Abstract
Difficult-to-treat and severe asthma are challenging clinical entities. In the face of suboptimal asthma control, the temptation for clinicians is to reflexively escalate asthma-directed therapy, including increasing exposure to corticosteroids and commencement of costly but potent biologic therapies. However, asthma control is objectively and subjectively assessed based on measurable parameters (such as exacerbations or variability in pulmonary physiology), symptoms and patient histories. Crucially, these features can be confounded by common untreated comorbidities, affecting clinicians’ assessment of asthma treatment efficacy.
Tweetable abstract
The prevalence of comorbidities in asthma is high. Untreated, these conditions can worsen asthma control, leading to reflexive escalation of therapy. Systematic assessment and management of these entities in severe asthma is of paramount importance. https://bit.ly/3LRGBPE
Educational aims
To review the prevalence of selected comorbidities in severe asthma.
To describe the role of comorbidities in contributing to poor asthma control and confounding treatment response.
To present the benefits of active management of comorbidities associated with difficult-to-treat and severe asthma.
Introduction
The 2007 National Institutes of Health (NIH) guidelines on asthma define asthma as “a common chronic disorder of the airways that involves a complex interaction of airflow obstruction, bronchial hyperresponsiveness and an underlying inflammation. This interaction can be highly variable among patients and within patients over time” [1]. Asthma is common, affecting 5–10% of the population; yet, confirming the diagnosis remains challenging, leading to issues with both under- and overdiagnosis [2, 3]. “Difficult-to-treat” asthma is defined as asthma that is not controlled despite high or medium doses of inhaled steroids [4] or in which high doses of treatment are required to maintain an adequate control of the symptoms and to reduce the risk of exacerbations [5, 6]. “Severe” asthma is defined by the Global Initiative for Asthma (GINA) as asthma which remains “uncontrolled despite adherence with maximal optimised [therapy] and management of contributory factors, or that worsens when high-dose treatment is decreased” [7], thereby representing a subset of difficult-to-treat asthma. Although difficult-to-treat and severe asthma only affect a small proportion of all patients living with asthma, their impact on patient outcomes, healthcare utilisation and associated cost is exponential [5, 8, 9]. The true prevalence of severe asthma is challenging to determine; however, it has been estimated to represent ∼5–10% of all patients living with asthma [10].
The aim of good asthma management is for each patient to receive the lowest dose of inhaled corticosteroid (ICS) required to maintain freedom from symptoms and exacerbations [7]. Factors associated with poor asthma control include suboptimal patient insight into their condition, as well as non-adherence to inhaled therapies or poor inhaler technique [11–13]. Moreover, numerous comorbidities can have an impact on asthma control, or indeed confound the subjective response to asthma treatments.
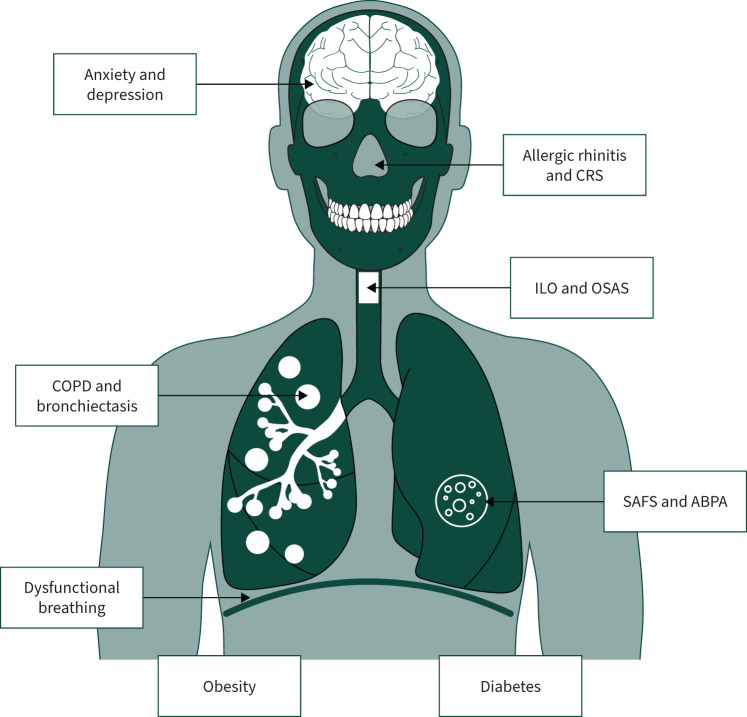
A meticulous review of comorbidities is therefore an essential part of a clinical assessment of asthma, helping to differentiate between undertreated asthma and undertreated comorbidities, and to avoid reflexive escalation of asthma-directed therapies in the first instance [5, 14–16]. This review provides the clinician with an overview of the most common comorbidities associated with asthma (table 1 and figure 1), their established and emerging treatment options, as well as new areas of interest.
TABLE 1.
Summary of significant comorbidities in difficult asthma
| Comorbidity | Impact on asthma | Diagnosis | Treatment | Treatment impact on asthma outcomes |
| COPD | Confounder of PROMs of asthma control, especially dyspnoea scores, cough and wheeze | Appropriate clinical context plus spirometry evidence of fixed airflow obstruction ±CT-evident emphysema |
As with asthma, LABA±LAMA±ICS Azithromycin prophylaxis ±Pulmonary rehabilitation ±LTOT/home NIV ±Lung volume reduction techniques |
ICS±LABA for ACO improves lung function LAMA and pulmonary rehabilitation may improve dyspnoea Anti-IL-4/IL-13 and anti-IL-5 receptor drugs may improve lung physiology and exacerbation frequency, respectively |
| Allergic rhinitis | May contribute to or worsen cough via post-nasal drip Direct effects on lower airway changes are unclear |
Clinical history (rhinorrhoea, sneezing, nasal obstruction, pruritus, conjunctivitis) Demonstration of allergen via skin prick/in vitro testing |
Trigger avoidance Nasal/oral antihistamines Nasal ICS LTRA Allergen-specific SCIT and SLIT |
Nasal ICS treatment may improve asthma control LTRA use improves nasal and asthma control Antihistamines can improve asthma symptoms and bronchial hyperresponsiveness |
| CRS with or without nasal polyposis | May contribute to or worsen cough via post-nasal drip Nasal polyposis raises possibility of AERD/NERD |
3 months of symptoms plus objective proof of mucosal inflammation (CT or nasal endoscopy) ±Visualisation of polyps |
Nasal irrigation Nasal ICS FESS, polypectomy or posterior nasal neurectomy in selected patients |
Improvement in quality of life and decrease in steroid and antibiotic dependency |
| ABPA | Worsens pulmonary function, cough, wheeze, mucus production, exacerbation frequency and steroid requirements | Various criteria exist; typically: 1) proven CF or asthma and 2) total IgE >1000 IU·mL−1 and 3) increased Aspergillus sensitisation (IgE) and 4) increased Aspergillus IgG/radiographic changes/peripheral blood eosinophilia | Glucocorticoids and/or azoles Defined best regimen lacks consensus Omalizumab Anti-IL-5/5R and IL-4α receptor antagonist |
Glucocorticoids and azoles decrease ABPA exacerbations and improve symptoms Biologics have been helpful in decreasing steroid dosing and decreasing exacerbation frequency |
| Bronchiectasis | Confounds symptoms of cough and wheeze Associated with frequent exacerbations, decline in lung function and poor quality of life |
CT imaging evidence of bronchiectatic airways While not diagnostic, sputum or bronchoscopy derived microbiology samples help guide treatment |
Airway clearance education Vaccination Targeted antibiotic therapy based on sputum culture and sensitivity Azithromycin prophylaxis |
Unclear: bronchiectasis management can be expected to reduce exacerbation frequency, but specific asthma outcomes have not been assessed |
| GORD | Responsible for poor control of asthma directly and indirectly Associated with obesity and OSAS |
History suggestive of symptomatic reflux Oesophageal pH manometry Endoscopy may demonstrate oesophagitis/laryngopharyngeal inflammation suggestive of GORD |
Empiric trial of PPI for 8 weeks Lifestyle modification (head of bed elevation, trigger avoidance, meal timing) Endoscopy in PPI non-responders Surgical management |
No asthma benefit for asymptomatic GORD treatment Treatment of symptomatic GORD in asthma patients reduces steroid and reliever use and may improve lung function |
| Obesity | Associated with high symptom burden, increased frequency of exacerbation and poor quality of life, and steroid resistance | Objective verification of BMI >30 kg·m−2 | Weight management strategies and bariatric surgery aiming for >5% body weight loss | >5% body weight loss has been shown to improve spirometry, peak flow and asthma control in adults and children Bariatric surgery has been associated with decreased dependency on asthma medication and reduced hospitalisation |
| OSAS | Independent risk factor for poor control of asthma | Gold standard is polysomnography, but limited sleep studies, overnight spirometry and validated questionnaire can be used as screening tools | Weight loss Optimisation of nasal/tonsillar disease Mandibular advancement devices CPAP Surgery |
CPAP use has shown benefit in asthma symptom control and improvement in lung function in some studies |
| T2DM | Insulin resistance and metabolic syndrome have been associated with both asthma development and increased exacerbation risk | Any two of: 1) 8-h fasting plasma glucose ≥7.0 mmol·L−1, 2) 2-h plasma glucose ≥11.1 mmol·L−1 during OGTT#, 3) HbA1c ≥6.5% (48 mmol·mol−1), 4) random plasma glucose ≥11.1 mmol·L−1 in a patient with classic symptoms of hyperglycaemia or hyperglycaemic crisis | Lifestyle and dietary optimisation Weight management Anti-hyperglycaemic therapy Comprehensive management of comorbidities such as hypertension and dyslipidaemia |
Metformin use in patients with concurrent T2DM and asthma has been associated with reduced asthma exacerbation rates and asthma-related hospitalisation GLP-1RA use is associated with lower asthma exacerbation rates in patients with T2DM and asthma compared with SGLT-2 inhibitors, DPP-4 inhibitors, basal insulin and sulfonylureas |
| ILO | Mimics poorly controlled asthma Non-response to ICS can result in reflex escalation of asthma therapies with high side-effect burden, including excessive corticosteroids and occasionally intubation |
Gold standard is direct laryngoscopy visualising excessive adduction of vocal cord or laryngeal structures ±provocation challenge Flattened inspiratory flow–volume loop at spirometry can suggest ILO Continuous laryngoscopy with exercise is gold standard for EILO |
Identifying and modifying environmental and occupational irritants, alongside speech and language therapy for throat relaxation and cough suppression Case series reports of low-dose amitriptyline, botulin toxin and surgical resection |
Speech and language therapy retraining interventions have been demonstrated to reduce symptom burden in those with chronic cough refractory to medical management; while such an intervention may therefore reduce corticosteroid use in patients with ILO and asthma, further trials are needed to guide management in ILO |
| Dysfunctional breathing | Results in disproportionate symptoms of breathlessness, confounding PROMs of asthma | Specialist physiotherapist assessment is required for diagnosis Nijmegen Questionnaire score ≥24 may support a diagnosis |
Physiotherapy-led breathing retraining, can be in-person or online | Significant improvements in mean Asthma Control Test and Asthma Quality of Life Questionnaire scores |
| Anxiety/depression | Reduce treatment adherence, worsen asthma control Panic disorders confounding PROMs of asthma control, namely dyspnoea |
World Health Organization International Classification of Diseases 11th Revision criteria | Assessment of symptom severity Risk assessment Education and psychosocial interventions Pharmacotherapy and short interval reassessment Specialist psychiatric assessment as deemed appropriate |
Mixed evidence regarding the role of cognitive behavioural and relaxation therapies in improving asthma-related outcomes among those with anxiety or depression No evidence for asthma-specific management strategies |
ABPA: allergic bronchopulmonary aspergillosis; ACO: asthma with COPD overlap; AERD/NERD: aspirin/nonsteroidal anti-inflammatory drug-exacerbated respiratory disease; BMI: body mass index; CF: cystic fibrosis; CPAP: continuous positive airway pressure; CRS: chronic rhinosinusitis; CT: computed tomography; DPP-4: dipeptidyl peptidase-4; EILO: exercise-induced laryngeal obstruction; FESS: functional endoscopic sinus surgery; GLP-1RA: glucagon-like peptide-1 receptor agonist; GORD: gastro-oesophageal reflux disease; HbA1c: haemoglobin A1c; ICS: inhaled corticosteroid; IL: interleukin; ILO: inducible laryngeal obstruction; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; LTOT: long-term oxygen therapy; LTRA: leukotriene receptor antagonist; NIV: noninvasive ventilation; OGTT: oral glucose tolerance test; OSAS: obstructive sleep apnoea syndrome; PPI: proton pump inhibitor; PROMs: patient-reported outcome measures; SCIT: subcutaneous immunotherapy; SGLT-2: sodium/glucose cotransporter-2; SLIT: sublingual immunotherapy; T2DM: type 2 diabetes mellitus. #: glucose load equivalent to 75 g anhydrous glucose dissolved in water, with baseline, 1-h and 2-h plasma blood glucose measurement.
FIGURE 1.
Common comorbidities encountered in difficult and severe asthma. CRS: chronic rhinosinusitis; ILO: inducible laryngeal obstruction; OSAS: obstructive sleep apnoea syndrome; SAFS: severe asthma with fungal sensitisation; ABPA: allergic bronchopulmonary aspergillosis.
Asthma–COPD overlap
Asthma and COPD are the two most common respiratory conditions globally [17]. Moreover, they are frequently mimics and confounders of each other, making their distinction in the clinical setting a common challenge. The entity of “asthma with COPD overlap” (ACO) remains a major diagnostic challenge and one for which a widely accepted definition remains elusive and disputed. ACO has been reported to be associated with higher asthma and COPD severity, decreased lung function and greater health burden compared with COPD or asthma alone [18].
Several clinical features are shared by asthma and COPD, including a history of current or past smoking, dyspnoea, wheeze and cough [19, 20]. The traditional and reductionist view that bronchodilator reversibility at spirometry denotes a diagnosis of asthma, and that fixed airflow obstruction with negative bronchodilator reversibility is supportive of COPD, is poorly predictive of underlying diagnosis [21]. Fixed airflow obstruction (ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC) less than the lower limit of normal or <0.7) after bronchodilator administration and negative bronchodilator reversibility is a common feature in asthmatic populations, and more so in severe asthma. Equally, significant post-bronchodilator reversibility in spirometry (i.e. >200 mL in FEV1 or FVC) is frequently described in COPD cohorts, although it is unusual to have a post-bronchodilator response of >400 mL without a concomitant diagnosis of asthma [22, 23]. Moreover, while eosinophilia has traditionally been thought of as a hallmark of asthma, the evidence supporting eosinophilia as a “treatable trait” in COPD is well established [24].
Much of the confusion surrounding ACO arises from the traditional view of considering asthma and COPD as two distinct diseases. While airway remodelling appears to differ significantly between individuals with asthma and COPD, those with a clinical diagnosis of ACO may demonstrate a distinct pattern of their own, with higher goblet cell numbers than in those with COPD, but lower smooth muscle thickness than in those with asthma [25]. Given the lack of established consensus on the best definitions of phenotypes in this area, there has been a growing tendency to shift away from fixed disease labels in obstructive airways disease, and towards identifying and treating individual treatable traits [26]. Given that established pharmacological classes in asthma and COPD overlap significantly (i.e. ICS, long-acting β-agonists (LABAs) and long-acting muscarinic antagonists (LAMAs)), the challenge becomes one of identifying the best-fit sequence of treatment escalation, meaning clinicians can be more focused on identifying treatable traits and appropriate clinical interventions.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines for the management of COPD suggest that where a coexistent diagnosis of asthma with COPD is suspected, treatment algorithms should in the first instance follow asthma guidelines [27], which fundamentally implies an ICS±LABA-based management plan [28]. Treatment with 3 months of ICS±LABA for ACO with mild to moderate airflow limitation has been shown to significantly improve lung function [29]. Thereafter, escalation of treatment for patients with ACO can be guided by consideration of the predominant symptoms. Dyspnoea, in the absence of typical indicators of asthma, might be best addressed by addition of a LAMA bronchodilator and pulmonary rehabilitation targeting the COPD component. While pulmonary rehabilitation has demonstrated benefits in COPD, there is growing evidence to support its use in asthma [30], suggesting a benefit in ACO is plausible. Conversely, if the predominant symptoms burden is typical of asthma, escalation along the GINA guidelines might be more appropriate. Finally, recent evidence from the BOREAS study demonstrated that treatment of individuals with COPD and type 2 (T2) inflammation with the anti-interleukin (IL)-4/IL-13-receptor drug dupilumab resulted in a reduction in severe exacerbations, improved FEV1 and improved symptoms compared to placebo [31]. Similarly, exacerbation rates in eosinophilic COPD have been shown to be modestly improved by the anti-IL-5 drug mepolizumab [32], although not by the IL-5-receptor antagonist benralizumab [33].
Allergic rhinitis, chronic rhinosinusitis and nasal polyposis
Allergic rhinitis and severe asthma are two respiratory conditions that may coexist in up to 40% of people with asthma. Allergic rhinitis is characterised by immunoglobulin E (IgE)-mediated inflammation of the nasal passages in response to allergens such as pollen, dust mites or pet dander, and is subdivided into intermittent and persistent allergic rhinitis based on the transient/seasonal or perennial exposure to causative allergens [34]. Common symptoms include sneezing, congestion, rhinorrhoea and associated allergic conjunctivitis. Allergic rhinitis may increase the likelihood of developing asthma [35] and eventual poor asthma control, including hospital attendance [36]. The diagnosis of allergic rhinitis is typically clinical, although in the setting of asthma, specific allergen testing for triggers may occur as part of clinical phenotyping by way of either allergy skin testing or in vitro tests for specific allergens.
Treatment of allergic rhinitis is usually pharmacological. Trigger avoidance is ideal, but not always feasible. First-line treatment is with oral antihistamines, especially in mild and intermittent disease. For moderate to severe symptoms or persistent allergic rhinitis, nasal ICS are indicated with or without the addition of inhaled topical antihistamines such as azelastine. Addition of the leukotriene receptor antagonist montelukast has demonstrated effectiveness in patients who have both allergic rhinitis and asthma, reducing asthma medication needs [37], exacerbations [38], and healthcare utilisation and cost [39]. Where sensitisation to certain allergens is proven, subcutaneous or sublingual immunotherapy has been shown to be efficacious in children and adults for both allergic rhinitis and asthma [40–43], although safety and efficacy evidence is less clear in severe asthma [44]. Biologic therapies such as anti-IgE, anti-IL-5/5R and anti-IL-4/13R are not indicated in allergic rhinitis but can provide benefit when administered for treatment of severe asthma.
Chronic rhinosinusitis (CRS) is defined as objectively assessed (by endoscopic or computed tomography (CT) examination) chronic inflammation of the paranasal sinuses or nasal airway tissues, accompanied by persistent symptoms of at least 3 months duration [45]. CRS is a disease of middle age, with peak incidence between the third and fifth decade of life, and prevalence varies between 5% and 17% in asthma patients [46], but has been reported in up to 35–60% in severe asthma cohorts [47–49]. CRS is further subclassified by the presence or absence of nasal polyposis. CRS with nasal polyposis is associated with T2 inflammation, in both upper and lower airways, as well as eosinophilic asthma [50]. The well described triad of aspirin intolerance, nasal polyposis and asthma, known as Samter's triad or aspirin/nonsteroidal anti-inflammatory drug-exacerbated respiratory disease (AERD or NERD), has a reported prevalence of 5–25% in asthma populations, and is associated with exacerbation-prone asthma and increased reliance on oral glucocorticoids [51]. CRS frequently coexists with asthma and correlates with asthma severity [52]. The pathophysiology of CRS is incompletely understood and varies by subtype, but is known to be multifactorial, including allergic, inflammatory and genetic causes. The available evidence suggests that where CRS occurs in conjunction with identifiable allergic triggers, severity is worse, especially when the allergic triggers are perennial [52].
Diagnosis is made based on symptoms and accompanying endoscopic or CT features. The diagnosis of AERD/NERD is made on the basis of CRS with nasal polyposis, asthma, and a history of dyspnoea or asthma after ingestion of aspirin or other nonsteroidal anti-inflammatories, where aspirin challenges may help clarify the latter feature [53]. Tools such as the Self-Administered Odor Questionnaire (SAOQ), Visual Analog Scale (VAS) and Sino-Nasal Outcome Test-22 (SNOT-22) may help improve the diagnosis of CRS in patients with asthma, but primarily track symptom severity.
Treatment of CRS is broadly divided into medical and surgical management. First-line treatment is nasal glucocorticoids, which help in reducing inflammation. This can be combined with nasal irrigation, with resultant decrease in dependency on oral corticosteroids and antibiotics. In selected patients, nasal irrigation with steroids is superior to nasal spray. Recent studies of targeted monoclonal antibodies for CRS with nasal polyposis, including dupilumab [54], omalizumab [55], mepolizumab [56], reslizumab [57, 58] and benralizumab [59–61], have shown positive results.
Surgical treatment with functional endoscopic sinus surgery (FESS) is effective in restoring sinus drainage, allowing maintenance sinusitis medications to work better; however, polyp recurrence is common. FESS may improve asthma outcomes post-operatively, although the evidence is conflicting [62, 63]. Posterior nasal neurectomy has been studied in a selected cohort of patients with combinations of allergic rhinitis, CRS and asthma, demonstrating improvements in symptoms of nasal congestion and quality of life, and some benefits relating to asthma control and pulmonary function [64–66].
Bronchiectasis
Bronchiectasis is a clinical syndrome characterised by cough and sputum production in the presence of abnormal thickening and dilation of the bronchial wall that is visible on lung imaging [67] and is a common finding in many lung conditions. In severe asthma, bronchiectasis affects 25–68% of individuals [5], with an apparent association with advancing age and exacerbation frequency [68]. The aetiology of bronchiectasis is highly variable. Among patients with severe asthma, T2 inflammation, eosinophilic degranulation and RNA expression of the Charcot–Leyden crystal protein have been shown to associate with a bronchiectatic phenotype [69], suggesting a possible shared pathobiology.
Asthma patients with coexisting bronchiectasis are at increased risk of exacerbations, worse lung function, more frequent hospitalisations, and poor quality of life. Coexistent bronchiectasis may increase mortality in patients with corticosteroid-dependent asthma [70], and the presence of asthma is associated with increased risk of relapse after 14 days in patients treated for bronchiectasis exacerbations [71]. Bronchiectasis is therefore an important and frequent comorbidity in severe asthma that should be considered in patients with severe asthma who are failing to respond to standard therapy, and those with persistent crepitations, clubbing, frequent pulmonary infection or complex sputum microbiology. High-resolution CT imaging of the lungs is the gold standard for diagnosis of bronchiectasis and the argument for greater use of CT for earlier detection in severe asthma has been made by some authors [72], while sputum or bronchial washing culture and sensitivities can help identify complicating microbiology. Identification of bronchiectasis in severe asthma should prompt consideration of the possibility of allergic bronchopulmonary aspergillosis (ABPA).
The mainstay of treatment is minimising acute infective exacerbations through empiric antibiotic therapy guided by prior cultures, augmenting airway clearance, attempting eradication of Pseudomonas aeruginosa in first-time isolates, and ensuring up-to-date vaccination status, although these recommendations are in line with general management of bronchiectasis, and not specific to asthma patients [67]. Patients with comorbid bronchiectasis and asthma are at increased risk of relapse and antibiotic duration should be 14 days, as advised in the European Respiratory Society and British Thoracic Society guidelines on the management of bronchiectasis [73, 74]. Low-dose macrolides, particularly azithromycin, share a therapeutic evidence base in both non-P. aeruginosa-colonised refractory bronchiectasis and exacerbation-prone asthma as maintenance therapy [75, 76], but there is a risk of antibiotic resistance, cardiotoxicity and ototoxicity. There is a lack of evidence for the role of combined ICS+LABA in bronchiectasis alone, but based on the coexistence of asthma, in particular severe asthma, there is an assumption of benefit for inclusion of ICS in management strategies [77]. There has been increased interest in the role of biologic therapies in improving bronchiectasis outcomes [78], particularly among asthma patients with a T2-high phenotype. Thus far, the anti-IL-5 mepolizumab [15, 79] and anti-IL-5R benralizumab [80, 81] therapies have shown clinical effectiveness in improving relevant clinical outcomes in patients with comorbid severe asthma and bronchiectasis [82].
ABPA and severe asthma with fungal sensitisation
Severe asthma with fungal sensitisation (SAFS) is defined as severe asthma driven by allergy to identifiable fungal triggers, but where diagnostic criteria for ABPA are not met. ABPA is a specific form of SAFS in which airway destruction occurs due to hypersensitivity reaction to filamentous fungi, most commonly Aspergillus species [83]. Aspergillus fumigatus is a common airborne pathogen that contributes to severe asthma [84]. The major predisposing factors for the development of ABPA are underlying asthma and cystic fibrosis (CF), with an estimated incidence of ABPA in asthma of 2.5–15%, or 4–5 million cases, worldwide [85–87]. The prevalence of Aspergillus sensitisation may be higher still in severe asthma [88]. Recognised manifestations of ABPA include worsening asthma control, wheeze, fleeting pulmonary infiltrates, eosinophilia, proximal bronchiectasis and mucus plug formation with impaction and evidence of hyperattenuating mucus on CT imaging [89, 90].
Various diagnostic criteria for ABPA have been proposed over the years [91, 92]. The 2013 International Society for Human and Animal Mycology (ISHAM) guidelines [93] suggest that a diagnosis of ABPA requires 1) the presence of asthma or CF as predisposing factors and 2) evidence of both specific Aspergillus sensitisation and an elevated total IgE >1000 IU·mL−1, as well as 3) any two of the following three minor criteria: precipitating serum antibodies to A. fumigatus/elevated A. fumigatus-specific IgG levels, radiographic findings consistent with ABPA, or a total peripheral eosinophil count >500 cells·μL−1.
The management of ABPA has historically focused on medium-term glucocorticoid therapy and systemic antifungal therapy, namely azoles. The exact best combination of these agents remains a point of debate, although the Infectious Diseases Society of America (IDSA) recommends the use of both glucocorticoids and antifungals in the acute stage [94]. In a 2004 meta-analysis of itraconazole use for ABPA in non-CF asthma patients, use of itraconazole was associated with an increased likelihood of reducing serum IgE by >25% [95], a finding that was also seen in a prospective double-blinded randomised trial examining the effectiveness of addition of itraconazole in steroid-dependent ABPA [96]. While the bulk of experience of azole use in ABPA lies with itraconazole, newer agents such as voriconazole and posaconzole have demonstrated efficacy in ABPA [97] and can offer practical advantages over itraconazole including tolerability and bioavailability. Indeed, a 2018 single-centre study suggested equivalent response can be seen when comparing voriconazole or glucocorticoid monotherapy in ABPA [98].
Typically, the dosing of glucocorticoids is in the range of 0.5 mg per kg body weight of prednisone equivalent for initially at least 2 weeks, followed by tapering over a number of months guided by the observed response in total serum IgE levels. The initial dosing, duration and tapering practices are all highly variable in the literature. In one series of 24 patients receiving 0.5 mg·kg−1 daily for 1 month with subsequent taper, a mean reduction in baseline serum IgE levels was ∼25% after 1 month and ∼60% after 2 months, although the variance in response was significant [99]. In one randomised control trial of 92 patients with ABPA assigned to either 0.5 mg·kg−1 or 0.75 mg·kg−1 at treatment outset, no significant differences in response were noted between groups and rates of ABPA exacerbations were similar at 1 year (50% versus 40%; p=0.59) [100].
High relapse rates, as well as the challenges and side-effects of long-term and repeated courses of azoles or glucocorticoids, have led to interest in the role of biologic therapies for the management of ABPA. Here, omalizumab has demonstrated good efficacy, improving exacerbation rates, glucocorticoid dosing, FEV1 and symptoms [101–104], although optimal dosing is unknown given the highly elevated IgE levels seen in ABPA. Similar effectiveness for anti-IL-5/5R therapy has been demonstrated in both ABPA and SAFS in a growing body of case series [105, 106], while evidence of a role for the IL-4α receptor antagonist dupilumab is also growing [107, 108].
Obesity
Obesity is a growing global health issue and it increases the incidence and prevalence of asthma and severe asthma [109–113]. The association between obesity and asthma has been suggested to arise even prior to birth. Maternal obesity and weight gain during pregnancy have been independently associated with a ∼15–30% increased risk of asthma in offspring [114, 115]. Thereafter, childhood peak weight velocity and body mass index (BMI) have been found to be independently associated with wheezing and incident asthma in children and adolescents [116, 117]. Moreover, among adults, the prevalence of asthma, risk of hospitalisation from asthma and previously associated negative outcomes (intensive care unit admission, mechanical ventilation, pneumonia) have been shown to be increased in individuals who are overweight and obese, relative to those with normal BMI [118–120], with asthma prevalence almost twice as high in female individuals who are obese compared to those with normal BMI (14.6% versus 7.9%) [121].
The typical severe asthma phenotype is associated with obesity, steroid resistance, airway inflammation and comorbidities [109, 122–124]. In a post hoc analysis of the TENOR study (The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens), unsupervised clustering methods were used to identify biological clusters among 518 children and 3612 adolescents/adults with severe asthma. Obesity was highly prevalent in all clusters, ranging between 20% and 35.6% among five clusters identified in children and between 52.1% and 62.7% among five clusters identified in adolescents/adults [125]. This suggests that obesity is strongly represented in severe asthma, regardless of the putative underlying pathobiology. Increased body fat leads to systemic inflammation and increased levels of adiponectin and leptin [124, 126, 127], which may skew towards a T2-low response [128, 129], although T2-skewed inflammation is commonly still seen in obese asthma patients. Indeed, a recent analysis of the German Asthma Net registry suggested that among individuals with severe asthma, obesity was associated with higher symptom burden and significant differences in gastro-oesophageal reflux disease (GORD), anxiety and lung volumes, but not in T2 markers of disease (peripheral eosinophils, exhaled nitic oxide or IgE levels) [130]. Meanwhile, in a study selecting for females with T2-low asthma who were obese, obesity was associated with increased airway hyperresponsiveness and markers of inflammation in serum and adipose tissue, but surprisingly not with airway inflammation, when compared to non-asthmatic obese controls [122].
The role for active weight loss interventions to improve asthma outcomes in individuals who are overweight or obese is therefore an area of interest. Lifestyle and dietary interventions have been shown to generally improve spirometry, peak expiratory flow rate and asthma control in both adults and children, as summarised previously by other authors [112]; however, a >5% body weight loss appears to be a necessary threshold above which improvements can be expected. One systematic review with meta-analysis of 15 studies suggested that metabolic and bariatric surgery leads to discontinuation of asthma medication in 50% of patients [131], while in a self-controlled study of 2261 patients with asthma who were obese, a baseline 22% annual rate of hospitalisation or emergency department visit due to asthma was reduced by nearly 60% after bariatric surgery [132]. Consideration and active management of weight in asthma is therefore of critical importance. Currently, a single-centre randomised, placebo-controlled, proof-of-concept study is underway to examine the effectiveness of the glucagon-like peptide-1 receptor agonist (GLP-1RA) semaglutide for the treatment of asthma in individuals who are obese (ClinicalTrials.gov identifier NCT05254314). Of note, in one study, treatment with biologic agents for severe eosinophilic asthma requiring intermittent or maintenance oral corticosteroids was shown to reduce not only steroid exposure but also BMI, highlighting the two-way relationship of asthma and obesity [133]. This effect was most notable and significant among asthma patients who were obese and requiring maintenance steroids at baseline, with 39% achieving a clinically significant >5% body weight reduction after starting treatment with biologics.
Diabetes mellitus
The relationship between asthma and diabetes mellitus has been examined previously, suggesting a small but significant increase in risk of incidence of diabetes in asthma cohorts [8], although how much this relationship is confounded by obesity is unclear. Nonetheless, pharmacotherapy for type 2 diabetes mellitus (T2DM) has been associated with improved asthma outcomes including reduced exacerbation rates and hospitalisation for diabetic asthma patients [134, 135]. Furthermore, treatment with GLP-1RAs has been reported to improve asthma exacerbation incidence rates compared to other T2DM pharmacotherapies, a finding that was independent of changes in BMI or haemoglobin A1c (HbA1c) [136]. Moreover, a small prospective study in patients with T2DM but without respiratory disease found improvements in FEV1 and FVC among subjects receiving metformin and GLP-1RA compared with those receiving metformin and insulin [137].
Obstructive sleep apnoea syndrome
Obstructive sleep apnoea syndrome (OSAS) is a condition characterised by episodic occlusion of the upper airway, resulting in periods of apnoea or a reduced airflow (hypopnoea) during sleep. OSAS has been identified as an independent risk factor for asthma, with the mechanism for this association likely explained in part by obesity and nasal obstruction arising from allergic rhinitis/CRS [138], as well as modifications in vagal tone, local and systemic inflammation, and hypoxia-mediated angiogenesis [139]. OSAS is common in asthma populations, and even more so in severe asthma cohorts, with estimates of prevalence between 30% and 90% [140–142]. OSAS has been reported to be independently associated with an increased risk of hospital readmission among hospitalised asthma patients [143]. In the Severe Asthma Research Program (SARP) cohort of severe asthma patients, OSAS was associated with worse symptoms, higher exacerbation rates and greater reliever inhaler use [142]. Moreover, OSAS with comorbid asthma is associated with lower nocturnal oxygen saturation and more frequent apnoeas than without asthma [140].
Gold standard diagnosis of OSAS is by way of polysomnography, but limited sleep studies, overnight oximetry and questionnaires such as the Epworth Sleepiness Scale (ESS) [144], STOP-Bang [145] or the Berlin Questionnaire may be useful for screening purposes.
The effect of continuous positive airway pressure (CPAP) for OSAS in asthma patients remains debated. CPAP has been reported to improve bronchial hyperresponsiveness within 7 days of use [146], while in a prospective multicentre study of 99 adults with asthma and OSAS, Serrano-Pariente et al. [147] reported that treatment with CPAP improved symptoms, exacerbation rates and pulmonary function, including exhaled nitric oxide, after 6 months. Conversely, Ng et al. [148] reported that among 37 adults with asthma who were randomised to CPAP or not, there were no differences in asthma control tests at 3 months. Summarily, the evidence suggests that active treatment of OSAS is likely to result in a benefit in quality of life and asthma-specific improvements in comorbid asthma, underlining the importance of its identification in difficult-to-treat and severe asthma cohorts [149].
Gastro-oesophageal reflux disease
GORD is the term used when the otherwise normal physiological retrograde passage of gastric contents into the oesophagus leads to troublesome reflux symptoms (heartburn, regurgitation and sometimes dysphagia/odynophagia, chest pain, hypersalivation, nausea or globus sensation) or complications such as oesophageal macroscopic damage [150]. The estimated prevalence of GORD in asthma patients varies widely, likely due to varying definitions of GORD and asthma; a systematic review found a sample size-weighted average prevalence of GORD symptoms in asthma patients of 59.2%, versus 38.1% in controls [151]. Conversely, asthma is also over-represented in GORD patients, amounting to a bidirectional interrelationship [152, 153]. GORD is consistently found as a significant comorbidity in exacerbation-prone severe asthma, including across the SARP and European U-BIOPRED (Unbiased Biomarkers for the Prediction of Respiratory Disease Outcomes) cohorts [154–157], but is also commonly found in other relevant comorbidities, namely obesity and OSAS, which can confound analyses of causal risk in asthma [158]. Mechanistically, GORD could give rise to worsened asthma, and perhaps more so in severe asthma patients [159], through inducing bronchial hyperreactivity, stimulating vagal tone or via the micro-aspiration of gastric refluxate composed of acid, pepsin, gas or duodenally transited bile acid and pancreatic enzymes [159–162].
Large multicentre trials have shown that treating asymptomatic GORD with proton pump inhibitors has no benefit for mild–moderate severity adult asthma patients or children with poorly controlled asthma regarding pulmonary function, asthma exacerbation rate, asthma-related quality of life or asthma symptoms [163, 164]. In contrast, adults with symptomatic GORD and moderate-to-severe asthma administered 30 mg lansoprazole twice daily for 24 weeks had a favourable oral corticosteroid-treated exacerbation rate of 4% in the active arm versus 13.9% in placebo-treated controls, consistent with the known link of GORD to exacerbation-prone asthma [154, 165]. An updated Cochrane review that included 23 randomised controlled trials found moderate-certainty evidence that GORD medications (proton pump inhibitors, nocturnal use of histamine 2 receptor antagonists) lead to small improvements in lung function and reduced need for rescue medications [166].
Guidelines suggest that patients with typical GORD symptoms take a proton pump inhibitor once daily, 30–60 min before a meal, as an empiric 8-week diagnostic/therapeutic trial. Endoscopy is indicated in proton pump inhibitor non-responders, those whose symptoms return when off treatment, or sooner in those with alarm symptoms (dysphagia, unintentional weight loss, bleeding, emesis or anaemia) [150]. Recommended dietary/lifestyle interventions include weight management if overweight/obese, eliminating offending foods/drinks, smoking cessation, elevating the head of the bed and avoiding late meals. When lifestyle, dietary and pharmacological interventions confer insufficient control of severe symptoms, or patients cannot commit to sustained medical therapy for various reasons (e.g. medication intolerance/non-adherence, personal preference), anti-reflux surgical procedures can be offered to appropriately selected patients. There is now a heightened rationale for future investigation of anti-reflux interventions for reflux symptoms comorbidly affecting uncontrolled asthma patients in larger well-designed studies, in light of a recent Mendelian randomisation analysis of the largest genome-wide association studies of asthma, GORD and atopic dermatitis showing a strong causal effect of genetically determined GORD on asthma risk [153].
Inducible laryngeal obstruction
Inducible laryngeal obstruction (ILO) has been suggested as the preferred umbrella term for describing the range of clinical conditions centred around abnormal function of the laryngeal structure, which can coexist with or mimic asthma [167]. ILOs encompass vocal cord dysfunction (VCD) and paradoxical vocal cord motion, among others, but commonly describe conditions in which a hyperfunctioning laryngeal reflex leads to abnormal paradoxical vocal cord adduction or glottic movement, resulting in obstructed airflow limitation. This classically occurs during inspiration but can also be during expiration [168].
Given the paucity of studies and variability of definitions and criteria, the true prevalence of ILO is difficult to determine, with most of the evidence pertaining to VCD. However, prevalence has been estimated to be 4–6% of the general population and as high as 32–50% among those with difficult asthma [169, 170]. There is a 2:1 to 3:1 female predominance in the literature, with presentations ranging from infancy into the ninth decade of life. Similarly, the prevalence of exercise-induced laryngeal obstruction (EILO) appears to increase from 5–8% in the general population to >20% in high-performing athletes and combat soldiers [171].
The pathophysiology of ILO remains incompletely understood, and whether all forms share the same mechanisms is unclear. Nonetheless, there is a suggestion that an exaggerated laryngeal protective reflex, possibly arising as a result of previous inflammatory insult, is a major component. In a study of young subjects with confirmed VCD, evidence of laryngopharyngeal reflux was seen in 95% of participants [172]. Similarly, in a recent study that included oesophageal pH studies, individuals with verified VCD were more likely to report symptoms of reflux and have more time with oesophageal pH <4 compared to participants without VCD [173]. Rhinosinusitis and chronic post-nasal drip have been shown to increase laryngeal sensitivity and hyperfunction, although a proven association with ILO or VCD has not been shown [170]. Irritant exposures causing VCD have been reported, frequently misdiagnosed as reactive airways dysfunction syndrome; in both conditions, symptoms can present within 24 h of exposure to the noxious agent [174–176]. Some VCD patients are described as having a psychosomatic or “functional” aetiology, with several studies demonstrating association between VCD and psychiatric illnesses such as anxiety, depression, personality disorders and post-traumatic stress disorder [170, 177], although emotional stresses can trigger VCD in the absence of any obvious psychiatric disorder.
Patients with ILO typically experience dyspnoea, wheeze, stridor, cough, throat tightness or changes in voice [5, 170]. Given the symptoms of ILO can mimic those of asthma, patients are frequently misdiagnosed as having severe, steroid-refractory asthma with frequent exacerbations, although coexistent objectively verified asthma is frequent, highlighting the challenges in this area. A study of 132 adult patients with VCD revealed 42% to be misdiagnosed as having asthma for an average of 9 years and 33% to have coexistent VCD and asthma [178]. Patients with ILO may present acutely in significant distress and have been intubated on the assumption of a severe asthma attack. In one case series, 34 out of 42 patients with VCD deemed not to have asthma had been receiving oral prednisone regularly at an average daily dose of 29.2 mg, while 28% had previously been intubated [168]. When intubated for presumed asthma, these patients may demonstrate relative ease of ventilation and low airway pressures, a finding which should prompt consideration of ILO.
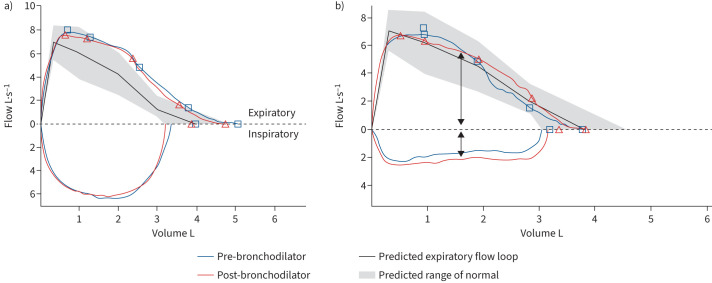
Diagnosis of ILO is difficult due to its nonspecific symptoms. A discrepancy between reliever medication use and lung function preservation, or delayed onset of action of relievers, may suggest ILO. Similarly, changes in phonation at the time of attacks, or subjective inspiratory rather than expiratory difficulty or “wheeze”, are suggestive. Among individuals with EILO, symptoms tend to be worse at maximal exertion, as opposed to symptoms of exercise-induced bronchoconstriction, which typically worsen for up to 1 h post-exercise. The Pittsburgh VCD Index is a short questionnaire with a reported 83% sensitivity and 95% specificity in distinguishing asthma from VCD [179], while the Newcastle laryngeal hypersensitivity questionnaire [180] also shows good ability to discriminate individuals with laryngeal hypersensitivity disorders from healthy controls. On spirometry, VCD is suggested by a flattened inspiratory flow–volume loop and an abnormal ratio of forced flow at 50% of expiration to forced flow at 50% of inspiration (figure 2). The frequency of inspiratory flow–volume loop flattening in VCD ranges from 23% to 46%.
FIGURE 2.
a) Spirometry demonstrating normal inspiratory and expiratory flow–volume curves. b) Spirometry flow loop demonstrating normal expiratory flow with inspiratory flow obstruction (black arrows), suggestive of variable extrathoracic obstruction.
The gold standard diagnostic test is direct laryngoscopy, enabling visualisation of excessive adduction of vocal cord or laryngeal structures either during inspiration or throughout the respiratory cycle. Given the episodic nature of ILO, however, laryngoscopy has been shown to be diagnostic in only 60% of adult patients [168]. Normal laryngoscopy in a case of suspected VCD should prompt consideration of a provocation challenge to induce obstruction, with evidence for both histamine [174] and methacholine [181], although standardised protocols are lacking. Resting laryngoscopy is usually normal in patients with only exercise-induced symptoms. EILO can be diagnosed with post-exercise laryngoscopy and/or continuous laryngoscopy with exercise (CLE), although access and availability of these diagnostic procedures is limited. CLE is well tolerated and can improve the yield of ILO in asymptomatic individuals, while also helping to differentiate exercise-induced VCD from other ILOs such as dynamic collapse of the supraglottic structures or laryngomalacia [181–183].
Finally, there are preliminary data to suggest that eucapnic voluntary hyperventilation [184, 185] and impulse oscillometry testing [186, 187] may have a role in the diagnosis of ILO, while dynamic CT scanning of the upper airways to diagnose VCD by assessing for reduction in vocal cord luminal area has also demonstrated utility in prospective studies but is not widely used in clinical practice yet [169, 188].
In acute severe presentation of ILO, first-line management is reassurance and breathing exercises such as pursed-lip or diaphragmatic breathing to relieve glottic obstruction. Case reports identify other treatment modalities, such as CPAP [189] or inhaled 80% helium/20% oxygen to decrease airflow turbulence and aid work of breathing, as well as nebulised lidocaine to anaesthetise the upper airway and decrease laryngeal constriction.
Chronic management of ILO is multidisciplinary [190], with roles for speech and language therapy (SLT), psychotherapy [191], pharmacotherapy and occasionally surgery. Identifying and modifying inhalational, often occupational, irritants or exposures is first-line management. SLT can provide techniques for throat relaxation and cough suppression, coaching the patient to control their laryngeal response during acute attacks. Speech therapy for VCD/ILO is typically recommended, and a 2022 systematic review determined that the majority of studies demonstrated a reduction in symptoms score, although the authors noted that none contained control arms, there was an overall high risk of bias, and interpretation and intervention description was inconsistent and poorly described in most [192].
Evidence to support specific pharmacotherapy in ILO is scarce [193]. Initial reports suggested that patients with EILO might benefit from inhaled ipratropium therapy [194], although more recent studies refute this finding [195]. One case series of 62 VCD patients demonstrated low-dose amitriptyline to be effective in 90% of cases [196], although randomised studies are lacking. Despite the association with GORD, treatment appears to have limited benefit for ILO. In a study of 62 VCD patients, 83.9% of whom had GORD, 24.2% reported improvement in the severity and frequency of VCD episodes after 8 weeks of acid suppression [197], although other authors have reported no significant benefit [197, 198]. In a subset of patients with supraglottic ILO, surgical resection may induce lasting benefit, while preliminary reports of botulin toxin use appear favourable [199, 200].
Depression and anxiety in severe asthma
Depression and anxiety, often coexisting, are significantly more common in people with asthma than in those without [201], with prevalence in the severe asthma population estimated to be around 25% for depression and 38% for anxiety [202]. Depression and anxiety are both associated with reduced treatment adherence, a major determinant of asthma control and outcomes [203, 204]. Anxiety and panic disorders may increase the subjective sensation of dyspnoea, confounding the assessment of asthma control, while also being frequently reported as a comorbidity in ILO.
While the direction of causality remains unclear, depression has been shown to be an independent risk factor for developing asthma. Depression has been reported to be associated with increased levels of pro-inflammatory cytokines including IL-1, IL-4, IL-6 and tumour necrosis factor-α and with sputum neutrophilia, which in turn may correlate with reduced bronchodilator response [205, 206]. This finding is supported by similar results in a survey study of 20 272 adult asthma patients, where major depression was associated with a 4.2% reduction in bronchodilator response [207], while in a study of 234 children and adolescents, stress was similarly associated with reduced bronchodilator response [208].
Regardless of the mechanisms of disease, screening for anxiety and depression [209] and subsequent treatment in asthma cohorts appear to be suboptimal [210], especially in light of the fact that these comorbidities are associated with worse asthma control, higher corticosteroid use, greater non-adherence and increased symptoms [202].
Studies on the management of anxiety and depression in people with asthma are limited. A 2021 systematic review of the effectiveness of exercise in asthma outcomes demonstrated overall positive effects, although anxiety and depression measures were only assessed in a few studies [211]. Cognitive behavioural therapy (CBT) and relaxation therapy may improve psychological outcomes (anxiety and depression) as well as asthma-related outcomes [202], although a 2016 Cochrane review found no evidence for a role for CBT in asthma, suggesting further research is needed [212]. More recent trials of CBT and music and relaxation therapies for panic disorder and comorbid asthma yielded more positive results, although dropout rates were high [213, 214]. The role for complimentary therapies also remains unclear [215].
Evidence for the best pharmacotherapy for depression/anxiety in the setting of asthma, and particularly severe asthma, is lacking [216, 217]. A recent meta-analysis identified six randomised controlled trials investigating the effectiveness and safety of pharmacological interventions in the treatment of psychological distress (primary major depressive disorder) in individuals with asthma. Meta-analysis of the pooled data from the four studies on selective serotonin reuptake inhibitors demonstrated no significant effect on depressive symptoms compared to control [217].
Dysfunctional breathing and severe asthma
Dysfunctional breathing (or breathing pattern disorder) is an umbrella term for heterogeneous disorders characterised by an abnormal and inefficient breathing pattern, resulting in symptoms of breathlessness typically disproportionate to the severity of airflow obstruction [218]. Dysfunctional breathing affects 24–29% of patients with asthma and 30–64% of patients with difficult asthma [218]. Moreover, dysfunctional breathing has been reported to be frequently associated with ILO/VCD [219].
The most well described dysfunctional breathing patterns are hyperventilation, deep sighing, thoracic-dominant breathing, mouth breathing, accessory muscle use at rest and thoraco-abdominal asynchrony, although overlap between these patterns may occur [218]. The Nijmegen Questionnaire can be used to aid in the diagnosis of dysfunctional breathing at a score of >23, although its use has only been validated for hyperventilation with a reported sensitivity of 92.7% and specificity of 91.6% at a score threshold of >17 [220]. Indeed, an observational study by Denton et al. [218] reviewed 35 patients from a difficult asthma clinic suspected clinically to have dysfunctional breathing. Following specialist physiotherapist assessment, 29 out of 35 were diagnosed with a range of dysfunctional breathing patterns but seven (24%) of these had normal (<24) Nijmegen scores. Regardless, more sensitive diagnostic questionnaires are required to detect forms of breathing pattern disorder other than hyperventilation.
Intervention by physiotherapy-led breathing retraining has been proven beneficial in difficult asthma, as well as in asthma in primary care. In the aforementioned study, the 29 patients with difficult asthma and dysfunctional breathing underwent one or more specialist physiotherapy-led breathing retraining sessions, composed of abdominal and nose breathing, postural training, relaxation and feedback to consciously decrease the respiratory rate. Follow-up demonstrated significant improvements in mean Asthma Control Test and Asthma Quality of Life Questionnaire (AQLQ) scores, with significant decrease in exacerbation frequency [218].
While access to specialist physiotherapy may be limited, there is promise in self-guided patient use of online resources. A randomised control trial in primary care of 655 patients aged 16–70 years with physician-diagnosed asthma with AQLQ score <5.5 demonstrated significant improvements in AQLQ score at 12 months following three face-to-face physiotherapy-led breathing retraining sessions or following self-guided intervention through a DVD and booklet, as compared to standard care, with no significant difference in outcome between the physiotherapy-led or self-guided interventions [221].
Conclusion
The recent consensus among clinicians and researchers that difficult-to-treat asthma is a more prevalent state than treatment-refractory severe asthma, the latter treated with expensive biologic medicines, mandates us to explore all clinical factors potentially holding back a therapeutic response in these patients. In addition to adherence and inhaler technique barriers, a range of well-established comorbidities are over-represented in uncontrolled asthma and, as evidence shows, can be remedied, often with resultant gains in asthma exacerbation frequency, asthma control, lung function and quality of life. Given how prevalent the comorbidities often are, it is prudent for healthcare professionals across the spectrum of asthma care, from severe asthma multidisciplinary teams to primary care teams, to take a uniform approach in suspecting and verifying such comorbidities. Future research is needed, employing standardised definitions of asthma/comorbid disease and objective, valid measures of disease severity, in tandem with emerging research methods, to allow better conclusions as to the therapeutic merits and cost-effectiveness in asthma of optimising such prevalent comorbid factors.
Self-evaluation questions
- A 49-year-old female with severe T2-high eosinophilic asthma with untreated intermittent allergic rhinitis, OSAS and obesity presents to your clinic reporting poor asthma control despite high-dose ICS. Which intervention would not be expected to have a positive impact on her asthma control?
- Treatment of allergic rhinitis with montelukast
- Empiric treatment for asymptomatic GORD with a proton pump inhibitor
- Initiation of benralizumab
- CPAP therapy for OSAS
- Weight loss of >5%
- An 18-year-old female never-smoker presents to your clinic. There is a query of a diagnosis of asthma made on the basis of clinical symptoms. She has completed 3 months of moderate-dose ICS+LABA, confirmed by electronic smart monitor. This has had no effect on her symptoms. During her episodes of dyspnoea, the onset is rapid within seconds and terminates quickly. She reports dysphonia, throat fullness and difficulty breathing in. Triggers for her dyspnoea include exertion (where dyspnoea is greatest at maximal exertion) and ingesting fluids. These episodes have been happening sporadically for the past 2 years. Her peripheral eosinophil count is 100 cells·μL−1 (reference range 50–500), her total IgE is in the normal range and an inhaled allergens panel is negative. The patient reports no childhood history of asthma, dermatitis or previous allergies and no relevant family history. She reports no nasal or gastro-oesophageal symptoms. Spirometry is normal with no bronchodilator response and an exhaled nitric oxide fraction of 7 ppb. What is the most likely diagnosis?
- T2-low intermittent asthma
- ILO
- COPD
- Dysfunctional breathing
- Post-nasal drip syndrome
- A 55-year-old female never-smoker with history of objectively verified T2-high asthma presents with a 2-year history of recurrent and worsening episodes of productive cough, wheezing and shortness of breath. Her medications include formoterol/budesonide 200/6 μg taken eight times daily, tiotropium 2.5 μg one puff daily and azithromycin 500 mg three times a week. She has been prescribed four courses of oral corticosteroids and antibiotics in the past 6 months. Her pulmonary function tests show an FEV1 of 60% predicted, FVC of 105% predicted and FEV1/FVC ratio of 0.59. Her peripheral eosinophil count is 700 cells·μL−1 (reference range 50–500). C-reactive protein levels are normal. High-resolution CT of the thorax demonstrates infiltrates and central bronchiectasis with mucus plugs and no evidence of emphysema. Her previous asthma workup reveals skin test positivity to ragweed, dog dander, house dust mite and A. fumigatus. Which of the following would be the single most important subsequent investigation?
- Exhaled nitric oxide fraction measurement
- Bronchoalveolar lavage for bacterial, viral and fungal culture
- Alpha-1 antitrypsin deficiency testing
- Inhaler adherence monitoring
- Total serum IgE
Suggested answers
b. Symptomatic GORD is a significant comorbidity but treating silent GORD has not been proven to improve asthma outcomes [222]. All other answers have been shown to improve asthma control.
b. ILO is a common mimic of asthma and is typified by inspiratory respiratory symptoms and wheeze [168, 170]. Our patient describes symptoms consistent with laryngeal sensitivity, and rapid onset and remission of inspiratory symptoms. Her exercise-induced symptoms occur most often at maximum exertion, as opposed to exercise-induced bronchoconstriction/asthma, which typically worsens after exercise for up to 1 h. Observed adherence to an ICS has made no difference to her symptoms, which is unusual for most asthma. The absence of inspiratory loop abnormalities on spirometry is not sensitive for ruling out ILO. Answers a and d are possibilities, but the clinical history is more consistent with ILO. Normal spirometry rules out COPD (option c), and there is no clinical indication of post-nasal drip (option e) in the vignette.
e. The combination of a predisposing factor such as asthma, central bronchiectasis with plugging and worsening recurrent exacerbations manifested by shortness of breath and cough should raise the suspicion of ABPA [93]. This patient has asthma and historic evidence of sensitisation to A. fumigatus, as well as peripheral eosinophilia and radiological features consistent with ABPA. Confirmation of a total serum IgE >1000 IU·mL−1 would fulfil the ISHAM criteria for diagnosis of ABPA. Exhaled nitric oxide fraction (option a) would provide surrogate evidence of eosinophilic airway inflammation, but this would not point to a specific cause. Option b, while clinically useful in general terms, would not aid in confirming the most pressing diagnosis of ABPA, as evidence of Aspergillus species is not required. Alpha-1 antitrypsin deficiency (option c) is not the most likely diagnosis, particularly on the basis that emphysema is not reported on CT. The association between alpha-1 antitrypsin deficiency and bronchiectasis has been recently debated [223]. Inhaler adherence monitoring (option d) is always useful in uncontrolled asthma, but improved adherence to ICS is unlikely to achieve control in active ABPA, meaning option e is the most important investigation in this case.
Footnotes
Conflict of interest: The authors report no conflict of interests.
References
- 1.National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. NIH Publication 07-4051. Bethesda, National Heart, Lung, and Blood Institute, 2007. [Google Scholar]
- 2.Louis R, Satia I, Ojanguren I, et al. European Respiratory Society guidelines for the diagnosis of asthma in adults. Eur Respir J 2022; 60: 2101585. doi: 10.1183/13993003.01585-2021 [DOI] [PubMed] [Google Scholar]
- 3.Larsson K, Kankaanranta H, Janson C, et al. Bringing asthma care into the twenty-first century. NPJ Prim Care Respir Med 2020; 30: 25. doi: 10.1038/s41533-020-0182-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol 2015; 15: 57–65. doi: 10.1038/nri3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porsbjerg C, Menzies-Gow A. Co-morbidities in severe asthma: clinical impact and management. Respirology 2017; 22: 651–661. doi: 10.1111/resp.13026 [DOI] [PubMed] [Google Scholar]
- 6.Panek M, Mokros Ł, Pietras T, et al. The epidemiology of asthma and its comorbidities in Poland – health problems of patients with severe asthma as evidenced in the Province of Lodz. Respir Med 2016; 112: 31–38. doi: 10.1016/j.rmed.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Asthma (GINA) . Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. 2023. https://ginasthma.org/wp-content/uploads/2023/09/GINA-Severe-Asthma-Guide-2023-WEB-WMS.pdf
- 8.Su X, Ren Y, Li M, et al. Prevalence of comorbidities in asthma and nonasthma patients: a meta-analysis. Medicine (Baltimore) 2016; 95: e3459. doi: 10.1097/MD.0000000000003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 2020; 55: 1900588. doi: 10.1183/13993003.00588-2019 [DOI] [PubMed] [Google Scholar]
- 10.Hekking P-PW, Wener RR, Amelink M, et al. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015; 135: 896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 11.Al-kalemji A, Johannesen H, Dam Petersen K, et al. Asthma from the patient's perspective. J Asthma 2014; 51: 209–220. doi: 10.3109/02770903.2013.860162 [DOI] [PubMed] [Google Scholar]
- 12.Guénette L, Breton M-C, Grégoire J-P, et al. Effectiveness of an asthma integrated care program on asthma control and adherence to inhaled corticosteroids. J Asthma 2015; 52: 638–645. doi: 10.3109/02770903.2014.999084 [DOI] [PubMed] [Google Scholar]
- 13.Dürr S, Hersberger KE, Zeller A, et al. The integrated care of asthma in Switzerland (INCAS)-study: patients’ perspective of received asthma care and their interest in asthma education. J Asthma 2016; 53: 955–963. doi: 10.3109/02770903.2016.1170140 [DOI] [PubMed] [Google Scholar]
- 14.Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy 2018; 73: 1369–1382. doi: 10.1111/all.13370 [DOI] [PubMed] [Google Scholar]
- 15.Carpagnano GE, Scioscia G, Lacedonia D, et al. Severe uncontrolled asthma with bronchiectasis: a pilot study of an emerging phenotype that responds to mepolizumab. J Asthma Allergy 2019; 12: 83–90. doi: 10.2147/JAA.S196200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Clemente M, Enríquez-Rodríguez AI, Iscar-Urrutia M, et al. Severe asthma and bronchiectasis. J Asthma 2020; 57: 505–509. doi: 10.1080/02770903.2019.1579832 [DOI] [PubMed] [Google Scholar]
- 17.GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020; 8: 585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendy A, Forno E, Niyonsenga T, et al. Prevalence and features of asthma-COPD overlap in the United States 2007–2012. Clin Respir J 2018; 12: 2369–2377. doi: 10.1111/crj.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang FP, Liu T, Wang G, et al. Asthma or asthma-COPD overlap syndrome? Respirology 2017; 22: 612. doi: 10.1111/resp.12993 [DOI] [PubMed] [Google Scholar]
- 20.Baarnes CB, Andersen ZJ, Tjønneland A, et al. Determinants of incident asthma-COPD overlap: a prospective study of 55,110 middle-aged adults. Clin Epidemiol 2018; 10: 1275–1287. doi: 10.2147/CLEP.S167269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest 1994; 105: 1042–1045. doi: 10.1378/chest.105.4.1042 [DOI] [PubMed] [Google Scholar]
- 22.Sin DD, Leung JM, Wechsler ME. Concern of underdiagnosing asthma–COPD overlap syndrome if age limit of 40 years for asthma is used. Eur Respir J 2017; 50: 1701120. doi: 10.1183/13993003.01120-2017 [DOI] [PubMed] [Google Scholar]
- 23.Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J 2008; 31: 742–750. doi: 10.1183/09031936.00129607 [DOI] [PubMed] [Google Scholar]
- 24.Kerkhof M, Voorham J, Dorinsky P, et al. Association between COPD exacerbations and lung function decline during maintenance therapy. Thorax 2020; 75: 744–753. doi: 10.1136/thoraxjnl-2019-214457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dey S, Lu W, Weber HC, et al. Differential airway remodeling changes were observed in patients with asthma COPD overlap compared to patients with asthma and COPD alone. Am J Physiol Lung Cell Mol Physiol 2022; 323: L473–L483. doi: 10.1152/ajplung.00137.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tay TR, Radhakrishna N, Hew M. Asthma or asthma-COPD overlap syndrome? Reply. Respirology 2017; 22: 612–613. doi: 10.1111/resp.12992 [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan P. GOLD COPD report: 2023 update. Lancet Respir Med 2023; 11: 18. doi: 10.1016/S2213-2600(22)00494-5 [DOI] [PubMed] [Google Scholar]
- 28.Bujarski S, Parulekar AD, Sharafkhaneh A, et al. The asthma COPD overlap syndrome (ACOS). Curr Allergy Asthma Rep 2015; 15: 509. doi: 10.1007/s11882-014-0509-6 [DOI] [PubMed] [Google Scholar]
- 29.Lee S-Y, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016; 11: 2797–2803. doi: 10.2147/COPD.S114964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zampogna E, Zappa M, Spanevello A, et al. Pulmonary rehabilitation and asthma. Front Pharmacol 2020; 11: 542. doi: 10.3389/fphar.2020.00542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt SP, Rabe KF, Hanania NA, et al. Dupilumab for COPD with type 2 inflammation indicated by eosinophil counts. N Engl J Med 2023; 389: 205–214. doi: 10.1056/NEJMoa2303951 [DOI] [PubMed] [Google Scholar]
- 32.Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 33.Criner GJ, Celli BR, Brightling CE, et al. Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 34.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy 2008; 63: Suppl. 86, 8–160. doi: 10.1111/j.1398-9995.2007.01620.x [DOI] [PubMed] [Google Scholar]
- 35.Settipane RJ, Hagy GW, Settipane GA. Long-term risk factors for developing asthma and allergic rhinitis: a 23-year follow-up study of college students. Allergy Proc 1994; 15: 21–25. doi: 10.2500/108854194778816634 [DOI] [PubMed] [Google Scholar]
- 36.Bousquet J, Gaugris S, Kocevar VS, et al. Increased risk of asthma attacks and emergency visits among asthma patients with allergic rhinitis: a subgroup analysis of the investigation of montelukast as a partner agent for complementary therapy. Clin Exp Allergy 2005; 35: 723–727. doi: 10.1111/j.1365-2222.2005.02251.x [DOI] [PubMed] [Google Scholar]
- 37.Virchow JC, Bachert C. Efficacy and safety of montelukast in adults with asthma and allergic rhinitis. Respir Med 2006; 100: 1952–1959. doi: 10.1016/j.rmed.2006.02.026 [DOI] [PubMed] [Google Scholar]
- 38.Borderias L, Mincewicz G, Paggiaro PL, et al. Asthma control in patients with asthma and allergic rhinitis receiving add-on montelukast therapy for 12 months: a retrospective observational study. Curr Med Res Opin 2007; 23: 721–730. doi: 10.1185/030079906X167606 [DOI] [PubMed] [Google Scholar]
- 39.Dal Negro R, Piskorz P, Vives R, et al. Healthcare utilisation and costs associated with adding montelukast to current therapy in patients with mild to moderate asthma and co-morbid allergic rhinitis: PRAACTICAL study. Pharmacoeconomics 2007; 25: 665–676. doi: 10.2165/00019053-200725080-00004 [DOI] [PubMed] [Google Scholar]
- 40.Calderon MA, Alves B, Jacobson M, et al. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev 2007; 1: CD001936. doi: 10.1002/14651858.CD001936.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross RN, Nelson HS, Finegold I. Effectiveness of specific immunotherapy in the treatment of allergic rhinitis: an analysis of randomized, prospective, single- or double-blind, placebo-controlled studies. Clin Ther 2000; 22: 342–350. doi: 10.1016/S0149-2918(00)80038-7 [DOI] [PubMed] [Google Scholar]
- 42.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev 2010; 8: CD001186. doi: 10.1002/14651858.CD001186.pub2 [DOI] [PubMed] [Google Scholar]
- 43.Kim JY, Jang MJ, Kim DY, et al. Efficacy of subcutaneous and sublingual immunotherapy for house dust mite allergy: a network meta-analysis-based comparison. J Allergy Clin Immunol Pract 2021; 9: 4450–4458.e6. doi: 10.1016/j.jaip.2021.08.018 [DOI] [PubMed] [Google Scholar]
- 44.Epstein TEG, Calabria CW. Is immunotherapy safe for treatment of severe asthma. Curr Opin Allergy Clin Immunol 2022; 22: 396–401. doi: 10.1097/ACI.0000000000000853 [DOI] [PubMed] [Google Scholar]
- 45.Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol 2021; 11: 213–739. doi: 10.1002/alr.22741 [DOI] [PubMed] [Google Scholar]
- 46.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy 2012; 67: 91–98. doi: 10.1111/j.1398-9995.2011.02709.x [DOI] [PubMed] [Google Scholar]
- 47.Annesi-Maesano I. Epidemiological evidence of the occurrence of rhinitis and sinusitis in asthmatics. Allergy 1999; 54: Suppl. 57, 7–13. doi: 10.1111/j.1398-9995.1999.tb04401.x [DOI] [PubMed] [Google Scholar]
- 48.Jani AL, Hamilos DL. Current thinking on the relationship between rhinosinusitis and asthma. J Asthma 2005; 42: 1–7. doi: 10.1081/JAS-200044744 [DOI] [PubMed] [Google Scholar]
- 49.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol 2007; 119: 405–413. doi: 10.1016/j.jaci.2006.11.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachert C, Bhattacharyya N, Desrosiers M, et al. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy 2021; 14: 127–134. doi: 10.2147/JAA.S290424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SD, Cho KS. Samter's triad: state of the art. Clin Exp Otorhinolaryngol 2018; 11: 71–80. doi: 10.21053/ceo.2017.01606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy 2016; 30: 44–47. doi: 10.2500/ajra.2016.30.4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dursun AB, Woessner KA, Simon RA, et al. Predicting outcomes of oral aspirin challenges in patients with asthma, nasal polyps, and chronic sinusitis. Ann Allergy Asthma Immunol 2008; 100: 420–425. doi: 10.1016/S1081-1206(10)60465-6 [DOI] [PubMed] [Google Scholar]
- 54.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019; 394: 1638–1650. doi: 10.1016/S0140-6736(19)31881-1 [DOI] [PubMed] [Google Scholar]
- 55.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020; 146: 595–605. doi: 10.1016/j.jaci.2020.05.032 [DOI] [PubMed] [Google Scholar]
- 56.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9: 1141–1153. doi: 10.1016/S2213-2600(21)00097-7 [DOI] [PubMed] [Google Scholar]
- 57.Weinstein SF, Katial RK, Bardin P, et al. Effects of reslizumab on asthma outcomes in a subgroup of eosinophilic asthma patients with self-reported chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract 2019; 7: 589–596.e3. doi: 10.1016/j.jaip.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 58.Boiko NV, Lodochkina OE, Kit MM, et al. [Impact of reslizumab on the course of chronic rhinosinusitis in patients with eosinophilic asthma]. Vestn Otorinolaringol 2021; 86: 43–48. doi: 10.17116/otorino20218602143 [DOI] [PubMed] [Google Scholar]
- 59.Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo-controlled trial. J Allergy Clin Immunol 2022; 149: 1309–1317.e12. doi: 10.1016/j.jaci.2021.08.030 [DOI] [PubMed] [Google Scholar]
- 60.Tversky J, Lane AP, Azar A. Benralizumab effect on severe chronic rhinosinusitis with nasal polyps (CRSwNP): a randomized double-blind placebo-controlled trial. Clin Exp Allergy 2021; 51: 836–844. doi: 10.1111/cea.13852 [DOI] [PubMed] [Google Scholar]
- 61.Takabayashi T, Asaka D, Okamoto Y, et al. A phase II, multicenter, randomized, placebo-controlled study of benralizumab, a humanized anti-IL-5R alpha monoclonal antibody, in patients with eosinophilic chronic rhinosinusitis. Am J Rhinol Allergy 2021; 35: 861–870. doi: 10.1177/19458924211009429 [DOI] [PubMed] [Google Scholar]
- 62.Dunlop G, Scadding GK, Lund VJ. The effect of endoscopic sinus surgery on asthma: management of patients with chronic rhinosinusitis, nasal polyposis, and asthma. Am J Rhinol 1999; 13: 261–265. doi: 10.2500/105065899782102809 [DOI] [PubMed] [Google Scholar]
- 63.Ragab S, Scadding GK, Lund VJ, et al. Treatment of chronic rhinosinusitis and its effects on asthma. Eur Respir J 2006; 28: 68–74. doi: 10.1183/09031936.06.00043305 [DOI] [PubMed] [Google Scholar]
- 64.Ai J, Xie Z, Qing X, et al. Clinical effect of endoscopic vidian neurectomy on bronchial asthma outcomes in patients with coexisting refractory allergic rhinitis and asthma. Am J Rhinol Allergy 2018; 32: 139–146. doi: 10.1177/1945892418764964 [DOI] [PubMed] [Google Scholar]
- 65.Qi Y, Liu J, Peng S, et al. Efficacy of selective vidian neurectomy for allergic rhinitis combined with chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec 2021; 83: 327–334. doi: 10.1159/000512083 [DOI] [PubMed] [Google Scholar]
- 66.Maimaitiaili G, Kahaer K, Tang L, et al. The effect of vidian neurectomy on pulmonary function in patients with allergic rhinitis and chronic rhinosinusitis with nasal polyps. Am J Med Sci 2020; 360: 137–145. doi: 10.1016/j.amjms.2020.04.024 [DOI] [PubMed] [Google Scholar]
- 67.O'Donnell AE. Bronchiectasis – a clinical review. N Engl J Med 2022; 387: 533–545. doi: 10.1056/NEJMra2202819 [DOI] [PubMed] [Google Scholar]
- 68.Ma D, Cruz M-J, Ojanguren I, et al. Risk factors for the development of bronchiectasis in patients with asthma. Sci Rep 2021; 11: 22820. doi: 10.1038/s41598-021-02332-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frøssing L, Von Bülow A, Porsbjerg C. Bronchiectasis in severe asthma is associated with eosinophilic airway inflammation and activation. J Allergy Clin Immunol Glob 2022; 2: 36–42. doi: 10.1016/j.jacig.2022.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi H, Lee H, Ryu J, et al. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: a nationwide population study. Ther Adv Respir Dis 2020; 14: 1753466620963030. doi: 10.1177/1753466620963030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill AR, Bedi P, Cartlidge MK, et al. Early exacerbation relapse is increased in patients with asthma and bronchiectasis (a post hoc analysis). Lung 2023; 201: 17–23. doi: 10.1007/s00408-023-00601-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol 2019; 19: 46–52. doi: 10.1097/ACI.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 73.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. doi: 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 74.Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019; 74: Suppl. 1, 1–69. doi: 10.1136/thoraxjnl-2018-212463 [DOI] [PubMed] [Google Scholar]
- 75.Kelly C, Chalmers JD, Crossingham I, et al. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst Rev 2018; 3: CD012406. doi: 10.1002/14651858.CD012406.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–668. doi: 10.1016/S0140-6736(17)31281-3 [DOI] [PubMed] [Google Scholar]
- 77.Martínez-García MÁ, Oscullo G, García-Ortega A, et al. Inhaled corticosteroids in adults with non-cystic fibrosis bronchiectasis: from bench to bedside. A narrative review. Drugs 2022; 82: 1453–1468. doi: 10.1007/s40265-022-01785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nigro M, Simonetta E, Martínez-García MÁ, et al. Biologics in bronchiectasis: a future treatment? Arch Bronconeumol 2023; 59: 139–141. doi: 10.1016/j.arbres.2022.12.008 [DOI] [PubMed] [Google Scholar]
- 79.Crimi C, Campisi R, Nolasco S, et al. Mepolizumab effectiveness in patients with severe eosinophilic asthma and co-presence of bronchiectasis: a real-world retrospective pilot study. Respir Med 2021; 185: 106491. doi: 10.1016/j.rmed.2021.106491 [DOI] [PubMed] [Google Scholar]
- 80.Campisi R, Nolasco S, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with co-presence of bronchiectasis: a real-world multicentre observational study. J Clin Med 2023; 12: 3953. doi: 10.3390/jcm12123953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nolasco S, Crimi C, Campisi R, et al. Rapid benralizumab effectiveness in patients with severe eosinophilic asthma and bronchiectasis. Eur Respir J 2020; 56: Suppl. 64, 2225. doi: 10.1183/13993003.congress-2020.2225 [DOI] [Google Scholar]
- 82.Bendien SA, Kroes JA, van Hal LHG, et al. Real-world effectiveness of IL-5/5Ra targeted biologics in severe eosinophilic asthma with comorbid bronchiectasis. J Allergy Clin Immunol Pract 2023; 11: 2724–2731.e2. doi: 10.1016/j.jaip.2023.05.041 [DOI] [PubMed] [Google Scholar]
- 83.Kurihara Y, Tashiro H, Takahashi K, et al. Clinical manifestations of allergic bronchopulmonary aspergillosis without major features of asthma diagnosed by the new criteria in Japan. Intern Med 2021; 60: 1251–1255. doi: 10.2169/internalmedicine.6072-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen H, Zhang X, Zhu L, et al. Clinical and immunological characteristics of Aspergillus fumigatus-sensitized asthma and allergic bronchopulmonary aspergillosis. Front Immunol 2022; 13: 939127. doi: 10.3389/fimmu.2022.939127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Agarwal R, Sehgal IS, Dhooria S, et al. Developments in the diagnosis and treatment of allergic bronchopulmonary aspergillosis. Expert Rev Respir Med 2016; 10: 1317–1334. doi: 10.1080/17476348.2016.1249853 [DOI] [PubMed] [Google Scholar]
- 86.Soundappan K, Muthu V, Dhooria S, et al. Population prevalence of allergic bronchopulmonary aspergillosis in asthma: an epidemiological study of 43,261 participants from North India. Clin Exp Allergy 2023; 53: 777–780. doi: 10.1111/cea.14299 [DOI] [PubMed] [Google Scholar]
- 87.Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol 2013; 51: 361–370. doi: 10.3109/13693786.2012.738312 [DOI] [PubMed] [Google Scholar]
- 88.Agarwal R, Nath A, Aggarwal AN, et al. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with acute severe asthma in a respiratory intensive care unit in North India. Mycoses 2010; 53: 138–143. doi: 10.1111/j.1439-0507.2008.01680.x [DOI] [PubMed] [Google Scholar]
- 89.Phuyal S, Garg MK, Agarwal R, et al. High-attenuation mucus impaction in patients with allergic bronchopulmonary aspergillosis: objective criteria on high-resolution computed tomography and correlation with serologic parameters. Curr Probl Diagn Radiol 2016; 45: 168–173. doi: 10.1067/j.cpradiol.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 90.Agarwal R, Gupta D, Aggarwal AN, et al. Clinical significance of hyperattenuating mucoid impaction in allergic bronchopulmonary aspergillosis: an analysis of 155 patients. Chest 2007; 132: 1183–1190. doi: 10.1378/chest.07-0808 [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg M, Patterson R, Mintzer R, et al. Clinical and immunologic criteria for the diagnosis of allergic bronchopulmonary aspergillosis. Ann Intern Med 1977; 86: 405–414. doi: 10.7326/0003-4819-86-4-405 [DOI] [PubMed] [Google Scholar]
- 92.Greenberger PA, Patterson R. Allergic bronchopulmonary aspergillosis and the evaluation of the patient with asthma. J Allergy Clin Immunol 1988; 81: 646–650. doi: 10.1016/0091-6749(88)91034-2 [DOI] [PubMed] [Google Scholar]
- 93.Agarwal R, Chakrabarti A, Shah A, et al. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43: 850–873. doi: 10.1111/cea.12141 [DOI] [PubMed] [Google Scholar]
- 94.Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63: e1–e60. doi: 10.1093/cid/ciw326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev 2004; 3: CD001108. doi: 10.1002/14651858.CD001108.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med 2000; 342: 756–762. doi: 10.1056/NEJM200003163421102 [DOI] [PubMed] [Google Scholar]
- 97.Chishimba L, Niven RM, Cooley J, et al. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma 2012; 49: 423–433. doi: 10.3109/02770903.2012.662568 [DOI] [PubMed] [Google Scholar]
- 98.Agarwal R, Dhooria S, Sehgal IS, et al. A randomised trial of voriconazole and prednisolone monotherapy in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2018; 52: 1801159. doi: 10.1183/13993003.01159-2018 [DOI] [PubMed] [Google Scholar]
- 99.Natarajan S, Subramanian P. Allergic bronchopulmonary aspergillosis: a clinical review of 24 patients: are we right in frequent serologic monitoring? Ann Thorac Med 2014; 9: 216–220. doi: 10.4103/1817-1737.140130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agarwal R, Aggarwal AN, Dhooria S, et al. A randomised trial of glucocorticoids in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2016; 47: 490–498. doi: 10.1183/13993003.01475-2015 [DOI] [PubMed] [Google Scholar]
- 101.Voskamp AL, Gillman A, Symons K, et al. Clinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 2015; 3: 192–199. doi: 10.1016/j.jaip.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 102.Tillie-Leblond I, Germaud P, Leroyer C, et al. Allergic bronchopulmonary aspergillosis and omalizumab. Allergy 2011; 66: 1254–1256. doi: 10.1111/j.1398-9995.2011.02599.x [DOI] [PubMed] [Google Scholar]
- 103.Tanou K, Zintzaras E, Kaditis AG. Omalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidence. Pediatr Pulmonol 2014; 49: 503–507. doi: 10.1002/ppul.22937 [DOI] [PubMed] [Google Scholar]
- 104.Tomomatsu K, Oguma T, Baba T, et al. Effectiveness and safety of omalizumab in patients with allergic bronchopulmonary aspergillosis complicated by chronic bacterial infection in the airways. Int Arch Allergy Immunol 2020; 181: 499–506. doi: 10.1159/000507216 [DOI] [PubMed] [Google Scholar]
- 105.Dhariwal J, Hearn AP, Kavanagh JE, et al. Real-world effectiveness of anti-IL-5/5R therapy in severe atopic eosinophilic asthma with fungal sensitization. J Allergy Clin Immunol Pract 2021; 9: 2315–2320.e1. doi: 10.1016/j.jaip.2021.02.048 [DOI] [PubMed] [Google Scholar]
- 106.Tolebeyan A, Mohammadi O, Vaezi Z, et al. Mepolizumab as possible treatment for allergic bronchopulmonary aspergillosis: a review of eight cases. Cureus 2020; 12: e9684. doi: 10.7759/cureus.9684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van der Veer T, Dallinga MA, van der Valk JPM, et al. Reduced exacerbation frequency and prednisone dose in patients with ABPA and asthma treated with dupilumab. Clin Transl Allergy 2021; 11: e12081. doi: 10.1002/clt2.12081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mikura S, Saraya T, Yoshida Y, et al. Successful treatment of mepolizumab- and prednisolone-resistant allergic bronchopulmonary aspergillosis with dupilumab. Intern Med 2021; 60: 2839–2842. doi: 10.2169/internalmedicine.6679-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tashiro H, Shore SA. Obesity and severe asthma. Allergol Int 2019; 68: 135–142. doi: 10.1016/j.alit.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol 2008; 121: 1087–1093. doi: 10.1016/j.jaci.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 111.Garcia-Rio F, Alvarez-Puebla MJ, Esteban-Gorgojo I, et al. Obesity and asthma: key clinical questions. J Investig Allergol Clin Immunol 2019; 29: 262–271. doi: 10.18176/jiaci.0316 [DOI] [PubMed] [Google Scholar]
- 112.Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol 2018; 141: 1169–1179. doi: 10.1016/j.jaci.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohan A, Grace J, Wang BR, et al. The effects of obesity in asthma. Curr Allergy Asthma Rep 2019; 19: 49. doi: 10.1007/s11882-019-0877-z [DOI] [PubMed] [Google Scholar]
- 114.Forno E, Young OM, Kumar R, et al. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014; 134: e535–e546. doi: 10.1542/peds.2014-0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dumas O, Varraso R, Gillman MW, et al. Longitudinal study of maternal body mass index, gestational weight gain, and offspring asthma. Allergy 2016; 71: 1295–1304. doi: 10.1111/all.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Casas M, den Dekker HT, Kruithof CJ, et al. Early childhood growth patterns and school-age respiratory resistance, fractional exhaled nitric oxide and asthma. Pediatr Allergy Immunol 2016; 27: 854–860. doi: 10.1111/pai.12645 [DOI] [PubMed] [Google Scholar]
- 117.Mebrahtu TF, Feltbower RG, Greenwood DC, et al. Childhood body mass index and wheezing disorders: a systematic review and meta-analysis. Pediatr Allergy Immunol 2015; 26: 62–72. doi: 10.1111/pai.12321 [DOI] [PubMed] [Google Scholar]
- 118.Hasegawa K, Tsugawa Y, Lopez BL, et al. Body mass index and risk of hospitalization among adults presenting with asthma exacerbation to the emergency department. Ann Am Thorac Soc 2014; 11: 1439–1444. doi: 10.1513/AnnalsATS.201406-270BC [DOI] [PubMed] [Google Scholar]
- 119.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007; 175: 661–666. doi: 10.1164/rccm.200611-1717OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holguin F, Bleecker ER, Busse WW, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol 2011; 127: 1486–1493.e2. doi: 10.1016/j.jaci.2011.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Akinbami LJ, Fryar CD. Current asthma prevalence by weight status among adults: United States, 2001–2014. NCHS Data Brief Number 239. Hyattsville, National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 122.Sideleva O, Suratt BT, Black KE, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med 2012; 186: 598–605. doi: 10.1164/rccm.201203-0573OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sutherland TJT, Cowan JO, Young S, et al. The association between obesity and asthma: interactions between systemic and airway inflammation. Am J Respir Crit Care Med 2008; 178: 469–475. doi: 10.1164/rccm.200802-301OC [DOI] [PubMed] [Google Scholar]
- 124.Marko M, Pawliczak R. Obesity and asthma: risk, control and treatment. Adv Dermatol Allergol 2018; 35: 563–571. doi: 10.5114/ada.2018.77607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schatz M, Hsu J-WY, Zeiger RS, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 2014; 133: 1549–1556. doi: 10.1016/j.jaci.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 126.Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med 2006; 174: 112–119. doi: 10.1164/rccm.200602-231PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liang L, Hur J, Kang JY, et al. Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model. Korean J Intern Med 2018; 33: 1210–1223. doi: 10.3904/kjim.2017.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Manuel S-S, Luis G-M. Nutrition, obesity and asthma inception in children. The role of lung function. Nutrients 2021; 13: 3837. doi: 10.3390/nu13113837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mhamed SC, Saad AB, Migaou A, et al. Asthme et obésité: relation et implications thérapeutiques auprès des patients asthmatiques du Service de Pneumologie de Monastir, Tunisie [Asthma and obesity: relationship and therapeutic implications in patients with asthma at the department of pneumology in Monastir, Tunisia]. Pan Afr Med J 2020; 36: 49. doi: 10.11604/pamj.2020.36.49.21098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bal C, Pohl W, Milger K, et al. Characterization of obesity in severe asthma in the German Asthma Net. J Allergy Clin Immunol Pract 2023; in press [ 10.1016/j.jaip.2023.06.049]. [DOI] [PubMed] [Google Scholar]
- 131.Xie L, Chandrasekhar A, DeSantis SM, et al. Discontinuation and reduction of asthma medications after metabolic and bariatric surgery: a systematic review and meta-analysis. Obes Rev 2023; 24: e13527. doi: 10.1111/obr.13527 [DOI] [PubMed] [Google Scholar]
- 132.Hasegawa K, Tsugawa Y, Chang Y, et al. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol 2015; 136: 288–294.e8. doi: 10.1016/j.jaci.2014.12.1931 [DOI] [PubMed] [Google Scholar]
- 133.Nanzer AM, Taylor V, Hearn AP, et al. The impact of steroid-sparing biologic therapies on weight loss in obese individuals with severe eosinophilic asthma. Eur Respir J 2023; 62: 2300245. doi: 10.1183/13993003.00245-2023 [DOI] [PubMed] [Google Scholar]
- 134.Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology 2016; 21: 1210–1218. doi: 10.1111/resp.12818 [DOI] [PubMed] [Google Scholar]
- 135.Wu TD, Keet CA, Fawzy A, et al. Association of metformin initiation and risk of asthma exacerbation. A claims-based cohort study. Ann Am Thorac Soc 2019; 16: 1527–1533. doi: 10.1513/AnnalsATS.201812-897OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Foer D, Beeler PE, Cui J, et al. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med 2021; 203: 831–840. doi: 10.1164/rccm.202004-0993OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forno E. A potential new treatment option for asthma in the setting of obesity or insulin resistance? Am J Respir Crit Care Med 2021; 203: 788–789. doi: 10.1164/rccm.202010-4017ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang RS, Liang KL, Hsin CH, et al. The impact of chronic rhinosinusitis on sleep-disordered breathing. Rhinology 2016; 54: 75–79. doi: 10.4193/Rhino15.204 [DOI] [PubMed] [Google Scholar]
- 139.Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med 2009; 5: 71–78. doi: 10.5664/jcsm.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Julien JY, Martin JG, Ernst P, et al. Prevalence of obstructive sleep apnea–hypopnea in severe versus moderate asthma. J Allergy Clin Immunol 2009; 124: 371–376. doi: 10.1016/j.jaci.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 141.Yigla M, Tov N, Solomonov A, et al. Difficult-to-control asthma and obstructive sleep apnea. J Asthma 2003; 40: 865–871. doi: 10.1081/JAS-120023577 [DOI] [PubMed] [Google Scholar]
- 142.Teodorescu M, Broytman O, Curran-Everett D, et al. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract 2015; 3: 566–575.e1. doi: 10.1016/j.jaip.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hirayama A, Goto T, Faridi MK, et al. Association of obstructive sleep apnea with all-cause readmissions after hospitalization for asthma exacerbation in adults aged 18–54 years: a population-based study, 2010–2013. J Asthma 2021; 58: 1176–1185. doi: 10.1080/02770903.2020.1781887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991; 14: 540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 145.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest 2016; 149: 631–638. doi: 10.1378/chest.15-0903 [DOI] [PubMed] [Google Scholar]
- 146.Busk M, Busk N, Puntenney P, et al. Use of continuous positive airway pressure reduces airway reactivity in adults with asthma. Eur Respir J 2013; 41: 317–322. doi: 10.1183/09031936.00059712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Serrano-Pariente J, Plaza V, Soriano JB, et al. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy 2017; 72: 802–812. doi: 10.1111/all.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ng SSS, Chan TO, To KW, et al. Continuous positive airway pressure for obstructive sleep apnoea does not improve asthma control. Respirology 2018; 23: 1055–1062. doi: 10.1111/resp.13363 [DOI] [PubMed] [Google Scholar]
- 149.Davies SE, Bishopp A, Wharton S, et al. Does continuous positive airway pressure (CPAP) treatment of obstructive sleep apnoea (OSA) improve asthma-related clinical outcomes in patients with co-existing conditions? A systematic review. Respir Med 2018; 143: 18–30. doi: 10.1016/j.rmed.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 150.Katz PO, Dunbar KB, Schnoll-Sussman FH, et al. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2022; 117: 27–56. doi: 10.14309/ajg.0000000000001538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut 2007; 56: 1654–1664. doi: 10.1136/gut.2007.122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim SY, Min C, Oh DJ, et al. Bidirectional association between GERD and asthma: two longitudinal follow-up studies using a national sample cohort. J Allergy Clin Immunol Pract 2020; 8: 1005–1013.e9. doi: 10.1016/j.jaip.2019.10.043 [DOI] [PubMed] [Google Scholar]
- 153.Ahn K, Penn RB, Rattan S, et al. Mendelian randomization analysis reveals a complex genetic interplay among atopic dermatitis, asthma, and gastroesophageal reflux disease. Am J Respir Crit Care Med 2023: 207: 130–137. doi: 10.1164/rccm.202205-0951OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Denlinger LC, Phillips BR, Ramratnam S, et al. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med 2017; 195: 302–313. doi: 10.1164/rccm.201602-0419OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.ten Brinke A, Sterk PJ, Masclee AA, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J 2005; 26: 812–818. doi: 10.1183/09031936.05.00037905 [DOI] [PubMed] [Google Scholar]
- 156.Price D, Wilson AM, Chisholm A, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy 2016: 9: 1–12. doi: 10.2147/JAA.S97973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tariq K, Schofield JPR, Nicholas BL, et al. Sputum proteomic signature of gastro-oesophageal reflux in patients with severe asthma. Respir Med 2019; 150: 66–73. doi: 10.1016/j.rmed.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 158.Althoff MD, Ghincea A, Wood LG, et al. Asthma and three colinear comorbidities: obesity, OSA, and GERD. J Allergy Clin Immunol Pract 2021; 9: 3877–3884. doi: 10.1016/j.jaip.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Perotin JM, Wheway G, Tariq K, et al. Vulnerability to acid reflux of the airway epithelium in severe asthma. Eur Respir J 2022; 60: 2101634. doi: 10.1183/13993003.01634-2021 [DOI] [PubMed] [Google Scholar]
- 160.Harding SM, Sontag SJ. Asthma and gastroesophageal reflux. Am J Gastroenterol 2000; 95: Suppl. 8, S23–S32. doi: 10.1016/S0002-9270(00)01075-3 [DOI] [PubMed] [Google Scholar]
- 161.Vincent D, Cohen-Jonathan AM, Leport J, et al. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J 1997; 10: 2255–2259. doi: 10.1183/09031936.97.10102255 [DOI] [PubMed] [Google Scholar]
- 162.Jack CI, Calverley PM, Donnelly RJ, et al. Simultaneous tracheal and oesophageal pH measurements in asthmatic patients with gastro-oesophageal reflux. Thorax 1995; 50: 201–204. doi: 10.1136/thx.50.2.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.American Lung Association Asthma Clinical Research Centers . Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med 2009; 360: 1487–1499. doi: 10.1056/NEJMoa0806290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Writing Committee for the American Lung Association Asthma Clinical Research Centers . Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA 2012; 307: 373–380. doi: 10.1001/jama.2011.2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Littner MR, Leung FW, Ballard ED 2nd, et al. Effects of 24 weeks of lansoprazole therapy on asthma symptoms, exacerbations, quality of life, and pulmonary function in adult asthmatic patients with acid reflux symptoms. Chest 2005; 128: 1128–1135. doi: 10.1378/chest.128.3.1128 [DOI] [PubMed] [Google Scholar]
- 166.Kopsaftis Z, Yap HS, Tin KS, et al. Pharmacological and surgical interventions for the treatment of gastro-oesophageal reflux in adults and children with asthma. Cochrane Database Syst Rev 2021; 5: CD001496. doi: 10.1002/14651858.CD001496.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Christensen PM, Heimdal JH, Christopher KL, et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur Respir Rev 2015; 24: 445–450. doi: 10.1183/16000617.00006513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Newman KB, Mason UG 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med 1995; 152: 1382–1386. doi: 10.1164/ajrccm.152.4.7551399 [DOI] [PubMed] [Google Scholar]
- 169.Low K, Lau KK, Holmes P, et al. Abnormal vocal cord function in difficult-to-treat asthma. Am J Respir Crit Care Med 2011; 184: 50–56. doi: 10.1164/rccm.201010-1604OC [DOI] [PubMed] [Google Scholar]
- 170.Idrees M, FitzGerald JM. Vocal cord dysfunction in bronchial asthma. A review article. J Asthma 2015; 52: 327–335. doi: 10.3109/02770903.2014.982288 [DOI] [PubMed] [Google Scholar]
- 171.Clemm HH, Olin JT, McIntosh C, et al. Exercise-induced laryngeal obstruction (EILO) in athletes: a narrative review by a subgroup of the IOC Consensus on ‘acute respiratory illness in the athlete’. Br J Sports Med 2022; 56: 622–629. doi: 10.1136/bjsports-2021-104704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg 2000; 126: 29–34. doi: 10.1001/archotol.126.1.29 [DOI] [PubMed] [Google Scholar]
- 173.Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol 2005; 114: 253–257. doi: 10.1177/000348940511400401 [DOI] [PubMed] [Google Scholar]
- 174.Bucca C, Rolla G, Brussino L, et al. Are asthma-like symptoms due to bronchial or extrathoracic airway dysfunction? Lancet 1995; 346: 791–795. doi: 10.1016/S0140-6736(95)91617-2 [DOI] [PubMed] [Google Scholar]
- 175.Galdi E, Perfetti L, Pagella F, et al. Irritant vocal cord dysfunction at first misdiagnosed as reactive airway dysfunction syndrome. Scand J Work Environ Health 2005; 31: 224–226. doi: 10.5271/sjweh.873 [DOI] [PubMed] [Google Scholar]
- 176.Perkner JJ, Fennelly KP, Balkissoon R, et al. Irritant-associated vocal cord dysfunction. J Occup Environ Med 1998; 40: 136–143. doi: 10.1097/00043764-199802000-00009 [DOI] [PubMed] [Google Scholar]
- 177.Kenn K, Balkissoon R. Vocal cord dysfunction: what do we know? Eur Respir J 2011; 37: 194–200. doi: 10.1183/09031936.00192809 [DOI] [PubMed] [Google Scholar]
- 178.Kenn K, Schmitz M. “Vocal Cord Dysfunction” (VCD), eine wichtige Differentialdiagnose zum schweren und inplausiblen Asthma bronchiale [Vocal cord dysfunction, an important differential diagnosis of severe and implausible bronchial asthma]. Pneumologie 1997; 51: 14–18. [PubMed] [Google Scholar]
- 179.Traister RS, Fajt ML, Landsittel D, et al. A novel scoring system to distinguish vocal cord dysfunction from asthma. J Allergy Clin Immunol Pract 2014; 2: 65–69. doi: 10.1016/j.jaip.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 180.Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle laryngeal hypersensitivity questionnaire. Cough 2014; 10: 1. doi: 10.1186/1745-9974-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Guss J, Mirza N. Methacholine challenge testing in the diagnosis of paradoxical vocal fold motion. Laryngoscope 2006; 116: 1558–1561. doi: 10.1097/01.mlg.0000228007.74561.33 [DOI] [PubMed] [Google Scholar]
- 182.Maat RC, Hilland M, Røksund OD, et al. Exercise-induced laryngeal obstruction: natural history and effect of surgical treatment. Eur Arch Otorhinolaryngol 2011; 268: 1485–1492. doi: 10.1007/s00405-011-1656-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tervonen H, Niskanen MM, Sovijärvi AR, et al. Fiberoptic videolaryngoscopy during bicycle ergometry: a diagnostic tool for exercise-induced vocal cord dysfunction. Laryngoscope 2009; 119: 1776–1780. doi: 10.1002/lary.20558 [DOI] [PubMed] [Google Scholar]
- 184.Christensen PM, Rasmussen N. Eucapnic voluntary hyperventilation in diagnosing exercise-induced laryngeal obstructions. Eur Arch Otorhinolaryngol 2013; 270: 3107–3113. doi: 10.1007/s00405-013-2571-4 [DOI] [PubMed] [Google Scholar]
- 185.Turmel J, Gagnon S, Bernier M, et al. Eucapnic voluntary hyperpnoea and exercise-induced vocal cord dysfunction. BMJ Open Sport Exerc Med 2015; 1: e000065. doi: 10.1136/bmjsem-2015-000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hira HS, Singh A. Significance of upper airway influence among patients of vocal cord dysfunction for its diagnosis: role of impulse oscillometry. Lung India 2009; 26: 5–8. doi: 10.4103/0970-2113.45197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Komarow HD, Young M, Nelson C, et al. Vocal cord dysfunction as demonstrated by impulse oscillometry. J Allergy Clin Immunol Pract 2013; 1: 387–393. doi: 10.1016/j.jaip.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Holmes PW, Lau KK, Crossett M, et al. Diagnosis of vocal cord dysfunction in asthma with high resolution dynamic volume computerized tomography of the larynx. Respirology 2009; 14: 1106–1113. doi: 10.1111/j.1440-1843.2009.01629.x [DOI] [PubMed] [Google Scholar]
- 189.Archer GJ, Hoyle JL, McCluskey A, et al. Inspiratory vocal cord dysfunction, a new approach in treatment. Eur Respir J 2000; 15: 617–618. doi: 10.1034/j.1399-3003.2000.15.30.x [DOI] [PubMed] [Google Scholar]
- 190.Ludlow S, Daly R, Elsey L, et al. Multidisciplinary management of inducible laryngeal obstruction and breathing pattern disorder. Breathe 2023; 19: 230088. 10.1183/20734735.0088-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andrianopoulos MV, Gallivan GJ, Gallivan KH. PVCM, PVCD, EPL, and irritable larynx syndrome: what are we talking about and how do we treat it? J Voice 2000; 14: 607–618. doi: 10.1016/S0892-1997(00)80016-8 [DOI] [PubMed] [Google Scholar]
- 192.Haines J, Smith JA, Wingfield-Digby J, et al. Systematic review of the effectiveness of non-pharmacological interventions used to treat adults with inducible laryngeal obstruction. BMJ Open Respir Res 2022; 9: e001199. doi: 10.1136/bmjresp-2022-001199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mahoney J, Hew M, Vertigan A, et al. Treatment effectiveness for vocal cord dysfunction in adults and adolescents: a systematic review. Clin Exp Allergy 2022; 52: 387–404. doi: 10.1111/cea.14036 [DOI] [PubMed] [Google Scholar]
- 194.Doshi DR, Weinberger MM. Long-term outcome of vocal cord dysfunction. Ann Allergy Asthma Immunol 2006; 96: 794–799. doi: 10.1016/S1081-1206(10)61341-5 [DOI] [PubMed] [Google Scholar]
- 195.Muralitharan P, Carlsen P, Hilland M, et al. Use of inhaled ipratropium bromide to improve exercise-induced laryngeal obstruction cannot be recommended. ERJ Open Res 2023; 9: 00308-2022. doi: 10.1183/23120541.00308-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Varney V, Parnell H, Evans J, et al. The successful treatment of vocal cord dysfunction with low-dose amitriptyline – including literature review. J Asthma Allergy 2009; 2: 105–110. doi: 10.2147/JAA.S6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Woolnough K, Blakey J, Pargeter N, et al. Acid suppression does not reduce symptoms from vocal cord dysfunction, where gastro-laryngeal reflux is a known trigger. Respirology 2013; 18: 553–554. doi: 10.1111/resp.12058 [DOI] [PubMed] [Google Scholar]
- 198.Maturo S, Hill C, Bunting G, et al. Pediatric paradoxical vocal-fold motion: presentation and natural history. Pediatrics 2011; 128: e1443–e1449. doi: 10.1542/peds.2011-1003 [DOI] [PubMed] [Google Scholar]
- 199.deSilva B, Crenshaw D, Matrka L, et al. Vocal fold botulinum toxin injection for refractory paradoxical vocal fold motion disorder. Laryngoscope 2019; 129: 808–811. doi: 10.1002/lary.27471 [DOI] [PubMed] [Google Scholar]
- 200.Woisard V, Liu X, Bes MC, et al. Botulinum toxin injection in laryngeal dyspnea. Eur Arch Otorhinolaryngol 2017; 274: 909–917. doi: 10.1007/s00405-016-4289-6 [DOI] [PubMed] [Google Scholar]
- 201.Ye G, Baldwin DS, Hou R. Anxiety in asthma: a systematic review and meta-analysis. Psychol Med 2021; 51: 11–20. doi: 10.1017/S0033291720005097 [DOI] [PubMed] [Google Scholar]
- 202.McLoughlin RF, McDonald VM. The management of extrapulmonary comorbidities and treatable traits; obesity, physical inactivity, anxiety, and depression, in adults with asthma. Front Allergy 2021; 2: 735030. doi: 10.3389/falgy.2021.735030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Smith JR, Mildenhall S, Noble M, et al. Clinician-assessed poor compliance identifies adults with severe asthma who are at risk of adverse outcomes. J Asthma 2005; 42: 437–445. doi: 10.1081/JAS-200067949 [DOI] [PubMed] [Google Scholar]
- 204.Rietveld S, Creer TL. Psychiatric factors in asthma: implications for diagnosis and therapy. Am J Respir Med 2003; 2: 1–10. doi: 10.1007/BF03256634 [DOI] [PubMed] [Google Scholar]
- 205.Zhang L, Zhang X, Zheng J, et al. Depressive symptom-associated IL-1beta and TNF-alpha release correlates with impaired bronchodilator response and neutrophilic airway inflammation in asthma. Clin Exp Allergy 2019; 49: 770–780. doi: 10.1111/cea.13346 [DOI] [PubMed] [Google Scholar]
- 206.Jiang M, Qin P, Yang X. Comorbidity between depression and asthma via immune-inflammatory pathways: a meta-analysis. J Affect Disord 2014; 166: 22–29. doi: 10.1016/j.jad.2014.04.027 [DOI] [PubMed] [Google Scholar]
- 207.Han YY, Forno E, Marsland AL, et al. Depression, asthma, and bronchodilator response in a nationwide study of US adults. J Allergy Clin Immunol Pract 2016; 4: 68–73.e1. doi: 10.1016/j.jaip.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brehm JM, Ramratnam SK, Tse SM, et al. Stress and bronchodilator response in children with asthma. Am J Respir Crit Care Med 2015; 192: 47–56. doi: 10.1164/rccm.201501-0037OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Clark VL, Gibson PG, Genn G, et al. Multidimensional assessment of severe asthma: a systematic review and meta-analysis. Respirology 2017; 22: 1262–1275. doi: 10.1111/resp.13134 [DOI] [PubMed] [Google Scholar]
- 210.Cordina M, Fenech AG, Vassallo J, et al. Anxiety and the management of asthma in an adult outpatient population. Ther Adv Respir Dis 2009; 3: 227–233. doi: 10.1177/1753465809347038 [DOI] [PubMed] [Google Scholar]
- 211.Kuder MM, Clark M, Cooley C, et al. A systematic review of the effect of physical activity on asthma outcomes. J Allergy Clin Immunol Pract 2021; 9: 3407–3421.e8. doi: 10.1016/j.jaip.2021.04.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kew KM, Nashed M, Dulay V, et al. Cognitive behavioural therapy (CBT) for adults and adolescents with asthma. Cochrane Database Syst Rev 2016; 9: CD011818. doi: 10.1002/14651858.CD011818.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Feldman JM, Matte L, Interian A, et al. Psychological treatment of comorbid asthma and panic disorder in Latino adults: results from a randomized controlled trial. Behav Res Ther 2016; 87: 142–154. doi: 10.1016/j.brat.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lehrer PM, Karavidas MK, Lu S-E, et al. Psychological treatment of comorbid asthma and panic disorder: a pilot study. J Anxiety Disord 2008; 22: 671–683. doi: 10.1016/j.janxdis.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mehl-Madrona L, Kligler B, Silverman S, et al. The impact of acupuncture and craniosacral therapy interventions on clinical outcomes in adults with asthma. Explore (NY) 2007; 3: 28–36. doi: 10.1016/j.explore.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 216.Cooley C, Park Y, Ajilore O, et al. Impact of interventions targeting anxiety and depression in adults with asthma. J Asthma 2022; 59: 273–287. doi: 10.1080/02770903.2020.1847927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tran L, Sharrad K, Kopsaftis Z, et al. Pharmacological interventions for the treatment of psychological distress in patients with asthma: a systematic review and meta-analysis. J Asthma 2021; 58: 759–769. doi: 10.1080/02770903.2020.1731826 [DOI] [PubMed] [Google Scholar]
- 218.Denton E, Bondarenko J, O'Hehir RE, et al. Breathing pattern disorder in difficult asthma: characteristics and improvement in asthma control and quality of life after breathing re-training. Allergy 2019; 74: 201–203. doi: 10.1111/all.13611 [DOI] [PubMed] [Google Scholar]
- 219.Lee J, Denton E, Hoy R, et al. Paradoxical vocal fold motion in difficult asthma is associated with dysfunctional breathing and preserved lung function. J Allergy Clin Immunol Pract 2020; 8: 2256–2262. doi: 10.1016/j.jaip.2020.02.037 [DOI] [PubMed] [Google Scholar]
- 220.Grammatopoulou EP, Skordilis EK, Georgoudis G, et al. Hyperventilation in asthma: a validation study of the Nijmegen Questionnaire – NQ. J Asthma 2014; 51: 839–846. doi: 10.3109/02770903.2014.922190 [DOI] [PubMed] [Google Scholar]
- 221.Bruton A, Lee A, Yardley L, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respir Med 2018; 6: 19–28. doi: 10.1016/S2213-2600(17)30474-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Riscili BP, Parsons JP, Mastronarde JG. Treating silent reflux disease does not improve poorly controlled asthma. Cleve Clin J Med 2010; 77: 155–160. doi: 10.3949/ccjm.77a.09111 [DOI] [PubMed] [Google Scholar]
- 223.Sanduzzi A, Ciasullo E, Capitelli L, et al. Alpha-1-antitrypsin deficiency and bronchiectasis: a concomitance or a real association? Int J Environ Res Public Health 2020; 17: 2294. doi: 10.3390/ijerph17072294 [DOI] [PMC free article] [PubMed] [Google Scholar]