Abstract
In the evolving molecular treatment landscape of metastatic colorectal cancer (mCRC), the identification of druggable alterations is pivotal to achieve the best therapeutic opportunity for each patient. Because the number of actionable targets is expanding, there is the need to timely detect their presence or emergence to guide the choice of different available treatment options. Liquid biopsy, through the analysis of circulating tumor DNA (ctDNA), has proven safe and effective as a complementary method to address cancer evolution while overcoming the limitations of tissue biopsy. Even though data are accumulating regarding the potential for ctDNA-guided treatments applied to targeted agents, still major gaps in knowledge exist as for their application to different areas of the continuum of care. In this review, we recapitulate how ctDNA information could be exploited to drive different targeted treatment strategies in mCRC patients, by refining molecular selection before treatment by addressing tumor heterogeneity beyond tumor tissue biopsy; longitudinally monitoring early-tumor response and resistance mechanisms to targeted agents, potentially leading to tailored, molecular-driven, therapeutic options; guiding the molecular triage towards rechallenge strategies with anti-EGFR agents, suggesting the best time for retreatment; and providing opportunities for an “enhanced rechallenge” through additional treatments or combos aimed at overcoming acquired resistance. Besides, we discuss future perspectives concerning the potential role of ctDNA to fine-tune investigational strategies such as immuno-oncology.
Introduction
The idea to detect and monitor tumor evolution in the blood of cancer patients through liquid biopsy started back in the 20th century (1). This opportunity progressively gained increasing relevance in several cancers, as many advantages over tissue biopsy became evident, like minimal invasiveness and lower costs (2). Several biological cancer footprints can be isolated from blood, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTC), etc (2). Interestingly, the amount of these biomarkers depends on the shedding capacity of different tumor types (3). Among others, colorectal cancer is one of the major ctDNA shedders, whereas CTCs are rare and challenging to collect hampering their integration in clinical practice (4).
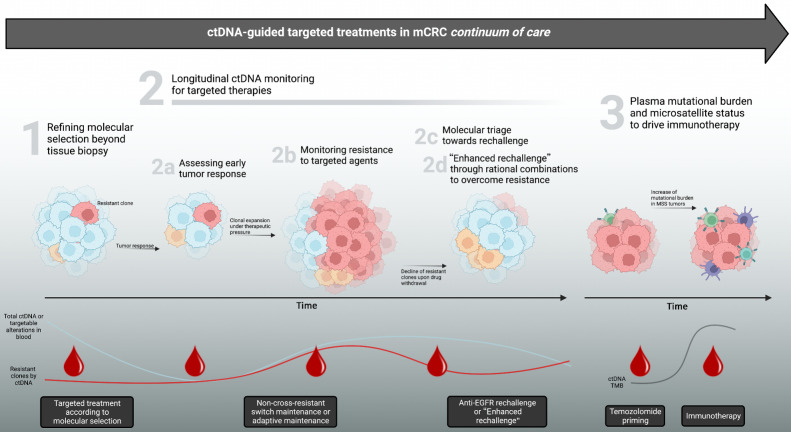
Colorectal cancer ranks third among tumors worldwide (5). The prognosis of patients diagnosed with metastatic colorectal cancer (mCRC) is poor as only 10% to 15% are alive at 5 years from diagnosis (5). During the last two decades, several targeted agents emerged for subsets of mCRC patients, starting from but not limited to anti-EGFR agents for RAS and BRAF wild-type disease (6, 7). In precision oncology, the identification of drug targets and resistance alterations is key to refine patients’ selection towards the best opportunity, and the dynamic evaluation through ctDNA could offer the chance to timely pick up the optimal targeted option within the continuum of care (8, 9). In this review (Fig. 1), we discuss the role of ctDNA in guiding a timely comprehensive treatment choice in mCRC patients, focusing on but not limited to EGFR-targeted treatments.
Figure 1.
Applications of ctDNA monitoring for driving targeted treatments in metastatic colorectal cancer. Different scenarios where ctDNA can effectively parallel therapeutic decision making during mCRC continuum of care. Potential impact in each scenario (1–3) is reported in gray boxes at the bottom and discussed in corresponding chapters of this review. ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; MSS, microsatellite stable; TMB, tumor mutational burden. (Adapted from an image created with BioRender.com.)
Refining Molecular Selection Beyond Tissue Biopsy with ctDNA
Cancers are characterized by the concomitant occurrence of different gene alterations leading to the phenomenon of spatial and temporal heterogeneity, that is particularly relevant in mCRC (2, 4). Tissue biopsy can recapitulate neither one nor the other, since it allows the molecular retrieval of only a tiny tumor area at a precise time. On the opposite, ctDNA offers a broader view of molecular features by allowing the analysis of DNA fragments that are shed from different tumor cells from all cancer lesions at specific time frames (2, 4).
Several studies demonstrated that ctDNA can effectively recapitulate tumor molecular findings with high concordance with tissue analysis and shorter turnaround times (8, 10–13). When large cohorts of colorectal cancer patients were genotyped both on tumor tissue and plasma, the overall concordance ranged between 85% and 100%. In addition, ctDNA could unveil low-allele frequency alterations, potentially leading to better molecular refinement (11, 14).
The mutational status of RAS and BRAF must be ascertained for the selection of mCRC patients towards anti-EGFR treatment, avoiding ineffective treatment in primary resistant mutant tumors with MAPK pathway activation downstream of EGFR (6, 15–19). Although pivotal studies were performed on tumor tissue, retrospective data support the hypothesis that ctDNA characterization might improve selection for anti-EGFR treatment through higher sensitivity (20).
Beyond RAS and BRAF mutations (accounting for about half of the resistant cases), different studies retrospectively broadened negative selection in the effort of optimizing tumor response. So far, studies of “negative ultraselection” encompassed alterations such as gene mutations of ERBB2, EGFR ectodomain (ECD), FGFR1, PDGFRA, PIK3CA, PTEN, AKT1 and MAP2K1, amplifications of KRAS, ERBB2 and MET, and fusions of ALK, ROS1, NTRK1–3 and RET (21–24). Among these, some EGFR ECD mutations (S492R, K467, and R451C) were shown to drive progression to cetuximab but not panitumumab and could potentially allow a therapeutic switch to the latter agent (25, 26). Moreover, further mutations occurring in the EGFR ECD (S464L, G465R, G465E, V441D, V441G) were reported to drive resistance to all anti-EGFR agents (25, 26). For most of these alterations, given their relatively low incidence, there is still limited and heterogeneous evidence to affirm a biologically and clinically relevant negative predictive effect towards anti-EGFR agents. For instance, the clinical validity of PIK3CA, PTEN and other gene alterations in the decision algorithm for anti-EGFR administration still remains to be elucidated ahead of inclusion into clinical guidelines recommendations, although data are progressively accumulating for some biomarkers as in the case of ERBB2 amplification (22, 23, 27–29). Importantly, ctDNA is regarded as an exquisite tool for the detection of these additional biomarkers of resistance, by comprehensively capturing heterogeneity together with a higher sensitivity for minor clones (30). Next-generation sequencing (NGS) was adopted by our group and others to show how genomic alterations associated with anti-EGFR primary resistance can be detected in plasma (8, 31), thus potentially leveraging ultra-selection through ctDNA. This concept was recently reinforced by a post hoc analysis of the PARADIGM trial, where ctDNA allowed negative ultra-selection to distinguish those patients experiencing greater benefit to chemotherapy and panitumumab (vs. bevacizumab) regardless of primary tumor sidedness (32). To validate this concept prospectively, the LIBImAb study (NCT04776655) was designed as a ctDNA-based, randomized phase III trial in RAS/BRAF wild-type mCRC patients, that will answer whether the retrieval of circulating RAS mutations hampers anti-EGFR efficacy and therefore favors otherwise an anti-VEGF (bevacizumab) combination with chemotherapy (FOLFIRI) in first line as compared with FOLFIRI-cetuximab.
Beyond EGFR targeting, ctDNA could be used to identify potential candidates to anti-HER2 regimens in up to 5% mCRC patients, as previously demonstrated (33, 34). In an exploratory analysis of the HERACLES study investigating trastuzumab and lapatinib in mCRC, we found that ERBB2 copy number by ctDNA is concordant with tissue in more than 96% of cases, although mild discrepancy was observed when evaluating similar data from the TRIUMPH trial, likely depending on the adopted molecular criteria to define HER2 positivity (35, 36). Consistently with HERACLES, the DESTINY-CRC01 trial with the antibody–drug conjugate (ADC) trastuzumab-deruxtecan confirmed higher overall response rate (ORR) and progression-free survival (PFS) in patients with greater levels of ERBB2 copy number in plasma (37). Likewise, ctDNA was applied as a predictive biomarker for BRAFV600E mutant mCRC patients receiving targeted therapy (anti-BRAF/EGFR with or without anti-MEK drugs). In this context, overall tissue–ctDNA accuracy was more than 90% in patients from the BEACON trial and another similar study (38, 39). Differently from ERBB2 amplification, the predictive effect of BRAFV600E was not quantitative but solely qualitative, since improved ORR to targeted BRAF-regimens as compared with chemotherapy were observed independently of mutant allele frequency (MAF) in plasma, with higher MAF worsening prognosis (38, 40). Finally, ctDNA allowed the retrieval of other actionable or potentially targetable biomarkers such as KRASG12C, but also NTRK1–3, RET, FGFR2–3, ALK, and ROS1 fusions (41–43). Relatively to nonfusion variants, fusions are more likely to be subclonal, and therefore MAF should be addressed for these alterations (42).
Longitudinal ctDNA Monitoring to Detect and Trade Off Acquired Alterations to Targeted Agents
Assessing early tumor response during treatment
Early assessment of tumor response is crucial to guide treatment decisions towards improved patient outcomes. Overall, several studies demonstrated that a decrease of ctDNA can predict tumor response to chemotherapy and anti-EGFR therapy as soon as 2 weeks after treatment administration in mCRC, usually remarkably anticipating response by conventional imaging and CEA standard biomarker (31, 44–46). Besides anti-EGFR agents and chemotherapy, the predictive value of ctDNA dynamic was also confirmed for other targeted strategies, such as anti-HER2, anti-BRAF/EGFR, and KRASG12C-directed regimens (36, 40, 47).
Monitoring resistance to targeted agents and ctDNA-driven switch maintenance
Apart from driving primary resistance when present as clonal, gene alterations can be acquired (or alternatively, selected from pre-existing subclones) during the course of targeted treatments, thus precluding cure despite initial response (8, 48, 49). In fact, EGFR blockade was shown to favor the occurrence of a selective sweep for some minor resistant subclones, eventually taking over the initial, vastly sensitive, clonal population and leading to acquired resistance and tumor progression (8). In this context, dynamic monitoring by ctDNA appears well suited to unveil acquired resistance mechanisms.
In 2012, we first reported together with Diaz and colleagues the emergence of KRAS mutant alleles in the blood of anti-EGFR treated mCRC patients, showing not only ctDNA–tissue concordance, but also the capacity of anticipating the emergence of secondary resistance (48, 49). We also reported that ctDNA has higher sensitivity for acquired RAS mutations as compared with paired tissue rebiopsy, with detection rates of 57.1% versus 9.5% after panitumumab (50). Besides, ctDNA tracking demonstrated that several other alterations emerge in plasma potentially driving acquired resistance, in genes such as ERBB2, BRAF, PIK3CA, EGFR ECD, MET, FLT3, and MAP2K1 (8, 11, 26, 27, 51–54). Recently, paired tissue–ctDNA analysis further broadened knowledge of many other mutations, copy gains, and fusions that emerge upon anti-EGFR therapy, both clonally and subclonally, underlining the complexity of heterogeneity regarding resistance mechanisms triggered by targeted agents (55). The evolutionary dynamics of resistance alterations was also reported to be heterogenous, with RAS being mutated earlier than EGFR (56). A comprehensive presentation of ctDNA studies regarding resistance alterations to anti-EGFR agents in mCRC is presented in Table 1. Finally, the analysis of mutational signatures on tissue and ctDNA recently emerged as a new class of cancer evolution predictor; in particular, single base substitution (SBS) 17B was enriched in EGFR Q61 mutant resistant clones and could be considered as a future resource if technical challenges linked to blood analysis are further unraveled (57).
Table 1.
Published studies of baseline and dynamic molecular monitoring by liquid biopsy (ctDNA) to unveil mechanisms of primary and acquired resistance to anti-EGFR–based regimens in metastatic colorectal cancer.
| Study/trial | Pts | Drugs | Line of therapy | ctDNA analysis | Resistance mechanisms | List of genes harboring resistance alterations |
|---|---|---|---|---|---|---|
| Misale et al. Nature 2012 (ref 48) | 3 | Cetuximab or panitumumab-based therapy | Any | BEAMing | Acquired | KRAS |
| Diaz et al. Nature 2012 (ref 49) | 24 | Panitumumab monotherapy | Refractory | BEAMing | Acquired | KRAS |
| Spindler et al. CCR 2012 (ref 97) | 108 | Cetuximab and irinotecan | 3rd line | qPCR | Primary | KRAS and BRAF |
| Montagut et al. Nat Med 2012 (ref 53) | 10 | Cetuximab-based therapy | Any | qPCR | Acquired | EGFR ECD |
| Siravegna et al. Nat Med 2015 (ref 8) | 100 | Cetuximab or panitumumab-based therapy | Any | ddPCR for RAS and BRAF Extended NGS analysis | Primary and acquired | KRAS, NRAS, MET, ERBB2, FLT3, EGFR ECD and MAP2K1 |
| Grasselli et al. Ann Oncol 2017 (ref 93) | 146 | Cetuximab or panitumumab-based therapy | Any | BEAMing | Primary | KRAS |
| Toledo et al. Oncotarget 2017 (ref 51) | 23 | Cetuximab and FOLFIRI | 1st line | BEAMing | Primary and acquired | KRAS, NRAS, BRAF and PIK3CA |
| Pietrantonio et al. CCR 2017 (ref 52) | 22 | Cetuximab or panitumumab | Any | ddPCR | Acquired | KRAS, BRAF, EGFR ECD and MET |
| Vidal et al. Ann Oncol 2017 (ref 102) | 115 | Cetuximab or panitumumab-based therapy | Any | BEAMing | Primary and acquired | KRAS and NRAS |
| Siena et al. Ann Oncol 2018 (ref 50) | 39 | Panitumumab and irinotecan | Refractory | BEAMing | Primary and acquired | KRAS and NRAS |
| Montagut et al. JAMA Oncol 2018 (ref 26) | 193 | Sym004 (futuximab and modotuximab) | Refractory | NGS and ddPCR | Primary and acquired | KRAS, NRAS, BRAF, EGFR ECD, ERBB2, MET |
| Normanno et al. Ann Oncol 2018 (ref 20) | 92 | Cetuximab and FOLFIRI | 1st line | BEAMing and ddPCR | Primary | KRAS and NRAS |
| Strickler et al. Cancer Discov 2018 (ref 11) | 24 | Cetuximab or panitumumab-based therapy | Any | NGS | Acquired | KRAS, NRAS, BRAF, EGFR ECD, MET, MAP2K1 |
| Maurel et al. JCO PO 2019 (ref 98) | 178 | Cetuximab or panitumumab-based therapy | 1st line | qPCR | Primary and acquired | KRAS, NRAS, and BRAF |
| Peeters et al. CCR 2019 (ref 54) | 261 | Panitumumab or panitumumab | Refractory | NGS | Primary and acquired | KRAS, NRAS, BRAF, MAP2K1, EGFR ECD, PI3KCA |
| Knebel et al. Cancers 2020 (ref 99) | 10 | Cetuximab or panitumumab-based therapy | Any | NGS | Primary and acquired | KRAS, NRAS, MAP2K1 and ERBB2 |
| Lim et al. Nature 2021 (ref 100) | 93 | Cetuximab-based therapy | 1st line | NGS | Primary and acquired | KRAS, NRAS, HRAS, BRAF, MAP2K1, ERBB2, PIK3CA, PTEN, MET, and ERBB3 |
| Yang et al. Front Oncol 2022 (ref 101) | 22 | Cetuximab-based therapy | Any | NGS | Primary and acquired | KRAS, NRAS, BRAF, MAP2K1, EGFR ECD, ERBB2, PIK3CA |
| Sartore-Bianchi et al. Nat Med 2022 (ref 27) | 52 | Panitumumab monotherapy | Refractory | NGS | Primary and acquired | KRAS, NRAS, BRAF, EGFR ECD, ERBB2, MAP2K1, PTEN, SMAD4, PIK3CA, PTEN, MET |
| Topham et al. JCO 2023 (ref 55) | 169 | Cetuximab or panitumumab | 3rd line | NGS | Acquired | EGFR ECD, KRAS, LRP1B, ZNF217, MAP2K1, PIK3CG, BRAF, NRAS, SMO, MET, FLT3, NOTCH4, ERBB2, FGFR1 |
Note: Some additional references to those previously cited in the manuscript are reported here (97–102).
Abbreviations: BEAMing, Beads, Emulsion, Amplification, Magnetics digital polymerase chain reaction; ctDNA, circulating tumor DNA; ddPCR, droplet digital polymerase chain reaction; ECD, ectodomain; EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; NGS, next generation sequencing; pts, patients; qPCR, qualitative polymerase chain reaction.
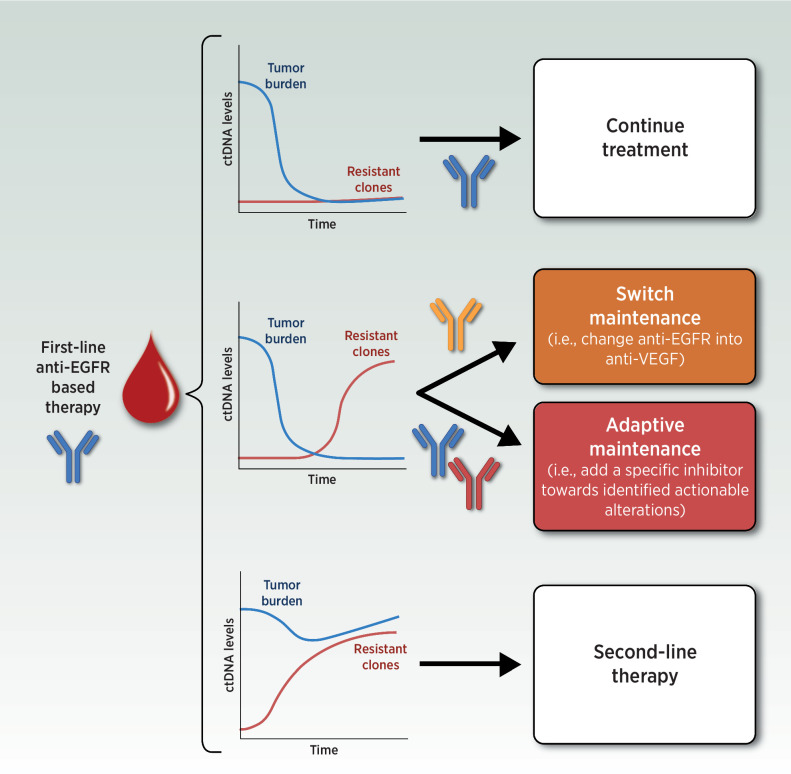
Based on this, research has moved forward to leveraging this knowledge to circumvent anti-EGFR acquired resistance. We might ask whether modulating anti-EGFR exposure based on ctDNA monitoring in first line could prevent the acquisition of massive molecular heterogeneity allowing longer time on treatment and survival. This is line with the concept of adaptive therapy, encompassing those therapeutic strategies aiming at maintaining control of the tumor burden by allowing a significant population of treatment-sensitive cells to survive (58). However, no data is available regarding ctDNA-driven approaches during first-line anti-EGFR treatment, as the only evidence of a switch-maintenance paradigm from the FIRE-4 trial (continuation of FOLFIRI-cetuximab until disease progression or switch to a maintenance of fluoropyrimidine-bevacizumab after induction) have been generated without ctDNA selection and provided negative results in an unselected mCRC population (59). In first line, ctDNA monitoring is congenial for driving ad hoc maintenance based on current molecular make-up of the tumor according to scenarios reported in Fig. 2, and in this direction adaptive/switch maintenance is being investigated in ongoing trials, such as MODUL (NCT02291289, tissue-based only, ctDNA-unguided adaptive maintenance; ref. 60), Rapid 1 (NCT04786600), LIBImAb (NCT04776655), and MoLiMoR (NCT04554836; ref. 61). However, potential limitations to this approach come from the results of two recent retrospective studies that agreed on the limited actionability of acquired mutations in first line. Indeed, alterations in the MAPK pathway as we know them could be less likely to be developed when chemotherapy is associated with anti-EGFR agents, as in the case of first line treatment regimens (62, 63). Different from resistance to single-agent anti-EGFR therapy, in this setting transcriptomic alterations might be the predominant drive of resistance (62, 63). Therefore, switch maintenance in first line may be applicable in less cases than previously hypothesized, significantly slowing accrual of ongoing trials.
Figure 2.
Clinical scenarios for an adaptive/switch maintenance ctDNA-guided approach following first-line induction treatment in metastatic colorectal cancer. ctDNA, circulating tumor DNA; EGFR, epidermal growth factor receptor. (Adapted from an image created with BioRender.com.)
ctDNA applications have recently expanded to actionable genomic alterations other than EGFR in mCRC, allowing the monitoring of resistance mechanisms in blood (33, 34). Considering ERBB2 amplification as a representative example, we and others extensively studied clonal evolution upon anti-HER2 exposure by ctDNA, and identified several mutations and copy number alterations associated with resistance (KRAS, NRAS, BRAF, ERBB2, EGFR, PIK3CA, MET and PTEN alterations), similarly to the previous experience with anti-EGFR agents (30, 35, 37, 64). Besides, we were able to measure the molecular contribution of individual metastasis in blood through ctDNA and post-mortem tissue analysis, supporting liquid biopsy as an advanced tool to track resistance alterations that are heterogeneously scattered across different tumor sites rather than ubiquitously detectable (30). Recently, novel anti-HER2/HER2 ADCs were also proposed to circumvent alterations of these genes as a mechanism of resistance to anti-EGFR drugs (65). Similarly, convergent patterns of genomic evolution in the MAPK pathway were demonstrated with other targeted therapies, like the anti-BRAF/EGFR combinations for BRAFV600E mutant mCRC patients, and ctDNA proved again to be the optimal tool for monitoring and detection (40).
ctDNA triage to guide anti-EGFR rechallenge
Rechallenge with anti-EGFR agents has long been adopted as an empiric therapeutic option for chemorefractory RAS wild-type mCRC patients after a wash-out time from previous anti-EGFR therapy (66). Indeed, resistant clones that emerge during EGFR blockade were shown to decline upon withdrawal of these agents, thereby conferring regained sensitivity to rechallenge strategies (8, 67, 68). In this regard, ctDNA has proven to be highly apt for the screening of patients that are candidates to rechallenge (8). This key concept was first demonstrated in 2015 by our group, showing how individuals who benefited from multiple anti-EGFR lines exhibited pulsatile levels of ctDNA RAS mutations, and therefore providing the molecular bases for the efficacy of rechallenge (8). In the clinic, the CRICKET and other trials then retrospectively revealed that RAS wild-type ctDNA at the time of rechallenge was a compulsory condition for response (69–72).
Based on this rationale, we designed the CHRONOS trial, the first phase II study of panitumumab rechallenge in mCRC guided by upfront prospective ctDNA mutational status of RAS, BRAF, and EGFR ECD (27). All patients were known to have RAS and BRAF wild-type mCRC on tissue analysis, having previously demonstrated sensitivity to an anti-EGFR–based therapy. Anti-EGFR rechallenge was proposed after a washout period of at least an intervening anti-EGFR–free line, on condition that RAS, BRAF, and EGFR mutant clones were undetectable by ctDNA screening. Overall, the clearing of all subclones at screening was 69% (36/52); time-to-clearance was as early as 4 months in a few patients, whereas in others resistance-conferring mutations were persistent up to 33 months. The trial included 27 patients showing 30% ORR with 8 of 27 partial responses and 63% disease control rate (DCR); median PFS and duration of response were 16 and 17 weeks. Altogether, ctDNA-driven anti-EGFR rechallenge compared favorably with standard third-line treatments and the historical 8% to 21% ORR of anti-EGFR rechallenge plus chemotherapy or immunotherapy in unselected patients. Hence, these results are the first prospective proof that ctDNA genotyping can effectively direct anti-EGFR rechallenge in mCRC management (27).
On the same track, other research groups are currently investigating ctDNA-driven rechallenge (Table 2; refs. 64, 73). The REMARRY and PURSUIT phase II trials prospectively investigated ctDNA RAS dynamics in the same setting as CHRONOS; at progression, 50 patients with ctDNA-negative RAS clones were rechallenged with a combination of cetuximab–irinotecan. ORR was however limited to 14% (7/50), with 80% DCR (40/50) and 3.6 months PFS (64). The lower-than-expected ORR in this study may be related to the fact that BRAF and EGFR mutations were not addressed by ctDNA, differently from CHRONOS (64). Besides, while the molecular criteria of the CHRONOS trial did not allow any minimal residual presence of circulating resistant clones, in the PURSUIT study ctDNA negativity was defined as a MAF <0.1, thereby potentially impacting on results (64). Finally, given the small sample size of these phase II studies, some variability in terms of confidence interval is expected, and the ORR of the PURSUIT trial is indeed in the lower range for ctDNA-informed rechallenge (64).
Table 2.
Published and ongoing studies prospectively investigating ctDNA-driven anti-EGFR rechallenge in metastatic colorectal cancer patients.
| Trial name/NCT | Phase | Pts | Drugs | Molecular ctDNA triage for inclusion | ORR (%) | DRC (%) | PFS (months) |
|---|---|---|---|---|---|---|---|
| Published | |||||||
| CHRONOS | II | 27 | Panitumumab | KRAS, NRAS, BRAF, and EGFR ECD wild type | 30 | 63 | 4.0 |
| PURSUIT | II | 50 | Cetuximab and irinotecan | KRAS and NRAS wild type | 14 | 80 | 3.6 |
| Ongoing | |||||||
| CAPRI 2-GOIM/ NCT05312398 | II | NA | Cetuximab and irinotecan | KRAS, NRAS, and BRAF wild type | NA | NA | NA |
| PARERE/NCT04787341 | II | NA | Panitumumab (randomized vs. regorafenib) | KRAS, NRAS, and BRAF wild type | NA | NA | NA |
| CAVE2-GOIM/ NCT05291156 | II | NA | Cetuximab and avelumab | KRAS, NRAS, and BRAF wild type | NA | NA | NA |
| NCT04509635 | III | NA | Cetuximab (vs. chemotherapy) | KRAS and NRAS wild type | NA | NA | NA |
| NCT04775862 | II | NA | Panitumumab or cetuximab-based rechallenge | KRAS and NRAS wild type | NA | NA | NA |
| CITRIC/EudraCT 2020–000443–3 | II | NA | Cetixumab and irinotecan (vs. anti-EGFR free regimens) | KRAS, NRAS, BRAF, and EGFR ECD wild type | NA | NA | NA |
Abbreviations: ctDNA, circulating tumor DNA; ECD, ectodomain; EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; pts, patients.
Overall, current data support the application of ctDNA-guided rechallenge in clinical practice, because this strategy favorably compared with other late line options (27). Further studies could clarify the limitations of the PURSUIT trial and suggest whether ERK activation or other mechanisms interfere with the proper inhibition of the MAPK pathway (64, 73).
“Enhanced rechallenge” through rational combinations to overcome resistance mechanisms
A relevant question for the optimization of rechallenge is whether resistance revealed by alterations in the MAPK pathways could be overcome by adding on top of anti-EGFR drugs another agent targeting that specific alteration. Indeed, resistant clones frequently display clinically actionable oncogenic events (8). ctDNA may rapidly identify these alterations and allow strategies of “enhanced rechallenge” encompassing not only EGFR blockade but also the prevention or treatment of the escaping refractory clones. This approach was recently investigated by Parseghian and colleagues by adopting a vertical double blockade with EGFR and MEK inhibitors (73). In this study, rechallenge with panitumumab monotherapy or panitumumab-trametinib was provided according to ctDNA status for RAS, BRAF or MAP2K1 mutant clones. Crossing over to the anti-EGFR/MEK combination was possible for ctDNA-negative patients in case of progression to monotherapy (73). Despite preclinical data supporting this approach (74, 75), the study led to a modest 18% ORR, 64% DCR, and 4.1-month PFS. Besides, the combination with MEK blockade failed to improve outcomes and did not overcome resistance when given at the time of crossover (73).
Other studies are further exploring this approach. The C-PRECISE-01 trial (NCT04495621) employs a ctDNA screening for the detection of PIK3CA mutations susceptible of drug targeting with a combination of MEN1611 (PI3K inhibitor) and cetuximab rechallenge, provided that no RAS and BRAF variants are detected in plasma and the tumor demonstrated previous sensitivity to anti-EGFR therapies. Also MET amplification has been associated with acquired anti-EGFR resistance (76), supporting a study of enhanced rechallenge with anti-MET (tepotinib) and cetuximab in ctDNA MET-driven acquired resistance (NCT04515394); however, the study was prematurely terminated due to operational challenges identifying suitable participants, highlighting the difficulties of precision oncology trials focused on low-prevalence molecular abnormalities. The OrigAMI-1 trial (NCT05379595) with the bispecific anti-EGFR/MET antibody amivantamab may provide some answers in this setting despite being molecular unselected for MET alterations, because it entails a cohort of anti-EGFR pretreated patients receiving amivantamab monotherapy and it incorporates ctDNA among the study procedures. Finally, rechallenge may expand beyond that of anti-EGFR agents alone to EGFR/BRAF dual inhibition, as demonstrated by a pilot case series of BRAFV600E mutant mCRC patients that gained clinical benefit by rechallenge after previous progression to cetuximab and encorafenib. In this situation, a MAF increase for BRAFV600E in ctDNA without the identification of any additional alterations was proposed to drive effective rechallenge with cetuximab and encorafenib (77).
Whether “enhanced rechallenge” may prove benefitting in the clinical setting is debatable; differences from preclinical data like the very low clonality of acquired alterations in patients may indeed be a relevant explanation for the negative results of Parseghian and colleagues (73–75). Besides, polyclonal resistance was reported in up to 21% of patients progressing to anti-EGFR therapy, meaning that several resistance alterations arose concomitantly upon progression (55). These discoveries potentially jeopardize the circumvention of acquired resistance by “enhanced rechallenge”, and further investigation is needed to provide answers.
Future Perspectives: Tumor Mutational Burden and Microsatellite Status in Blood
Another forthcoming application of ctDNA is related to immuno-oncology. Since immune checkpoint inhibitors (ICI) proved dramatically effective in mCRC with microsatellite instability (MSI), several research efforts are trying to turn immunologically “cold”, ICI-unresponsive, microsatellite stable (MSS) tumors into “hot” ones through alkylating agent-mediated transformation (78). Indeed, in the ARETHUSA trial cytotoxic priming with temozolomide (TMZ) increased tumor mutational burden (TMB) and blood TMB (bTMB) in a subset of MGMT methylated MSS mCRC, possibly gaining sensitivity to immunotherapy (79). Although the role of TMB in mCRC is still debated (80), our recent translational findings demonstrated that ctDNA could reliably measure bTMB and predicted potential benefit to pembrolizumab (with prolonged stabilization without tumor response), consistently with another study (79, 81). In this regard, ctDNA was also capable of identifying the occurrence of acquired MSH6 p.T1219I variant, which was suggested as a potential marker of TMZ molecular efficacy (79).
In this setting, methodology and cut-offs for bTMB and TMB assessment still require standardization (82). As for today, MSI remains the main biomarker predicting immunotherapy sensitivity in mCRC, and it is typically addressed by tissue analysis. Apart from immunohistochemistry and PCR, different possibilities are emerging for inferring MSI status using NGS data (83, 84) on tissue and, in particular, some methods have been FDA authorized (85). Because the mismatch repair (MMR) status can be heterogeneous within time and space (86), ctDNA was proposed for MMR characterization and provided accuracy above 98% in some studies (87–89), although NGS methods present several limitations due to low and unbalanced tumor purities in liquid biopsy samples (90). Prospective evaluation is ongoing (NCT03594448).
Conclusions
Several applications of ctDNA are emerging for the care of colorectal cancer patients according to the results of an increasing number of studies, as witnessed by the effort of clinical and multidisciplinary task forces to promote ctDNA for biomarker testing (91, 92). In this review, we focused on metastatic disease and described how ctDNA could (Fig. 1): 1) refine the molecular selection of mCRC patients at baseline by addressing tumor heterogeneity that is beyond the capacity of tissue biopsy; and dynamically 2a) assess early-tumor response; 2b) monitor resistance mechanisms, potentially leading to tailored, molecular-driven, therapeutic solutions such as switch maintenance to non-cross-resistant agents; 2c) guide the molecular triage towards rechallenge strategies, by acknowledging the clearance of resistant clones and suggesting the best time for retreatment; 2d) provide opportunities for enhanced rechallenge strategies through additional treatments or combos so as to overcome resistance. Besides, we discussed 3) promising applications of ctDNA in the field of immuno-oncology regarding MSI detection and TMB monitoring.
It is important for clinicians to be aware of these ctDNA-related opportunities, as it is increasingly more common to examine ctDNA reports in the clinical practice. When available, we recommend medical oncologists to propose ctDNA assessment to patients before anti-EGFR rechallenge, in order to limit ineffective treatments and spare unnecessary toxicity, as it is now also suggested in the updated 2022 ESMO Guidelines (6). So far, there is no evidence of an optimal time for rechallenge; however, we suggest considering it as soon as after progression to an intervening anti-EGFR-free regimen in the chemorefractory setting. Indeed, we found no correlation between the time interval from previous anti-EGFR therapy and the likelihood of response to rechallenge (27). We consider a patient candidate to anti-EGFR rechallenge from a molecular standpoint when the ctDNA assay is completely negative before starting treatment, according to the “zero mutation ctDNA” criteria for KRAS, NRAS, BRAF and EGFR ectodomain mutations (27). We suggest to analyze ctDNA by PCR (with some technical limitations) or NGS with barcoding to improve limit of detection, as proposed in the CHRONOS trial (27, 69). Then, in case of a “wild type” result, we advise commencing panitumumab withing 4 weeks; yet no data limit the administration at a later time. In the instance of a mutation precluding rechallenge, dynamic assessment of ctDNA is supported by available data about clonal evolution, and we feel an intervening line of treatment could be enough to reassess whether resistant clones have been wiped out (8, 27).
Addressing ctDNA limitations is as important as listing its huge potential. Previous publications and reviews on this topic already thoroughly dissected the main technical and translational limitations of ctDNA in mCRC (2, 4, 9). Concerning sensitivity, the amount of ctDNA shed into the bloodstream greatly varies according to the primary tumor location and metastatic sites, and false negatives are not a remote possibility (3, 12, 93, 94). Moreover, clonal hematopoiesis, reported in around 10% of tumor-free patients aged 70 or older, can lead to ctDNA false-positive results (95). Finally, limited availability of ctDNA assays was reported outside academic or comprehensive cancer centers, although this issue could be pragmatically solved by referring to companion diagnostic tests (96).
Summarizing, we believe that, aiming at overcoming the majority of ctDNA limitations towards a broaden translatability into the real-world setting, more interventional trials based on ctDNA analysis are mandatory. Indeed, as suggested by the CHRONOS trial, only prospective data from controlled trials could prove ctDNA as a reliable biomarker to be widely exploited for mCRC patients’ management. Eventually, considering intrinsic limitations beyond well-known advantages, we envision ctDNA analysis as a vehicle to improve currently available prognostic and predictive tools rather than replacing them.
Acknowledgments
The authors are supported by Fondazione Oncologia Niguarda Onlus (G. Patelli, G. Mauri, A. Sartore-Bianchi) and Fondazione AIRC per la ricerca sul cancro ETS (ID 21091 program; S. Marsoni, A. Bardelli, S. Siena, A. Sartore-Bianchi). G. Patelli and G. Mauri are PhD students within the European School of Molecular Medicine (SEMM).
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authors' Disclosures
F. Di Nicolantonio reports grants from Fondazione AIRC IG 21407, Fondazione AIRC 5 per Mille 2018 - ID. 21091 programme, and Ministero della Salute during the conduct of the study as well as personal fees from Pierre Fabre and Illumina outside the submitted work; in addition, F. Di Nicolantonio has a patent for a method for detecting colorectal cancer (Application 102017000072650) pending to University of Turin. A. Bardelli reports personal fees from Guardant Health and Inivata during the conduct of the study as well as grants from AstraZeneca, Boehringer-Ingelheim, and Neophore outside the submitted work; in addition, A. Bardelli is a shareholder of Neophore and Kither. S. Siena is an advisory board member for Agenus, AstraZeneca, Bayer, BMS, CheckmAb, Daiichi-Sankyo, Guardant Health, Merck, Novartis, Roche-Genentech, and Seagen. A. Sartore-Bianchi reports personal fees from Amgen, Bayer, Servier, Guardant Health, and Novartis during the conduct of the study. No disclosures were reported by the other authors.
References
- 1. Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res 1977;37:646–50. [PubMed] [Google Scholar]
- 2. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531–48. [DOI] [PubMed] [Google Scholar]
- 3. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patelli G, Vaghi C, Tosi F, Mauri G, Amatu A, Massihnia D, et al. Liquid biopsy for prognosis and treatment in metastatic colorectal cancer: circulating tumor cells vs circulating tumor DNA. Target Oncol 2021;16:309–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cancer of the colon and rectum - cancer stat facts [Internet]. SEER [cited 2020 Apr 14]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html
- 6. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:10–32. [DOI] [PubMed] [Google Scholar]
- 7. Sartore-Bianchi A, Agostara AG, Patelli G, Mauri G, Pizzutilo EG, Siena S. Application of histology-agnostic treatments in metastatic colorectal cancer. Dig Liver Dis 2022;54:1291–303. [DOI] [PubMed] [Google Scholar]
- 8. Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med 2015;21:827. [DOI] [PubMed] [Google Scholar]
- 9. Mauri G, Vitiello PP, Sogari A, Crisafulli G, Sartore-Bianchi A, Marsoni S, et al. Liquid biopsies to monitor and direct cancer treatment in colorectal cancer. Br J Cancer 2022;127:394–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Nicolantonio F, Vitiello PP, Marsoni S, Siena S, Tabernero J, Trusolino L, et al. Precision oncology in metastatic colorectal cancer - from biology to medicine. Nat Rev Clin Oncol 2021;18:506–25. [DOI] [PubMed] [Google Scholar]
- 11. Strickler JH, Loree JM, Ahronian LG, Parikh AR, Niedzwiecki D, Pereira AAL, et al. Genomic landscape of cell-free DNA in patients with colorectal cancer. Cancer Discov 2018;8:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bachet JB, Bouché O, Taieb J, Dubreuil O, Garcia ML, Meurisse A, et al. RAS mutation analysis in circulating tumor DNA from patients with metastatic colorectal cancer: the AGEO RASANC prospective multicenter study. Ann Oncol 2018;29:1211–9. [DOI] [PubMed] [Google Scholar]
- 13. Kim TW, Peeters M, Thomas A, Gibbs P, Hool K, Zhang J, et al. Impact of emergent circulating tumor DNA RAS mutation in panitumumab-treated chemoresistant metastatic colorectal cancer. Clin Cancer Res 2018;24:5602–9. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura Y, Taniguchi H, Ikeda M, Bando H, Kato K, Morizane C, et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat Med 2020;26:1859–64. [DOI] [PubMed] [Google Scholar]
- 15. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34. [DOI] [PubMed] [Google Scholar]
- 16. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65. [DOI] [PubMed] [Google Scholar]
- 17. Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705–12. [DOI] [PubMed] [Google Scholar]
- 18. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55. [DOI] [PubMed] [Google Scholar]
- 19. Van Cutsem E, Köhne C-H, Hitre E, Zaluski J, Chang Chien C-R, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408–17. [DOI] [PubMed] [Google Scholar]
- 20. Normanno N, Esposito Abate R, Lambiase M, Forgione L, Cardone C, Iannaccone A, et al. RAS testing of liquid biopsy correlates with the outcome of metastatic colorectal cancer patients treated with first-line FOLFIRI plus cetuximab in the CAPRI-GOIM trial. Ann Oncol 2018;29:112–8. [DOI] [PubMed] [Google Scholar]
- 21. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 2014;4:1269–80. [DOI] [PubMed] [Google Scholar]
- 22. Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One 2009;4:e7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Randon G, Maddalena G, Germani MM, Pircher CC, Manca P, Bergamo F, et al. Negative ultraselection of patients with RAS/BRAF wild-type, microsatellite-stable metastatic colorectal cancer receiving anti-EGFR-based therapy. JCO Precis Oncol 2022;6:e2200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mauri G, Patelli G, Gori V, Lauricella C, Mussolin B, Amatu A, et al. Corrigendum: case report: MAP2K1 K57N mutation is associated with primary resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer. Front Oncol 2023;13:1147497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arena S, Bellosillo B, Siravegna G, Martínez A, Cañadas I, Lazzari L, et al. Emergence of Multiple EGFR Extracellular Mutations during Cetuximab Treatment in Colorectal Cancer. Clin Cancer Res 2015;21:2157–66. [DOI] [PubMed] [Google Scholar]
- 26. Montagut C, Argilés G, Ciardiello F, Poulsen TT, Dienstmann R, Kragh M, et al. Efficacy of sym004 in patients with metastatic colorectal cancer with acquired resistance to anti-EGFR therapy and molecularly selected by circulating tumor DNA analyses: a phase 2 randomized clinical trial. JAMA Oncol 2018;4:e175245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Crisafulli G, et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: the phase 2 CHRONOS trial. Nat Med 2022;28:1612–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raghav K, Loree JM, Morris JS, Overman MJ, Yu R, Meric-Bernstam F, et al. Validation of HER2 amplification as a predictive biomarker for anti-epidermal growth factor receptor antibody therapy in metastatic colorectal cancer. JCO Precis Oncol 2019;3:1–13. [DOI] [PubMed] [Google Scholar]
- 29. Martin V, Landi L, Molinari F, Fountzilas G, Geva R, Riva A, et al. HER2 gene copy number status may influence clinical efficacy to anti-EGFR monoclonal antibodies in metastatic colorectal cancer patients. Br J Cancer 2013;108:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siravegna G, Lazzari L, Crisafulli G, Sartore-Bianchi A, Mussolin B, Cassingena A, et al. Radiologic and genomic evolution of individual metastases during HER2 blockade in colorectal cancer. Cancer Cell 2018;34:148–62. [DOI] [PubMed] [Google Scholar]
- 31. Vidal J, Fernández-Rodríguez MC, Casadevall D, García-Alfonso P, Páez D, Guix M, et al. Liquid biopsy detects early molecular response and predicts benefit to first-line chemotherapy plus cetuximab in metastatic colorectal cancer: PLATFORM-B study. Clin Cancer Res 2023;29:379–88. [DOI] [PubMed] [Google Scholar]
- 32. Shitara K, Muro K, Watanabe J, Yamazaki K, Ohori H, Shiozawa M, et al. Negative hyperselection of patients with RAS wild-type metastatic colorectal cancer for panitumumab: a biomarker study of the phase III PARADIGM trial. J Clin Oncol 2023;41:11.35944238 [Google Scholar]
- 33. Schrock AB, Pavlick D, Klempner SJ, Chung JH, Forcier B, Welsh A, et al. Hybrid capture-based genomic profiling of circulating tumor DNA from patients with advanced cancers of the gastrointestinal tract or anus. Clin Cancer Res 2018;24:1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takegawa N, Yonesaka K, Sakai K, Ueda H, Watanabe S, Nonagase Y, et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget 2016;7:3453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siravegna G, Sartore-Bianchi A, Nagy RJ, Raghav K, Odegaard JI, Lanman RB, et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin Cancer Res 2019;25:3046–53. [DOI] [PubMed] [Google Scholar]
- 36. Nakamura Y, Okamoto W, Kato T, Esaki T, Kato K, Komatsu Y, et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med 2021;27:1899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siena S. Exploratory biomarker analysis of DESTINY-CRC01, a phase II, multicenter, open-label study of trastuzumab deruxtecan (T-DXd, DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC). Ann Oncol 2021 [DOI] [PubMed] [Google Scholar]
- 38. Kopetz S, Murphy DA, Pu J, Yaeger R, Ciardiello F, Desai J, et al. Evaluation of baseline BRAF V600E mutation in circulating tumor DNA and efficacy response from the BEACON study. J Clin Oncol 2022;40:162. [Google Scholar]
- 39. Mas L, Bachet J-B, Taly V, Bouché O, Taieb J, Cohen R, et al. BRAF mutation status in circulating tumor DNA from patients with metastatic colorectal cancer: extended mutation analysis from the AGEO RASANC study. Cancers 2019;11:998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan L, Tran B, Tie J, Markman B, Ananda S, Tebbutt NC, et al. A phase Ib/II trial of combined BRAF and EGFR inhibition in BRAF V600E positive metastatic colorectal cancer and other cancers: the EVICT (Erlotinib and Vemurafenib In Combination Trial) study. Clin Cancer Res 2023;29:1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thein KZ, Biter AB, Banks KC, Duda AW, Saam J, Roszik J, et al. Identification of KRASG12C mutations in circulating tumor DNA in patients with cancer. JCO Precis Oncol 2022;6:e2100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clifton K, Rich TA, Parseghian C, Raymond VM, Dasari A, Pereira AAL, et al. Identification of actionable fusions as an anti-EGFR resistance mechanism using a circulating tumor DNA assay. JCO Precis Oncol 2019;3:PO.19.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cao W, Xu Y, Chang L, Gong Y, Li L, Mo X, et al. Genotyping of circulating tumor DNA reveals the clinically actionable mutation landscape of advanced colorectal cancer. Mol Cancer Ther 2019;18:1158–67. [DOI] [PubMed] [Google Scholar]
- 44. Tie J, Kinde I, Wang Y, Wong HL, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015;26:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garlan F, Laurent-Puig P, Sefrioui D, Siauve N, Didelot A, Sarafan-Vasseur N, et al. Early evaluation of circulating tumor DNA as marker of therapeutic efficacy in metastatic colorectal cancer patients (PLACOL Study). Clin Cancer Res 2017;23:5416–25. [DOI] [PubMed] [Google Scholar]
- 46. Parikh AR, Mojtahed A, Schneider JL, Kanter K, Van Seventer EE, Fetter IJ, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res 2020;26:1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yaeger R, Weiss J, Pelster MS, Spira AI, Barve M, Ou SI, et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N Engl J Med 2023;388:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012;486:532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012;486:537–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Siena S, Sartore-Bianchi A, Garcia-Carbonero R, Karthaus M, Smith D, Tabernero J, et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann Oncol 2018;29:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toledo RA, Cubillo A, Vega E, Garralda E, Alvarez R, de la Varga LU, et al. Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab. Oncotarget 2017;8:35289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res 2017;23:2414–22. [DOI] [PubMed] [Google Scholar]
- 53. Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, et al. Identification of a mutation in the extracellular domain of the epidermal growth factor receptor conferring cetuximab resistance in colorectal cancer. Nat Med 2012;18:221–3. [DOI] [PubMed] [Google Scholar]
- 54. Peeters M, Price T, Boedigheimer M, Kim TW, Ruff P, Gibbs P, et al. Evaluation of emergent mutations in circulating cell-free DNA and clinical outcomes in patients with metastatic colorectal cancer treated with panitumumab in the ASPECCT study. Clin Cancer Res 2019;25:1216–25. [DOI] [PubMed] [Google Scholar]
- 55. Topham JT, O'Callaghan CJ, Feilotter H, Kennecke HF, Lee YS, Li W, et al. Circulating tumor DNA identifies diverse landscape of acquired resistance to anti-epidermal growth factor receptor therapy in metastatic colorectal cancer. J Clin Oncol 2023;41:485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Emburgh BO, Arena S, Siravegna G, Lazzari L, Crisafulli G, Corti G, et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun 2016;7:13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woolston A, Barber LJ, Griffiths B, Pich O, Lopez-Bigas N, Matthews N, et al. Mutational signatures impact the evolution of anti-EGFR antibody resistance in colorectal cancer. Nat Ecol Evol 2021;5:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. West J, You L, Zhang J, Gatenby RA, Brown JS, Newton PK, et al. Towards multidrug adaptive therapy. Cancer Res 2020;80:1578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stintzing S, von Weikersthal L, Fuchs M, Kaiser F, Heinrich K, Modest DP, et al. Randomized study to investigate a switch maintenance concept with 5-FU plus bevacizumab after FOLFIRI plus cetuximab induction treatment versus continued treatment with FOLFIRI plus cetuximab: Report of a secondary endpoint of the phase-III FIRE-4 study (AIO KRK-0114). J Clin Oncol 2022;40:3519. [Google Scholar]
- 60. Ducreux M, Tabernero J, Grothey A, Arnold D, O'Dwyer PJ, Gilberg F, et al. Clinical and exploratory biomarker findings from the MODUL trial (Cohorts 1, 3 and 4) of biomarker-driven maintenance therapy for metastatic colorectal cancer. Eur J Cancer 2023;184:137–50. [DOI] [PubMed] [Google Scholar]
- 61. Schmoll H-J, Arnold D, de Gramont A, Ducreux M, Grothey A, O'Dwyer PJ, et al. MODUL-a multicenter randomized clinical trial of biomarker-driven maintenance therapy following first-line standard induction treatment of metastatic colorectal cancer: an adaptable signal-seeking approach. J Cancer Res Clin Oncol 2018;144:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parseghian CM, Sun R, Woods M, Napolitano S, Lee HM, Alshenaifi J, et al. Resistance mechanisms to anti-epidermal growth factor receptor therapy in RAS/RAF wild-type colorectal cancer vary by regimen and line of therapy. J Clin Oncol 2023;41:460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Raghav K, Ou F-S, Venook AP, Innocenti F, Sun R, Lenz H-J, et al. Acquired genomic alterations on first-line chemotherapy with cetuximab in advanced colorectal cancer: circulating tumor DNA analysis of the CALGB/SWOG-80405 trial (Alliance). J Clin Oncol 2023;41:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kagawa Y, Kotani D, Bando H, Takahashi N, Hamaguchi T, Kanazawa A, et al. Plasma RAS dynamics and anti-EGFR rechallenge efficacy in patients with RAS/BRAF wild-type metastatic colorectal cancer: REMARRY and PURSUIT trials. J Clin Oncol 2022;40:3518. [Google Scholar]
- 65. Yonesaka K. HER2-/HER3-targeting antibody-drug conjugates for treating lung and colorectal cancers resistant to EGFR inhibitors. Cancers. 2021;13:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mauri G, Pizzutilo EG, Amatu A, Bencardino K, Palmeri L, Bonazzina EF, et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: systematic review of different strategies. Cancer Treat Rev 2019;73:41–53. [DOI] [PubMed] [Google Scholar]
- 67. Parseghian CM, Loree JM, Morris VK, Liu X, Clifton KK, Napolitano S, et al. Anti-EGFR-resistant clones decay exponentially after progression: implications for anti-EGFR re-challenge. Ann Oncol 2019;30:243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan KH, Cunningham D, Werner B, Vlachogiannis G, Spiteri I, Heide T, et al. Longitudinal liquid biopsy and mathematical modeling of clonal evolution forecast time to treatment failure in the PROSPECT-C phase II colorectal cancer clinical trial. Cancer Discov 2018;8:1270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol 2019;5:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS mutations in circulating tumor DNA and clinical outcomes of rechallenge treatment with anti-EGFR antibodies in patients with metastatic colorectal cancer. JCO Precis Oncol 2020;4:898–911. [DOI] [PubMed] [Google Scholar]
- 71. Nakamura M. MO3–12–5 - phase II study of cetuximab rechallenge in patients with RAS wild-type metastatic colorectal cancer: E-rechallenge trial. Ann Oncol 2019;30:vi116. [Google Scholar]
- 72. Martinelli E, Martini G, Famiglietti V, Troiani T, Napolitano S, Pietrantonio F, et al. Cetuximab rechallenge plus avelumab in pretreated patients with RAS wild-type metastatic colorectal cancer: the phase 2 single-arm clinical CAVE trial. JAMA Oncol 2021;7:1529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Parseghian CM, Vilar Sanchez E, Sun R, Eluri M, Morris VK, Johnson B, et al. Phase 2 study of anti-EGFR rechallenge therapy with panitumumab with or without trametinib in advanced colorectal cancer. J Clin Oncol 2022;40:3520.35537102 [Google Scholar]
- 74. Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med 2014;6:224ra26. [DOI] [PubMed] [Google Scholar]
- 75. Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, et al. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res 2014;20:3775–86. [DOI] [PubMed] [Google Scholar]
- 76. Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov 2013;3:658–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ji J, Wang C, Fakih M. Rechallenge with BRAF and anti-EGFR inhibitors in patients with metastatic colorectal cancer harboring BRAFV600E mutation who progressed on cetuximab and encorafenib with or without binimetinib: a case series. Clin Colorectal Cancer 2022;21:267–71. [DOI] [PubMed] [Google Scholar]
- 78. Marmorino F, Boccaccino A, Germani MM, Falcone A, Cremolini C. Immune checkpoint inhibitors in pMMR metastatic colorectal cancer: a tough challenge. Cancers 2020;12:E2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Crisafulli G, Sartore-Bianchi A, Lazzari L, Pietrantonio F, Amatu A, Macagno M, et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients. Cancer Discov 2022;12:1656–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rousseau B, Foote MB, Maron SB, Diplas BH, Lu S, Argilés G, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med 2021;384:1168–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: the Canadian cancer trials group CO.26 study. JAMA Oncol 2020;6:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stenzinger A, Allen JD, Maas J, Stewart MD, Merino DM, Wempe MM, et al. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer 2019;58:578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jia P, Yang X, Guo L, Liu B, Lin J, Liang H, et al. MSIsensor-pro: fast, accurate, and matched-normal-sample-free detection of microsatellite instability. Genomics Proteomics Bioinformatics 2020;18:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kautto EA, Bonneville R, Miya J, Yu L, Krook MA, Reeser JW, et al. Performance evaluation for rapid detection of pan-cancer microsatellite instability with MANTIS. Oncotarget 2017;8:7452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. He W-Z, Hu W-M, Wang F, Rong Y-M, Yang L, Xie Q-K, et al. Comparison of mismatch repair status between primary and matched metastatic sites in patients with colorectal cancer. J Natl Compr Cancer Netw 2019;17:1174–83. [DOI] [PubMed] [Google Scholar]
- 87. Willis J, Lefterova MI, Artyomenko A, Kasi PM, Nakamura Y, Mody K, et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin Cancer Res 2019;25:7035–45. [DOI] [PubMed] [Google Scholar]
- 88. Silveira AB, Bidard F-C, Kasperek A, Melaabi S, Tanguy M-L, Rodrigues M, et al. High-accuracy determination of microsatellite instability compatible with liquid biopsies. Clin Chem 2020;66:606–13. [DOI] [PubMed] [Google Scholar]
- 89. Deng A, Yang J, Lang J, Jiang Z, Wang W, Yuan D, et al. Monitoring microsatellite instability (MSI) in circulating tumor DNA by next-generation DNA-seq. J Clin Oncol 2018;36:12025. [Google Scholar]
- 90. Baudrin LG, Deleuze J-F, How-Kit A. Molecular and computational methods for the detection of microsatellite instability in cancer. Front Oncol 2018;8:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dasari A, Morris VK, Allegra CJ, Atreya C, Benson AB, Boland P, et al. ctDNA applications and integration in colorectal cancer: an NCI colon and rectal-anal task forces whitepaper. Nat Rev Clin Oncol 2020;17:757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pascual J, Attard G, Bidard F-C, Curigliano G, De Mattos-Arruda L, Diehn M, et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO precision medicine working group. Ann Oncol 2022;33:750–68. [DOI] [PubMed] [Google Scholar]
- 93. Grasselli J, Elez E, Caratù G, Matito J, Santos C, Macarulla T, et al. Concordance of blood- and tumor-based detection of RAS mutations to guide anti-EGFR therapy in metastatic colorectal cancer. Ann Oncol 2017;28:1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bando H, Nakamura Y, Taniguchi H, Shiozawa M, Yasui H, Esaki T, et al. Impact of a metastatic site on circulating tumor DNA (ctDNA) analysis in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2021;39:3554. [Google Scholar]
- 95. Chan HT, Chin YM, Nakamura Y, Low S-K. Clonal hematopoiesis in liquid biopsy: from biological noise to valuable clinical implications. Cancers 2020;12:E2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Downing A, Morris EJ, Corrigan N, Sebag-Montefiore D, Finan PJ, Thomas JD, et al. High hospital research participation and improved colorectal cancer survival outcomes: a population-based study. Gut 2017;66:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Spindler K-LG, Pallisgaard N, Vogelius I, Jakobsen A. Quantitative cell-free DNA, KRAS, and BRAF mutations in plasma from patients with metastatic colorectal cancer during treatment with cetuximab and irinotecan. Clin Cancer Res 2012;18:1177–85. [DOI] [PubMed] [Google Scholar]
- 98. Maurel J, Alonso V, Escudero P, Fernández-Martos C, Salud A, Méndez M, et al. Clinical impact of circulating tumor RAS and BRAF mutation dynamics in patients with metastatic colorectal cancer treated with first-line chemotherapy plus anti-epidermal growth factor receptor therapy. JCO Precis Oncol 2019;3:1–16. [DOI] [PubMed] [Google Scholar]
- 99. Knebel FH, Barber LJ, Newey A, Kleftogiannis D, Woolston A, Griffiths B, et al. Circulating tumour DNA sequencing identifies a genetic resistance-gap in colorectal cancers with acquired resistance to EGFR-antibodies and chemotherapy. Cancers. 2020;12:E3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lim Y, Kim S, Kang J-K, Kim H-P, Jang H, Han H, et al. Circulating tumor DNA sequencing in colorectal cancer patients treated with first-line chemotherapy with anti-EGFR. Sci Rep 2021;11:16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yang W, Zou J, Li Y, Liu R, Yan Z, Chen S, et al. Longitudinal circulating tumor DNA profiling in metastatic colorectal cancer during anti-EGFR therapy. Front Oncol 2022;12:830816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vidal J, Muinelo L, Dalmases A, Jones F, Edelstein D, Iglesias M, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol 2017;28:1325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]