Acute lymphoblastic leukemia (ALL) and outcomes of refractory/relapsed disease with conventional chemotherapy approach
ALL is the most common pediatric cancer with approximately 6,000 cases diagnosed annually in the United States, representing nearly 25% of pediatric cancer diagnoses (1). Based on immune phenotype, ALL is classified to B-ALL or T-ALL, with B-ALL accounting for approximately 85% of cases. Over the last half-century, the 5-year overall survival (OS) rate has remarkably improved to above 90% in high-income countries with the implementation of improved diagnostic and disease monitoring methods, optimization of multi-agent risk-stratified chemotherapeutic regimens, and better supportive care.
However, approximately 10–15% of patients still relapse and 2% are refractory to induction therapy (2). Risk factors that predict worse outcome after the first relapse include time from diagnosis to relapse (shorter is worse, especially within 36 months from diagnosis), site of relapse (isolated marrow relapses are worse, followed by combined marrow and extramedullary, and then isolated extramedullary), immunophenotype (T-ALL is worse than B-ALL), and minimal residual disease (MRD) response to reinduction therapy. Unfortunately, the 5-year OS of the patients after first relapse is approximately 50%, and that in patients with multiply-relapsed disease, especially after allogeneic hematopoietic stem cell transplantation (HSCT), is dismal at 30% or less (2). Therefore, novel therapeutic approaches are necessary to improve survival in patients with relapsed or refractory disease.
Immunotherapy in ALL and use of chimeric antigen receptor (CAR) T-cells
Immunotherapies typically target antigens that are expressed on the cell surface of cancer cells and have been incorporated into B-ALL treatment by targeting B-cell specific antigens, such as CD19 and CD22 (1,3). There are 3 main approaches: antibody-based therapy that includes bispecific antibodies (e.g., blinatumomab), antibody-drug conjugates (e.g., inotuzumab ozogamicin), and CAR T-cells. Although these approaches cause variable degrees and durations of B-cell aplasia and hypogammaglobulinemia, supplemental immunoglobulin can be administered to maintain the humoral immune system.
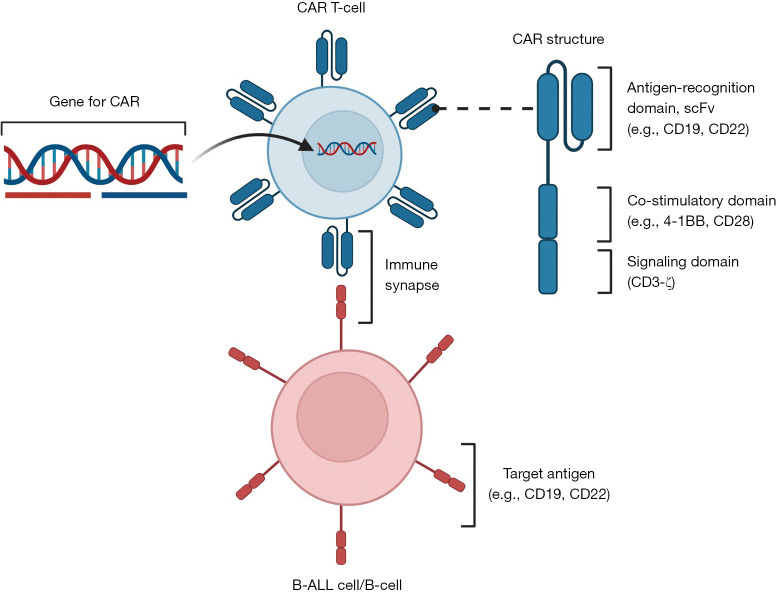
CAR T-cells are engineered T cells that exhibit powerful anti-leukemia effects and can provide curative responses (Figure 1). CAR T-cell therapy was first approved by the Food and Drug Administration (FDA) in 2017 for patients (up to 25 years of age) with B-ALL that is refractory to treatment or in second or later relapse. Historically, adoptive T-cell transfer, such as the use of autologous tumor-infiltrating lymphocytes and post-HSCT donor lymphocyte infusions for patients with solid tumor malignancies (e.g., melanoma) and leukemia, respectively, has been performed, although they are not antigen-specific. The first-generation CAR T-cells only consisted of a chimeric immunoreceptor composed of a CD19-specific extracellular antigen recognition domain of the single-chain Fragment variant (scFv) and a CD3-ζ intracellular signaling domain (19-z CAR T-cells) (4). However, the cytotoxicity and proliferation were not adequate, as first-generation CAR T-cells depended on co-stimulatory signal by the ligands of 4-1BB and CD28, which are largely not expressed on B-ALL cells. To overcome this, a co-stimulatory molecule such as 4-1BB (CD137) or CD28 was added to the CAR construct (second-generation CAR T-cells), 19-BBz CAR T-cells and 19-28z CAR T-cells, respectively, which showed remarkably improved anti-leukemic activity when compared to first-generation CAR T-cells (5,6). For example, CAR T-cells that express 4-1BB co-stimulatory molecule exponentially expanded and eliminated B-ALL cells co-cultured with bone marrow mesenchymal stromal cells, regardless of chemotherapy sensitivity profile (5). This provided strong pre-clinical evidence to develop clinical trials in patients with refractory or relapsed B-ALL who need more effective therapies.
Figure 1.
Structure of chimeric antigen receptor T-cells and mechanisms of action. The figure was created with the assistance of BioRender. CAR, chimeric antigen receptor; scFv, single-chain variable fragment; ALL, acute lymphoblastic leukemia.
Early clinical studies of 19-BBz and 19-28z CAR T-cells
The first successful use of this novel 19-BBz CAR T-cell was reported in a patient with progressive chronic lymphocytic leukemia (7). Infused CAR T-cells, at a dose of 1.46×105 cells per kilogram of body weight, expanded more than 1,000 times by day 21, and the patient had a complete response within 28 days, which had been sustained for 10 months at the time of report. Following this, 19-BBz CAR T-cells were infused in two pediatric patients with relapsed and refractory B-ALL. Again, at a dose of 1.2×105 and 1.4×105 cells per kilogram, respectively, these cells expanded more than 1,000 times by day 20 in the peripheral blood, and CAR T-cells were observed not only in peripheral blood and bone marrow but also in the cerebrospinal fluid (CSF). Both patients developed B-cell aplasia and cytokine release syndrome, with one patient treated with anti-interleukin (IL)-6 inhibitor tocilizumab. Both had a morphologic remission at approximately 1 month after infusion, with one having been in remission for 11 months at the time of report and the other relapsed with loss of CD19 antigen expression at 2 months (8).
The efficacy was confirmed with a single-center phase I/IIA study of 30 children and adults with relapsed or refractory CD19+ B-ALL having 90% complete remission rate and 6-month event-free survival (EFS) of 67% and OS of 78% (9) (Table S1). Among 30 patients, 19 had received HSCT prior to receiving CAR T-cell therapy, and there was no difference in EFS or OS between patients who had undergone HSCT and those who had not. Only 3 patients received HSCT after CAR T-cell therapy. Two major mechanisms of relapse after CAR T-cell therapy were observed; loss of B-cell aplasia (which suggests loss of CAR T-cell activity/persistence) and loss of CD19 expression on ALL cells (despite persistence of CAR T-cells). All patients developed cytokine-release syndrome of which 27% was severe (grades 3 and 4 in the Penn grading scale), which was associated with high leukemia burden and tocilizumab was confirmed as an effective treatment. Neurotoxicity was manifested as encephalopathy with aphasia, confusion, delirium, hallucinations, or seizure. In 27 patients with a response, CAR T-cells were detected with the median high peak proportion of 39.8% (range, 4.4% to 69.3%) among CD3-positive peripheral blood cells by flow cytometry and remained detectable up to 11 months. With quantitative polymerase-chain-reaction, all patients had peak levels of greater than 5,000 copies per microgram of genomic DNA, and 26 had greater than 15,000 copies. The probability of persistence at 6 months was 68% and DNA remained detectable in some patients for 2 years. All patients who had response to CAR T-cell therapy had B-cell aplasia, which lasted for up to 1 year after CAR T-cells became undetectable by flow cytometry. These patients developed hypogammaglobulinemia and received intravenous immunoglobulin (IVIG) supplements.
CAR T-cells with alternative CD28 co-stimulatory domain, 19-28z CAR T-cell, were also evaluated (10-12). Two phase 1 studies in children and young adults with CD19+ relapsed or refractory B-ALL [single institution study in National Cancer Institute (50 ALL patients) and multi-institutional ZUMA-4 study (24 patients)] showed complete remission rates of 62% and 67%, respectively, and severe [grade ≥3 in the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 and 4.03, respectively] cytokine release syndrome was seen in 18% and 33%, respectively. In both studies, the durations of B-cell aplasia or CAR T-cell detection were short, mostly lost at day 28 or at 3 months, respectively. These findings suggested that 19-28z CAR T-cells are effective but can be considered as bridging therapy to HSCT because their persistence is short compared with 19-BBz CAR T-cells.
Multi-center global study of 19-BBz CAR T-cell, tisagenlecleucel, ELIANA: early report
Following the success of the single-center trial with the 19-BBz CAR T-cell, named tisagenlecleucel, a phase II multi-center global study, ELIANA, was conducted and the first report included 75 children and young adults (≤21 years old) with CD19+ relapsed or refractory B-ALL (13) (Tables 1,2). The results of previous studies were confirmed; the overall remission rate was 81% within 3 months and all were negative for MRD as assessed by flow cytometry. At the time of this first report with median follow-up of 13.1 months, the EFS and OS were 73% and 90% at 6 months and 50% and 76% at 12 months, respectively. Eight patients underwent HSCT, and all were alive at the time of report.
Table 1. Finding of the ELIANA trial (13,14).
| Variables | Outcomes |
|---|---|
| Enrollment | 97 pediatric and young adults with relapsed/refractory B-ALL |
| Treated patients | 79 patients (81% of enrolled patients) |
| Median age of patients | 11 years (3 to 24 years) |
| Previous line of therapy | 3 (range, 1 to 8) |
| Previous HSCT | 48 patients (61%) |
| Median blasts prior to tisagenlecleucel therapy | 74% (range, 5% to 99%) |
| Median follow-up | 38.8 months |
| Overall remission | 65 of 79 (82%) (95% CI, 72% to 90%) |
| Negative minimal residual disease | 64 of 65 patients who had remission (98%) |
| HSCT after remission | 11 of 65 (17%) |
| 3-year event-free survival | 44% (95% CI, 31% to 57%) |
| 3-year overall survival | 63% (95% CI, 51% to 73%) |
| 3-year relapse-free survival (n=66) | 52% (95% CI, 37% to 66%)* and 48% (95% CI, 34% to 60%)** |
| 3-year relapse-free survival in responders, no additional therapy (n=32) | 76% (95% CI, 56% to 88%) |
| Median B-cell recovery among responders (n=66) | 35.3 months (95% CI, 22.9 months to not estimable) |
| Toxicities | |
| Any adverse events | Grade 3: 23 (29%) and grade 4: 31 (39%) |
| Cytokine release syndrome | Grade 3: 17 (22%) and grade 4: 21 (27%) |
| Neurologic events | Grade 3: 10 (13%) and grade 4: 0 (0%) |
| Infection | Grade 3: 16 (20%) and grade 4: 3 (4%) |
| ≤8 weeks post infusion (n=71) | Grade 3: 13 (18%) and grade 4: 2 (3%) |
| >1 year post infusion (n=48) | Grade 3: 8 (17%) and grade 4: 2 (4%) |
*, censored for HSCT and/or other additional therapy; **, no censoring. ALL, acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; CI, confidence interval.
Table 2. Lessons learned from ELIANA trial and future directions of CAR T-cell therapy.
| Issues learned from this study |
| Tisagenlecleucel is effective in pediatric and young adults with relapsed/refractory B-ALL |
| No reported relapses after HSCT with limited number of follow-up (n=8) and some patients can be cured with tisagenlecleucel treatment only |
| Although majority of relapses occurred within 12 months after tisagenlecleucel infusion, 6 of 24 (25%) relapses occurred at >12 months |
| Loss of target antigen (CD19) and loss of CAR T-cell persistence and/or B-cell aplasia are the major mechanisms of relapse |
| Cytokine release syndrome and neurologic events are commonly seen within 8 weeks of infusion |
| No new severe adverse effects in long-term, mostly happening within 8 weeks after infusion |
| B-cell aplasia can persist for a long time, which duration is associated with remission, and hypogammaglobulinemia and infections are seen |
| Evaluation of health-related quality of life shows continuous improvement |
| Future directions |
| Development of CAR T-cells that target multiple antigens (e.g., CD19 and 22) or co-administration of multiple CAR T-cells |
| Devise the CAR T-cells with prolonged persistence: co-stimulatory molecule, humanized molecule, epigenetic alternation |
| Optimal lymphodepleting chemotherapy (e.g., exposure to fludarabine) |
| Confirmation of efficacy in the extramedullary disease (e.g., central nervous system, testis) |
| Establishment of risk criteria after CAR T-cell therapy (e.g., next-generation sequencing MRD, monitoring of B-cell aplasia) for subsequent therapy |
CAR, chimeric antigen receptor; ALL, acute lymphoblastic leukemia; HSCT, hematopoietic stem cell transplantation; MRD, minimal residual disease.
Tisagenlecleucel maximally expanded at median 10 days for responders and persisted in blood for median 168 days (range, 20 to 617 days). B-cell aplasia was seen in all responders to CAR T-cell therapy and probability of B-cell aplasia was 83% at 6 months after infusion. Grade ≥3 (in CTCAE version 4.03) cytokine release syndrome occurred in 35 patients (47%), requiring care in the intensive care unit (ICU), 28 (37%) received tocilizumab, and 19 (25%) were treated with high-dose vasopressors. Median days of onset was 3 days (range, 1 to 22 days) and duration was 8 days (range, 1 to 22 days). Transient increases in serum levels of IL-6, interferon gamma, ferritin, IL-10, IL12p70, IL-1β, and IL-2 were also observed. Grade 3 neurologic events such as encephalopathy, confusion, delirium, tremor, agitation, and somnolence occurred in 10 patients (13%). One patient had seizure and no patient developed cerebral edema. Most of the neurotoxicity events were seen during the cytokine release syndrome or shortly after it resolved.
This initial study report describes that tisagenlecleucel is effective at providing durable remissions in patients that needed more effective therapies. However, it is associated with transient high-grade toxicities that mostly occur within the first 8 weeks after infusion and must be closely monitored by a multidisciplinary team that may require an intensivist. Some patients remained in remission with 19-BBz CAR T-cell without HSCT.
ELIANA long-term follow-up report
Although tisagenlecleucel is a great option for treating children and young adults with relapsed and refractory B-ALL, it is important to confirm the long-term efficacy and safety. In a recently published article, Laetsch et al. reported outcomes of 79 patients (median 11 years old) in ELIANA trial at median 38.8 months (14) (Tables 1,2). The median number of previous regimens was 3 and 48 patients (61%) had a prior HSCT. The overall remission rate was 82% within 3 months, and 3-year EFS and OS were 44% and 63%, respectively. Although majority of relapses occurred within 12 months after tisagenlecleucel infusion, 6 of 24 (25%) relapses occurred >12 months, and the latest one was 33 months after remission. Seventeen (22%) patients underwent HSCT after infusion, with 11 being in a tisagenlecleucel-mediated remission (MRD negative by flow cytometry in 5, positive in 4, and unknown in 2), and no patient with available follow-up data (n=8) relapsed afterwards, regardless of disease status before HSCT, with a median follow-up of 18 months. The 32 responders who did not receive additional therapy while in remission had relapse-free survival of 76% at 3 years. These results are encouraging, as no other treatments have shown equivalent or superior outcomes in this high-risk relapsed and refractory patient population. B-cell recovery among responders was seen for a median 35.3 months, which reflects the persistence of tisagenlecleucel, and the duration of B-cell aplasia correlated with duration of remission.
Adverse events following infusion were mostly reported previously, with most grade 3 and 4 events occurring within the first year, especially within the first 8 weeks after infusion. Those that most frequently occurred after the first year were infections and skin disorders, in 20.4% and 6.1% of patients, respectively. Infections were likely attributed to hypogammaglobulinemia (immunoglobulin G <400 mg/dL), which is associated with prolonged B-cell aplasia, and IVIG replacement was given to 91% of patients for a median of 200 days to mitigate this risk. Furthermore, the health-related quality of life continued to improve throughout this 3-year follow-up.
With this longer follow-up analysis, 19-BBz CAR T-cell, tisagenlecleucel, continues to show promising results with durable responses as a potential curative therapy with manageable adverse events and improvement of quality of life, although B-cell aplasia associated hypogammaglobulinemia and infection are seen. The role and indication of HSCT in patients who received tisagenlecleucel was not assessed in this study, although many of the responsive patients did not receive HSCT.
Recent updates and future direction of CAR T-cell therapy
Although tisagenlecleucel is clearly efficacious, up to 50% of patients still relapse mostly secondary to down-regulation or loss of target antigen (e.g., CD19) or loss of CAR T-cell persistence and/or B-cell aplasia (3). CD19 antigen down-regulation or loss despite the presence of CAR T-cells can be caused by genetic mutations in CD19 exons 2‒5, alternative splicing at exon 2, alterations of the chaperone protein CD81, trogocytosis of CD19 by CAR T-cells, and lineage switch. Expression levels of CD19 on ALL cells can affect CAR T-cell engraftment and proliferation, with low expression being associated with a shorter duration of B-cell aplasia (15). Previous exposure to blinatumomab in patients of CAR T-cell therapy is associated with low CD19 expression and patients who did not previously respond to blinatumomab therapy have worse outcomes (16). The use of multi-modal therapy has been a tenet of ALL treatment and has led to significant improvements in survival. Therefore, CAR T-cells that target multiple antigens may further improve outcomes by overcoming antigen modulation. The bispecific CAR T-cell targeting both CD19 and CD22 with 4-1BB co-stimulatory domain (19-22-BBz CAR T-cells), axicabtagene ciloleucel, was safe and efficacious (17). Similarly, patients who received co-administration of CD19 and CD22-specific CAR T-cells attained durable remissions, which were augmented by subsequent HSCT (18).
Loss of CAR T-cell persistence and B-cell aplasia is affected by the co-stimulatory molecule (4-1BB showing longer duration of B-cell aplasia than CD28), immune-mediated rejection to murine molecular component, lymphodepleting therapy, and exhaustion of T cells (3). Whether third-generation CAR T-cells with two costimulatory molecules can improve their persistence is not known. A humanized CAR T-cell product has shown to persist long-term and induced durable remissions in children and young adults with relapsed or refractory B-ALL, including those who had failures to the previous CAR T-cell therapy (non-humanized product) without CD19 antigen loss (19). When lymphodepletion chemotherapy with fludarabine (30 mg/m2 per day for 4 days) and cyclophosphamide (500 mg/m2 per days for 2 days) were administered prior to CAR T-cell infusion, fludarabine exposure with an area under the curve of ≥13.8 mg × h/L was associated with decreased risks of relapse and loss of B-cell aplasia (20). CAR T-cell exhaustion can be caused by epigenetic repression of multipotent developmental potential of T cells with DNA methyltransferase 3 alpha (DNMT3A) (21). Deletion of the DNMT3A in CAR T-cells preserved proliferation and antitumor responses during prolonged tumor/antigen exposure.
Other factors that are associated with worse outcomes are high-leukemia burden defined by ≥5% bone marrow blasts, central nervous system (CNS) involvement as CNS3 (white blood cell counts of 5/µL or more with blasts), or non-CNS extramedullary disease prior to CAR T-cell therapy (22). However, it is possible to use CAR T-cell therapy in patients with extramedullary relapses because they have been detected in CSF, although proliferation can be limited in this body component, as well as in peripheral blood (8). CAR T-cells can be effective in controlling disease in patients with central nervous system or testicular relapses (18), who have been typically treated with cranial or testicular irradiation, respectively. Therefore, patients who receive CAR T-cell therapy in this setting may not require radiation therapy, which is associated with significant late effects. Furthermore, CAR T-cell therapy can induce remissions regardless of cytogenetic risk categories based on responses to conventional chemotherapy, including high-risk cytogenetics such as KMT2A rearranged infant ALL (23).
Although HSCT after CAR T-cells seems to be associated with better outcomes, there are patients who remain in remission after CAR T-cell therapy alone. MRD detection with next-generation sequencing of immunoglobulin or T-cell receptor gene rearrangements is sensitive at the level of 1 in ≤106 cells and specific to detect ALL cells even after CD19/CD22 antigen loss. Any detectable next-generation sequencing MRD after CAR T-cell therapy are associated with increased risk of relapse in addition to early loss (especially before 6 months) of B-cell aplasia (24). By incorporating these data and patient clinical status, new risk criteria for consolidative HSCT or other additional therapies need to be established.
There are several limitations for CAR T-cell therapy. Cell collection in smaller children can be difficult because of poor venous access, limited inlet rates due to small venous catheters, relatively large extracorporeal volume required by the cell separator device, and metabolic complications such as citrate toxicity (25). Heavily pre-treated patients, especially those who received lymphodepleting therapy such as clofarabine and fludarabine, may have low quantity and quality of T-cells to produce optimal CAR T-cells (26). Furthermore, although there has been continued optimization of the manufacturing process of tisagenlecleucel, especially with commercialization, the median time from leukapheresis to infusion remains at 23 days (range, 21–37 days), which is not feasible for patients with rapidly progressive disease (27). Further refinement of this process is ongoing, including the selection of efficient and non-exhausted T-cell subpopulations, addition of cytokine cocktails, and creation of off-the-shelf allogeneic CAR T-cells (28). Finally, socioeconomic status impacts whether a patient will receive CAR T-cell therapy (29). Children with a high disease burden exposed to poverty, as defined by public insurance, or those from low opportunity neighborhoods, as defined by the Childhood Opportunity Index, received this therapy less frequently, when compared to those with commercial insurance or from high opportunity neighborhoods, respectively. Complete remission rate and OS of these patients were comparable between these groups.
Conclusions
CAR T-cell therapy has brought a curative option for patients who have dismal outcomes with conventional chemotherapy. This is a great example of bench to bedside research making a great stride in ALL treatment. It is encouraging that the efficacy lasts for a long-term, with no new severe adverse effects after the first few months as reported by Laetsch et al. (14). However, further improvements in efficacy, management of toxicity, and risk stratification will be necessary through collaborative research and future clinical trials can assess whether CAR T-cell therapy can be brought to the upfront therapy.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank Vani Shanker, PhD, Department of Scientific Editing, St. Jude Children’s Research Hospital, for editing the manuscript.
Funding: This work was supported by the National Cancer Institute (Cancer Center Support CORE Grant CA21765 to BJM and HI) and by the American Lebanese Syrian Associated Charities (to BJM and HI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-23-366/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-23-366/coif). HI reports that he received research support from Servier, Amgen, and Incyte, received consulting fees from Amgen, Servier, and Jazz Pharmaceuticals and honoraria from Amgen, and participated in the advisory board for Jazz Pharmaceuticals. BJM has no conflicts of interest to declare.
References
- 1.Inaba H, Pui CH. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J Clin Med 2021;10:1926. 10.3390/jcm10091926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood 2020;136:1803-12. 10.1182/blood.2019004043 [DOI] [PubMed] [Google Scholar]
- 3.Inaba H, Pui CH. Immunotherapy in pediatric acute lymphoblastic leukemia. Cancer Metastasis Rev 2019;38:595-610. 10.1007/s10555-019-09834-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper LJ, Topp MS, Serrano LM, et al. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood 2003;101:1637-44. 10.1182/blood-2002-07-1989 [DOI] [PubMed] [Google Scholar]
- 5.Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676-84. 10.1038/sj.leu.2403302 [DOI] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13:5426-35. 10.1158/1078-0432.CCR-07-0674 [DOI] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517-28. 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NN, Lee DW, Yates B, et al. Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. J Clin Oncol 2021;39:1650-9. 10.1200/JCO.20.02262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wayne AS, Huynh V, Hijiya N, et al. Three-year results from phase I of ZUMA-4: KTE-X19 in pediatric relapsed/refractory acute lymphoblastic leukemia. Haematologica 2023;108:747-60. 10.3324/haematol.2022.280678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 2018;378:439-48. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laetsch TW, Maude SL, Rives S, et al. Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J Clin Oncol 2023;41:1664-9. 10.1200/JCO.22.00642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017;129:3322-31. 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myers RM, Taraseviciute A, Steinberg SM, et al. Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL. J Clin Oncol 2022;40:932-44. 10.1200/JCO.21.01405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med 2021;27:1419-31. 10.1038/s41591-021-01436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Tang Y, Cai J, et al. Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia: A Single-Arm, Multicenter, Phase II Trial. J Clin Oncol 2023;41:1670-83. 10.1200/JCO.22.01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 2021;39:3044-55. 10.1200/JCO.20.03458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrizio VA, Boelens JJ, Mauguen A, et al. Optimal fludarabine lymphodepletion is associated with improved outcomes after CAR T-cell therapy. Blood Adv 2022;6:1961-8. 10.1182/bloodadvances.2021006418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prinzing B, Zebley CC, Petersen CT, et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med 2021;13:eabh0272. 10.1126/scitranslmed.abh0272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schultz LM, Baggott C, Prabhu S, et al. Disease Burden Affects Outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia After Commercial Tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J Clin Oncol 2022;40:945-55. 10.1200/JCO.20.03585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leahy AB, Devine KJ, Li Y, et al. Impact of high-risk cytogenetics on outcomes for children and young adults receiving CD19-directed CAR T-cell therapy. Blood 2022;139:2173-85. 10.1182/blood.2021012727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov 2022;3:66-81. 10.1158/2643-3230.BCD-21-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Fante C, Seghatchian J, Perotti C. Reflections on methodical approaches to hematopoietic stem cell collection in children. Transfus Apher Sci 2018;57:425-7. 10.1016/j.transci.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 26.Hutt D, Bielorai B, Baturov B, et al. Feasibility of leukapheresis for CAR T-cell production in heavily pre-treated pediatric patients. Transfus Apher Sci 2020;59:102769. 10.1016/j.transci.2020.102769 [DOI] [PubMed] [Google Scholar]
- 27.Tyagarajan S, Spencer T, Smith J. Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials. Mol Ther Methods Clin Dev 2020;16:136-44. 10.1016/j.omtm.2019.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blache U, Popp G, Dünkel A, et al. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat Commun 2022;13:5225. 10.1038/s41467-022-32866-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman H, Li Y, Liu H, et al. Impact of poverty and neighborhood opportunity on outcomes for children treated with CD19-directed CAR T-cell therapy. Blood 2023;141:609-19. 10.1182/blood.2022017866 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as