Abstract
Purpose:
This interim report of the GARNET phase I trial presents efficacy and safety of dostarlimab in patients with advanced or recurrent endometrial cancer (EC), with an analysis of tumor biomarkers as prognostic indicators.
Patients and Methods:
A total of 153 patients with mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) and 161 patients with mismatch repair proficient (MMRp)/microsatellite stable (MSS) EC were enrolled and dosed. Patients received 500 mg dostarlimab every 3 weeks for four cycles, then 1,000 mg every 6 weeks until progression. Primary endpoints were objective response rate (ORR) and duration of response (DOR).
Results:
A total of 143 patients with dMMR/MSI-H EC and 156 patients with MMRp/MSS EC were evaluated for efficacy. ORR was 45.5% (n = 65) and 15.4% (n = 24) for dMMR/MSI-H EC and MMRp/MSS EC, respectively. Median DOR for dMMR/MSI-H EC was not met (median follow-up, 27.6 months); median DOR for MMRp/MSS EC was 19.4 months. The ORRs by combined positive score (CPS) ≥1 status were 54.9% and 21.7% for dMMR/MSI-H EC and MMRp/MSS EC, respectively. ORRs by high tumor mutational burden (≥10 mutations/Mb) were 47.8% (43/90) and 45.5% (5/11) for dMMR/MSI-H EC and MMRp/MSS EC, respectively. ORR in TP53mut or POLεmut molecular subgroups was 18.1% (17/94) and 40.0% (2/5), respectively. The safety profile of dostarlimab was consistent with previous reports.
Conclusions:
Dostarlimab demonstrated durable antitumor activity and safety in patients with dMMR/MSI-H EC. Biomarkers associated with EC may identify patients likely to respond to dostarlimab.
Translational Relevance.
Patients with advanced or recurrent endometrial cancer (EC) have relatively poor prognoses and limited treatment options following disease progression on frontline therapy. Dostarlimab is an immune checkpoint inhibitor that targets the PD-(L)1 pathway, thereby reducing T-cell inhibition and enhancing antitumor immune activity. In this interim analysis of the phase I GARNET trial, patients with advanced or recurrent mismatch repair deficient/microsatellite instability-high or mismatch repair proficient/microsatellite stable EC that had progressed on or after platinum therapy demonstrated durable responses to dostarlimab with low toxicity. In addition, we analyzed clinical and molecular biomarkers associated with EC, including PD-L1 (as determined by combined positive score), tumor mutational burden, and mutations in the TP53 and POLε genes. Utilization of these biomarkers may aid in identifying patients who are likely to benefit from immune checkpoint inhibitor monotherapy and provide rationale for combination therapies and expanding the effective treatment options available to patients.
Introduction
Endometrial cancer (EC), the most common gynecologic malignant neoplasm in the developed world, has increased in incidence as well as mortality over the past three decades (1, 2). Deaths are predominantly from recurrent or advanced EC, which has limited treatment options beyond platinum-based chemotherapy (3). Approximately 30% of ECs are classified as DNA mismatch repair deficient (dMMR) and are thus potentially suitable for treatment with immunotherapy (4, 5), as neoantigens expressed by dMMR tumors render them highly immunogenic (6). Recurrent or advanced tumors can evade T-cell immune surveillance by activating programmed death 1 (PD-1) signaling via upregulation of programmed death ligand 1 (PD-L1) expression (7). Antibodies targeting PD-(L)1 can restore the immunogenicity of these tumors and have been shown to be effective and well-tolerated treatments (8). Moreover, tumor MMR status is a promising biomarker for predicting response to treatment with anti–PD-1 and PD-L1 therapies in EC (9).
In clinical practice, MMR status is established by the presence of four MMR proteins, MLH1, MSH2, MSH6, and PMS2, as determined by IHC. When all four proteins are present, the tumor is classed as MMR proficient (MMRp); when one or more is absent, the tumor is dMMR. Loss of expression of one or more of the MMR proteins due to genetic alteration or epigenetic silencing is associated with microsatellite instability (MSI), an accumulation of DNA replication errors at microsatellite regions. High MSI (MSI-H) and microsatellite stability (MSS) are detected by PCR or next-generation sequencing (NGS).
Dostarlimab is an IgG4-k humanized monoclonal antibody that binds with high affinity to PD-1, resulting in the inhibition of PD-L1 and PD-L2 binding. In the United States, dostarlimab is approved as a monotherapy in adult patients with dMMR recurrent or advanced EC that has progressed on or after a platinum-containing regimen (10). In the European Union and United Kingdom, dostarlimab (JEMPERLI) is approved as a monotherapy in adult patients with recurrent or advanced dMMR/MSI-H EC that has progressed on or after treatment with a platinum-containing regimen (11). These approvals were based on the earlier interim analyses of the phase I GARNET trial (NCT02715284; refs. 12, 13).
In this third and final interim analysis of GARNET, we report objective response rate (ORR), duration of response (DOR), progression-free survival (PFS), and overall survival (OS) for dostarlimab in two separate EC cohorts, patients with dMMR/MSI-H EC (cohort A1) and patients with MMRp/MSS EC (cohort A2). Given the need for biomarkers to reliably identify those patients likely to respond to anti–PD-(L)1 pathway-targeted therapies, we also present the results of post hoc exploratory analyses of putative clinical and molecular biomarkers, including PD-L1 expression as determined by combined positive score (CPS), tumor mutational burden (TMB), and tumor molecular profiles, as an aid to predicting response to immunotherapy in patients with EC.
Patients and Methods
Study design
GARNET is a phase I single-arm study of dostarlimab monotherapy in patients with advanced and recurrent solid tumors from 123 international sites, as described previously (12). The initial parts of the trial (Parts 1 and 2A) determined the recommended therapeutic dose to be 500 mg by intravenous infusion every 3 weeks for four cycles, then 1,000 mg by intravenous infusion every 6 weeks until disease progression. Part 2B of the trial explored the antitumor activity and safety of dostarlimab in prespecified cohorts, including EC (cohorts A1 and A2). Data from the first and second prespecified interim analyses including patients in the EC cohorts have been published prior to this third and final interim analysis (12, 13).
For the EC cohorts, inclusion criteria included recurrent EC that progressed on or after platinum doublet therapy, ≤2 prior lines of treatment for recurrent or advanced disease, measurable disease at baseline confirmed by central radiology review, and anti–PD-(L)1 naive. All histologic subtypes except sarcoma and carcinosarcoma were eligible. The trial was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practices, and all local laws. The study was overseen by an independent data and safety monitoring committee. The study protocol and all amendments were approved by an institutional ethics committee, institutional review board, and/or appropriate authorities at each site. All patients provided written informed consent.
Patients were prospectively screened on the basis of MMR/MSI testing results obtained using IHC, PCR, or NGS performed in a certified local laboratory. For patients enrolled after protocol amendment 5 (13), eligibility was determined by IHC performed in a local laboratory or by central testing if local IHC testing was not available. Central confirmation was not required for patients with local IHC results. When results from more than one test (MMR or MSI) were available, patients were classified by their MMR status. Patients screened using MMR IHC testing were not required to have MSI testing performed. However, in cases where MMR testing was inconclusive (MMR unknown [MMRunk]), patients were classified by their MSI status. Patients with MSI-H and MMRunk EC were grouped with the patients with dMMR EC, and patients with MSS and MMRunk EC were grouped with the patients with MMRp EC.
Sample size
As described previously, cohort A1 was designed to enroll approximately 100 patients with dMMR/MSI-H EC, with the potential for up to 165 patients (12). Cohort A2 was designed to enroll approximately 125 patients with MMRp/MSS EC, with the potential for up to 250 patients (12).
For cohort A1, the null hypothesis (H0) that the true response rate is ≤20% (P ≤ 0.2) was tested against a one-sided alternative hypothesis (HA) of ≥40% (P ≥ 0.4). With 65 patients treated, the cohort has a 92% power to rule out H0 ≤20% ORR when the true ORR is 40% at the 2.5% type I error rate (one-sided). The sample size of cohort A1 was increased to 100 patients, which allowed the lower-limit boundary of the exact 95% confidence interval (CI) to exclude a response rate of 25% or less, assuming the observed ORR is 35%.
For cohort A2, a two-stage design was used. The null hypothesis that the true response rate is ≤5% (H0: P ≤ 0.05) was tested against a one-sided alternative of ≥15% (HA: P ≥ 0.15). In the first stage, 25 patients were accrued. Because two or more responses were observed, approximately 40 additional patients were accrued for an approximate total of 65 patients. This design yields a type I error rate of 10% (one-sided) and a power of 87% when the true response rate is 15%. On the basis of favorable clinical activity observed in cohort A2 during the first stage, the sample size was increased as described above to allow for more precise estimates of ORR with the lower limit of the exact 95% CI, excluding a response rate of 15% or less.
Prespecified endpoints
The primary endpoints for both cohorts included evaluation of antitumor activity of dostarlimab per ORR and DOR by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1).
The prespecified secondary endpoints for both cohorts included PFS, OS, immune-related ORR (irORR), immune-related disease control rate (irDCR), immune-related PFS (irPFS), and irDOR based on investigators’ assessment using immune-related RECIST (irRECIST) and DCR based on BICR using RECIST v1.1.
Safety variables
Safety analyses included incidence of treatment-emergent adverse events (TEAE); treatment-related adverse events (TRAE); immune-related adverse events of interest (irAEI), including hypothyroidism, increased alanine transaminase (ALT), increased aspartate transferase (AST), arthralgia, and pneumonitis; and serious adverse events (SAE) occurring while patients were on treatment or up to 90 days after the end of treatment. Any changes in clinical laboratory parameters (hematology, chemistry, thyroid function, coagulation, urinalysis) and Common Terminology Criteria for Adverse Events (CTCAE) v4.03–graded laboratory toxicities, vital signs, Eastern Cooperative Oncology Group performance status, electrocardiogram parameters, physical examinations, and usage of concomitant medications were recorded. No formal hypothesis-testing analysis of adverse event incidence rates was performed.
Translational analyses
The objective of the post hoc analyses was to evaluate the effect of dostarlimab activity according to EC tumor CPS and molecular profiling as predictors of response to anti–PD-(L)1 therapy. High TMB (TMB-H) was defined as ≥10 mutations/Mb as determined by the Foundation One test (Foundation Medicine, Inc.). CPS was determined by VENTANA PD-L1 assay (Roche Diagnostics), with CPS ≥1 corresponding to positive PD-L1 expression. Molecular classification, including mutations in the POLε exonuclease (POLε) genes and TP53, was performed using the Foundation One test. Patients were classified according to molecular subtype, in a manner similar to The Cancer Genome Atlas algorithm—first patients with tumor POLε gene mutations [POLεmut; missense alterations identified in the exonuclease domain of the POLε gene (between amino acid residues 268–471, which includes common hotspot POLε mutations P286R, S297F, V411L, A456P, and S459F)], then those remaining were classified by MMR/MSI status (dMMR/MSI-H), and then those without POLε mutations or MMR defects were classified by TP53 mutation status (TP53mut; ref. 14). Patients with available data who lacked tumor POLεmut, MMR defects, or TP53mut were classified as having no specific molecular profile (NSMP).
Statistical analysis
All analyses included summary statistics, including the number and percentage for categorical variables and the number of participants, mean, SD, median, minimum, and maximum for continuous variables. Two-sided 95% CIs are provided as appropriate. Time-to-event analyses were performed using Kaplan–Meier (KM) methods. All analyses were performed individually for each MMR tumor status (dMMR, MMRp, and MMRunk).
All patients who received at least one dose of dostarlimab by the data cutoff date were included in the safety analysis population. All patients who received at least one dose of dostarlimab, had measurable disease at baseline (defined as the existence of at least one target lesion at baseline tumor assessment by BICR), and were followed for at least 6 months as of the data cutoff date were included in the efficacy-evaluable population, regardless of whether the patient had a postbaseline tumor assessment.
Data availability
Study documents and data will be made available within 6 months of product approval/trial termination or publication obligation fulfilled date (whichever is later) at https://vivli.org/ourmember/gsk/. Enquiries about the availability of clinical studies that are not currently listed on the site can be made via the enquiry form found at https://vivli.org/ourmember/gsk/.
Results
Patients
The study was initiated on April 10, 2017, with enrollment in both cohorts now complete. Data analysis was performed using a data cutoff date of November 1, 2021. In total, 153 patients with dMMR or MSI-H/MMRunk EC (cohort A1) and 161 patients with MMRp or MSS/MMRunk EC (cohort A2) were enrolled and dosed with dostarlimab (Supplementary Fig. S1).
The demographic characteristics of the patients were generally representative of patients with advanced or recurrent EC (Supplementary Table S1). Patient demographics were similar between the 2 cohorts, except for disease histology (Supplementary Table S2). Consistent with previous reports, low-grade endometrioid tumors were the most common in the dMMR/MSI-H cohort (64.3%), and most patients in the MMRp/MSS cohort had a high-grade tumor type, with serous histology being the most common histologic subtype (40.4%).
Antitumor activity
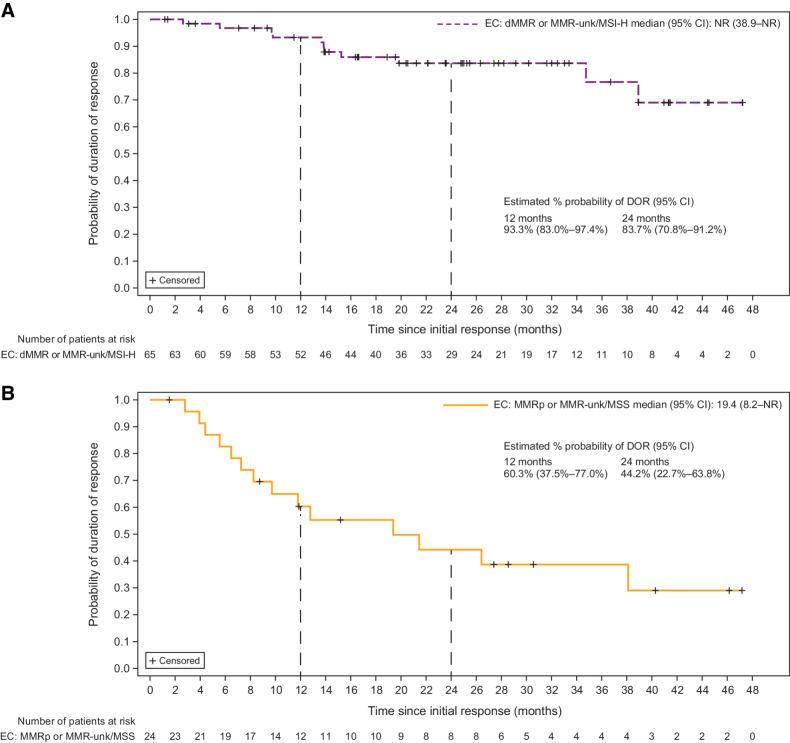
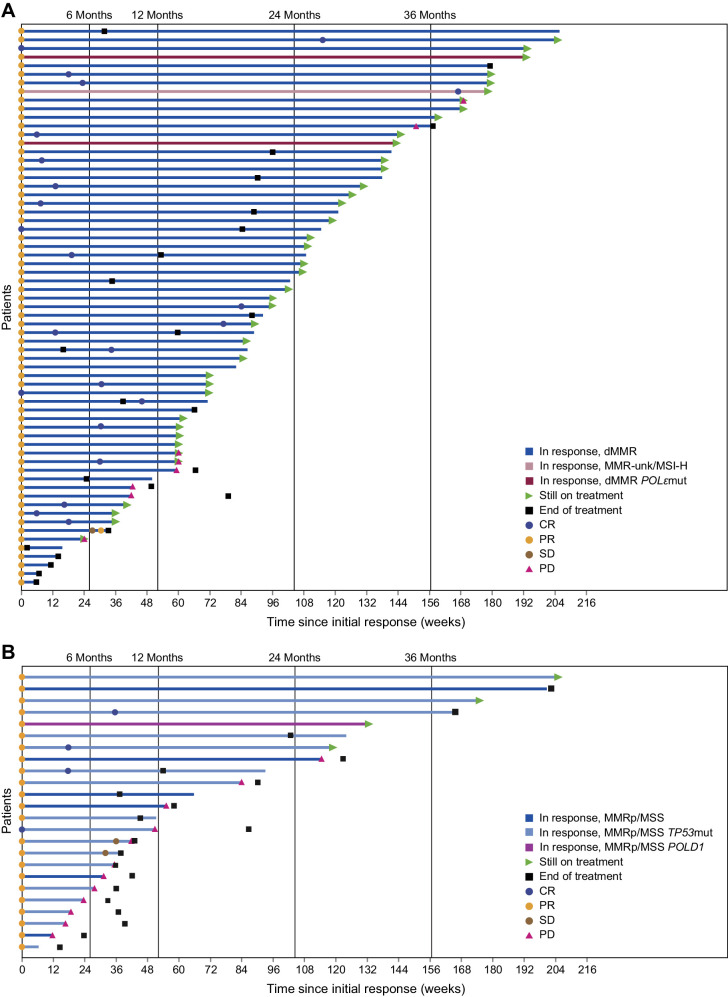
The prespecified primary endpoint analysis is shown in Table 1. The ORR was 45.5% in patients with dMMR/MSI-H EC and 15.4% in patients with MMRp/MSS EC. The median DOR for patients with dMMR/MSI-H EC had not been reached as of the data cutoff date (median follow-up, 27.6 months), whereas the median DOR for patients with MMRp/MSS EC was 19.4 months (Table 1). Patients in both cohorts had durable responses; patients with dMMR/MSI-H EC had a 93.3% probability of maintaining a response for ≥12 months, and patients with MMRp/MSS EC had a 60.3% probability of maintaining a response for ≥12 months (Fig. 1). DOR among responders for each cohort is shown in Fig. 2, and best percent change from baseline in target lesion size is shown in Supplementary Fig. S2. PFS and OS for each cohort are shown in Supplementary Figs S3 and S4, respectively. ORR was consistent regardless of histology (Supplementary Fig. S5).
Table 1.
Primary endpoint analysis.
| dMMR | dMMR/MSI-H | |
|---|---|---|
| dMMR/MSI-H EC | (N = 141) | (N = 143) |
| Median follow-up, months | 27.6 | |
| ORR, n, % (95% CI) | 64, 45.4% | 65, 45.5% |
| (37.0–54.0) | (37.1–54.0) | |
| Best confirmed response, n (%) | ||
| CR | 22 (15.6) | 23 (16.1) |
| PR | 42 (29.8) | 42 (29.4) |
| SD | 21 (14.9) | 21 (14.7) |
| PD | 51 (36.2) | 51 (35.7) |
| NE | 5 (3.5) | 6 (4.2) |
| DCR, n (%) | 85 (60.3) | 86 (60.1) |
| Median DOR (95% CI), months | NR (38.9–NR) | NR (38.9–NR) |
| Duration ≥12 months, n (%) | 51 (79.7) | 52 (80.0) |
| Duration ≥24 months, n (%) | 28 (43.8) | 29 (44.6) |
| Probability of maintaining response (95% CI) | ||
| At 12 months | 93.1 (82.7–97.4) | 93.3 (83.0–97.4) |
| At 24 months | 83.4 (70.3–91.0) | 83.7 (70.8–91.2) |
| MMRp | MMRp/MSS | |
|---|---|---|
| MMRp/MSS EC | (N = 142) | (N = 156) |
| Median follow-up, months | 33.0 | |
| ORR, n, % (95% CI) | 21, 14.8% (9.4–21.7) | 24, 15.4% (10.1–22.0) |
| Best confirmed response, n (%) | ||
| CR | 4 (2.8) | 4 (2.6) |
| PR | 17 (12.0) | 20 (12.8) |
| SD | 28 (19.7) | 29 (18.6) |
| PD | 80 (56.3) | 88 (56.4) |
| NE | 13 (9.1) | 15 (9.6) |
| DCR, n (%) | 49 (34.5) | 53 (34.0) |
| Median DOR (95% CI), months | 19.4 (7.3–38.1) | 19.4 (8.2–NR) |
| Duration ≥12 months, n (%) | 10 (47.6) | 12 (50.0) |
| Duration ≥24 months, n (%) | 6 (28.6) | 8 (33.3) |
| Probability of maintaining response (95% CI) | ||
| At 12 months | 59.2 (34.7–77.2) | 60.3 (37.5–77.0) |
| At 24 months | 40.0 (17.7–61.5) | 44.2 (22.7–63.8) |
Abbreviations: CR, complete response; NE, not evaluable, includes patients with a best response of not done; NR, not reached; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 1.
Duration of response by cohort. Duration of response in patients with advanced or recurrent EC treated with dostarlimab monotherapy. Patients with (A) dMMR/MSI-H EC (Cohort A1) and (B) MMRp/MSS EC (Cohort A2). NR, not reached.
Figure 2.
Duration of response among responders with advanced or recurrent EC treated with dostarlimab monotherapy. Patients with (A) dMMR/MSI-H EC (Cohort A1) and (B) MMRp/MSS EC (Cohort A2). Time since initial response and first and subsequent responses are shown.
A post hoc exploratory analysis in the dMMR/MSI-H and MMRp/MSS cohorts of patients who received prior treatment only in the adjuvant or neoadjuvant setting was also conducted. ORR was consistent with that of the individual overall cohorts (Supplementary Table S3).
Post hoc biomarker subgroup analyses
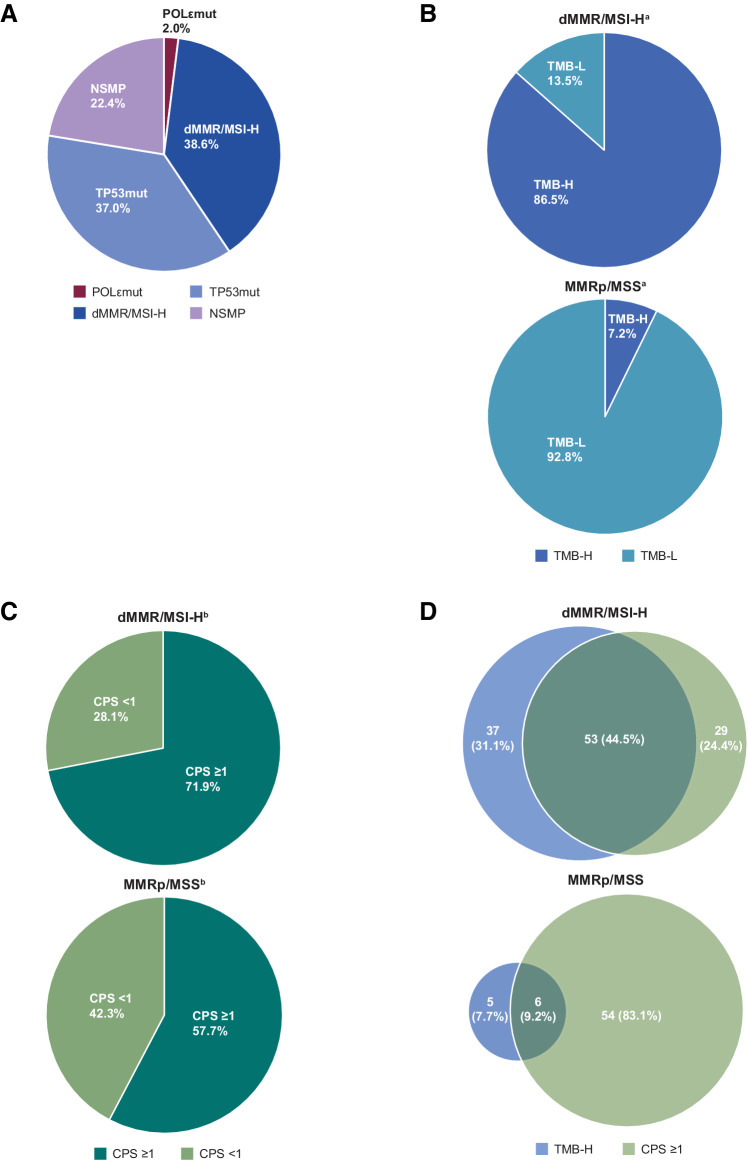
We analyzed the efficacy of dostarlimab according to molecular subtype when biomarker data were available. A total of 5 patients were identified as POLεmut subtype—3 patients with dMMR/MSI-H EC and 2 patients with MMRp/MSS EC. Patients in the POLεmut molecular subgroup or dMMR/MSI-H molecular subgroup showed strong responses, 40.0% (2/5) and 43.9% (43/98), respectively (Table 2). However, both patients in the POLεmut molecular subgroup who experienced a response were also dMMR. For patients in both cohorts, 38.6% were dMMR/MSI-H, 37.0% were TP53mut, and 22.4% were NSMP (Fig. 3A).
Table 2.
ORR stratified according to biomarkers.
| Molecular classification, n/N (%; 95% CI)a | ||
|---|---|---|
| POLεmut | 2/5 (40.0%; 5.3–85.3)b | |
| dMMR/MSI-H | 43/98 (43.9%; 33.9–54.3) | |
| TP53mut | 17/94 (18.1%; 10.9–27.4) | |
| NSMP | 7/57 (12.3%; 5.1–23.7) | |
| dMMR/MSI-H EC | MMRp/MSS EC | |
|---|---|---|
| TMB, n/N (%; 95% CI) | ||
| TMB-H | 48/101 (47.5%; 37.5–57.7) | |
| TMB-H by cohort | 43/90 (47.8%; 37.1–58.6) | 5/11 (45.5%; 16.7–76.7) |
| TMB-L | 22/155 (14.2%; 9.1–20.7) | |
| TMB-L by cohort | 3/14 (21.4%; 4.7–50.8) | 19/141 (13.5%; 8.3–20.2) |
| CPS (PD-L1 expression), n/N (%; 95% CI) | ||
| ≥1 | 58/142 (40.8%; 32.7–49.4) | |
| ≥1 by cohort | 45/82 (54.9%; 43.5–65.9) | 13/60 (21.7%; 12.1–34.2) |
| <1 | 13/76 (17.1%; 9.4–27.5) | |
| <1 by cohort | 10/32 (31.3%; 16.1–50.0) | 3/44 (6.8%; 1.4–18.7) |
| TMB and CPS, n/N (%; 95% CI) | ||
| TMB-H and CPS ≥ 1 | 32/53 (60.4%; 46.0–73.5) | 4/6 (66.7%; 22.3–95.7) |
| TMB-H and CPS < 1 | 5/17 (29.4%; 10.3–56.0) | 0/1 |
| TMB-L and CPS ≥ 1 | 2/5 (40.0%; 5.3–85.3) | 9/54 (16.7%; 7.9–29.3) |
| TMB-L and CPS < 1 | 1/5 (20.0%; 0.5–71.6) | 3/42 (7.1%; 1.5–19.5) |
aPatients with missing data for a subgroup were not included in calculations.
bBoth patients with POLεmut tumors who responded also had dMMR/MSI-H tumors.
Figure 3.
Biomarker distribution in patients with advanced or recurrent EC treated with dostarlimab monotherapy. A, Percentage of EC tumors with molecular biomarkers in merged cohorts, including POLεmut, TP53mut, NSMP, or dMMR/MSI-H. B, TMB status of tumors in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). C, CPS of tumors in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). D, Venn diagrams showing percentages of tumors with TMB-H or CPS ≥1 or both in patients with dMMR/MSI-H EC (top) or patients with MMRp/MSS EC (bottom). aTMB status was available for 104 patients with dMMR/MSI-H tumors and 152 patients with MMRp/MSS tumors. bCPS status was available for 114 patients with dMMR/MSI-H tumors and 104 patients with MMRp/MSS tumors. TMB-NA, tumor mutational burden results were not available or undetermined.
Response rates in the TP53mut and the NSMP molecular subgroups were low at 18.1% (17/94) and 12.3% (7/57), respectively (Table 2). As shown in Supplementary Fig. S6, DOR and PFS stratified by these subgroups indicated that patients with dMMR/MSI-H had a relatively increased probability of maintaining a response at 12 and 24 months compared with TP53 and NSMP molecular subtypes.
Information on TMB status was available for 97.4% of patients with MMRp/MSS EC, but only for 62.7% of patients with dMMR/MSI-H EC. TMB-H was more common in the dMMR/MSI-H cohort; of the patients with TMB status available, 86.5% (90/104) in the dMMR/MSI-H cohort were TMB-H compared with 7.2% (11/152) in the MMRp/MSS cohort (Fig. 3B). Although TMB-H was infrequent in MMRp/MSS tumors, ORRs in patients with TMB-H were high in both the dMMR/MSI-H and MMRp/MSS cohorts, 47.8% (43/90) and 45.5% (5/11), respectively (Table 2). Of the 11 patients in the MMRp/MSS cohort who had TMB-H tumors, none were POLεmut although 1 had a mutation in POLD1.
Molecular and clinical characteristics of MMRp/MSS tumors that exhibited a response are detailed in Table 3. Among the 24 responders with MMRp/MSS EC, 5 were TMB-H; there were 2 POLεmut patients in the MMRp/MSS cohort and neither responded.
Table 3.
Molecular and clinical characteristics of MMRp/MSS tumors that exhibited a response.
| Responder | BOR | DOR (days) | MMR status | MSI statusa | TMB score | CPS | Histology | Molecular subgroup |
|---|---|---|---|---|---|---|---|---|
| 1 | PR | 804 | MMRp | MSS | 6.3 | 3% | Clear cell carcinoma | NSMP |
| 2 | PR | 389 | MMRp | MSS | 7.57 | 15% | Clear cell carcinoma | NSMP |
| 3 | PR | 590 | MMRp | MSS | 3.78 | 5% | Mixed | TP53mut |
| 4 | PR | 869+ | MMRunk | MSS | 3.78 | 30% | Serous | TP53mut |
| 5 | PR | 930+ | MMRp | MSS | 83.22 | 10% | Endometrioid type I | NSMPb |
| 6 | PR | 296 | MMRp | MSS | 5.04 | NA | Endometrioid type II | TP53mut |
| 7 | PR | 1,436+ | MMRunk | MSS | 16.39 | NA | Adenocarcinoma | TP53mut |
| 8 | CR | 834+ | MMRp | MSS | 0 | 2% | Serous | TP53mut |
| 9 | CR | 359 | MMRp | MSS | 1.26 | 1% | Serous | TP53mut |
| 10 | PR | 197 | MMRp | Unknown | 3.78 | 1% | Clear cell carcinoma | TP53mut |
| 11 | PR | 1,405+ | MMRp | MSS | 13.87 | 70% | Mixed carcinoma | NSMP |
| 12 | PR | 1,226+ | MMRp | MSS | 7.57 | NA | Undifferentiated | TP53mut |
| 13 | CR | 1,160 | MMRp | MSS | 5.04 | NA | Serous | TP53mut |
| 14 | PR | 134 | MMRp | MSS | 1.26 | NA | Serous | TP53mut |
| 15 | PR | 462+ | MMRp | MSS | 2.52 | <1% | Endometrioid type I | NSMP |
| 16 | PR | 251 | MMRp | MSS | 7.57 | 30% | Serous | TP53mut |
| 17 | PR | 47+ | MMRp | MSS | 2.52 | 20% | Serous | TP53mut |
| 18 | PR | 120 | MMRunk | MSS | 3.78 | NA | Endometrioid type I | TP53mut |
| 19 | PR | 169 | MMRp | MSS | 5.04 | NA | Serous | TP53mut |
| 20 | CR | 653 | MMRp | MSS | 2.52 | NA | Serous | TP53mut |
| 21 | PR | 85 | MMRp | MSS | 13.87 | 1% | Serous | NSMP |
| 22 | PR | 221 | MMRp | MSS | 5.04 | 0% | Endometrioid type I | NSMP |
| 23 | PR | 266+ | MMRp | MSS | 3.78 | <1% | Serous | TP53mut |
| 24 | PR | 361+ | MMRp | MSS | 10.09 | 80% | Mixed carcinoma | TP53mut |
Abbreviation: BOR, best overall response.
aMSI status was determined by FoundationOne CDX.
bTumor also had a mutation in POLD1.
CPS ≥1 was frequent in both cohorts. For patients with CPS status available, CPS ≥1 was observed in 71.9% (82/114) of patients with dMMR/MSI-H EC and 57.7% (60/104) of patients with MMRp/MSS EC (Fig. 3C). Within each cohort, there was a nonsignificant trend toward increased response rate for tumors with CPS ≥1 [54.9% (95% CI, 43.5–65.9; 45/82) for dMMR/MSI-H EC, and 21.7% (95% CI, 12.1–34.2; 13/60) for MMRp/MSS EC, Table 2].
When patients were stratified according to both TMB and CPS status, patients whose tumors were TMB-H and CPS ≥1 had high ORR regardless of MMR status—60.4% in dMMR/MSI-H EC (32/53) and 66.7% in MMRp/MSS EC (4/6). Likewise, those that were TMB-low (TMB-L) and CPS <1 had low ORR—20.0% in dMMR/MSI-H EC (1/5) and 7.1% in MMRp/MSS EC (3/42). Patients with one marker (TMB-H or CPS ≥1) had intermediate response rates, 47.8% (43/90) and 54.9% (45/82) in dMMR/MSI-H EC, respectively, and 45.5% (5/11) and 21.7% (13/60) in MMRp/MSS EC, respectively (Table 2).
Safety
The safety population included 314 patients with EC who had received at least one dose of dostarlimab (Supplementary Table S4). Dostarlimab was well tolerated, with an AE profile characteristic of other anti–PD-1 therapies. There were no new safety signals identified since the second interim analysis (12). Most TRAEs were grade 1 or 2 and manageable, with fatigue (17.8%), diarrhea (14.6%), and nausea (13.7%) being the most common. The most common irTRAEs were hypothyroidism (8.3%), arthralgia (3.2%), increased ALT (2.5%), and increased AST (2.2%). Grade ≥3 TRAEs occurred in 17.6% of the 153 patients with dMMR/MSI-H EC and 20.5% of the 161 patients with MMRp/MSS EC, with anemia being the most frequent, observed in 3.2% of patients overall. Other grade ≥3 TRAEs were asymptomatic elevation of ALT, AST, lipase, and glucose. Grade ≥3 pneumonitis and fatigue occurred in 1.0% and 1.3% of patients, respectively. Of note, only 8.6% of patients discontinued dostarlimab because of a TRAE. No deaths were associated with dostarlimab in these cohorts.
Discussion
To our knowledge, this interim analysis comprises the largest number of patients with advanced or recurrent EC despite prior platinum-based doublet chemotherapy that were treated with immune monotherapy reported to date. The results demonstrate that dostarlimab monotherapy in dMMR/MSI-H EC achieves clinically meaningful outcomes and durable responses in patients who had previously been treated with chemotherapy. Although the median DOR was not reached for patients with dMMR/MSI-H EC, our results indicate that responses were durable regardless of MMR status and achieved with low toxicity.
Both PFS and OS were numerically lower in the MMRp/MSS cohort; however, in responders, the median DOR was 19.4 months. Thus, for patients who responded to dostarlimab, the responses were durable regardless of MMR status. The wide tails of the DOR curves in both cohorts, as well as the PFS curve in the dMMR/MSI-H cohort, are driven by the durability of responses (15–17).
The relatively low ORR (20.0%) observed in patients with dMMR/MSI-H EC with TMB-L and CPS <1 tumors is a finding of interest. Similarly, the higher ORR (66.7%) observed in MMRp/MSS patients with TMB-H and CPS ≥1 is a finding of interest. Although the numbers in these subgroups are small, they suggest that the response to immunotherapy is more complex than the presence of a single biomarker.
Despite the limitations of cross-trial comparisons, it is valuable to examine our results in the context of other treatment options for this patient population. Second-line chemotherapy with paclitaxel in paclitaxel-naive patients with recurrent EC was reported as achieving an ORR of 27.3% (95% CI, 15%–42.8%), DOR of 4.2 months, and OS of 10.3 months, with a grade ≥3 toxicity of 67% (18). Bevacizumab and paclitaxel combination therapy in the second-line setting with no biomarker selection achieved an ORR of 13.5% (90% CI, 6.5%–27%), median PFS of 4.2 months, and OS of 10.5 months, with grade ≥3 toxicity of 46% (19). The patients in the GARNET dMMR/MSI-H EC cohort had similar characteristics to the 79 patients with dMMR EC treated with pembrolizumab monotherapy every 3 weeks in the KEYNOTE-158 single-arm trial (20). The responses and toxicity reported were comparable with those achieved with dostarlimab monotherapy in this study. Combination of pembrolizumab with lenvatinib also achieved high ORR in 65 patients with dMMR EC enrolled in the phase III randomized controlled KEYNOTE-775 trial (16); however, the high toxicity reported may not justify use of this combination in patients with dMMR EC. Studies of other immune therapies have comprised only a small number of patients with dMMR EC (n = 13–35) with short-term follow-up (21–23). Given the durable responses achieved and low toxicity observed with dostarlimab in this study, initiating a randomized control trial with second-line chemotherapy as a comparator would be ethically questionable.
No new safety signals were observed during the follow-up period in this study; the safety profile of dostarlimab remained favorable and consistent with prior interim analyses and with the known safety profile for this drug class. Discontinuations due to TRAEs were low (8.6%). When the efficacy and safety profiles are considered, dostarlimab monotherapy offers a favorable benefit:risk profile in dMMR/MSI-H EC.
Since the GARNET trial was designed, there has been a shift in how EC is categorized, with molecular profiling becoming increasingly important (24–26). As dMMR is the second largest molecular subgroup of EC with the second worst prognosis (27), the long-term efficacy and safety results derived from the GARNET dMMR population is encouraging. Notably, dostarlimab is the only anti–PD-1 therapy to date to report efficacy and clinical safety based on a 6-week dosing regimen, which has the potential to reduce burden on patients, caregivers, and the healthcare system.
TMB has been shown to be a predictor of response to anti–PD-1 therapies across various solid tumor types (28). Our biomarker analysis suggests that TMB-H may be a reliable predictor of response to dostarlimab, independent of MMR status. TMB and PD-L1 expression have been used independently as predictors of response to anti–PD-1 therapies; however, considering these markers together may provide improved predictive utility (29). Elevated PD-L1 expression (CPS ≥1) is often used as a predictive biomarker of response to anti–PD-L1 therapies in other tumor types; however, CPS has not been widely used clinically for patients with EC (30, 31). There was a trend toward improved response in patients with TMB-H and CPS ≥1 relative to those with TMB-L or CPS <1, regardless of MMR status, suggesting that presence of both tumor neoantigens as a consequence of TMB-H status and a permissive tumor microenvironment due to CPS ≥1 enhance the likelihood of response to dostarlimab. POLεmut has been indicated as a potential prognostic marker in EC (32, 33); however, no association could be made in the present analysis, as a limited number of patients had POLεmut tumors (n = 5) and all responders were dMMR/MSI-H.
There are some important limitations in the analyses presented. First, GARNET was launched as a phase I single-arm trial without a comparator arm in patients with EC that had progressed beyond platinum-based doublet chemotherapy. As the dMMR/MSI-H and MMRp/MSS cohorts were enrolled separately, the distribution of patients is not intended to be representative of the distribution of these biomarkers in the general EC population. Beyond dMMR/MSI-H and MMRp/MSS, data from the other biomarkers reported are from post hoc analyses that were not sufficiently powered to determine a difference in response rate between subgroups. Finally, not all patients had a complete biomarker profile available.
Conclusion
The efficacy and safety data from this large trial of immunotherapy in patients with dMMR EC that had progressed on or after platinum-based chemotherapy establishes dostarlimab monotherapy as a valuable treatment option for this patient population. Predicting responses in patients with MMRp/MSS EC remains a challenge; however, additional biomarker analyses, including PD-L1 expression, TMB, or tumor profiling, may identify those most likely to benefit from PD-1 inhibitor monotherapies. Beyond this, combination therapies may expand treatment options available to patients with MMRp/MSS EC. The ongoing phase III RUBY part 1 trial (NCT03981796), which evaluates carboplatin-paclitaxel in combination with dostarlimab versus carboplatin-paclitaxel alone in frontline primary advanced or recurrent EC regardless of MMR status, met its primary endpoint and showed significantly improved PFS outcomes in this population with substantial benefit seen in dMMR/MSI-H tumors (34).
Supplementary Material
This includes supplementary tables and figures
Acknowledgments
The GARNET trial (NCT02715284) was originally designed and funded by Tesaro Inc. (acquired by GSK in 2018) in collaboration with the authors. The authors performed the collection, analysis, and interpretation of the data, and had the final decision to submit the manuscript for publication. GSK had a role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. The manuscript was written with medical writing assistance funded by GSK. Authors employed by GSK, in coordination with all authors, were involved in the preparation, review, approval, and decision to submit the manuscript.
We thank the patients and their families for participating in the study. We are also grateful to the clinical investigators, site personnel, and the members of the trial-specific independent data and safety monitoring committees. Medical writing and editorial support, funded by GSK and coordinated by Heather Ostendorff-Bach, PhD, of GSK, was provided by Sandra B. Munro, PhD, Shannon Morgan-Pelosi, PhD, Nicole Renner, PhD, and Jen Robertson, PhD, of Ashfield MedComms, an Inizio Company.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This article is featured in Selected Articles from This Issue, p. 4519
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A. Oaknin reports personal fees from Agenus, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, EMD Serono, Genmab, GSK, ImmunoGen, Itheos, Merck Sharp & Dohme, Mersana Therapeutics, Novocure, Shattuck Labs, Seagen, and Sutro Biopharma and personal fees and other support from AstraZeneca, F. Hoffmann-La Roche, and PharmaMar outside the submitted work. B. Pothuri reports grants and personal fees from GSK during the conduct of the study as well as grants and personal fees from AstraZeneca, Merck, Sutro, and Seagan; personal fees from Eisai, Eli Lilly, and Signatera; and grants from Immunogen, Mersana, Toray, VBL Therapeutics, Karyopharm Therapeutics, Genetech, Clovis Oncology, Incyte, and Celsion outside the submitted work. L. Gilbert reports personal fees from GSK, Merck, Eisai, Eisai -Merck, and Novocure and grants from AstraZeneca, Pfizer, Merck Sharp & Dohme, Karyopharm, Tesaro, Alkermes, ImmunoGen Inc., Roche, Mersana, Esperas, Novocure GmbH, OncoQuest, K-Goup Beta Inc., and GSK outside the submitted work. R. Sabatier reports personal fees and nonfinancial support from GSK, personal fees from Novartis and Eisai, grants from AstraZeneca, and nonfinancial support from MSD outside the submitted work. J. Brown reports other support from GSK/Tesaro outside the submitted work. S. Ghamande reports grants from GSK during the conduct of the study as well as personal fees from GSK and Eisai and other support from Seagen Consulting outside the submitted work. C. Mathews reports grants from GSK during the conduct of the study as well as grants from Astellas Pharma, Avenge Bio, AstraZeneca, Deciphera, EMD Serono, Genentech, Genmab, Merck, Moderna, Regeneron, Seagen, Syros, GSK, and National Cancer Institute outside the submitted work. D.M. O'Malley reports grants, personal fees, and nonfinancial support from GSK during the conduct of the study as well as grants from AbbVie, Advaxis, Agenus, Inc., Alkermes Aravive, Arcus Biosciences, AstraZeneca, BeiGene USA, Boston Biomedical, Bristol Myers Squibb, Clovis Oncology, Deciphera Pharma, Eisai, EMD, Serono, Exelixis, Genentech, Genmab, GSK, GOG Foundation, Hoffmann-La Roche, ImmunoGen, Incyte Corporation, IOVANCE Biotherapeutics, Karyopharm, Leap Therapeutics, Ludwig Institute for Ca Merck & Co., Merck Sharp & Dohme Corp., Mersana Therapeutics, NCI, Novartis, NovoCure, NRG Oncology, OncoC4, OncoQuest, Pfizer, Precision Therapeutics, Prelude Therapeutics, Regeneron Pharmaceuticals, RTOG, Rubius Therapeutics, Seattle Genetics (SeaGen), Sutro Biopharma, SWOG, Tesaro, and Verastem and personal fees from AbbVie, AdaptImmune, Agenus, Arquer Diagnostics, Arcus Biosciences, AstraZeneca, Atossa Therapeutics, Boston Biomedical, Cardiff Oncology, Celcuity, Clovis Oncology, Corcept Therapeutics, Duality Bio, Eisai, Elevar, Exelixis, Genentech Genelux, GSK, GOG Foundation, Hoffmann-La Roche, ImmunoGen, Imvax, InterVenn, INXMED, IOVANCE Biotherapeutics, Janssen, Jazz Pharmaceuticals, Laekna, Leap Therapeutics, Luzsana Biotechology, Merck & Co., Merck Sharp & Dohme Corp., Mersana Therapeutics, Myriad, Novartis, NovoCure, OncoC4, Onconova, Regeneron Pharmaceuticals, RepImmune, R Pharm, Roche Diagnostics, Seattle Genetics (SeaGen), Sorrento, Sutro Biopharma, Tarveda Therapeutics, Toray, Trillium, Umoja, Verastem, VBL Therapeutics, Vincerx Pharma, Xencor, and Zentalis outside the submitted work. R. Kristeleit reports personal fees from GSK outside the submitted work. V. Boni reports other support from Abbvie, AVEO, Adaptimmune, Amcure, Amgen, Amunix, AstraZeneca, Bicycle, BMS Cytomx, GSK, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kura, Lilly, Loxo, Nektar, Macrogenics, Menarini, Merck, Merus, Nanobiotix, Novartis, Pfizer, PharmaMar, Principia, PUMA, Ryvu, Ribbon, Sanofi, Taiho, Tesaro, BeiGene, Transgene, Takeda, Incyte, Innovio, MSD, PsiOxus, Seattle Genetics, Mersana, Daiichi, Nektar, Astellas, ORCA, Boston Therapeutics, Dynavax, DebioPharm, Boehringer Ingelheim, Regeneron, Rigontec, Millennium, Seagen, Synthon, Spectrum, Urogen, and Zenith and personal fees from Puma Biotechnology, Ideaya Biosciences, Loxo Therapeutics, CytomX Therapeutics, Guidepoint, Oncoart, Lilly, Janssen, Eli Lilly, MSD, Solti, Tactics, Getthi, and Gedefo outside the submitted work. A. Gravina reports nonfinancial support from Pfizer outside the submitted work. S. Banerjee reports grants and personal fees from AstraZeneca and GSK and personal fees from Amgen, Clovis, Immunogen, MSD, Mersana, Pfizer, Roche, Takeda, Novacure, Oncxerna, Seagen, Shattuck Labs, Regeneron, Epsilogen, and Merck Serono outside the submitted work; in addition, S. Banerjee is PI of ENGOT0v60-GOG3052/RAMP201 Verastem sponsored trial. R. Miller reports grants and personal fees from GSK, personal fees and nonfinancial support from AstraZeneca, and personal fees from Clovis Oncology and Ellipses outside the submitted work. M. Mirza reports grants from GSK during the conduct of the study as well as personal fees from GSK, AstraZeneca, Merck, Karyopharm, Roche, Zailab, and BioNTech and grants from AstraZeneca, Ultimovacs, Apexigen, and GSK outside the submitted work. E. Zografos reports other support from GSK during the conduct of the study as well as other support from GSK outside the submitted work. J. Veneris reports other support from GSK during the conduct of the study. A.V. Tinker reports personal fees from GSK, Merck, Eisai, and AstraZeneca outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
A. Oaknin: Conceptualization, formal analysis, investigation, writing–original draft, writing–review and editing. B. Pothuri: Conceptualization, formal analysis, writing–original draft, writing–review and editing. L. Gilbert: Conceptualization, formal analysis, writing–original draft, writing–review and editing. R. Sabatier: Conceptualization, formal analysis, writing–original draft, writing–review and editing. J. Brown: Conceptualization, formal analysis, writing–original draft, writing–review and editing. S. Ghamande: Conceptualization, formal analysis, writing–original draft, writing–review and editing. C. Mathews: Conceptualization, formal analysis, writing–original draft, writing–review and editing. D.M. O'Malley: Conceptualization, formal analysis, writing–original draft, writing–review and editing. R. Kristeleit: Conceptualization, formal analysis, writing–original draft, writing–review and editing. V. Boni: Conceptualization, formal analysis, writing–original draft, writing–review and editing. A. Gravina: Conceptualization, formal analysis, writing–original draft, writing–review and editing. S. Banerjee: Conceptualization, formal analysis, writing–original draft, writing–review and editing. R. Miller: Conceptualization, formal analysis, writing–original draft, writing–review and editing. J. Pikiel: Conceptualization, formal analysis, writing–original draft, writing–review and editing. M.R. Mirza: Conceptualization, formal analysis, writing–original draft, writing–review and editing. N. Dewal: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. G. Antony: Conceptualization, formal analysis, methodology, writing–original draft, writing–review and editing. Y. Dong: Conceptualization, formal analysis, visualization, methodology, writing–original draft, writing–review and editing. E. Zografos: Conceptualization, formal analysis, supervision, visualization, methodology, writing–original draft, writing–review and editing. J. Veneris: Conceptualization, formal analysis, supervision, visualization, methodology, writing–original draft, project administration, writing–review and editing. A.V. Tinker: Conceptualization, formal analysis, writing–original draft, writing–review and editing.
References
- 1. Gu B, Shang X, Yan M, Li X, Wang W, Wang Qet al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol Oncol 2021;161:573–80. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 3. Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: molecular markers and management of advanced stage disease. Gynecol Oncol 2018;150:569–80. [DOI] [PubMed] [Google Scholar]
- 4. Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen H-Zet al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LKet al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shao C, Li G, Huang L, Pruitt S, Castellanos E, Frampton Get al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Netw Open 2020;3:e2025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Green AK, Feinberg J, Makker V. A review of immune checkpoint blockade therapy in endometrial cancer. Am Soc Clin Oncol Educ Book 2020;40:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga Met al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 2019;16:563–80. [DOI] [PubMed] [Google Scholar]
- 9. Viale G, Trapani D, Curigliano G. Mismatch repair deficiency as a predictive biomarker for immunotherapy efficacy. Biomed Res Int 2017;2017:4719194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Food & Drug Administration. FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021.
- 11. GSK. European Commission approves GSK's JEMPERLI (dostarlimab), the first anti-PD-1 therapy approved for recurrent or advanced endometrial cancer. https://www.gsk.com/en-gb/media/press-releases/european-commission-approves-gsk-s-jemperli-dostarlimab-the-first-anti-pd-1-therapy-approved-for-recurrent-or-advanced-endometrial-cancer/.
- 12. Oaknin A, Gilbert L, Tinker AV, Brown J, Mathews C, Press Jet al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. J Immunother Cancer 2022;10:e003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oaknin A, Tinker AV, Gilbert L, Samouëlian V, Mathews C, Brown Jet al. Clinical activity and safety of the anti-programmed death 1 monoclonal antibody dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol 2020;6:1766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alexa M, Hasenburg A, Battista MJ. The TCGA molecular classification of endometrial cancer and its possible impact on adjuvant treatment decisions. Cancers 2021;13:1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McMeekin DS, Filiaci VL, Thigpen JT, Gallion HH, Fleming GF, Rodgers WH. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: a Gynecologic Oncology Group study. Gynecol Oncol 2007;106:16–22. [DOI] [PubMed] [Google Scholar]
- 16. Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DSet al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 2022;386:437–48. [DOI] [PubMed] [Google Scholar]
- 17. Halla K. Emerging treatment options for advanced or recurrent endometrial cancer. J Adv Pract Oncol 2022;13:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a gynecologic oncology group study. Gynecol Oncol 2003;88:277–81. [DOI] [PubMed] [Google Scholar]
- 19. Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PGet al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol 2011;29:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jet al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol 2022;40:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Konstantinopoulos PA, Luo W, Liu JF, Gulhan DC, Krasner C, Ishizuka JJet al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol 2019;37:2786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azad NS, Gray RJ, Overman MJ, Schoenfeld JD, Mitchell EP, Zwiebel JAet al. Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH (EAY131) study. J Clin Oncol 2020;38:214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Antill Y, Kok PS, Robledo K, Yip S, Cummins M, Smith Det al. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J Immunother Cancer 2021;9:e002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levine DA, Getz G, Gabriel SB, Cibulskis K, Lander E, Sivachenko Aet al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mittica G, Ghisoni E, Giannone G, Aglietta M, Genta S, Valabrega G. Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget 2017;8:90532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz Jet al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802–13. [DOI] [PubMed] [Google Scholar]
- 27. Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill Jet al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 2018;29:1180–8. [DOI] [PubMed] [Google Scholar]
- 28. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa Ket al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 2020;21:1353–65. [DOI] [PubMed] [Google Scholar]
- 29. Shiravand Y, Khodadadi F, Kashani SMA, Hosseini-Fard SR, Hosseini S, Sadeghirad Het al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr Oncol 2022;29:3044–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engerud H, Berg HF, Myrvold M, Halle MK, Bjorge L, Haldorsen ISet al. High degree of heterogeneity of PD-L1 and PD-1 from primary to metastatic endometrial cancer. Gynecol Oncol 2020;157:260–7. [DOI] [PubMed] [Google Scholar]
- 31. Mamat Yusof MN, Chew KT, Kampan N, Abd Aziz NH, Md Zin RR, Tan GCet al. PD-L1 expression in endometrial cancer and its association with clinicopathological features: a systematic review and meta-analysis. Cancers (Basel) 2022;14:3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teo MY, Seier K, Ostrovnaya I, Regazzi AM, Kania BE, Moran MMet al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J Clin Oncol 2018;36:1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang F, Zhao Q, Wang YN, Jin Y, He MM, Liu ZXet al. Evaluation of POLE and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol 2019;5:1504–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mirza MR, Chase DM, Slomovitz BM, Christensen RD, Novak Z, Black D, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med 2023;388:2145–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This includes supplementary tables and figures
Data Availability Statement
Study documents and data will be made available within 6 months of product approval/trial termination or publication obligation fulfilled date (whichever is later) at https://vivli.org/ourmember/gsk/. Enquiries about the availability of clinical studies that are not currently listed on the site can be made via the enquiry form found at https://vivli.org/ourmember/gsk/.