doi: 10.1016/j.dld.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 3. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of fda-approved immune checkpoint inhibitors per nccn guidelines with the level of evidence. Cancers (Basel) (2020) 12(3):738. doi: 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer (2019) 19(3):133–50. doi: 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochim Biophys Acta Rev Cancer (2021) 1876(2):188615. doi: 10.1016/j.bbcan.2021.188615 [DOI] [PubMed] [Google Scholar]
- 6. Sundar R, Smyth EC, Peng S, Yeong JPS, Tan P. Predictive biomarkers of immune checkpoint inhibition in gastroesophageal cancers. Front Oncol (2020) 10:763. doi: 10.3389/fonc.2020.00763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin W-W, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest (2007) 117(5):1175–83. doi: 10.1172/jci31537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer (2004) 4(1):11–22. doi: 10.1038/nrc1252 [DOI] [PubMed] [Google Scholar]
- 9. Melero I, Normanno N. Harmful cytokines in cancer immunology and immunotherapy: biomarkers and targets? Ann Oncol (2021) 32(11):1311–3. doi: 10.1016/j.annonc.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 10. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res (2022) 42(6):2977–87. doi: 10.21873/anticanres.15781 [DOI] [PubMed] [Google Scholar]
- 11. Ozawa Y, Amano Y, Kanata K, Hasegwa H, Matsui T, Kakutani T, et al. Impact of early inflammatory cytokine elevation after commencement of pd-1 inhibitors to predict efficacy in patients with non-small cell lung cancer. Med Oncol (2019) 36(4):6. doi: 10.1007/s12032-019-1255-3 [DOI] [PubMed] [Google Scholar]
- 12. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol (1982) 5(6):649–55. doi: 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised recist guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg SA. Il-2: the first effective immunotherapy for human cancer. J Immunol (2014) 192(12):5451–8. doi: 10.4049/jimmunol.1490019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shokrzadeh M, Mohammadpour A, Hoseini V, Abediankenari S, Ghassemi-Barghi N, Tabari YS. Serum cytokine of il-2, il-10 and il-12 levels in patients with stomach adenocarcinoma. Arquivos gastroenterologia (2018) 55(4):385–9. doi: 10.1590/s0004-2803.201800000-83 [DOI] [PubMed] [Google Scholar]
- 16. Forones NM, Mandowsky SV, Lourenco LG. Serum levels of interleukin-2 and tumor necrosis factor-alpha correlate to tumor progression in gastric cancer. Hepato-Gastroenterology (2001) 48(40):1199–201. [PubMed] [Google Scholar]
- 17. Li Z, Chen L, Qin Z. Paradoxical roles of il-4 in tumor immunity. Cell Mol Immunol (2009) 6(6):415–22. doi: 10.1038/cmi.2009.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gocher AM, Workman CJ, Vignali DAA. Interferon-gamma: teammate or opponent in the tumour microenvironment? Nat Rev Immunol (2022) 22(3):158–72. doi: 10.1038/s41577-021-00566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kumari N, Agrawal U, Mishra AK, Kumar A, Vasudeva P, Mohanty NK, et al. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumor Biol (2017) 39(4):14. doi: 10.1177/1010428317697552 [DOI] [PubMed] [Google Scholar]
- 20. Sánchez-Zauco N, Torres J, Gómez A, Camorlinga-Ponce M, Muñoz-Pérez L, Herrera-Goepfert R, et al. Circulating blood levels of il-6, ifn-Γ, and il-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer (2017) 17(1):384. doi: 10.1186/s12885-017-3310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oka M, Yamamoto K, Takahashi M, Hakozaki M, Abe T, Iizuka N, et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res (1996) 56(12):2776–80. [PubMed] [Google Scholar]
- 22. Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer (2009) 9:155. doi: 10.1186/1471-2407-9-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Łukaszewicz-Zając M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Szmitkowski M. Higher importance of interleukin 6 than classic tumor markers (Carcinoembryonic antigen and squamous cell cancer antigen) in the diagnosis of esophageal cancer patients. Dis Esophagus (2012) 25(3):242–9. doi: 10.1111/j.1442-2050.2011.01242.x [DOI] [PubMed] [Google Scholar]
- 24. Lippitz BE. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol (2013) 14(6):E218–E28. doi: 10.1016/s1470-2045(12)70582-x [DOI] [PubMed] [Google Scholar]
- 25. Taniguchi K, Karin M. Il-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol (2014) 26(1):54–74. doi: 10.1016/j.smim.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 26. Yamagiwa Y, Meng F, Patel T. Interleukin-6 decreases senescence and increases telomerase activity in Malignant human cholangiocytes. Life Sci (2006) 78(21):2494–502. doi: 10.1016/j.lfs.2005.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: A counterpoint. J Leukocyte Biol (2005) 78(5):1043–51. doi: 10.1189/jlb.0705358 [DOI] [PubMed] [Google Scholar]
- 28. O'Garra A, Murphy KM. From il-10 to il-12: how pathogens and their products stimulate apcs to induce T(H)1 development. Nat Immunol (2009) 10(9):929–32. doi: 10.1038/ni0909-929 [DOI] [PubMed] [Google Scholar]
- 29. Gong K, Guo G, Beckley N, Zhang Y, Yang X, Sharma M, et al. Tumor necrosis factor in lung cancer: complex roles in biology and resistance to treatment. Neoplasia (2021) 23(2):189–96. doi: 10.1016/j.neo.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (Tnf-A) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr) (2020) 43(1):1–18. doi: 10.1007/s13402-019-00489-1 [DOI] [PubMed] [Google Scholar]
- 31. Mercogliano MF, Bruni S, Elizalde PV, Schillaci R. Tumor necrosis factor A Blockade: an opportunity to tackle breast cancer. Front Oncol (2020) 10:584. doi: 10.3389/fonc.2020.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang M, Liu J, Yang D, Tross D, Li P, Chen F, et al. A tnfr2 antibody by countering immunosuppression cooperates with hmgn1 and R848 immune stimulants to inhibit murine colon cancer. Int Immunopharmacol (2021) 101(Pt A):108345. doi: 10.1016/j.intimp.2021.108345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, Ségui B. The tnf paradox in cancer progression and immunotherapy. Front Immunol (2019) 10:1818. doi: 10.3389/fimmu.2019.01818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ji X, Peng Z, Li X, Yan Z, Yang Y, Qiao Z, et al. Neutralization of tnfα in tumor with a novel nanobody potentiates paclitaxel-therapy and inhibits metastasis in breast cancer. Cancer Lett (2017) 386:24–34. doi: 10.1016/j.canlet.2016.10.031 [DOI] [PubMed] [Google Scholar]
- 35. Yu M, Zhou X, Niu L, Lin G, Huang J, Zhou W, et al. Targeting transmembrane tnf-A Suppresses breast cancer growth. Cancer Res (2013) 73(13):4061–74. doi: 10.1158/0008-5472.Can-12-3946 [DOI] [PubMed] [Google Scholar]
- 36. Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus erysipelas and the bacillus prodigiosus). Proc R Soc Med (1910) 3(Surg Sect):1–48. doi: 10.1177/003591571000301601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiong Q, Huang Z, Xin L, Qin B, Zhao X, Zhang J, et al. Post-treatment neutrophil-to-lymphocyte ratio (Nlr) predicts response to anti-pd-1/pd-L1 antibody in sclc patients at early phase. Cancer Immunol Immunother (2021) 70(3):713–20. doi: 10.1007/s00262-020-02706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen T, Song C, Liang G, Xu X, Wang C, Zhang Z, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their variations as a basis for a prediction model in advanced nsclc patients receiving anlotinib. Dis Markers (2022) 2022:5879137. doi: 10.1155/2022/5879137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang P, Zhang Y, Ru B, Yang Y, Trang V, Paul R, et al. Systematic investigation of cytokine signaling activity at the tissue and single-cell levels. Nat Methods (2021) 18(10):1181. doi: 10.1038/s41592-021-01274-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (Il-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/il-12 synthesis in accessory cells. J Exp Med (1993) 178(3):1041–8. doi: 10.1084/jem.178.3.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wurtz O, Bajenoff M, Guerder S. Il-4-mediated inhibition of ifn-gamma production by cd4(+) T cells proceeds by several developmentally regulated mechanisms. Int Immunol (2004) 16(3):501–8. doi: 10.1093/intimm/dxh050 [DOI] [PubMed] [Google Scholar]
- 42. Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ. Interleukin 2-mediated immune interferon (Ifn-gamma) production by human T cells and T cell subsets. J Immunol (Baltimore Md 1950) (1983) 130(4):1784–9. doi: 10.4049/jimmunol.130.4.1784 [DOI] [PubMed] [Google Scholar]
- 43. Honda K, Wada H, Nakamura M, Nakamoto K, Inui T, Sada M, et al. Il-17a synergistically stimulates tnf-alpha-induced il-8 production in human airway epithelial cells: A potential role in amplifying airway inflammation. Exp Lung Res (2016) 42(4):205–16. doi: 10.1080/01902148.2016.1190796 [DOI] [PubMed] [Google Scholar]
- 44. Pol JG, Caudana P, Paillet J, Piaggio E, Kroemer G. Effects of interleukin-2 in immunostimulation and immunosuppression. J Exp Med (2020) 217(1):e20191247. doi: 10.1084/jem.20191247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garrelds IM, van Meeteren ME, Meijssen MA, Zijlstra FJ. Interleukin-2-deficient mice: effect on cytokines and inflammatory cells in chronic colonic disease. Dig Dis Sci (2002) 47(3):503–10. doi: 10.1023/a:1017995414697 [DOI] [PubMed] [Google Scholar]
- 46. Holcomb EA, Zou W. A forced marriage of il-2 and pd-1 antibody nurtures tumor-infiltrating T cells. J Clin Invest (2022) 132(3):e156628. doi: 10.1172/jci156628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Langan EA, Kümpers C, Graetz V, Perner S, Zillikens D, Terheyden P. Intralesional interleukin-2: A novel option to maximize response to systemic immune checkpoint therapy in loco-regional metastatic melanoma. Dermatol Ther (2019) 32(3):e12901. doi: 10.1111/dth.12901 [DOI] [PubMed] [Google Scholar]
- 48. Yu Y, Wang S, Su N, Pan S, Tu B, Zhao J, et al. Increased circulating levels of crp and il-6 and decreased frequencies of T and B lymphocyte subsets are associated with immune-related adverse events during combination therapy with pd-1 inhibitors for liver cancer. Front Oncol (2022) 12:906824. doi: 10.3389/fonc.2022.906824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. George DJ, Halabi S, Shepard TF, Sanford B, Vogelzang NJ, Small EJ, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: results from cancer and leukemia group B 9480. Clin Cancer Res (2005) 11(5):1815–20. doi: 10.1158/1078-0432.Ccr-04-1560 [DOI] [PubMed] [Google Scholar]
- 51. Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R, Gadducci A, et al. Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. Br J Cancer (1995) 71(2):354–6. doi: 10.1038/bjc.1995.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, et al. Combined blockade of il6 and pd-1/pd-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res (2018) 78(17):5011–22. doi: 10.1158/0008-5472.Can-18-0118 [DOI] [PubMed] [Google Scholar]
- 53. Ohno Y, Toyoshima Y, Yurino H, Monma N, Xiang H, Sumida K, et al. Lack of interleukin-6 in the tumor microenvironment augments type-1 immunity and increases the efficacy of cancer immunotherapy. Cancer Sci (2017) 108(10):1959–66. doi: 10.1111/cas.13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kang JH, Park S, Rho J, Hong EJ, Cho YE, Won YS, et al. Il-17a promotes helicobacter pylori-induced gastric carcinogenesis via interactions with il-17rc. Gastric Cancer (2023) 26(1):82–94. doi: 10.1007/s10120-022-01342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Karstens KF, Kempski J, Giannou AD, Freiwald E, Reeh M, Tachezy M, et al. Systemic interleukin 10 levels indicate advanced stages while interleukin 17a levels correlate with reduced survival in esophageal adenocarcinomas. PloS One (2020) 15(4):e0231833. doi: 10.1371/journal.pone.0231833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen J, Ye X, Pitmon E, Lu M, Wan J, Jellison ER, et al. Il-17 inhibits cxcl9/10-mediated recruitment of cd8(+) cytotoxic T cells and regulatory T cells to colorectal tumors. J Immunother Cancer (2019) 7(1):324. doi: 10.1186/s40425-019-0757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, et al. Blocking il-17a enhances tumor response to anti-pd-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer (2021) 9(1):e001895. doi: 10.1136/jitc-2020-001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Llosa NJ, Luber B, Tam AJ, Smith KN, Siegel N, Awan AH, et al. Intratumoral adaptive immunosuppression and type 17 immunity in mismatch repair proficient colorectal tumors. Clin Cancer Res (2019) 25(17):5250–9. doi: 10.1158/1078-0432.Ccr-19-0114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guo Y, Xiang D, Wan J, Yang L, Zheng C. Focus on the dynamics of neutrophil-to-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: A meta-analysis and systematic review. Cancers (Basel) (2022) 14(21):5297. doi: 10.3390/cancers14215297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory interactions between neutrophils, tumor cells and T cells. Front Immunol (2019) 10:1690. doi: 10.3389/fimmu.2019.01690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol (2016) 51(5):610–7. doi: 10.3109/00365521.2015.1121515 [DOI] [PubMed] [Google Scholar]
- 62. Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep (2012) 28(2):453–8. doi: 10.3892/or.2012.1812 [DOI] [PubMed] [Google Scholar]
- 63. Kitajima M, Ziegler SF. Cutting edge: identification of the thymic stromal lymphopoietin-responsive dendritic cell subset critical for initiation of type 2 contact hypersensitivity. J Immunol (2013) 191(10):4903–7. doi: 10.4049/jimmunol.1302175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, et al. Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res (2013) 73(8):2480–92. doi: 10.1158/0008-5472.Can-12-3542 [DOI] [PubMed] [Google Scholar]
- 65. Rahimi Kalateh Shah Mohammad G, Ghahremanloo A, Soltani A, Fathi E, Hashemy SI. Cytokines as potential combination agents with pd-1/pd-L1 blockade for cancer treatment. J Cell Physiol (2020) 235(7-8):5449–60. doi: 10.1002/jcp.29491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.