Abstract
Platelet-rich plasma (PRP) is gaining more and more attention in regenerative medicine as an innovative and efficient therapeutic approach. The regenerative properties of PRP rely on the numerous bioactive molecules released by the platelets: growth factors are involved in proliferation and differentiation of endothelial cells and fibroblasts, angiogenesis and extracellular matrix formation, while cytokines are mainly involved in immune cell recruitment and inflammation modulation. Attempts are ongoing to improve the therapeutic potential of PRP by combining it with agents able to promote regenerative processes. Two interesting candidates are ozone, administered at low doses as gaseous oxygen-ozone mixtures, and procaine. In the present study, we investigated the effects induced on platelets by the in vitro treatment of PRP with ozone or procaine, or both. We combined transmission electron microscopy to obtain information on platelet modifications and bioanalytical assays to quantify the secreted factors. The results demonstrate that, although platelets were already activated by the procedure to prepare PRP, both ozone and procaine induced differential morpho-functional modifications in platelets resulting in an increased release of factors. In detail, ozone induced an increase in surface protrusions and open canalicular system dilation suggestive of a marked α-granule release, while procaine caused a decrease in surface protrusions and open canalicular system dilation but a remarkable increase in microvesicle release suggestive of high secretory activity. Consistently, nine of the thirteen platelet-derived factors analysed in the PRP serum significantly increased after treatment with ozone and/or procaine. Therefore, ozone and procaine proved to have a remarkable stimulating potential without causing any damage to platelets, probably because they act through physiological, although different, secretory pathways.
Key words: platelet activation, platelet growth factors, cytokines, ozone therapy, transmission electron microscopy
Introduction
The advent of regenerative medicine has transformed the landscape of healthcare, offering innovative strategies to harness the body’s inherent healing mechanisms for tissue repair and functional restoration.
Among the myriad of therapeutic approaches, platelet-rich plasma (PRP) has emerged as front-runner for its high regenerative properties, attributed to the platelet reservoir of hundreds of bioactive molecules1,2 involved not only in haemostasis but also in inflammation modulation, angiogenesis, and cellular proliferation and differentiation.3-5
PRP has beneficial effects in diverse clinical applications, including orthopaedics, sports medicine, dermatology, and pain management. Positive outcomes have been obtained in conditions such as tennis elbow6 and knee osteoarthritis.7 In dermatology, PRP is utilized for skin rejuvenation, scar revision, and hair restoration.8,9 Furthermore, PRP has demonstrated efficacy in wound healing of chronic skin lesions such as diabetic foot ulcers and venous leg ulcers.10,11 In dental and oral surgery, PRP has been used to enhance postoperative healing, promote bone regeneration, and improve the outcomes of implant dentistry and periodontal procedures.12-14
Attempts are ongoing to improve the therapeutic potential of PRP by combining it with agents able to promote inflammation modulation and regenerative processes.
An interesting candidate is ozone (O3), which is used at low doses as gaseous O2-O3 mixtures for complementary therapeutic purposes in various medical disciplines including orthopaedics, dermatology, and dentistry (recent reviews in15-19). The therapeutic efficacy of low-dose O3 would rely on the induction of an oxidative “eustress”20 that stimulates the cell antioxidant and cytoprotective pathways through activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) without inducing injury or inflammation.21-24 Moreover, low O3 concentrations have been demonstrated to promote angiogenesis, wound healing and tissue preservation from injury,25-30 all key events in regenerative medicine. Remarkably, injections of PRP in association with O3 proved to give better results than PRP alone in treating osteoarthritis31,32 or tendon-tobone healing33 in both humans and animals.
Procaine (Pr), a local anaesthetic agent, has attracted attention for its potential role in tissue regeneration beyond its analgesic properties. In fact, at low concentrations Pr demonstrated antioxidant and cytoprotective properties in various disease states such as inflammation, sepsis, intoxication, atherogenesis and neurodegeneration, resulting in tissue repair and, ultimately, in “anti-ageing” effects.34 In addition, Pr is the preferred local anaesthetic in neural therapy (a complementary treatment designed to resolve chronic pain) thanks to its ability to influence cytokine metabolism, such as Interleukin 6 (IL-6) and Tumour Necrosis Factor α (TNF-α), and promote antiinflammatory response.35-37 These properties have led to the exploration of Pr therapeutic potential in comparison with PRP38 and the combination of the two treatments to enhance the regenerative processes is currently under discussion among clinicians.
In this context, in the present study we provide evidence concerning the effects of the in vitro treatment of PRP with O3 or Pr or both O3 and Pr. The study was carried out with PRP obtained from healthy subjects using a combined approach that included a comparative analysis of the ultrastructural features of platelets, providing information on their structural integrity and functional modifications, as well as a quantitative evaluation of growth factors and cytokines secreted, which play an important role in the therapeutic efficacy of PRP.
Materials and Methods
PRP samples were prepared from blood drawn from three healthy non-smoker volunteers (one male and two females aged 31-70 years, i.e., the authors MAL, OA and MM), who did not take any drug for at least two weeks and did not undergo ozone therapy for at least three months. This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013, and all participants signed an informed consent prior to blood sampling and investigation. From each subject, 36 mL of blood were collected in a syringe containing 4 mL of 3.8% sodium citrate (Fidia Spa, San Mauro Torinese, TO, Italy) as anticoagulant. It is known that sodium citrate is unable to activate platelets39 and significantly reduces platelet aggregation in blood ozonation in comparison to heparin.40 PRP was obtained by using the cell concentration unit Duografter II with its specific Push-Out tubes and syringes (Fidia Spa), according to the manufacturer’s double spin method. Briefly, the blood was distributed in the tubes and centrifuged at 180 rpm for 8 min. The supernatant (plasma) and the upper cell pellet corresponding to the platelet-rich fraction and buffy coat were aspirated and put in a second tube that was centrifuged at 200 rpm for 10 min. After that, the platelet pellet and the lower half of plasma were collected as PRP. For each experiment 3 mL PRP samples were put in 20 mL polypropylene (O3-resistant) syringes and treated with: i) a gaseous mixture of O2-O3 (16 μg O3/mL O2) in the proportion of 1:1 (i.e., 3 mL PRP + 3mL gas) or ii) 3 mL of 2% Pr hydrochloride (Fidia Spa) (final Pr concentration 1%) or iii) 3 mL of 2% Pr hydrochloride plus 16 μg O3/mL O2 in the proportion of 1:1. For sake of simplicity, these experimental conditions hereinafter will be referred as O3-treated, Pr-treated and Pr+O3-treated samples, respectively.
O2-O3 gas mixtures were generated from medical-grade O2 by an OZO2 FUTURA apparatus (Alnitec Srl. Cremosano, CR, Italy). The concentration of O3 was chosen as the most commonly used in the treatment of many orthopaedic and dermatologic diseases, while 1% Pr is the standard concentration for this anaesthetic. Control samples were treated with air. The treatments took 20 min, and then each sample was processed for both transmission electron microscopy and bioanalytical assays.
Ultrastructural analysis of platelets
Morphological analyses were carried out at transmission electron microscopy, in order to analyse the effects of the exposure to O3, Pr and Pr+O3 on the fine features of platelets. To avoid possible morphological alterations due to pre-fixation cell handling, platelets were fixed in their plasma by adding an equal amount of fixative solution made of 5% glutaraldehyde and 4% paraformaldehyde in PBS. In detail, after treatment, the pellets were fixed for 2 h at 4°C; then, the liquid was removed and the samples were washed with PBS, post-fixed with 1% OsO4 and 1.5% K4Fe(CN)6 for 1 h at 4°C, dehydrated in acetone and embedded in Epon resin.
Semithin sections (2 μm in thickness) were stained with 1% aqueous toluidine blue and observed in an Olympus BX51 microscope (Olympus Italia Srl, Segrate, MI, Italy) equipped with a QICAM Fast 1394 Digital Camera (QImaging, Surrey, BC, Canada) for image acquisition.
Ultrathin sections (70-90 nm in thickness) were stained with Reynolds’ lead citrate and observed in a Philips Morgagni transmission electron microscope (FEI Company Italia Srl, Milan, Italy) operating at 80kV and equipped with a Megaview III camera (FEI Company Italia Srl) for digital image acquisition.
Growth factor and cytokine secretion
Aliquots (200 μL) of the plasmatic fraction of PRP collected 20 min after gas and/or Pr exposure were frozen in liquid nitrogen and stored at -80°C until analysis. The amount of 13 plateletderived factors was evaluated. The analysed growth factors were: Epidermal Growth Factor (EGF), Fibroblast Growth Factor 2 (FGF-2, also known as bFGF or FGF-β), Platelet-Derived Growth Factor (PDGF-AB), Vascular Endothelial Growth Factor (VEGF). The cytokines were: Interleukin 1 Receptor antagonist (IL-1Ra), IL-1β, IL-2, IL-6, IL-10, IL-13, Interferon α (INF-α), IFN-γ and TNF-α. Quantitation of the factors was conducted with a Luminex™ FLEXMAP 3D™ instrument (Bio-Rad Laboratories, Segrate, MI, Italy) coupled to the software Bio-Plex 6.2. Briefly, 180 μL aliquots of plasma were put in a 96-well plate. Superparamagnetic microspheres conjugated with fluorophores and antibodies against the target factors were added to the assay wells, then the plate was loaded into the Luminex system for reading and signal quantitation. Samples were run in duplicate.
Values of concentrations (expressed as pg/mL) were transformed into percentages based on the relative control values in order to make comparable the results obtained in the three subjects.
Statistical analysis
For each analysed factor, the Kolmogorov-Smirnov test was performed in order to verify the hypothesis of identical distributions in subjects for the same factor. Since this test confirmed equal distributions, the data for each factor were pooled according to the experimental condition (i.e., control, O3-treated, Pr-treated and Pr+O3-treated samples) and the mean ± standard error (SE) were calculated. Statistical comparison was performed by the one-way analysis of variance (ANOVA) test followed by Dunn’s test for pairwise comparisons (Bonferroni corrected p-values). Statistical significance was set at p≤0.05.
Results
Ultrastructural features of platelets
At light microscopy, all PRP samples appeared to be mostly composed of closely packed platelets, while leukocytes occurred in low amounts (Figure 1). At transmission electron microscopy, the morphological features of platelets from the three subjects were similar in each experimental condition. No damaged or abnormal platelets were found in any sample.
In control samples platelets showed irregular shape due to many elongated protrusions (Figure 2). They contained numerous α-granules, characterised by a homogeneous, moderately electron dense content, and a few dense (δ) granules, characterised by a dense core surrounded by a clear space.41,42 The open canalicular system, a surface-connected complex tubular network derived from plasma membrane invagination,43 was well developed and often dilated; some platelets showed tubular dilations containing finely granular material suggestive of secretory activity via exocytosis. Mitochondria and endoplasmic reticulum were well preserved, glycogen granules were abundant. Microvesicles were rarely found among platelets, suggesting a scarce secretory activity via vesicle release.
Platelets of O3-treated samples resembled the control ones but they were richer in elongated protrusions and in tubular dilations containing finely granular material (Figure 3), while microvesicles were rarely observed, thus suggesting a high secretory activity through exocytosis rather than vesicle release.
Platelets of Pr-treated samples showed a more regular shape in comparison to control, with a few short protrusions (Figure 4). The α-granules were numerous and dense granules were frequently observed. The open canalicular system was well developed and composed of small tubules often grouped in clusters whereas dilations were occasionally found, suggesting a low exocytic activity. Mitochondria and endoplasmic reticulum were well preserved, and glycogen was abundant. Many microvesicles protruding from the platelet surface were observed; moreover, microvesicle clusters were frequently found enclosed in vacuoles inside platelets, protruding from their surface and free in the extracellular environment, thus suggesting a marked secretory activity through vesicle release. Platelets of Pr+O3-treated samples resembled the Pr-treated ones, but they showed more irregular shapes due to some elongated protrusions (Figure 5). The open canalicular system frequently showed dilations with finely granular material suggestive of high exocytic activity, and only rare clusters of small tubules were observed. Similarly to Pr-treated samples, numerous microvesicles and microvesicle clusters occurred, suggesting a high secretory activity through vesicle release.
Figure 1.
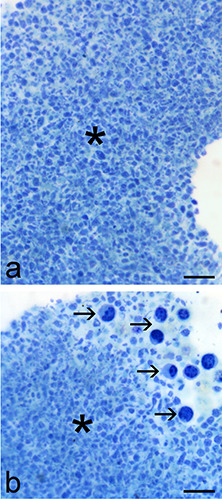
Light microscopy images of PRP pellets. a) The large majority of the sample is made of platelets (asterisks). b) Some leukocytes (arrows) sometimes occur at the border of the platelet pellet. Scale bars: 20 μm.
Growth factor and cytokine secretion
Significant differences were found in the amount of most platelet-derived factors considered in the PRP plasma from samples treated with O3 and/or Pr in comparison to control (Figures 6 and 7). As for growth factors, EGF and FGF-2 showed a significant increase after all treatments, with values significantly higher in Pr+O3-treated samples than in the O3- or Pr-treated ones (Figure 6 a,b). VEGF increased significantly after O3 treatment, whereas Pr and Pr+O3 did not induce any effect (Figure 6c). PDGF-AB did not undergo any change after all treatments (Figure 6d). Among cytokines, IL-1β, IL-6, IL-10 and TNF-α (Figure 7 a-d) showed a significant increase after treatment with both Pr and Pr+O3, whereas they did not change after O3 exposure. IL-2 and INF-α significantly increased after all treatments, with values significantly higher in Pr- and Pr+O3-treated samples than in the O3-treated ones (Figure 7 e,f). Finally, the values of IFN-γ, IL1-Ra and IL-13 were below the detection limit in all samples.
Discussion
Our morphological and bioanalytical study of the effect of O3 and Pr on PRP revealed significant changes in platelet structure as well as plasmatic concentrations of platelet-derived factors.
Figure 2.
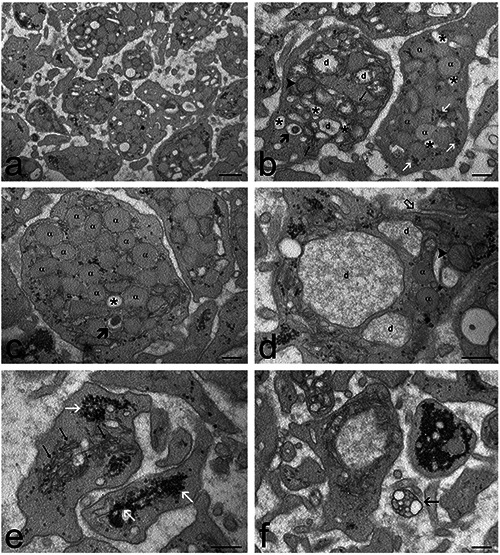
Transmission electron micrographs of control PRP samples. a) General view of the sample: the platelets show features typical of activation such as elongated surface protrusions and enlarged open canalicular system. b) Platelets containing α-granules (α), dense granules (thick arrow), mitochondria (arrowhead), endoplasmic reticulum (thin arrows), glycogen granules (white arrows), and a developed open canalicular system (asterisks) with evident dilations (d). c,d) Two platelets showing different activation stages: in c) α-granules (α) are numerous and the open canalicular system is not dilated (asterisks), a dense granule is indicated by a thick arrow; in d) canalicular dilations (d) are very evident and some α-granules (α) occur in close proximity. Note the tubule belonging to the open canalicular system that opens to the platelet surface (open arrow). A mitochondrion is indicated by an arrowhead. e) High magnification detail showing the well-developed endoplasmic reticulum (thin arrows) and glycogen clusters (white arrows). f) A rare microvesicle cluster (arrow) occurs in the extracellular space. Scale bars: a) 500 nm; b-f) 200 nm.
Platelets of control samples showed the typical morphological signs of activation compared to resting platelets, such as many elongated protrusions, and well-developed and dilated open canalicular system.42,44,45 Some platelets showed remarkable canalicular dilations containing a finely granular material corresponding to the content of α-granules released in the surface-connected open canalicular system.46 Occasionally, single microvesicles and clusters of microvesicles were observed, according to the heterogeneous types of membrane vesicles known to be released by platelets.45,47,48 Taken together, these observations demonstrate a remarkable secretory activity carried out mainly by α-granules excretion rather than microvesicle release. The activated status of control platelets is consistent with the double spin procedure used to prepare PRP, aimed at yielding highly concentrated plateletderived factors by stimulating platelet activation. Notably, this procedure did not induce any structural damage, as highlighted by transmission electron microscopy.
O3 treatment did not change the general morphology of platelets but made their shape even more irregular with respect to control and increased the number of large canalicular dilations with α-granule content. On the other hand, microvesicle release remained scarce. This suggests that O3 stimulates platelet secretory activity mostly via α-granule exocytosis in the open canalicular system but not via microvesicle release. Low O3 concentrations (such as 16 μg/mL) are known to induce mild oxidative stress unable to cause cell damage but able to influence several -although not fully understood- cell functions (reviews24,49). Interestingly, mild ozonation can affect the molecular organization of cytoskeleton, 50,51 which plays a key role in cell protrusion formation, organelle trafficking and endo-exocytosis, as well as it occurs in platelets.52-56 Accordingly, it may be hypothesized that, under our experimental conditions, O3 affects cytoskeletal proteins promoting the formation of new protrusions at the platelet surface as well as α-granule exocytosis, consistently with the higher concentration of some platelet-derived factors secreted in the plasma. The optimal preservation of cytoplasmic organelles demonstrates that this O3-driven stimulus did not induce any structural damage to platelets.
Figure 3.
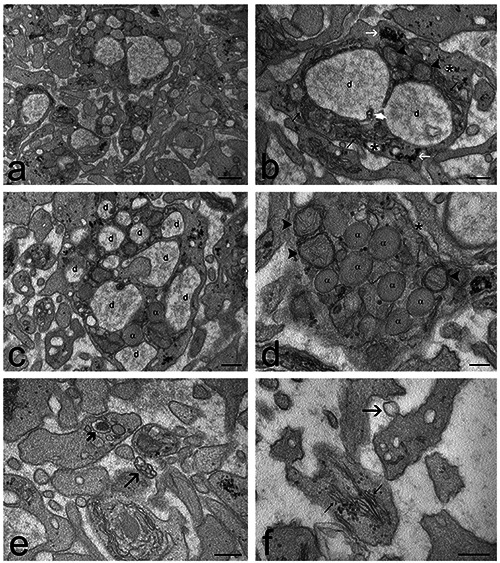
Transmission electron micrographs of O3-treated PRP samples. a) General view of the sample: the platelets show features typical of activation, with many elongated surface protrusions and enlarged open canalicular system. b) A platelet showing two large, intercommunicating (white arrowhead) canalicular dilations (d), tubules (asterisks) belonging to the open canalicular system, mitochondria (arrowheads), endoplasmic reticulum (thin arrows) and glycogen clusters (white arrows). c) Most platelets show many canalicular dilations (d) and a few α-granules (α). d) Some platelets appear less activated, containing many α-granules (α) and moderately enlarged open canalicular system (asterisk); mitochondria are indicated by arrowheads. e) A rare microvesicle cluster (arrow) occurs in the extracellular space. A dense granule is indicated by the thick arrow. f) A microvesicle (arrow) budding from the platelet surface. Note the well-developed endoplasmic reticulum (thin arrows). Scale bars: a) 500 nm; b-f) 200 nm.
The treatment with Pr markedly changed the general aspect of platelets, reducing surface protrusions and canalicular dilations. Pr is a well-known inhibitor of platelets activation by reducing calcium mobilization from intraplatelet storage pools and Ca2+ efflux across membranes.57-59 This is consistent with the small amount of elongated surface protrusions that represent a marker for platelet activation,42,44,45 observed in our Pr-treated samples. Pr also reduces α-granule exocytosis;60 consistently, in our Pr-treated platelets the reduction in dilation of the open canalicular system and the occurrence of many small tubules often grouped in clusters clearly indicate a decreased exocytic activity. However, the overall secretory activity of Pr-treated platelets was not reduced, as demonstrated by both microscopy and bioanalytical data. In fact, ultrastructural observations showed that platelets greatly increased microvesicle release, both as single units but especially as clusters, and most of the analysed platelet-derived factors occurred in higher amount in Pr-treated PRP than in control samples. It is known that Pr changes cell membrane fluidity inducing blebbing61 and this could facilitate microvesicle release. Notably, despite the evident morphological modifications induced by Pr, no platelet damage was observed, demonstrating the safety of this treatment.
Figure 4.
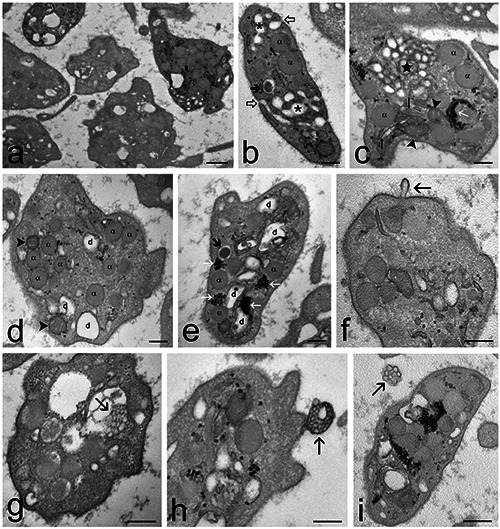
Transmission electron micrographs of Pr-treated PRP samples. a) General view of the sample: the platelets appear as activated but the surface protrusions are few and short, and the open canalicular system is moderately enlarged. b) A platelet containing α-granules (α) and dense granules (thick arrow). Tubules of the canalicular system (asterisks) open to the platelet surface (open arrowheads). c) The open canalicular system often forms clusters of small tubules (star). The platelet contains also α-granules (α), mitochondria (arrowheads), endoplasmic reticulum (thin arrows) and glycogen clusters (white arrow). d,e) Some platelets show moderate canalicular dilations (d). α- granules (α); dense granules (thick arrow); mitochondria (arrowheads); glycogen clusters (white arrows). f) A microvesicle (arrow) budding from the platelet surface. g-i) Microvesicle clusters (arrows) occurring g) inside a canalicular dilation, h) budding from the platelet surface and i) free in the extracellular space. Scale bars: a) 500 nm; b-i) 200 nm.
The combination of Pr and O3 treatments resulted in platelets similar to Pr-treated samples but showing more elongated surface protrusions and more canalicular dilations containing finely granular material; in addition, the clusters of small tubules disappeared. These features are an index of increased α-granules secretion46 and are probably due to the above-discussed effects of O3. Notably, Pr+O3-treated platelets maintained also a high secretory activity via microvesicles, as demonstrated by ultrastructural observations. This is probably the reason for the high plasmatic concentration of most of the secreted factors analysed.
Taken together, ultrastructural observations demonstrated that O3 and Pr differentially affect platelets structural features, but both of them exert a stimulating effect on the release pathways. Consistently, O3 and Pr treatments applied to PRP increased factor release in the plasma.
Figure 5.
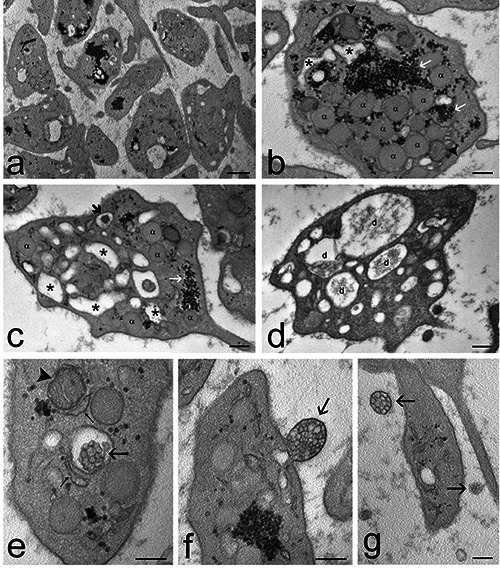
Transmission electron micrographs of Pr+O3-treated PRP samples. a) General view of the sample: the platelets show few and short surface protrusions and many dilations of the open canalicular system. b,c) Some platelets contain many α-granules (α), dense granules (thick arrow) and moderately dilated open canalicular system (asterisks). Mitochondria (arrowheads); endoplasmic reticulum (thin arrows); glycogen clusters (white arrows). d) Some platelets show many evident dilations (d) of the open canalicular system. e-g) Microvesicle clusters (arrows) e) occur inside canalicular dilations, f) bud from the platelet surface, and g) are distributed in the extracellular space. A mitochondrion is indicated by an arrowhead; endoplasmic reticulum cisternae are indicated by thin arrows. Scale bars: a) 500 nm; b-g) 200 nm.
Figure 6.
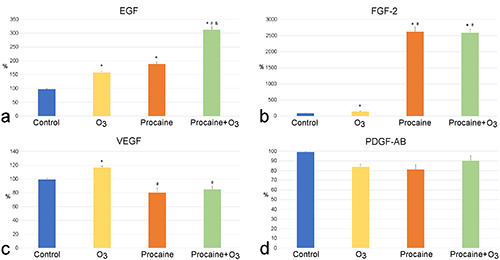
Mean value ± SE of platelet-derived growth factors detected in the PRP plasma of control, O3-treated, Pr-treated and Pr+O3- treated PRP samples after 20 min from treatment (three experiments in duplicate). Control values were set at 100%; *significant difference with control; #significant difference with O3-treated samples; &significant difference with Pr-treated samples.
Figure 7.
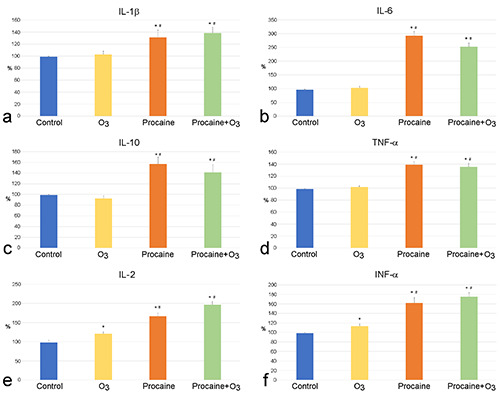
Mean value ± SE of platelet-derived cytokines detected in the PRP plasma of control, O3-treated, Pr-treated and Pr+O3-treated PRP samples after 20 min from treatment (three experiments in duplicate). Control values were set at 100%; *significant difference with control; #significant difference with O3-treated samples.
The thirteen factors analysed in our study are known to be present in PRP,3,62,63 although some inconsistencies are present in the literature due to different experimental conditions (e.g., method to obtain PRP, sample volumes, anticoagulant factors, platelet concentration). These platelet-derived factors play key roles in many physiological and regenerative processes: growth factors (EGF, FGF-2, PDGF, VEGF) are involved in growth, proliferation and differentiation of endothelial cells and fibroblasts, vessel wall permeability and angiogenesis, and extracellular matrix formation, while cytokines (IL1-Ra, IL-1β, IL-2, IL-6, IL-10, IL-13, INFα, IFN-γ, TNF-α) are mainly involved in immune cell recruitment and inflammation modulation (recent review in5). Therefore, these platelet-derived factors are of primary importance for the therapeutic potential of PRP since they are essential for wound healing, bone regeneration and cartilage repair.5
In our study, most of the analysed factors were found in all plasma samples, with the exception of IL1-Ra, IL-13 and IFN-γ, which were below the detection limit. Nine of the ten detected factors showed significant modifications in their plasmatic concentration following at least one treatment.
Pr- and Pr+O3 induced a significant increase of IL-1β, IL-6, IL- 10 and TNF-α release in comparison to control, whereas O3 did not influence the secretion of these factors. IL-1β, IL-6 and TNF-α are all released via both α-granules and microvesicles64,65 and, although scarce information is available about IL-10 in PRP, its presence has been reported in platelets secretion62,63 suggesting similar secretory mechanisms. It may be hypothesized that the increased microvesicle release induced by Pr is responsible for the increased secretion of these factors. On the other hand, O3 alone proved to be unable to induce an incremental effect on the secretion of these factors. It cannot be excluded that the O3-driven stimulus would require a time longer than 20 min, as demonstrated for other platelet-derived factors in a previous in vitro study on ozonated human platelets.66
IL-2, INF-α and FGF-2 showed a significant increase in all treated samples in comparison to control, with values of Pr- and Pr+O3-treated samples significantly higher than O3-treated ones. The three factors are known to be secreted by platelets via both α- granules and microvesicles5,41 and the above-discussed ability of O3 and Pr to stimulate both release pathways is probably the reason for the observed plasmatic increment of these factors. Concerning in particular IL-2, it has been demonstrated that low O3 concentrations stimulate IL-2 release by lymphocytes,67 which occur in PRP although in low amounts, and IL-2 stimulates platelet secretory activity:68 this could contribute to the increased amount of IL-2 found in O3-treated samples. The strikingly augmented microvesicle release induced by Pr could explain the higher increase of IL- 2, INF-α and FGF-2 in both Pr- and Pr+O3-treated samples.
EGF showed a similar increase in both O3- and Pr-treated samples in comparison to control, suggesting that the release of both α- granules and microvesicles play a similar role in the secretion of this growth factor.5,41 Consistently, when the O3 and Pr are combined, they act synergically thus significantly improving the amount of EGF in comparison to samples treated by O3 or Pr only.
VEGF showed a significant increase only in O3-treated samples in comparison to control. This growth factor has been found in both α-granules and microvesicles5,41,46 but probably the increase in α- granules release induced by O3 is more efficient than microvesicle release in incrementing the plasmatic content of this factor.
The plasmatic value of PDGF-AB was not affected by any treatment, suggesting that platelet activation due to PRP preparation promoted its strong release and no further implementation could be obtained by O3 or Pr. A previous study reported that in vitro ozonisation of human platelets increased PDGF-AB release;66 however, this discrepancy may be explained by the different experimental conditions applied, with special reference to the higher O3 concentration used (40-80 μg O3/mL).
In conclusion, the results of this study demonstrate that in vitro treatment of PRP with 16 μg O3/mL O2 or 1% Pr or both is able to induce differential morpho-functional modifications in platelets that always result in an increased release of factors. It is worth noting that the treatments were performed on PRP i.e., a concentrate of platelets already activated through the preparation protocol; therefore, the modifications observed in the treated samples represent additional effects occurring during the 20-min exposure to O3 and/or Pr. Hence, O3 and Pr proved to have a remarkable stimulating potential without causing any damage to platelets, probably because they act through physiological -although different- secretory pathways. It is worth noting that the absence of cell damage is of primary importance to avoid inflammatory reactions in patients. We are aware that this work has been conducted on a limited number of subjects and must be considered as a pilot study, but the interesting results obtained make O3 and Pr promising candidates for further research aimed at improving the therapeutic potential of PRP.
Acknowledgements
We thank Mrs. Marika Ceresa for skilful technical assistance in PRP preparation.
References
- 1.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004;103:2096-104. [DOI] [PubMed] [Google Scholar]
- 2.Piersma SR, Broxterman HJ, Kapci M, de Haas RR, Hoekman K, Verheul HMW, Jimenez CR. Proteomics of the TRAPinduced platelet releasate. J Proteomics 2009;72:91-109. [DOI] [PubMed] [Google Scholar]
- 3.Amable PR, Carias RB, Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, Borojevic R. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013;4:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Plateletrich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci 2020;21:7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spakova T, Janockova J, Rosocha J. Characterization and therapeutic use of extracellular vesicles derived from platelets. Int J Mol Sci 2021;22:9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet-rich plasma. Curr Pharm Biotechnol 2012;13:1185-95. [DOI] [PubMed] [Google Scholar]
- 7.Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 2012;13:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg 2014;7:189-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord 2018;4:18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cervelli V, Gentile P, Grimaldi M. Regenerative surgery: use of fat grafting combined with platelet-rich plasma for chronic lower-extremity ulcers. Aesthetic Plast Surg 2009;33:340-5. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex, Plateltex aktivated. Vox Sang 2009;97:110-8. [DOI] [PubMed] [Google Scholar]
- 12.Dori F, Nikolidakis D, Huszar T, Arweiler NB, Gera I, Sculean A. Effect of platelet-rich plasma on the healing of intrabony defects treated with an enamel matrix protein derivative and a natural bone mineral. J Clin Periodontol 2008;35:44-50. [DOI] [PubMed] [Google Scholar]
- 13.Del Corso M, Vervelle A, Simonpieri A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Curr Pharm Biotechnol 2012;13:1207-30. [DOI] [PubMed] [Google Scholar]
- 14.Simonpieri A, Del Corso M, Vervelle A, Jimbo R, Inchingolo F, Sammartino G, Dohan Ehrenfest DM. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Curr Pharm Biotechnol 2012;13:1231-56. [DOI] [PubMed] [Google Scholar]
- 15.Deepthi R, Bilichodmath S. Ozone therapy in periodontics: a meta-analysis. Contemp Clin Dent 2020;11:108-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anil U, Markus DH, Hurley ET, Manjunath AK, Alaia MJ, Campbell KA, et al. The efficacy of intra-articular injections in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. Knee 2021;32:173-82. [DOI] [PubMed] [Google Scholar]
- 17.Masan J, Sramka M, Rabarova D. The possibilities of using the effects of ozone therapy in neurology. Neuro Endocrinol Lett 2021;42:13-21. [PubMed] [Google Scholar]
- 18.Machado AU, Contri RV. Effectiveness and safety of ozone therapy for dermatological disorders: a literature review of clinical trials. Indian J Dermatol 2022;67:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlMogbel AA, Albarrak MI, AlNumair SF. Ozone therapy in the management and prevention of caries. Cureus 2023;15: e37510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niki E. Oxidative stress and antioxidants: distress or eustress? Arch Biochem Biophys 2016;595:19-24. [DOI] [PubMed] [Google Scholar]
- 21.Re L, Mawsouf MN, Menendez S, Leon OS, Sanchez GM, Hernandez F. Ozone therapy: clinical and basic evidence of its therapeutic potential. Arch Med Res 2008;39:17-26. [DOI] [PubMed] [Google Scholar]
- 22.Bocci V, Valacchi G. Nrf2 activation as target to implement therapeutic treatments. Front Chem 2015;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galie M, Costanzo M, Nodari A, Boschi F, Calderan L, Mannucci S, et al. Mild ozonisation activates antioxidant cell response by the Keap1/Nrf2 dependent pathway. Free Radic Biol Med 2018;124:114-21. [DOI] [PubMed] [Google Scholar]
- 24.Galie M, Covi V, Tabaracci G, Malatesta M. The role of Nrf2 in the antioxidant cellular response to medical ozone exposure. Int J Mol Sci 2019;20:4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Chen H, Liu XH, Chen ZY, Weng XD, Qiu T, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med 2014;8:1764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izadi M, Kheirjou R, Mohammadpour R, Aliyoldashi MH, Moghadam SJ, Khorvash F, et al. Efficacy of comprehensive ozone therapy in diabetic foot ulcer healing. Diabetes Metab Syndr 2019;13:822-5. [DOI] [PubMed] [Google Scholar]
- 27.Cisterna B, Costanzo M, Nodari A, Galie M, Zanzoni S, Bernardi P, et al. Ozone activates the Nrf2 pathway and improves preservation of explanted adipose tissue in vitro. Antioxidants 2020;9:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pchepiorka R, Moreira MS, Lascane NADS, Catalani LH, Allegrini S Jr, de Lima NB, Goncalves EF. Effect of ozone therapy on wound healing in the buccal mucosa of rats. Arch Oral Biol 2020;119:104889. [DOI] [PubMed] [Google Scholar]
- 29.Sen S, Sen S. Ozone therapy a new vista in dentistry: integrated review. Med Gas Res 2020;10:189-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang R, Liu F, Huang P, Zhang Y, He J, Pang X, et al. Ozone preconditioning protects rabbit heart against global ischemiareperfusion injury in vitro by up-regulating HIF-1α. Biomed Pharmacother 2022;150:113033. [DOI] [PubMed] [Google Scholar]
- 31.Dernek B, Kesiktas FN. Efficacy of combined ozone and platelet-rich-plasma treatment versus platelet-rich-plasma treatment alone in early stage knee osteoarthritis. J Back Musculoskelet Rehabil 2019;32:305-11. [DOI] [PubMed] [Google Scholar]
- 32.Huang P, Wang R, Pang X, Yang Y, Guan Y, Zhang D. Plateletrich plasma combined with ozone prevents cartilage destruction and improves weight-bearing asymmetry in a surgeryinduced osteoarthritis rabbit model. Ann Palliat Med. 2022;11:442-51. [DOI] [PubMed] [Google Scholar]
- 33.Gurger M, Once G, Yilmaz E, Demir S, Calik I, Say Y, et al. The effect of the platelet-rich plasma and ozone therapy on tendon-to-bone healing in the rabbit rotator cuff repair model. J Orthop Surg Res 2021;16:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gradinaru D, Ungurianu A, Margina D, Moreno-Villanueva M, Burkle A. Procaine-the controversial geroprotector candidate: new insights regarding its molecular and cellular effects. Oxid Med Cell Longev 2021;2021:3617042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egli S, Pfister M, Ludin SM, Puente de la Vega K, Busato A, Fischer L. Long-term results of therapeutic local anesthesia (neural therapy) in 280 referred refractory chronic pain patients. BMC Complement Altern Med 2015;15:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rey Novoa M, Munoz-Sellart M, Catalan Soriano M, Vinyes D. Treatment of localized vulvar pain with neural therapy: a case series and literature review. Complement Med Res 2021;28:571-7. [DOI] [PubMed] [Google Scholar]
- 37.Weinschenk S, Benrath J, Kessler E, Strowitzki T, Feisst M. Therapy with local anesthetics to treat vulvodynia. A pilot study. Sex Med 202210:100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin J. Platelet-rich plasma injection in the treatment of frozen shoulder: a randomized controlled trial with 6-month followup. Int J Clin Pharmacol Ther 2018;56:366-71. [DOI] [PubMed] [Google Scholar]
- 39.Almhanawi BH, Khalid B, Ibrahim TA, Tohit ERM. A transmission electron microscopy study of anticoagulant-induced platelet vesiculation. Porto Biomed J 2017;2:23-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bocci V, Valacchi G, Rossi R, Giustarini D, Paccagnini E, Pucci AM, Di Simplicio P. Studies on the biological effects of ozone: 9. Effects of ozone on human platelets. Platelets 1999;10:110-6. [DOI] [PubMed] [Google Scholar]
- 41.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 2009;23:177-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neumuller J, Meisslitzer-Ruppitsch C, Ellinger A, Pavelka M, Jungbauer C, Renz R, et al. Monitoring of platelet activation in platelet concentrates using transmission electron microscopy. Transfus Med Hemother 2013;40:101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selvadurai MV, Hamilton JR. Structure and function of the open canalicular system - the platelet’s specialized internal membrane network. Platelets 2018;29:319-25. [DOI] [PubMed] [Google Scholar]
- 44.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001;12: 261-73. [DOI] [PubMed] [Google Scholar]
- 45.Neumuller J, Ellinger A, Wagner T. Transmission Electron Microscopy of Platelets from Apheresis and Buffy-Coat- Derived Platelet Concentrates. In: Khan M. editor. The Transmission Electron Microscope - Theory and Applications. London: IntechOpen; 2015. p. 255-84. [Google Scholar]
- 46.Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev 1993;7:52-62. [DOI] [PubMed] [Google Scholar]
- 47.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999;94:3791-9. [PubMed] [Google Scholar]
- 48.Boilard E, Duchez AC, Brisson A. The diversity of platelet microparticles. Curr Opin Hematol 2015;22:437-44. [DOI] [PubMed] [Google Scholar]
- 49.Sagai M, Bocci V. Mechanisms of action involved in ozone therapy: is healing induced via a mild oxidative stress? Med Gas Res 2011;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costanzo M, Cisterna B, Vella A, Cestari T, Covi V, Tabaracci G, Malatesta M. Low ozone concentrations stimulate cytoskeletal organization, mitochondrial activity and nuclear transcription. Eur J Histochem 2015;59(2):2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cisterna B, Costanzo M, Lacavalla MA, Galie M, Angelini O, Tabaracci G, Malatesta M. Low ozone concentrations differentially affect the structural and functional features of non-activated and activated fibroblasts in vitro. Int J Mol Sci 2021;22:10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flaumenhaft R, Dilks JR, Rozenvayn N, Monahan-Earley RA, Feng D, Dvorak AM. The actin cytoskeleton differentially regulates platelet a-granule and dense-granule secretion. Blood 2005;105: 3879–87. [DOI] [PubMed] [Google Scholar]
- 53.Fox JE. The platelet cytoskeleton. Thromb Haemost 1993;70:884-93. [PubMed] [Google Scholar]
- 54.Hartwig JH. The platelet: form and function. Semin Hematol 2006;43:S94-100. [DOI] [PubMed] [Google Scholar]
- 55.Ge S, White JG, Haynes CL. Cytoskeletal F-actin, not the circumferential coil of microtubules, regulates platelet densebody granule secretion. Platelets 2012;23:259-63. [DOI] [PubMed] [Google Scholar]
- 56.Pertuy F, Eckly A, Weber J, Proamer F, Rinckel J-Y, Lanza F, et al. Myosin IIA is critical for organelle distribution and Factin organization in megakaryocytes and platelets. Blood 2014;123:1261–9. [DOI] [PubMed] [Google Scholar]
- 57.Iwamura M, Ishimori T, Makino M, Yasuda K, Izumi A, Himori N. Drug-induced inhibition of guinea pig platelet aggregation unrelated to their beta-adrenolytic actions. Jpn J Pharmacol 1983;33:219-26. [DOI] [PubMed] [Google Scholar]
- 58.Watala C, Boncler M, Golański J, Koziołkiewicz W, Walkowiak B, Cierniewski CS. Release of calcium and Pselectin from intraplatelet granules is hampered by procaine. Thromb Res 1999;94:1-11. [DOI] [PubMed] [Google Scholar]
- 59.Frangopol PT, Mihăilescu D. Interactions of some local anesthetics and alcohols with membranes. Colloids Surf B Biointerfaces 2001;22:3-22. [DOI] [PubMed] [Google Scholar]
- 60.Prowse C, Pepper D, Dawes J. Prevention of the platelet alphagranule release reaction by membrane-active drugs. Thromb Res 1982;25:219-27. [DOI] [PubMed] [Google Scholar]
- 61.Yau TM. Procaine-mediated modification of membranes and of the response to X irradiation and hyperthermia in mammalian cells. Radiat Res 1979;80:523-41. [PubMed] [Google Scholar]
- 62.Mussano F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine, chemokine, and growth factor profile of platelet-rich plasma. Platelets 2016;27:467-71. [DOI] [PubMed] [Google Scholar]
- 63.Dregalla RC, Herrera JA, Donner EJ. Soluble factors differ in platelets derived from separate niches: a pilot study comparing the secretome of peripheral blood and bone marrow platelets. Cytotherapy 2021;23:677-82. [DOI] [PubMed] [Google Scholar]
- 64.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost 2003;1:1897-905 [DOI] [PubMed] [Google Scholar]
- 65.Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, et al. Escaping the nuclear confines: signaldependent pre-mRNA splicing in anucleate platelets. Cell 2005;122:379-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valacchi G, Bocci V. Studies on the biological effects of ozone: 10. Release of factors from ozonated human platelets. Mediators Inflamm 1999;8:205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cappellozza E, Costanzo M, Calderan L, Galie M, Angelini O, Tabaracci G, Malatesta M. Low ozone concentrations affect the structural and functional features of Jurkat T cells. Processes 2021;9:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oleksowicz L, Paciucci PA, Zuckerman D, Colorito A, Rand JH, Holland JF. Alterations of platelet function induced by interleukin-2. J Immunother 1991;10:363-70. [DOI] [PubMed] [Google Scholar]


