Abstract
Background
Postpartum contraception improves the health of mothers and children by lengthening birth intervals. For lactating women, contraception choices are limited by concerns about hormonal effects on milk quality and quantity and passage of hormones to the infant. Ideally, the contraceptive chosen should not interfere with lactation or infant growth. Timing of contraception initiation is also important. Immediately postpartum, most women have contact with a health professional, but many do not return for follow‐up contraceptive counseling. However, immediate initiation of hormonal methods may disrupt the onset of milk production.
Objectives
To determine the effects of hormonal contraceptives on lactation and infant growth
Search methods
We searched for eligible trials until 2 March 2015. Sources included the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, POPLINE, Web of Science, LILACS, ClinicalTrials.gov, and ICTRP. We also examined review articles and contacted investigators.
Selection criteria
We sought randomized controlled trials in any language that compared hormonal contraception versus another form of hormonal contraception, nonhormonal contraception, or placebo during lactation. Hormonal contraception includes combined or progestin‐only oral contraceptives, injectable contraceptives, implants, and intrauterine devices.
Trials had to have one of our primary outcomes: breast milk quantity or biochemical composition; lactation initiation, maintenance, or duration; infant growth; or timing of contraception initiation and effect on lactation. Secondary outcomes included contraceptive efficacy while breastfeeding and birth interval.
Data collection and analysis
For continuous variables, we calculated the mean difference (MD) with 95% confidence interval (CI). For dichotomous outcomes, we computed the Mantel‐Haenszel odds ratio (OR) with 95% CI. Due to differing interventions and outcome measures, we did not aggregate the data in a meta‐analysis.
Main results
In 2014, we added seven trials for a new total of 11. Five reports were published before 1985 and six from 2005 to 2014. They included 1482 women. Four trials examined combined oral contraceptives (COCs), and three studied a levonorgestrel‐releasing intrauterine system (LNG‐IUS). We found two trials of progestin‐only pills (POPs) and two of the etonogestrel‐releasing implant. Older studies often lacked quantified results. Most trials did not report significant differences between the study arms in breastfeeding duration, breast milk composition, or infant growth. Exceptions were seen mainly in older studies with limited information.
For breastfeeding duration, two of eight trials indicated a negative effect on lactation. A COC study reported a negative effect on lactation duration compared to placebo but did not quantify results. Another trial showed a lower percentage of the LNG‐IUS group breastfeeding at 75 days versus the nonhormonal IUD group (reported P < 0.05) but no significant difference at one year.
For breast milk volume, two older studies indicated lower volume for the COC group versus the placebo group. One trial did not quantify results. The other showed lower means (mL) for the COC group, e.g. at 16 weeks (MD ‐24.00, 95% CI ‐34.53 to ‐13.47) and at 24 weeks (MD ‐24.90, 95% CI ‐36.01 to ‐13.79). Another four trials did not report any significant difference between the study groups in milk volume or composition with two POPs, a COC, or the etonogestrel implant.
Seven trials studied infant growth; one showed greater weight gain (grams) for the etonogestrel implant versus no method for six weeks (MD 426.00, 95% CI 58.94 to 793.06) but less compared with depot medroxyprogesterone acetate (DMPA) from 6 to 12 weeks (MD ‐271.00, 95% CI ‐355.10 to ‐186.90). The others studied POPs, COCs versus POPs, or an LNG‐IUS.
Authors' conclusions
Results were not consistent across the 11 trials. The evidence was limited for any particular hormonal method. The quality of evidence was moderate overall and low for three of four placebo‐controlled trials of COCs or POPs. The sensitivity analysis included six trials with moderate quality evidence and sufficient outcome data. Five trials indicated no significant difference between groups in breastfeeding duration (etonogestrel implant insertion times, COC versus POP, and LNG‐IUS). For breast milk volume or composition, a COC study showed a negative effect, while an implant trial showed no significant difference. Of four trials that assessed infant growth, three indicated no significant difference between groups. One showed greater weight gain in the etonogestrel implant group versus no method but less versus DMPA.
Keywords: Female; Humans; Infant; Breast Feeding; Breast Feeding/statistics & numerical data; Child Development; Child Development/drug effects; Contraception; Contraception/methods; Contraceptives, Oral, Combined; Contraceptives, Oral, Combined/pharmacology; Contraceptives, Oral, Hormonal; Contraceptives, Oral, Hormonal/pharmacology; Desogestrel; Desogestrel/pharmacology; Lactation; Lactation/drug effects; Levonorgestrel; Levonorgestrel/pharmacology; Milk, Human; Milk, Human/drug effects; Progestins; Progestins/pharmacology; Randomized Controlled Trials as Topic
Plain language summary
Hormonal and nonhormonal birth control during breastfeeding
Birth control for women who are breastfeeding is important worldwide. Delaying the next pregnancy improves the health of women and children. Each year, millions of women decide whether to use birth control after having a baby. The decision includes the birth control type and when to start using it. Researchers and health care providers debate these issues. Some worry that hormones could affect the breast milk and therefore the baby's growth. Ideally, the birth control would not affect the type or amount of breast milk or the baby's growth. Identifying the best time to start birth control is also important. When monthly cycles return is uncertain, and the woman could get pregnant again.
Combined birth control methods contain the hormones estrogen and progestin. Other types of birth control contain only progestin or no hormones. We looked at whether combined birth control or methods with only progestin affect breastfeeding more than other methods. We ran computer searches for randomized trials of birth control used during breastfeeding until 2 March 2015. These trials compared hormonal methods to other hormonal methods or to placebo ('dummy' method). We also looked at reference lists to find trials. For the initial review, we wrote to researchers to find other studies.
We included 11 studies with a total of 1482 women. These trials looked at many methods: pills, an implant, the injectable 'Depo,' and a hormonal intrauterine device (IUD). Some older reports did not have much data. Most trials showed no major difference due to hormonal birth control use. Two of eight trials noted less breastfeeding among women using hormonal birth control. One was a combined pill with few results and the other a hormonal IUD. In one study, the implant group infants gained more weight than those in the no‐method group but less weight than infants in the 'Depo' group. Two trials noted that a combined pill had a negative effect on breast milk volume or content. One report did not have much data. The other showed lower volume for combined pill users than for women taking pills with only progestin.
We found little information on any specific birth control method, with usually two studies per method. Results were not consistent across all trials. The data were of moderate quality overall. The results of better quality showed little effect on breastfeeding or infant growth.
Background
Description of the condition
Contraception for women who are breastfeeding is a public health issue of global importance. According to early Demographic and Health Surveys (DHS), nearly two‐thirds of women in their first postpartum year have an unmet need for family planning (WHO 2013). More recent DHS indicate that unmet need for modern contraceptive methods is 32% among married women in general, and one‐third to one‐half of unmarried women have an unmet need for such methods (Westhoff 2012). Each year more than 100 million women make decisions about beginning or resuming contraception after childbirth (Tsui 1997). These decisions include the choice of contraceptive method and the time at which to begin use. For women who are breastfeeding, the choice and timing of hormonal contraception may influence both lactation and infant growth.
Breastfeeding has well‐established health benefits. It provides the infant with complete nutrition up to six months, a safe food source, and immunological defense against infectious diseases (USAID 2009; AAP 2012). Breastfeeding has economic benefits from conserving funds that would be spent on milk substitutes (AAP 2012). Lactation is associated with reducing a woman's risk of type 2 diabetes and ovarian and breast cancer (Ip 2007). Infants exclusively breastfed for the first six months appear to have better health outcomes than those partially breastfed as of three or four months of age (Kramer 2012).
Breastfeeding influences the need for and timing of postpartum contraception. An interval of anovulation occurs after delivery, and the length of time until ovulation resumes depends on breastfeeding patterns, biological variation, nutrition, geography, culture, and socioeconomic factors (Knijff 2000). Lactation itself can be an effective form of temporary contraception, known as the lactational amenorrhea method (LAM) (Van der Wijden 2003). For LAM to be effective, the woman must fully (or nearly fully) breastfeed, have no menstrual bleeding, and be within six months of delivery (Kennedy 2011; K4 Health 2014). Return of menstruation and ovulation can be unpredictable in breastfeeding women. Therefore, the timing of contraception initiation is important. Ideally, the contraceptive method chosen should not interfere with lactation.
Description of the intervention
Contraception after childbirth improves the health of mothers and infants by lengthening birth intervals. Women are more likely to report births or pregnancies as unintended when they occur within an interval of 24 months or less (Tsui 1997). Preventing such unintended pregnancies reduces health risks and helps avoid associated financial and psychological costs. A longer birth interval of 18 to 27 months decreases the risk of major maternal complications including death, third‐trimester bleeding, puerperal endometritis, and anemia (Conde‐Agudelo 2000; WHO 2005). To reduce risk for poor maternal and infant health outcomes, the World Health Organization has recommended waiting 24 months before attempting the next pregnancy (WHO 2005).
Hormonal contraception includes combined methods that contain both estrogen and progestin as well as progestin‐only methods. Combined hormonal contraceptives include combined oral contraceptives (COCs), combined injectables, and the combined vaginal ring and transdermal patch. Progestin‐only methods include progestin‐only pills (POPs), the injectable depot medroxyprogesterone acetate (DMPA), levonorgestrel and etonogestrel implants, and the levonorgestrel intrauterine system. A progesterone vaginal ring was developed for use during lactation and is available in some Latin American countries (RamaRao 2013).
How the intervention might work
Hormonal contraceptives, especially those containing estrogen, may impair lactation through their effect on prolactin, the hormone responsible for production of milk. During pregnancy, prolactin levels rise and they peak at delivery. However, during pregnancy, both estrogen and progesterone block the effect of prolactin on the breasts. After delivery, levels of both estrogen and progesterone drop markedly, and, without their inhibitory effects, prolactin initiates milk production. Infant suckling stimulates more prolactin, which then sustains milk production. Breast engorgement and full milk secretion start three to four days after delivery, when estrogen and progesterone have sufficiently cleared from the maternal circulation (Speroff 2004; Pang 2007).
Choices of contraception may be limited for lactating women because of concerns about potential negative hormonal effects on quality and quantity of milk, passage of hormones to the infant, and infant growth and development. Some studies have found negative effects on lactation from COCs but not from progestin‐only contraception (Kapp 2010a; Kapp 2010b; Kennedy 2011). However, those studies had various ways of measuring effects on milk production, yielded inconsistent results, and generally did not show negative effects on infants. Theoretical concerns about milk production focus on the early postpartum period, i.e. the onset of lactogenesis (CDC 2011; Gurtcheff 2011). Gonadal steroids may also impact milk supply in established lactation, as suggested by evidence of slower growth among children who continue to breastfeed when their mothers conceive another pregnancy (Bohler 1996). To determine whether hormonal contraception affects milk production, studies ideally would quantify the rate of exclusive or full breastfeeding, as well as the supplementation that infants receive, among women using different contraceptive methods. In the setting of mixed feeding, comparisons of infant growth are difficult to interpret because mothers can compensate for differences in milk supply by formula supplementation.
Due to concerns about the effect on lactation, combined hormonal contraceptives are considered category 4 for breastfeeding women up to six weeks postpartum and category 3 for six weeks to six months (WHO 2009; CDC 2011). Category 3 means that the theoretical or proven risks usually outweigh the advantages of using the method. The method is not usually recommended unless more appropriate methods are unavailable or unacceptable (WHO 2009). Most progestin‐only methods are considered category 3 for less than six weeks postpartum (WHO 2009). For the first month postpartum in the United States, they are considered category 2, i.e. the advantages of using the method generally outweigh the risks (CDC 2011).
Why it is important to do this review
Despite potential adverse effects of COCs on lactation, many women prefer this method (Erwin 1994). Combined oral contraceptives have many benefits, including familiarity with the method, effectiveness, safety, reversibility, excellent cycle control, a decrease in menstrual cramps and pain, decreased days of bleeding and amount of blood loss, and other noncontraceptive benefits. Other hormonal methods, including the progestin‐only pill, may not offer all of these advantages (Raymond 2011). Progestin‐only pill use is estimated at only 0.4% in the United States, according to an analysis of data from the National Survey of Family Growth (Hall 2012). The percentage may be much higher for breastfeeding women, but the sample size was very small for postpartum women. Some women quit breastfeeding early so they can start the COC (Erwin 1994).
Examining the impact of long‐acting reversible contraception (LARC) on lactation is also important. The levonorgestrel‐releasing intrauterine system (LNG‐IUS) and the etonogestrel‐releasing implant (ETG implant) provide highly effective birth control; both are progesterone‐only methods.
Clinical recommendations must be evidence‐based if women are to make informed choices concerning contraception while breastfeeding. This updated review examined the effects of combined hormonal contraceptives and progestin‐only contraceptives on lactation and infant growth.
Objectives
To determine the effects of hormonal contraceptives on lactation and infant growth
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) reported in any language that compared hormonal contraception during lactation versus other hormonal contraception, nonhormonal contraception, or placebo.
Types of participants
Breastfeeding women of any age or parity who desired contraception
Types of interventions
Any form of hormonal contraception compared with another form of hormonal contraception, nonhormonal contraception, or placebo. Combined hormonal contraceptives include oral or injectable methods, vaginal rings and transdermal patches. Progestin‐only contraceptives include oral and injectable methods, subdermal implants, and hormonal intrauterine devices.
Types of outcome measures
For the 2015 update, we separated the original list of outcomes into primary and secondary outcomes. Trials must have reported on at least one of the primary outcomes, which focus on the effect of the hormonal contraceptive on lactation.
Primary outcomes
Quantity of milk
Biochemical analysis of milk composition
Initiation, maintenance and duration of lactation (any or fully breastfeeding)
Infant growth
Timing of contraception initiation and its effects on lactation
Secondary outcomes
Efficacy of contraceptive method while breastfeeding (pregnancy)
Birth interval
Search methods for identification of studies
Electronic searches
We searched for eligible RCTs until 2 March 2015. Sources included the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, POPLINE, Web of Science, and LILACS. We also searched for recent trials via ClinicalTrials.gov and the search portal of the International Clinical Trials Registry Platform (ICTRP). Search strategies are shown in Appendix 1. The initial review and the 2005 and 2008 updates also included EMBASE (Appendix 2).
Searching other resources
For the initial review, we began with review articles. We also examined reference lists of relevant articles and book chapters to seek publications comparing different forms of contraception in breastfeeding women and their effects on lactation. We contacted other investigators in the field to find publications that might have been missed, including unpublished reports. For the 2015 update, we also examined reference lists of reviews and relevant articles for other trials.
Data collection and analysis
2003: For the initial review, the authors read titles and abstracts from the searches to assess whether trials appeared to meet the inclusion criteria. We retrieved the full text when necessary. The authors verified that included references were satisfactory and reviewed others that could have met the inclusion criteria. They resolved disagreements by consensus. They sought additional information from investigators of the original included trials. Two investigators responded to questions about randomization methods and blinding (Miller 1970; WHO 1984).
2005 to 2010: The authors reviewed titles and abstracts from the database searches to determine whether trials appeared to be eligible. We retrieved the full text when necessary to determine whether the trial met the inclusion criteria.
2014: We describe below the data collection and analysis methods used in the current version of this review.
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches with no language limitation.
Data extraction and management
Two authors independently abstracted the data. One author entered the data into Review Manager (RevMan 2014), and a second author verified the accuracy. We resolved discrepancies through discussion. In Characteristics of included studies, we focused on primary and secondary outcomes for this review, which may not include all outcomes from each study.
Assessment of risk of bias in included studies
We examined studies for methodological quality, according to recommended principles (Higgins 2011). We considered factors such as study design, methods used to generate the randomization sequence, allocation concealment, blinding, and losses to follow‐up and to early discontinuation. We also examined the methods used for outcome assessment.
Measures of treatment effect
For continuous variables, we computed the mean difference (MD) with 95% confidence interval (CI) using a fixed‐effect model. RevMan uses the inverse variance approach. For dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% CI using a fixed‐effect model. When multivariate analysis was conducted, we presented the results as reported by the trial investigators.
Dealing with missing data
We wrote to investigators to request missing data, such as sample sizes for analysis and actual numbers for outcomes presented in figures. However, we limited our requests to studies less than 10 years old, unless a report was produced within the past five years. Investigators are unlikely to have access to data from 10 years ago.
Data synthesis
To assess evidence quality and to address confidence in the effect estimates, we applied principles from GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (Balshem 2011; Higgins 2011). When meta‐analysis is not viable because of varied interventions, a Summary of findings table is not feasible. In this review, experimental and comparison interventions differed across the included trials. Outcomes assessed also varied, e.g. breast milk composition, reports of breastfeeding, or change in infant weight. Because of the heterogeneity of interventions and outcomes, we did not conduct a formal GRADE assessment with an evidence profile and Summary of findings table (Guyatt 2011).
Our quality assessment was based on the quality of evidence from the individual studies, which could be rated as high, moderate, low, or very low. We considered the evidence from RCTs to be of high quality initially, then downgraded it for each of the following: (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) follow‐up less than 8 weeks for infant growth or less than 12 weeks for breastfeeding; (4) losses greater than 20%; and (5) information missing on both blinding and losses.
Sensitivity analysis
We examined a subgroup of trials that provided evidence of moderate or high quality and reported sufficient outcome data. Most of the older trials did not quantify results, limiting the interpretation of effect. Even recent trials might not have reported sufficient detail for interpreting the outcome data presented.
Results
Description of studies
Results of the search
The initial search in 2003 identified 50 articles as potentially eligible for inclusion. Seven were included and 43 were excluded, as indicated below. Searches in 2005, 2008, and 2010 did not yield any eligible studies.
In 2014, we identified a study that was eligible but was not include earlier. Therefore, we ran searches starting from September 2001. This revised search, completed in 2015, produced 215 unduplicated references (Figure 1). Duplicates removed totaled 109 (93 identified electronically and 16 by hand). With four citations identified from other sources, we had 219 unduplicated references. We reviewed the full text of 14 items (9 primary and 5 secondary). From recent clinical trial listings, we obtained 15 unduplicated trials. We located a conference abstract for a completed trial, which we included, and we listed another trial in Ongoing studies. In this review, we included seven new primary reports plus two secondary articles. We excluded two new primary reports plus one secondary article. We also discarded two more secondary articles related to an included trial; the abstracts indicated they did not meet our criteria.
1.
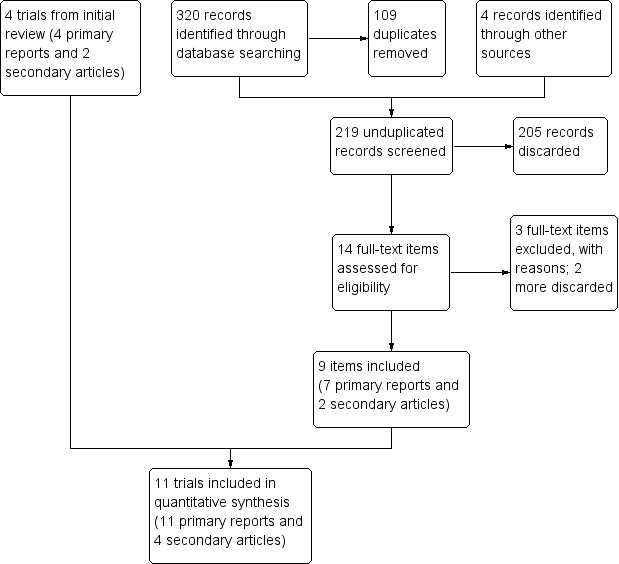
Study flow diagram (2014).
Included studies
A total of 11 trials met our inclusion criteria. The seven trials added in 2014 included three recent reports (Gurtcheff 2011; Espey 2012; Dutta 2013), a recent abstract (Stuart 2014), and three older reports missed during earlier searches (Heikkilä 1982; Shaamash 2005; Brito 2009).
The 11 trials included a total of 1482 women. Sample sizes ranged from 20 to 320 with a median of 80. Only four provided information on sample size calculations. Outcomes of focus were breastfeeding (Shaamash 2005; Gurtcheff 2011; Espey 2012) and infant growth (Shaamash 2005); one was designed to assess change in the woman's weight (Brito 2009).
Contraceptive methods examined
Seven trials studied just progestin‐only methods.
Two compared progestin‐only pills (POPs) versus placebo (Giner Velazquez 1976; Dutta 2013).
Three studied a progestin‐only intrauterine systems (IUS); two compared a hormonal IUS versus a nonhormonal IUD (Heikkilä 1982; Shaamash 2005), and one examined different insertion times for the same hormonal IUS (Stuart 2014).
Two focused on a progestin‐only implant; one compared the implant versus no contraceptive for the first six weeks followed by a progestin‐only injectable (Brito 2009), and the other examined different insertion times for the same implant (Gurtcheff 2011).
Four trials examined COCs.
Two studied a COC versus placebo (Semm 1966; Miller 1970).
Two compared a COC versus a POP (WHO 1984; Espey 2012).
Outcomes assessed
For breast milk, two studies examined quantity (Semm 1966; Miller 1970) and four reported on quantity and composition (Giner Velazquez 1976; WHO 1984; Gurtcheff 2011; Dutta 2013).
Seven trials assessed any or full (exclusive) breastfeeding (Miller 1970; Heikkilä 1982; Shaamash 2005; Brito 2009; Gurtcheff 2011; Espey 2012; Stuart 2014).
Seven studies reported infant growth (Giner Velazquez 1976; Heikkilä 1982; WHO 1984; Shaamash 2005; Brito 2009; Espey 2012; Dutta 2013).
Excluded studies
The initial review excluded 43 reports. Currently, we list articles as 'excluded' if the full text was needed to determine eligibility; otherwise, we 'discard' the abstract. Of the original 43 studies, 14 did not require full‐text review. We removed those 14 studies from 'excluded' to shorten the list, and listed them for reference in this update (Appendix 3). Most of the remaining articles had been excluded because communication with investigators indicated the trials were not RCTs, or because the method of participant allocation was unclear. In addition, Drury 1986 and Gellen 1984 were subgroup analyses that examined similar outcomes from WHO 1984 and were dropped from consideration.
In 2014, we divided the outcomes into primary and secondary and required one of the primary outcome measures. An earlier included trial was no longer eligible (Were 1997). The study had no outcomes addressing lactation; no pregnancy occurred in either group. We excluded three additional trials (Rodrigues da Cunha 2001; Chen 2011; Shaaban 2013) for design issues or for lack of our primary outcomes (Characteristics of excluded studies).
Risk of bias in included studies
The included trials span a publication period of nearly 50 years; earlier reports typically have more reporting limitations than later ones. The quality of evidence is discussed below and is summarized later (Table 9). In addition, Figure 2 presents the risk of bias for each study, and Figure 3 summarizes the risk of bias for all included trials.
1. Evidence quality.
| Study | Comparison groups | Inadequate randomization and allocation concealment | No blinding | Follow‐up: < 8 weeks for infant growth; < 12 weeks for lactation | Loss to follow‐up > 20% | Quality of evidencea |
| Progestin‐only pills (POPs) versus placebo | ||||||
| Giner Velazquez 1976 |
|
‐1 | ‐‐‐ | ‐1 | No information | Low |
| Dutta 2013 |
|
‐1 | No information | ‐‐‐ | No information | Low |
| Levonorgestrel‐releasing intrauterine system (LNG‐IUS) | ||||||
| Heikkilä 1982 |
|
‐1 | ‐‐‐ | ‐‐‐ | ‐‐‐ | Moderate |
| Shaamash 2005 |
|
‐‐‐ | No information | ‐‐‐ | No information | Moderate |
| Stuart 2014 | LNG‐IUS (20 μg), insertion times
|
‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | Moderate |
| Etonogestrel‐releasing (ETG) implant, early versus standard insertion | ||||||
| Brito 2009 |
|
‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | Moderate |
| Gurtcheff 2011 | ETG implant, postpartum insertion time
|
‐‐‐ | ‐1 | ‐‐‐ | ‐‐‐ | Moderate |
| Combined oral contraceptive (COC) versus placebo | ||||||
| Semm 1966 |
|
‐1 | No information | ‐1 | No information | Very low |
| Miller 1970 |
|
‐1 | ‐‐‐ | ‐‐‐ | ‐‐‐‐ | Moderate |
| Combined oral contraceptive versus progestin‐only pill | ||||||
| WHO 1984 |
|
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | Moderate |
| Espey 2012 |
|
‐‐‐ | ‐‐‐ | ‐‐‐ | ‐1 | Moderate |
| Overall quality of evidence | Moderate | |||||
aGrade levels were high, moderate, low, or very low. RCTs were downgraded by one level for each of the following: (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) follow‐up < 8 weeks for infant growth or < 12 weeks for lactation; (4) loss to follow‐up > 20%; (5) information missing for both blinding and losses.
2.
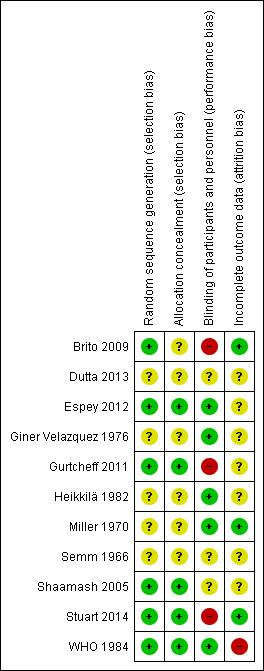
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
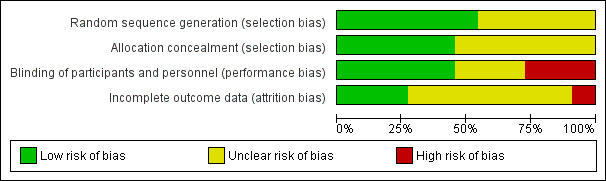
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Four of the recent trials mentioned some type of computer‐generated sequence (Shaamash 2005; Brito 2009; Gurtcheff 2011; Espey 2012), as did one earlier report (WHO 1984). Four of the five early trials and a recent preliminary report did not specify the method used to generate the random sequence (Semm 1966; Miller 1970; Giner Velazquez 1976; Heikkilä 1982; Dutta 2013). Additionally, Miller 1970 stratified the 'randomization' by gender of the infant and showed a disparity in baseline characteristics (primiparas) unlikely to result from a random process. Heikkilä 1982 also appeared to have an imbalance by parity, but the difference reportedly was not significant.
Allocation
Reporting of allocation concealment varied. One trial used pharmacy‐blinded pill packages (Espey 2012). Two used sealed, sequentially numbered, opaque envelopes (Shaamash 2005; Gurtcheff 2011). We determined through communication with an investigator that another also used sealed, opaque envelopes (WHO 1984). The remaining trials did not report the method of allocation concealment.
Blinding
Four trials of oral contraceptives (OCs) had placebos; two used identically labeled placebos (Semm 1966; Miller 1970). Miller 1970 and Giner Velazquez 1976 mentioned double‐blinding but did not describe the specifics. Written communication from Miller 1970 indicated that participants and clinicians were kept unaware of participant treatment assignments. Dutta 2013 did not mention blinding.
Two trials compared combined and progestin‐only oral contraceptives. Espey 2012 used pharmacy‐blinded pill packages. Written correspondence from an investigator in WHO 1984 (Tankeyoon) indicated that participants and clinicians were kept unaware of treatment assignments.
The two implant studies were not blinded. Brito 2009 was identified as an open trial of different contraceptive methods. Gurtcheff 2011 noted that blinding was not feasible because implants were inserted at different time points.
Of the three LNG‐IUS studies, Heikkilä 1982 noted that participants were unaware of which IUD was inserted, Stuart 2014 was listed as open label, and Shaamash 2005 did not mention blinding.
Incomplete outcome data
Four trials did not mention any losses to follow‐up, exclusions, or discontinuations (Semm 1966; Giner Velazquez 1976; Shaamash 2005; Dutta 2013). Heikkilä 1982 had no loss to follow‐up. Three trials had losses less than 20% (Miller 1970; Brito 2009; Gurtcheff 2011).
Losses were greater than 20% in two trials (WHO 1984; Espey 2012). In WHO 1984, the disposition of participants in the randomized arms was unclear. One table indicated that 50 participants in each arm completed the study, yet tables with outcome data showed data for 57 and 58 participants in the study arms at 24 weeks (trial completion) (WHO 1984). Losses at 16 weeks were 22% for the COC group and 14% for the POP group. At 24 weeks, from 32% to 34% of participants in each randomized arm were not included in the analysis. The investigators stated that participants who discontinued or were lost to follow‐up were analyzed via noncompeting risk life‐table procedures, and at least one participant was excluded after randomization.
Effects of interventions
Progestin‐only pills (POPs) versus placebo
These two studies did not provide sufficient data for analysis. The older trial did not provide actual values for the outcomes and the newer trial report did not have sample sizes for analysis or information on losses. Both studies noted no significant differences between the study groups in breast milk volume and composition and in infant growth.
Giner Velazquez 1976 (n = 20) compared a POP containing norethindrone 350 μg versus a placebo for 14 days, starting 48 hours postpartum. Results were presented in figures without actual numbers. The investigators reported no significant differences between the study groups in milk volume and composition, nor in infant growth.
In Dutta 2013 (n = 400), a POP containing desogestrel 75 μg was compared with placebo, starting six weeks postpartum. This 'preliminary report' stated the intervention was provided for six months. Results were presented in tables without time frames for assessments or specific sample sizes for analysis. The groups were not significantly different for amount or composition of breast milk nor for percent of infants with 'normal growth' (weight, length, and head circumference) (reported P > 0.15). Pregnancy was 0.5% in the POP group and 4% for the placebo group (reported P = 0.018).
Progestin‐only IUS versus nonhormonal IUD or different insertion times
Two studies compared a levonorgestrel‐releasing intrauterine system (LNG‐IUS) versus a nonhormonal intrauterine device (IUD). Neither provided sample sizes for analysis; results are presented as reported by the investigators.
-
Heikkilä 1982 (n = 80) compared an LNG‐IUS (30 μg) versus the Nova T IUD. Insertion was done at six weeks postpartum with a range from 29 to 56 days. Sample sizes were lacking in most tables.
The source of breastfeeding data was unclear (record or recall); one analysis date (75 days after insertion) did not correspond to clinic visit times (3, 6, and 12 months after insertion). The investigators did not mention whether women were asked to record days of breastfeeding as they did for bleeding and spotting. The percentage of women breastfeeding at 75 days was lower in the LNG‐IUS group than in the Nova T group (Analysis 1.1). However, the groups did not differ significantly in mean days of breastfeeding during the study (Analysis 1.2).
Women were asked to have their infants weighed and measured monthly and to record those numbers on a special card; results were shown in figures without actual numbers. Reportedly, infant growth did not differ between the two study groups.
A secondary article noted that no pregnancies occurred during the study.
Shaamash 2005 (n = 320) compared the LNG‐IUS (20 μg) versus the CuT 308A IUD. The article did not provide sample sizes for analysis or information on losses. Means and standard deviations (SDs) or standard errors (SEs) are presented as available for the 6‐ and 12‐month assessments. The groups did not differ significantly for mean infant weight or length or in the Kaplan‐Meier rates for full breastfeeding or continued use of the IUS or IUD (Analysis 2.1 to Analysis 2.4). Sample size for the study was based on detecting differences in breastfeeding rate and infant weight gain. No pregnancy occurred in either group.
In Stuart 2014 (n = 35), the LNG‐IUS was inserted either within 48 hours of delivery or four to eight weeks after delivery. The trial was stopped early because expulsion rates met a priori stopping rules. Only 35 women were randomized; 190 had been planned. At the six‐month study visit, the groups were not significantly different for any breastfeeding, but the sample size was much smaller than was intended (Analysis 3.1).
1.1. Analysis.
Comparison 1 LNG‐IUS (30 μg) versus nonhormonal IUD (Nova T), Outcome 1 Breastfeeding continuation at 75 days.
| Breastfeeding continuation at 75 days | |||
|---|---|---|---|
| Study | LNG‐IUS | Nova T | Reported P |
| Heikkilä 1982 | 56% | 79% | < .05 |
1.2. Analysis.
Comparison 1 LNG‐IUS (30 μg) versus nonhormonal IUD (Nova T), Outcome 2 Mean days of breastfeeding (over 12 months).
| Mean days of breastfeeding (over 12 months) | |||
|---|---|---|---|
| Study | LNG‐IUS (mean + SD) | Nova T (mean + SD) | Reported P |
| Heikkilä 1982 | 197 + 141 | 208 + 104 | not significant (NS) |
2.1. Analysis.
Comparison 2 LNG‐IUS (20 μg) versus nonhormonal IUD (CuT 380A), Outcome 1 Reported mean infant weight (g).
| Reported mean infant weight (g) | ||||
|---|---|---|---|---|
| Study | Time frame | LNG‐IUS (mean + SD) | CuT 380A (mean + SD) | Reported P |
| Shaamash 2005 | 6 months | 7225 + 810 | 7157 + 759 | NS |
| Shaamash 2005 | 12 months | 9284 + 423 | 9183 + 421 | NS |
2.4. Analysis.
Comparison 2 LNG‐IUS (20 μg) versus nonhormonal IUD (CuT 380A), Outcome 4 Reported net cumulative rate for continuation of IUS or IUD.
| Reported net cumulative rate for continuation of IUS or IUD | |||
|---|---|---|---|
| Study | Time frame | LNG‐IUS (mean + SE) | CuT 380A (mean + SE) |
| Shaamash 2005 | 6 months | 90.6 + 2.3 | 93.5 + 2.0 |
| Shaamash 2005 | 12 months | 89.3 + 2.5 | 90.9 + 2.3 |
3.1. Analysis.
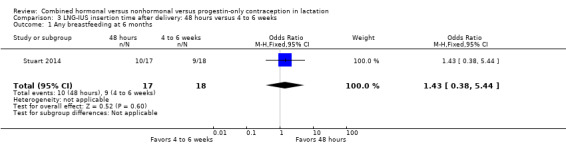
Comparison 3 LNG‐IUS insertion time after delivery: 48 hours versus 4 to 6 weeks, Outcome 1 Any breastfeeding at 6 months.
Progestin‐only implant versus no method or delayed method
Two studies examined the etonogestrel‐releasing (ETG) implant. Brito 2009 compared the implant versus no contraceptive for the first six weeks, then DMPA initiated at postpartum week six. In Gurtcheff 2011, both groups received the ETG implant, either by early insertion (one to three days postpartum) or standard insertion (four to eight weeks postpartum).
In Brito 2009 (n = 40), the sample size calculation was based on the women's weight change and a metabolic measure, which are not outcomes for our review. Women with a body mass index ≥ 30 kg/m2 were excluded from the trial, potentially affecting generalizability. The study groups did not differ significantly in proportions of women fully breastfeeding at 6 or 12 weeks postpartum (Analysis 4.1). None of the participants completely ceased breastfeeding during the study. Those who were not fully breastfeeding had started supplemental feeding. For infant weight, mean change (grams) by six weeks was greater in the ETG implant group than in the no‐contraceptive group (MD 426.00, 95% CI 58.94 to 793.06; Analysis 4.2). The wide CI indicates imprecision in the estimate; the standard deviations were large. Mean change between 6 and 12 weeks was lower for the ETG implant group than for the group that received DMPA (MD ‐271.00, 95% CI ‐355.10 to ‐186.90; Analysis 4.2). The report did not provide the actual infant weights.
For Gurtcheff 2011 (n = 69), the main outcomes were lactation failure and time to lactogenesis stage II (copious milk secretion). Sample size was based on establishing non‐inferiority in those outcomes. Non‐inferiority margins were a 15% increase in lactation failure and an additional eight hours to lactogenesis II in the early insertion group, as assessed through the 95% CI. Study groups were not significantly different for lactation failure (Analysis 6.1), mean time to lactogenesis (Analysis 6.2), or mean creamatocrit of breast milk at six weeks (Analysis 6.3). See Characteristics of included studies for explanations of these outcomes. However, our results differed from the non‐inferiority results for time to lactogenesis; our 95% CI exceeded the prespecified margin of an additional eight hours (MD ‐0.90, 95% CI ‐9.90 to 8.10). For breastfeeding (full or any), the investigator provided the actual numbers for analysis. Early and standard insertion groups did not differ significantly for full breastfeeding (Analysis 6.4) or for any breastfeeding (Analysis 6.5) at any time point. About half of study participants were fully breastfeeding at two weeks.
4.1. Analysis.
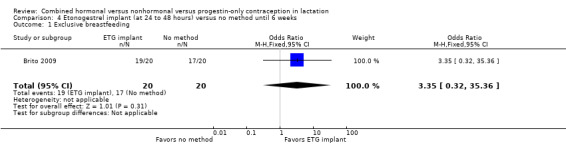
Comparison 4 Etonogestrel implant (at 24 to 48 hours) versus no method until 6 weeks, Outcome 1 Exclusive breastfeeding.
4.2. Analysis.
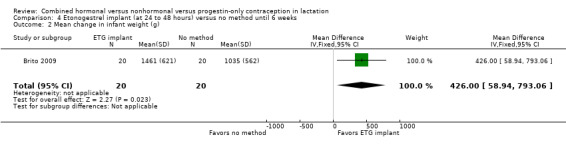
Comparison 4 Etonogestrel implant (at 24 to 48 hours) versus no method until 6 weeks, Outcome 2 Mean change in infant weight (g).
6.1. Analysis.
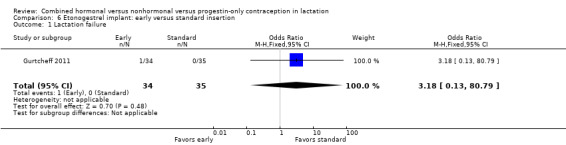
Comparison 6 Etonogestrel implant: early versus standard insertion, Outcome 1 Lactation failure.
6.2. Analysis.
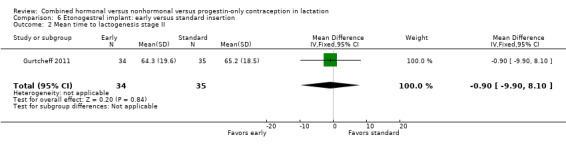
Comparison 6 Etonogestrel implant: early versus standard insertion, Outcome 2 Mean time to lactogenesis stage II.
6.3. Analysis.
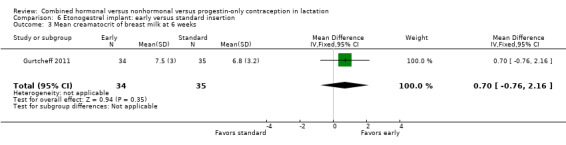
Comparison 6 Etonogestrel implant: early versus standard insertion, Outcome 3 Mean creamatocrit of breast milk at 6 weeks.
6.4. Analysis.
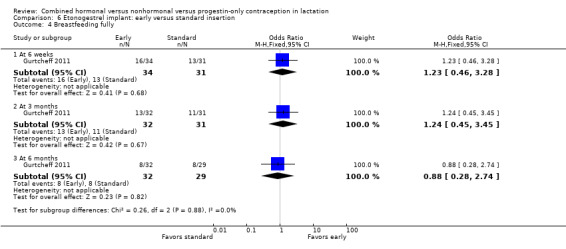
Comparison 6 Etonogestrel implant: early versus standard insertion, Outcome 4 Breastfeeding fully.
6.5. Analysis.
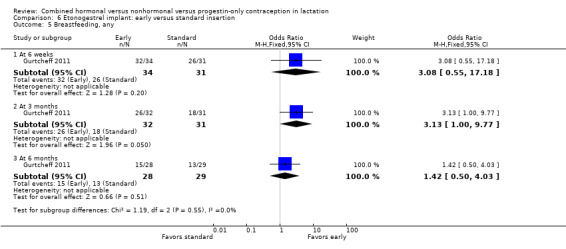
Comparison 6 Etonogestrel implant: early versus standard insertion, Outcome 5 Breastfeeding, any.
Combined oral contraceptive versus placebo
Findings from Miller 1970 and Semm 1966 were conflicting. Results were presented in figures without actual numbers. Neither trial quantified the outcomes, making interpretation difficult.
Miller 1970 (n = 50) examined a COC containing norethindrone 1 mg plus mestranol 80 μg versus placebo. The investigators noted inhibitory effects on milk volume and lactation duration from COC use. They indirectly measured milk volume by assessing the subjective need for supplemental infant feeds and infant weight as a proxy for milk adequacy. Only general estimates were given for the effects of the COC on lactation duration.
Semm 1966 (n = 100) compared a COC containing lynestrenol 2.5 mg plus mestranol 75 μg versus placebo. The investigators found no differences in milk volume or lactation initiation during the first 10 days postpartum. Hormone doses were larger than those in currently marketed COCs; therefore generalizability is limited.
Combined versus progestin‐only oral contraceptives
The two studies in this group included an older multisite trial of OCs containing different progestins (WHO 1984) and a recent trial that compared OCs containing the same progestin (Espey 2012). The report of WHO 1984 provided data on milk volume, but did not have sufficient data to analyze infant growth in this review. Also, data on milk composition were presented by center, not for the full trial. Espey 2012 examined infant growth and breastfeeding continuation.
WHO 1984 (n = 171) compared a COC containing levonorgestrel 150 μg plus ethinyl estradiol 30 μg versus a POP containing norgestrel 75 μg. Breast milk volume was determined by pump expression using standardized procedures. The participants breastfed their infants in the morning, waited two hours, and pumped milk while simultaneously nursing from the other breast for 20 minutes. The process was repeated two hours later, using the opposite breast for pumping. The 'average' amount was then presented. Mean milk volume (mL) was lower for the COC group than for the POP group at 9 weeks (MD ‐17.80, 95% CI ‐28.80 to ‐6.80), 16 weeks (MD ‐24.00, 95% CI ‐34.53 to ‐13.47), and 24 weeks (MD ‐24.90, 95% CI ‐36.01 to ‐13.79) (Analysis 7.1). Declines began after study initiation at six weeks postpartum and continued throughout the trial. From week 6 through week 24, average milk volume for COC users declined by 42% versus 12% among POP users. The randomized groups were not significantly different in infant weight change; sample sizes were not available for analysis (Analysis 7.2). Biochemical composition of breast milk was presented by center, not for the total sample. Differences within centers were small and inconsistent. Increases in milk lipid among combined contraceptive users in one center are of unknown clinical significance.
Espey 2012 (n = 197) compared a COC containing norethindrone 1 mg plus ethinyl estradiol 35 μg versus a POP containing norethindrone 350 μg. The trial was powered to assess differences in continuation of breastfeeding. The investigator provided means and standard deviations for actual change and percentage change in infant weight and length. The study groups did not differ significantly for mean change in infant weight (Analysis 8.1) or in infant length (Analysis 8.2) from week two to week eight. The groups did not differ significantly at two or eight weeks for supplementing with formula. Results for the Cox proportional hazard regression indicate that the intervention group was not statistically associated with breastfeeding discontinuation (Analysis 8.3). The regression included the covariates of OC history and breastfeeding history due to baseline differences. However, formula supplementation and concerns about milk supply were associated with breastfeeding discontinuation (reported P = 0.033 and 0.005, respectively). No pregnancies were reported by eight weeks.
7.1. Analysis.
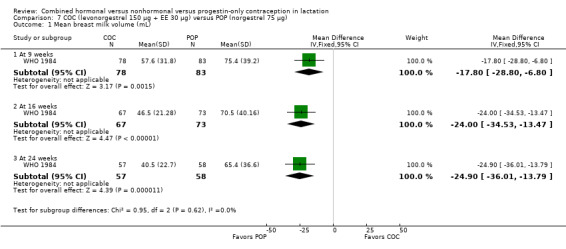
Comparison 7 COC (levonorgestrel 150 μg + EE 30 μg) versus POP (norgestrel 75 μg), Outcome 1 Mean breast milk volume (mL).
7.2. Analysis.
Comparison 7 COC (levonorgestrel 150 μg + EE 30 μg) versus POP (norgestrel 75 μg), Outcome 2 Mean change in infant weight (g).
| Mean change in infant weight (g) | |||
|---|---|---|---|
| Study | Visit interval (postpartum) | COC | POP |
| WHO 1984 | Mean + standard error (SE) | Mean + SE | |
| WHO 1984 | 6 to 9 weeks | 561.9 + 24.4 | 623.5 + 23.5 |
| WHO 1984 | 9 to 12 weeks | 523.0 + 24.3 | 531.9 + 23.2 |
| WHO 1984 | 20 to 24 weeks | 354.6 + 19.9 | 395.7 + 19.8 |
8.1. Analysis.
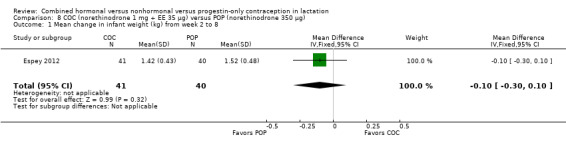
Comparison 8 COC (norethinodrone 1 mg + EE 35 μg) versus POP (norethinodrone 350 μg), Outcome 1 Mean change in infant weight (kg) from week 2 to 8.
8.2. Analysis.
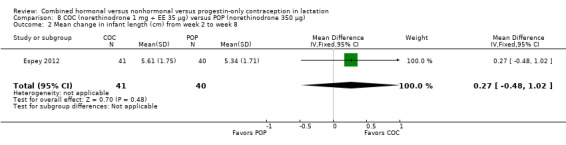
Comparison 8 COC (norethinodrone 1 mg + EE 35 μg) versus POP (norethinodrone 350 μg), Outcome 2 Mean change in infant length (cm) from week 2 to week 8.
8.3. Analysis.
Comparison 8 COC (norethinodrone 1 mg + EE 35 μg) versus POP (norethinodrone 350 μg), Outcome 3 Breastfeeding discontinuation by 6 months.
| Breastfeeding discontinuation by 6 months | ||||
|---|---|---|---|---|
| Study | Variable | Reported hazard ratio | Reported 95% CI | Reported P value |
| Espey 2012 | Group: COC versus POP | 1.42 | 0.76 to 2.65 | .270 |
| Espey 2012 | Supplementing: yes versus no | 2.81 | 1.09 to 7.23 | .033 |
| Espey 2012 | Milk concerns: yes versus no | 2.07 | 1.37 to 5.91 | .005 |
Discussion
Summary of main results
Most trials did not show or report significant differences between study arms in breastfeeding duration, breast milk composition, or infant growth (Table 10). The few exceptions were seen mainly in older studies with limited reporting.
2. Outcome summary.
| Study | Comparison groupsa | N | Postpartum initiation | Lactationb | Breast milkc | Infant growthd | Pregnancy |
| Progestin‐only pill (POP) versus placebo | |||||||
| Giner Velazquez 1976 |
|
20 | 48 hours | ‐‐‐ | NSe; not quantified | NSe; not quantified | ‐‐‐ |
| Dutta 2013 |
|
400 | 6 weeks | ‐‐‐ | NSe | NSe | POP < placeboe |
| Levonorgestrel‐releasing intrauterine system (LNG‐IUS) | |||||||
| Heikkilä 1982 |
|
80 | 6 weeks | BFe: LNG‐IUS < Nova T IUD | ‐‐‐ | NSe; not quantified | None |
| Shaamash 2005 |
|
320 | 6 to 8 weeks | NSe | ‐‐‐ | NSe | None |
| Stuart 2014 | LNG‐IUS (20 μg), insertion times
|
35 | 48 hours vs 4 to 8 weeks | NS | ‐‐‐ | ‐‐‐ | ‐‐‐ |
| Etonogestrel‐releasing (ETG) implant: early versus standard insertion | |||||||
| Brito 2009 |
|
40 | 24 to 48 hours vs 6 weeks | NS | ‐‐‐ | Weight: 6 weeks, implant > no method; 12 weeks, DMPA > implant | ‐‐‐ |
| Gurtcheff 2011 | ETG implant insertion
|
69 | 1 to 3 days vs 4 to 8 weeks | NS | NS | ‐‐‐ | ‐‐‐ |
| Combined oral contraceptive (COC) versus placebo | |||||||
| Semm 1966 |
|
100 | 1 day | NSe; not quantified | NSe; not quantified | ‐‐‐ | ‐‐‐ |
| Miller 1970 |
|
50 | 14 days | BFe: COC < placebo; unclear, not quantified | Volumee: COC < placebo; unclear, not quantified, | ‐‐‐ | ‐‐‐ |
| Combined versus progestin‐only oral contraceptive | |||||||
| WHO 1984 |
|
171 | 6 weeks | ‐‐‐ | Volume: COC < POP |
NSe | ‐‐‐ |
| Espey 2012 |
|
197 | 2 weeks | NS | ‐‐‐ | NS | None |
aTable shows direction of significant differences (reported or analyzed); NS = not significantly different. bInitiation, failure, breastfeeding (BF) fully or any. cTime to lactogenesis II, volume, composition. dWeight or length. eReported; insufficient data for analysis in this review.
Breastfeeding continuation was studied in eight trials. One older study noted a negative effect of a combined oral contraceptive (COC) on lactation duration but did not quantify results. An early trial of a levonorgestrel‐releasing intrauterine system (LNG‐IUS) showed a lower percentage of the LNG‐IUS group breastfeeding at 75 days but no significant difference at one year. The other five trials indicated no significant differences between the study arms. Of those five, two studied insertion times for the etonogestrel‐releasing (ETG) implant, one compared a COC versus a progestin‐only pill (POP), and two examined the LNG‐IUS. Of the latter, one compared the LNG‐IUS versus a nonhormonal intrauterine device (IUD); the other compared insertion times and was stopped early because of expulsion rates.
Milk volume or composition was examined in six trials. Two older studies indicated conflicting effects of a COC on milk volume, but neither quantified results. Another older trial showed lower volume for the COC versus POP group. Two placebo‐controlled trials reported no significant effect of a POP on breast milk volume or composition, although one did not quantify results, and the other did not provide sample sizes for analysis. An ETG implant study showed no significant effect of early versus standard insertion.
Infant growth was assessed in seven trials. One showed greater infant weight gain for the ETG implant group than for the no‐method group during the first six weeks. The implant group had less weight gain from 6 to 12 weeks when compared to those given depot medroxyprogesterone acetate (DMPA). The other six studied POPs only, COCs versus POPs, or the LNG‐IUS, and indicated no significant differences between groups. Trial reports did not present the amount of supplemental feeding provided to the infants.
Pregnancy was self‐reported. One trial showed a lower percentage in the POP group. Three had no pregnancy in either group; they examined an LNG‐IUS or a COC and POP.
Sensitivity analysis
This analysis was restricted to six studies that provided sufficient data and moderate‐quality evidence (Table 11). Two examined the LNG‐IUS, with one comparing it with a nonhormonal IUD and one examining insertion times. Two trials studied insertion times for the ETG implant, and two examined a COC versus a POP. Five of the six trials were published since 2005.
3. Sensitivity analysis.
| Studya | Comparison groups | N | Postpartum initiation | Lactationb | Breast milkc | Infant growth |
| Levonorgestrel‐releasing intrauterine system (LNG‐IUS) | ||||||
| Shaamash 2005 |
|
320 | 6 to 8 weeks | NSd: full BF (Analysis 2.3) | ‐‐‐ | NSd: weight (Analysis 2.1) and length Analysis 2.2) |
| Stuart 2014 | LNG‐IUS (20 μg) insertion times
|
35 | 48 hours vs 4 to 8 weeks | NS: any BF (Analysis 3.1) | ‐‐‐ | ‐‐‐ |
| Etonogestrel‐releasing (ETG) implant: early versus standard insertion | ||||||
| Brito 2009 |
|
40 | 24 to 48 hours vs 6 weeks | NS: full BF (Analysis 4.1) | ‐‐‐ | Weight: 6 weeks, ETG implant > no method (Analysis 4.2); 12 weeks, DMPA > ETG implant (Analysis 5.2) |
| Gurtcheff 2011 | ETG implant, postpartum insertion
|
65 | 1 to 3 days vs 4 to 8 weeks | NS: failure (Analysis 6.1); full BF (Analysis 6.4); any BF (Analysis 6.5) | NS: lactogenesis II (Analysis 6.2); creamatocrit (Analysis 6.3) | ‐‐‐ |
| Combined (COC) versus progestin‐only (POP) oral contraceptive | ||||||
| WHO 1984 |
|
171 | 6 weeks | ‐‐‐ | Volume: COC < POP (Analysis 7.1) | NSd: weight (Analysis 8.3) |
| Espey 2012 |
|
179 | 2 weeks | NS: BF (Analysis 8.3) | ‐‐‐ | NS: weight (Analysis 8.1) and length (Analysis 8.2) |
aIncludes studies with moderate‐quality evidence (Table 9) and sufficient outcome data (Table 10). Direction of significant differences shown (reported or analyzed); NS = not significantly different. bInitiation; failure; breastfeeding (BF) full, any, or discontinuation. cTime to lactogenesis II; milk volume or composition. dReported; insufficient data for analysis in this review.
Lactation duration: The five trials that examined breastfeeding duration indicated no significant differences between the study groups.
Breast milk volume or composition: One study showed a negative effect of the COC versus a POP on milk volume (mL expressed) through 24 weeks postpartum (mean difference (MD) ‐24.90, 95% confidence interval (CI) ‐36.01 to ‐13.79), and a recent implant trial showed no significant difference between insertion times.
Infant growth: Three trials indicated no significant differences in weight or length. One trial showed greater weight gain in the ETG implant group compared with the no‐method group at six weeks (MD 426.00, 95% CI 58.94 to 793.06) but less gain compared with the DMPA group at 12 weeks (MD ‐271.00, 95% CI ‐355.10 to ‐186.90).
Overall completeness and applicability of evidence
Most studies assessed breastfeeding duration (full or any) or breast milk volume or composition as well as infant growth. Four examined self‐reported pregnancy. Time frames for contraceptive initiation in the experimental group varied: one to three days after delivery in five trials (implant, LNG‐IUS, and OCs); two weeks postpartum in two trials (OCs); and approximately six weeks postpartum in four trials (OCs or IUS). Follow‐up ranged from 10 days (oldest trial) to one year (more recent studies).
Studies were conducted in Asia, Europe, North Africa, and the Americas. Five trials published before 1985 included a multicenter trial conducted in Hungary and Thailand and four other trials from Finland, Germany, Mexico, and the United States. Six newer trials, published from 2005 to 2014, were conducted in Brazil, Egypt, India, and the United States (three studies).
Inclusion and exclusion criteria varied among trials. Most studies were limited to healthy women with birth at 37 weeks gestation or later. Some trials included any woman intending to initiate breastfeeding, whereas others were limited to women who planned to breastfeed exclusively (Brito 2009) or for a minimum duration (Miller 1970; Stuart 2014), had previously breastfed successfully (WHO 1984), or were exclusively breastfeeding at the time of randomization (Shaamash 2005).
The evidence was limited for any particular hormonal method. Four trials examined COCs: two versus placebo and two versus a POP. Three of the four studies were at least 30 years old and had limited reporting. Older studies used hormonal preparations and doses that may be applicable to contemporary practice. Seven trials focused on progestin‐only methods. Two studied a POP versus placebo; the recent one was a preliminary report. Two trials compared ETG implant insertion times. Three examined an LNG‐IUS (one had an older IUS); two compared the LNG‐IUS versus a nonhormonal IUD, and one compared insertion times. We did not find any RCTs of a vaginal ring or a transdermal patch among lactating women. A trial of emergency contraception as a backup for the lactational amenorrhea method did not include data on our primary outcomes (Shaaban 2013).
This review examined infant growth rather than development or other health outcomes. Two included trials assessed infant health issues at 12 months. In Heikkilä 1982, the LNG‐IUS and Nova T groups did not differ significantly for incidence of otitis media and respiratory infection as reported by the mothers. Shaamash 2005 assessed 19 infant development items, grouped as gross motor, vision and fine motor, hearing and language, self‐help skills, and social skills (WHO 1986). No significant difference was noted between the LNG‐IUS and CuT 380A groups in time to pass any test. An earlier non‐randomized study of progestin‐only methods used the same 19 items (WHO 1994). The investigators found a few differences that were mainly site‐specific but no consistent trends. The progestin‐only methods apparently had no negative effect on infant development. A systematic review of mainly observational studies came to the same conclusion (Kapp 2010a). In a review of combined oral contraceptives, the limited evidence indicated no adverse health outcomes in infants (Kapp 2010b).
Quality of the evidence
The quality of reporting varied over time. The earlier publications often provided little detail on methodology and provided insufficient data on results. Standards for publishing trials were developed in the late 1990s, and the CONSORT statement was widely adopted in 2010 (Schulz 2010). We summarized the quality of evidence from the individual trials (Table 9). Overall, the quality was considered moderate. The lower‐quality evidence, often due to limited reporting, came from three of the four placebo‐controlled trials of oral contraceptives.
Half of the trials provided inadequate information on randomization and allocation concealment. Two trials had high losses to follow‐up (WHO 1984; Espey 2012). Loss to follow‐up greater than 20% threatens trial validity (Strauss 2005). Two trials with moderate‐quality evidence provided insufficient or inconsistent data (Miller 1970; Heikkilä 1982). One trial was terminated early because expulsion rates met a priori stopping rules (Stuart 2014). The resulting sample size was much smaller than was planned, so the trial was underpowered to detect differences between groups in breastfeeding outcomes.
Agreements and disagreements with other studies or reviews
Two systematic reviews of hormonal contraceptives and lactation included studies of various designs (Kapp 2010a; Kapp 2010b). Progestin‐only methods did not appear to negatively affect lactation (Kapp 2010a). The evidence was limited and inconsistent for COCs and breastfeeding (Kapp 2010b). For infant growth and health, the evidence for progestin‐only methods was consistent though limited but did not appear to show an adverse effect. The evidence for COCs was considered inadequate at the time for assessing the effect on infant health. Another systematic review found limited evidence regarding medroxyprogesterone use and breastfeeding at less than six weeks postpartum (Brownell 2012). Only three studies of limited quality were eligible and none adjusted for potential confounding. A non‐randomized study of hormonal contraceptives and lactation examined a COC, the LNG‐IUS, the etonogestrel implant, and a nonhormonal IUD (CuT 380A) initiated at 42 days postpartum (Bahamondes 2013). Participants had previously breastfed and were willing to breastfeed exclusively and on demand for the study duration. The investigators reported no significant differences between the study groups from 42 days to 63 days postpartum in infant milk intake, infant weight or height, or breastfeeding (also assessed at six months).
We did not review the evidence for venous thromboembolism (VTE) risk, which was a factor in developing medical eligibility criteria for hormonal contraceptive use by postpartum women (WHO 2009; CDC 2011). Physiological risk of VTE is high in the postpartum period, especially for the first three weeks, and declines by six weeks' postpartum (WHO 2010). However, direct evidence is lacking for risk of VTE with combined hormonal contraception.
Authors' conclusions
Implications for practice.
We found limited information on any particular hormonal method and lactation. The six trials of higher quality examined the levonorgestrel‐releasing IUS, the etonogestrel‐releasing implant, and two COCs versus progestin‐only pills. Of those six trials, an older one showed a negative effect of the COC on breast milk volume. A later study within those six indicated greater infant weight gain with the implant versus no contraceptive but less gain compared with the injectable DMPA. Volume of supplementation was not assessed.
As noted earlier, because of concerns about the effect on lactation, combined hormonal contraceptives are considered category 4 for breastfeeding women up to six weeks postpartum, and category 3 for six weeks to six months (WHO 2009; CDC 2011). Category 3 means that the risks outweigh the advantages. Progestin‐only methods are considered category 3 for less than six weeks' postpartum in global guidelines (WHO 2009), and category 2 for the first month postpartum in the USA (CDC 2010). The latter means that the advantages generally outweigh the risks.
Implications for research.
The body of evidence regarding the effects of hormonal contraceptives on lactation has grown considerably. Six RCTs were published after a 20‐year gap. The 11 trials in this review examined a range of contraceptive methods; consequently, evidence was limited for any specific method. Most early studies focused on oral contraceptives, often compared with placebo. Most of the later trials examined the levonorgestrel‐releasing intrauterine system or the etonogestrel‐releasing implant, with several comparing insertion times. The overall quality of evidence was moderate. Five of the six newer trials provided moderate‐quality data, as did one older multisite trial.
Further research on progestin‐only methods and lactation would be beneficial, especially because some are long‐acting methods. The field could benefit from additional research into the effects of initiation time on lactation and infant health. To more accurately assess those outcomes, future research should assess the mother's breastfeeding intention, duration of full and any breastfeeding, and amount of supplemental feeding.
Feedback
Combined hormonal versus nonhormonal versus progestin‐only contraception
Summary
The exclusion of the study by Diaz et al (Contraception 1983; 27: 1‐11) is not justified. In the review it is stated: "Per correspondence with the author, study participants received personal choice of intervention, not random allocation". In the methods division of this study the authors wrote: "Women requesting an oral contraceptive were assigned at random to the contraceptive pill under study or to an oral placebo on a patient‐blind basis. Both pills were offered as a low‐dose O.C. with no demonstrated effects upon lactation". I wrote to dr Diaz and asked her why the Cochrane review could come to a different conclusion. She answered me that the person doing the Cochrane review asked her only in general about all the studies on breastfeeding and contraception. In all the other trials women had the free choice of contraceptive method. Because the reviewer did not ask in detail about every trial she forgot to mention that in one trial the treatment was randomized.
In the discussion of the review is stated: " No trial to date has documented an adverse effect of hormonal contraceptives on infant growth". This statement is wrong because in the trial by Diaz et al (1983) the oral contraceptive group showed a significantly lower average absolute weight at days 61 and 91 postpartum and a significantly lower average daily weight increase during the first month of treatment.
In the discussion of the Cochrane review, the review authors criticized several trials because the putative effects of hormonal contraceptives on lactation could have been masked by the influence of supplemental foods. The review authors apparently did not realize that it is impossible to do a month long trial on breastfeeding and forbid the mothers to give supplements to their babies if they consider that the babies get insufficient food.
In the implications for research, the authors of the review suggest that investigators should do a trial of contraceptives versus placebo: "such a trial might enroll women who are not at risk of pregnancy because of a sterilization procedure (i.e. postpartum sterilization or partner vasectomy)."This proposal would imply that researchers would have to ask mothers who were planning to breastfeed their infants during several months to take a pill that could potentially deteriorate their lactation, while this medication could not offer her or her baby any advantage. Is there any ethical committee that would approve such a proposal?
I certify that I have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of my criticisms.
February, 2004.
Reply
Response to Treffers re: Dr. Treffers suggests that we incorrectly excluded the report by Diaz et al. (Contraception 1983;27:1‐11) and thus reached the wrong conclusion about the effect of combined oral contraceptives on infant growth. We stand by our exclusion for several reasons. First, we contacted Dr. Diaz and were advised by her in writing on April 1, 2002, that randomization had not been used in her study. Second, the report appears to describe a cohort study, with another group (not randomized) having received an intramuscular placebo. Third, the disparity in sample size between the ostensibly randomized groups (oral contraceptive, 103 participants; oral placebo, 79 participants) is unlikely to have resulted from simple randomization. By binomial theorem, the likelihood of getting a difference this large or larger due to chance is 4%. Stated alternatively, one can be 96% sure that randomization did not yield this result. Hence, we conclude that the Diaz 1983 study was not a randomized controlled trial, and our interpretation of the literature stands.
Given the absence of any demonstrable adverse effect of oral contraceptives on infant growth, the age and limited quality of existing studies, and the public health importance of the question, we believe a proper trial is both appropriate and ethical.
Contributors
Treffers, Pieter
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2015 | New citation required but conclusions have not changed | Searches updated |
| 4 August 2014 | Amended | Summaries of evidence quality (Table 9) and outcomes (Table 10) and a sensitivity analysis (Table 11) added |
| 31 July 2014 | New search has been performed | 7 trials (Heikkilä 1982; Shaamash 2005; Brito 2009; Gurtcheff 2011; Espey 2012; Dutta 2013; Stuart 2014) and 1 ongoing trial (Turok 2014) added |
| 14 July 2014 | Amended | Types of outcome measures separated into primary (effect on lactation) and secondary (contraceptive efficacy). Trials must report a primary outcome; Were 1997 determined to be no longer eligible (Excluded studies) |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 17 December 2012 | New search has been performed | Study added to 'awaiting classification' (Espey 2012). |
| 2 November 2010 | New search has been performed | Searches were updated for MEDLINE, CENTRAL, and POPLINE. New searches were added for ClinicalTrials.gov and ICTRP. No new trials met the inclusion criteria. |
| 7 May 2008 | New search has been performed | Searches were updated; no new trials were found. |
| 3 October 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of FHI 360 assisted with the original literature searches.
Appendices
Appendix 1. Search 2015
PubMed (1 September 2001 to 6 January 2015)
((breastfeeding OR lactation) AND (contraceptive devices, female OR contraceptive agents, female)) NOT (cancer OR cows) Article types: Clinical Trial
CENTRAL (2015, Issue 2 (on 2 March 2015))
Title, Abstract, Keywords: lactat* or breastfeed* OR breast‐feed* AND Title, Abstract, Keywords: contracept* Publication Date from 2001 to 2015 Limited to Trials
POPLINE (2001 to 8 July 2014)
Two strategies:
Keyword: Contraception AND Keyword: Lactation Filter by Keyword: Research report Years: 2001 to 2014
Keyword: Contraception AND Keyword: Breastfeeding AND Keyword: Clinical research Years: 2001 to 2014
Web of Science (11 December 2014)
TOPIC: (contracept*) AND TOPIC: (lactat*)
Refined by: WEB OF SCIENCE CATEGORIES: ( OBSTETRICS GYNECOLOGY OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH OR PUBLIC ENVIRONMENTAL OCCUPATIONAL HEALTH SSCI )
AND DOCUMENT TYPES: ( ARTICLE OR MEETING ABSTRACT OR REVIEW )
Timespan: 2001‐2014
LILACS (4 March 2015)
contraceptive agents, female or agentes anticonceptivos femeninos or anticoncepcionais femeninos [Words] and ("LACTATION" ) or "BREASTFEEDING" [Words]
ClinicalTrials.gov (14 January 2015)
Condition: breastfeeding OR lactation Intervention: contraception OR contraceptive Study type: Interventional studies First received: from 01 June 2010
ICTRP (14 January 2015)
Title: breast‐feed* OR breastfeed* OR lactat* Condition: contraception OR contraceptive Recruitment status: All
Date of registration: from 01 June 2010 to 14 January 2015
Appendix 2. Prior searches
2010 update
MEDLINE (via PubMed) (to 29 October 2014)
((breastfeeding OR lactation) AND (contraceptive devices, female OR contraceptive agents, female)) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ("latin square" [tw]) OR placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR follow‐up studies [mh] OR prospective studies [mh] OR cross‐over studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (cancer OR cows)
POPLINE (2006 to 1 November 2010)
(lactating / lactation / breastfed / breastfeed / breastfeeding / breastfeeding / breastfeeders / breastmilk) & contraception & clinical trials
CENTRAL (2008 to 29 October 2010)
lactat* or breastfeed* in Title, Abstract, or Keywords AND contracept* in Title, Abstract, or Keywords
ClinicalTrials.gov (to 1 November 2010)
(lactat* or breastfeed*) AND contracept*
ICTRP (to 1 November 2010)
Condition: lactating OR lactation OR breastfeed OR breastfeeding Intervention: contraception OR contraceptive
Initial review and updates in 2005 and 2008
Strategies for MEDLINE, POPLINE, and CENTRAL were those listed above for 2010. In addition, EMBASE and LILACS were searched as shown below. The 2005 searches were run to 5 April 2005, and the 2008 searches were run to 6 May 2008.
EMBASE
1. contracep? 2. contraception! 3. 1 or 2 4. breast(W)milk OR breastmilk 5. breastfeed? OR breast(W)feed? 6. lactation 7. 4 OR 5 OR 6 8. clinical trial! 9. controlled study! 10. 8 OR 9 11. 3 AND 7 AND 10 12. 11/human
LILACS
lactation, breastfeeding, contraception/contraceptives, clinical trials
Appendix 3. Non‐randomized studies (previously listed as excluded)
Full‐text review was not needed to determine that these studies were not eligible. In 2014, we classified them as 'discarded' to shorten the list of Excluded studies.
1. Bjarnadottir RI, Gottfredsdottir H, Sigurdardottir K, Geirsson RT, Dieben TO. Comparative study of the effects of a progestogen‐only pill containing desogestrel and an intrauterine contraceptive device in lactating women. British Journal of Obstetrics and Gynaecology 2001;108:1174‐80.
2. Chen JH, Wu SC, Shao WQ, Zou MH, Hu J, Cong L, et al. The comparative trial of TCu 380A IUD and progesterone‐releasing vaginal ring used by lactating women. Contraception 1998;57:371‐9.
3. Coutinho EM, Athayde C, Dantas C, Hirsch C, Barbosa I. Use of a single implant of elcometrine (ST‐1435), a nonorally active progestin, as a long acting contraceptive for postpartum nursing women. Contraception 1999;59:115‐22.
4. Danli S, Qingxiang S, Guowei S. A multicentered clinical trial of the long‐acting injectable contraceptive Depo Provera in Chinese women. Contraception 2000;62:15‐8.
5. Dunson TR, McLaurin VL, Grubb GS, Rosman AW. A multicenter clinical trial of a progestin‐only oral contraceptive in lactating women. Contraception 1993;47:23‐35.
6. Hannon PR, Duggan AK, Serwint JR, Vogelhut JW, Witter F, DeAngelis C. The influence of medroxyprogesterone on the duration of breast‐feeding in mothers in an urban community. Archives of Pediatrics & Adolescent Medicine 1997;151:490‐6.
7. Lönnerdal B, Forsum E, Hambraeus L. Effect of oral contraceptives on composition and volume of breast milk. American Journal of Clinical Nutriton 1980;33:816‐24.
8. Massai MR, Diaz S, Quinteros E, Reyes MV, Herreros C, Zepeda A, et al. Contraceptive efficacy and clinical performance of Nestorone implants in postpartum women. Contraception 2001;64:369‐76.
9. McCann MF, Moggia AV, Higgins JE, Potts M, Becker C. The effects of a progestin‐only oral contraceptive (levonorgestrel 0.03 mg) on breast‐feeding. Contraception 1989;40:635‐48.
10. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, Thaithumyanon P, Punnahitananda S, Tosukhowong P, et al. Effects of the etonogestrel‐releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception 2000;62:239‐46.
11. Virutamasen P, Leepipatpaiboon S, Kriengsinyot R, Vichaidith P, Muia PN, Sekadde‐Kigondu CB, et al. Pharmacodynamic effects of depot‐medroxyprogesterone acetate (DMPA) administered to lactating women and their male infants. Contraception 1996;54:153‐7.
12. World Health Organization Task Force for Epidemiological Research on Reproductive Health; Special Programme of Research, Development, and Research Training in Human Reproduction. Progestogen‐only contraceptives during lactation: I. infant growth. Contraception 1994;50:35‐53.
13. Zacharias S, Aguilera E, Assenzo JR, Zanartu J. Effects of hormonal and nonhormonal contraceptives on lactation and incidence of pregnancy. Contraception 1986;33:203‐12.
14. Zanartu J, Aguilera E, Munoz‐Pinto G. Maintenance of lactation by means of continuous low‐dose progestogen given post‐partum as a contraceptive. Contraception 1976;13:313‐8.
Data and analyses
Comparison 1. LNG‐IUS (30 μg) versus nonhormonal IUD (Nova T).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breastfeeding continuation at 75 days | Other data | No numeric data | ||
| 2 Mean days of breastfeeding (over 12 months) | Other data | No numeric data |
Comparison 2. LNG‐IUS (20 μg) versus nonhormonal IUD (CuT 380A).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reported mean infant weight (g) | Other data | No numeric data | ||
| 2 Reported mean infant length (cm) | Other data | No numeric data | ||
| 3 Reported net cumulative rate for full breastfeeding | Other data | No numeric data | ||
| 4 Reported net cumulative rate for continuation of IUS or IUD | Other data | No numeric data |
2.2. Analysis.
Comparison 2 LNG‐IUS (20 μg) versus nonhormonal IUD (CuT 380A), Outcome 2 Reported mean infant length (cm).
| Reported mean infant length (cm) | ||||
|---|---|---|---|---|
| Study | Time frame | LNG‐IUS (mean + SD) | CuT 380A (mean + SD) | Reported P |
| Shaamash 2005 | 6 months | 65.1 + 3.2 | 64.4 + 3.0 | NS |
| Shaamash 2005 | 12 months | 72.6 + 3.2 | 72.3 + 3.9 | NS |
2.3. Analysis.
Comparison 2 LNG‐IUS (20 μg) versus nonhormonal IUD (CuT 380A), Outcome 3 Reported net cumulative rate for full breastfeeding.
| Reported net cumulative rate for full breastfeeding | |||
|---|---|---|---|
| Study | Time frame | LNG‐IUS (mean + SE) | CuT 380A (mean + SE) |
| Shaamash 2005 | 6 months | 16.5 + 3.4 | 19.9 + 3.6 |
| Shaamash 2005 | 12 months | 0.3 + 0.4 | 0.0 + 0.0 |
Comparison 3. LNG‐IUS insertion time after delivery: 48 hours versus 4 to 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any breastfeeding at 6 months | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.38, 5.44] |
Comparison 4. Etonogestrel implant (at 24 to 48 hours) versus no method until 6 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.32, 35.36] |
| 2 Mean change in infant weight (g) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 426.0 [58.94, 793.06] |
Comparison 5. ETG implant (at 24 to 48 hours) versus DMPA from 6 to 12 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exclusive breastfeeding | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.38, 9.27] |
| 2 Mean change in infant weight (g) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐271.0 [‐355.10, ‐186.90] |
5.1. Analysis.
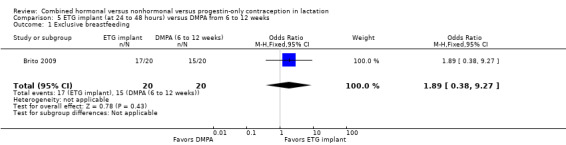
Comparison 5 ETG implant (at 24 to 48 hours) versus DMPA from 6 to 12 weeks, Outcome 1 Exclusive breastfeeding.
5.2. Analysis.
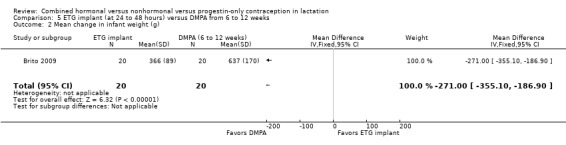
Comparison 5 ETG implant (at 24 to 48 hours) versus DMPA from 6 to 12 weeks, Outcome 2 Mean change in infant weight (g).
Comparison 6. Etonogestrel implant: early versus standard insertion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lactation failure | 1 | 69 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.13, 80.79] |
| 2 Mean time to lactogenesis stage II | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐9.90, 8.10] |
| 3 Mean creamatocrit of breast milk at 6 weeks | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.76, 2.16] |
| 4 Breastfeeding fully | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 weeks | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.46, 3.28] |
| 4.2 At 3 months | 1 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.45, 3.45] |
| 4.3 At 6 months | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.28, 2.74] |
| 5 Breastfeeding, any | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 6 weeks | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.55, 17.18] |
| 5.2 At 3 months | 1 | 63 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [1.00, 9.77] |
| 5.3 At 6 months | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.50, 4.03] |
Comparison 7. COC (levonorgestrel 150 μg + EE 30 μg) versus POP (norgestrel 75 μg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean breast milk volume (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 9 weeks | 1 | 161 | Mean Difference (IV, Fixed, 95% CI) | ‐17.80 [‐28.80, ‐6.80] |
| 1.2 At 16 weeks | 1 | 140 | Mean Difference (IV, Fixed, 95% CI) | ‐24.0 [‐34.53, ‐13.47] |
| 1.3 At 24 weeks | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐24.90 [‐36.01, ‐13.79] |
| 2 Mean change in infant weight (g) | Other data | No numeric data |
Comparison 8. COC (norethinodrone 1 mg + EE 35 μg) versus POP (norethinodrone 350 μg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in infant weight (kg) from week 2 to 8 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.30, 0.10] |
| 2 Mean change in infant length (cm) from week 2 to week 8 | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [‐0.48, 1.02] |
| 3 Breastfeeding discontinuation by 6 months | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brito 2009.
| Methods | Time frame and location: recruitment from September 2007 to July 2008; São Paulo, Brazil Described as a pilot study. Further details on methods were provided in later publication (Brito 2012) Sample size calculation and outcomes of focus: women's body weight and resistance to activated protein C (not outcomes for this review); difference of 1 SD in first 6 weeks; 20 per group due to potential loss during study; power not reported |
|
| Participants | General with N and source: 40 women in prenatal care program Inclusion criteria: 18 and 35 years old, exclusively breastfeeding and desires long‐acting contraception after delivery Exclusion criteria: smoker, alcoholic, or user of recreational drugs; body mass index (BMI) (kg/m2) ≥ 30 or systemic disease (diabetes mellitus, cardiovascular disease, liver disease, thyroid disease, autoimmune disease); history (personal or family) of thromboembolic events; alterations in hepatic enzymes or allergy to local anesthetics (xylocaine) |
|
| Interventions | Treatment: etonogestrel‐releasing implant, early insertion (24 to 48 hours after delivery) Comparison: no contraceptive for 6 weeks after delivery, then depot medroxyprogesterone acetate at week 6 postpartum Timing: as noted above |
|
| Outcomes | Measures: infant weight gain; maintenance of exclusive lactation to week 12 postpartum Assessments: 6 and 12 weeks |
|
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized; 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | According to communication with investigator, randomized groups were stored in sealed envelopes by one investigator and only opened by another investigator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open; ethical reasons |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses or discontinuations, according to study flow chart |
Dutta 2013.
| Methods | Time frame and location: conducted from January 2010 through June 2011 in Kalyani, Nadia, West Bengal (India) Limited details about methods; published as a preliminary report Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N and source: 400 lactating women from clinic Inclusion criteria: normal lipid profile, blood sugar levels, and thyroid stimulating hormone Exclusion criteria: family history or past history of thromboembolism |
|
| Interventions | Treatment: oral contraceptive (OC) containing desogestrel 75 μg for 6 months Comparison: placebo Timing: starting 6 weeks after delivery |
|
| Outcomes | Measures: milk volume and composition, pregnancy, normal infant growth (see below); time frames and samples sizes for analysis not specified (Normal infant growth defined as weight doubled by 4 months and tripled by 1 year, height 50 cm at birth increased to 62 cm by 6 months and 75 cm by 1 year, head circumference increased by 2 cm per month until l year.) Assessments: 8, 12, 16, and 24 weeks; 1 year |
|
| Notes | Investigator provided some design information; we were unable to obtain additional data for analysis and interpretation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Interventions were provided "in a random sequence." Communication with investigator indicated that this was an RCT. |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention |
Espey 2012.
| Methods | Time frame and location: conducted from January 2005 to June 2008 in Albuquerque, New Mexico (USA) Sample size calculation and outcome of focus: breastfeeding continuation at 8 weeks; 120 participants for 80% power to detect continuation difference of 35% in COC group versus 60% in POP group (hazard ratio = 2). Assumed 50% breastfeeding at 8 weeks. With potential 20% loss to follow‐up, 150 needed; increased to 200 because of high loss between enrollment and randomization. |
|
| Participants | General with N and source: 197 postpartum women in hospital after delivery Inclusion criteria: age 15 to 45 years; delivered at University of New Mexico Hospital; intended to breastfeed; planned to use oral contraceptives as family planning method Exclusion criteria: medical contraindication to combined pills, including history of venous thromboembolism, uncontrolled hypertension, or complex migraine headaches; preterm birth (< 37 weeks); newborn small for gestational age (< 2500 g) or large for gestational age (> 4500 g); newborn had major congenital anomaly |
|
| Interventions | Treatment: combined oral contraceptive (COC) containing norethindrone 1 mg plus ethinyl estradiol (EE) 35 μg for 21 days and placebo for 7 days Comparison: OC containing norethindrone 350 μg daily Timing: starting 2 weeks postpartum |
|
| Outcomes | Measures: breastfeeding continuation; infant weight, length, and head circumference (in figures without actual numbers); self‐reported pregnancy Assessments: phone call 3 to 7 weeks postpartum and 4 to 6 months postpartum; hospital visit 2 months postpartum |
|
| Notes | Investigator provided means and SD for change in infant weight and length. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated in randomly‐permuted blocks of 6. |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐blinded packages; pills placed in red capsules; identical monthly pill dispensers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: COC 23% (15/64); POP 22% (14/63) Women who stopped breastfeeding before 8 weeks were discontinued from the study. Infant growth was not obtained at 8 weeks: COC 12.5% (8/64); POP 14% (9/63). |
Giner Velazquez 1976.
| Methods | Time frame and location: no time frame; Mexico Limited details about methods Sample size calculation and outcome of focus: no mention; small sample size limits power |
|
| Participants | General with N and source: 20 women selected immediately after birth Inclusion criteria: age 18 to 36 years; adequate health and nutrition during pregnancy Exclusion criteria: not specified |
|
| Interventions | Treatment: OC containing norethindrone 350 μg Comparison: placebo Timing: starting 48 hours postpartum |
|
| Outcomes | Measures: average milk production; biochemical composition of milk; infant weights; results primarily in figures without actual numbers Assessments: 14 days |
|
| Notes | Information from report translation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Divided randomly (12 treatment and 8 control) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind; no detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of discontinuation, loss to follow‐up or exclusions |
Gurtcheff 2011.
| Methods | Time frame and location: January to October 2009 in Salt Lake City, Utah (USA) Described as non‐inferiority trial Sample size calculation and outcomes of focus:
|
|
| Participants | General with N and source: 69 healthy peripartum women Inclusion criteria: healthy; intended to breastfeed Exclusion criteria: onset of lactogenesis before randomization; hemorrhage requiring transfusion; severe pregnancy‐induced hypertension; prolonged hospitalization; coagulopathy; liver disease; undiagnosed genital bleeding; other contraindication to etonogestrel implant insertion; taking inducers of hepatic enzymes |
|
| Interventions | Etonogestrel implant with different insertion times Treatment: early insertion, 1 to 3 days postpartum Comparison: standard insertion, 4 to 8 weeks postpartum Timing: See above |
|
| Outcomes | Measures: lactation failure (assessed 3 times daily in hospital and at least daily after discharge; diagnosed by 120 hours); time to lactogenesis stage II (copious milk secretion); breastfeeding, full or any (shown in figure without actual numbers); creamatocrit for fat and energy content of milk sample from 6‐week visit Assessments: 2 and 6 weeks, 3 and 6 months postpartum |
|
| Notes | Investigator provided data for breastfeeding continuation (full or any). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in varying blocks of 2, 4, 6 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded; considered not feasible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: early insertion 17% (6/35); standard insertion 18% (6/34) |
Heikkilä 1982.
| Methods | Time frame and location: no time frame; conducted in Helsinki, Finland Some methods from secondary article Sample size calculation and outcome of focus: not specified |
|
| Participants | General with N and source: 80 women, delivered at State Maternity Hospital Inclusion criteria: breastfeeding and amenorrheic Exclusion criteria: not specified |
|
| Interventions | Treatment: levonorgestrel‐releasing IUD (30 μg) Comparison: Nova T intrauterine device (IUD) (copper releasing) Timing: inserted 6 weeks postpartum; range 29 to 56 days in one report, and 32 to 56 days in another |
|
| Outcomes | Measures: breastfeeding duration (no sample sizes for analysis); infant growth measured outside of study and presented in figure without actual numbers Assessment: unclear source and time frame; 75 days after insertion for analysis of breastfeeding data; secondary report notes recording of bleeding and spotting on cards and clinic visits at 3, 6, 12 months |
|
| Notes | Third group was added later (not randomized); received levonorgestrel‐releasing IUD (10 μg) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Divided at random" into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants did not know which IUD they received (30 μg/day LNG‐IUD or Nova T). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: none
Discontinuation (expulsion or removal): 4 LNG‐IUD 30 μg; 4 Nova T. Also, 2 excluded from study due to uterine perforation at insertion (group not specified). Sample sizes for analysis not specified or inconsistent across reports. |
Miller 1970.
| Methods | Time frame and location: September 1967 to June 1968 in Iowa City, Iowa (USA) Limited detail about methods Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N and source: 50 women delivering healthy term infants at city hospital. Inclusion criteria: desire to nurse for 3 months and use oral contraceptives while nursing Exclusion criteria: none specified |
|
| Interventions | Treatment: COC containing norethindrone 1 mg plus mestranol 80 μg daily for 21 days Comparison: identically‐labeled placebos for 21 days Timing: postpartum day 14; all received COC starting 6 weeks postpartum |
|
| Outcomes | Measures: lactation duration, milk volume production. Results presented in figures without actual numbers; graphic variations not identified. Assessments: 3 weeks, 5 weeks, and 3 months postpartum |
|
| Notes | ‐‐‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up: 1 in each arm; 1 discontinuation in placebo arm |
Semm 1966.
| Methods | Time frame and location: no time frame; Munich, Germany Limited detail about methods Sample size calculation and outcome of focus: no mention |
|
| Participants | General with N and source: 100 women delivering with a definite desire to lactate Inclusion criteria: women in "child‐bed"; with definite desire to lactate Exclusion criteria: not specified |
|
| Interventions | Treatment: COC containing lynestrenol 2.5 mg plus mestranol 75 μg daily Comparison: identically labeled placebos Timing: postpartum day 1 |
|
| Outcomes | Measures: lactation initiation and milk volume yield; results in figure without actual numbers Assessments: daily for 10 days; women also weighed babies at home in following 3 weeks, but data not shown |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Distribution of 2 types of tablets was "purely random" |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up: no mention, nor any mention of discontinuation or exclusions |
Shaamash 2005.
| Methods | Time frame and location: conducted from June 2001 to June 2003 in Assiut, Egypt Sample size calculation and outcomes of focus:
|
|
| Participants | General with N and source: 320 exclusively breastfeeding women; university hospital clinic Inclusion criteria: gave birth to healthy term baby with no health problem; had no contraindication to the use of LNG or copper IUD contraceptives; planning to breastfeed for at least 1 year Exclusion criteria: not specified |
|
| Interventions | Treatment: levonorgestrel‐releasing IUS (20 μg) Comparison: Copper T 380A IUD Timing: inserted 6 to 8 weeks postpartum |
|
| Outcomes | Measures: breastfeeding (full, partial); infant growth (weight, length, head circumference, mid‐arm circumference, triceps skinfold); pregnancy (presumably self‐reported) No sample sizes for analysis Assessments: 3, 6, 9, 12 months postpartum |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization scheme was computer generated. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes; opened in sequential order at time of enrollment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No mention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on losses or sample sizes for analysis |
Stuart 2014.
| Methods | Time frame and location: March 2012 to June 2013; Chapel Hill, NC (USA) RCT, phase 4 Sample size calculation and outcome of focus: no information. Planned to enroll 190; randomized 35. Study stopped early; expulsion rate met a priori stopping rules. |
|
| Participants | General with N and source: 35 women, 18 to 45 years old; Women's Hospital of North Carolina Inclusion criteria (for antepartum enrollment): Pregnant and ≥ 24 weeks of estimated gestational age; intent to breastfeed for at least 6 months; plan to use LNG‐IUS postpartum; anticipation of vaginal delivery; HIV negative; intention to stay in Chapel Hill area for at least 6 months after birth; no medical or personal conditions that in the judgment of study staff preclude participation; no allergies to any component of LNG‐IUS; no known uterine anomalies; fluent in English No history of ectopic pregnancy, carcinoma of the breast, acute liver disease or liver tumor (benign or malignant); uterine or cervical neoplasia or unresolved abnormal pap smear; active pelvic inflammatory disease; hypersensitivity to component of LNG‐IUS; genital bleeding of unknown etiology; solid organ transplantation Additional eligibility criteria for trial entry (assessed postpartum): No endometritis or chorioamnionitis; membranes ruptured < 24 hours before delivery; no fever ≥ 38°C during intrapartum or postpartum period; did not receive medication other than pitocin or misoprostol to control postpartum bleeding; did not have blood loss > 750 mL intrapartum, blood transfusion for postpartum hemorrhage, third or fourth degree laceration at delivery; infant > 35 weeks gestation as determined by physical exam; weight at least 2727 grams; singleton birth; not in intensive care nursery; not diagnosed with condition that precludes long‐term feeding Exclusion criteria: no other mention |
|
| Interventions | Levonorgestrel‐releasing IUS (20 μg) with different insertion times Treatment: inserted within 48 hours of delivery Comparison: inserted 4 to 8 weeks after delivery |
|
| Outcomes | Measures: any breastfeeding Assessments: 6 months |
|
| Notes | Information from published conference abstract, ClinicalTrials.gov listing, and communication with investigator Full report to be submitted for publication by mid‐September, according to investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 allocation Computer generated by someone not involved with study activities, according to communication with investigator |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes, according to communication with investigator |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six‐month analysis for LNG‐IUS continuation included all women randomized. |
WHO 1984.
| Methods | Time frame and location: no time frame; conducted in Szeged, Hungary; Bangkok, Thailand; Khon Kaen, Thailand Multicenter trial Sample size calculation and outcome of focus: no information |
|
| Participants | General with N and source: 171 women delivering and chose to use OCs Inclusion criteria: age 20 to 35 years; 2 to 4 live births; prior successful breastfeeding (3 months); hemoglobin 10 to 12 g/dL; normal gestation and uncomplicated singleton delivery (2700 g to 3700 g); no breast abnormalities; desiring to use hormonal contraception during lactation Exclusion criteria: none specified |
|
| Interventions | Treatment: COC containing levonorgestrel 150 μg plus ethinyl estradiol 30 μg Comparison: OC containing norgestrel 75 μg Timing: initiated at 6 weeks postpartum (± 3 days) Study length: 24 weeks |
|
| Outcomes | Measures:
Assessments: 6 (baseline), 9, 12, 16, 20, 24 weeks postpartum |
|
| Notes | 341 women were recruited; only 171 women were randomized. Others chose depot medroxyprogesterone acetate (DMPA) or nonhormonal methods. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence from WHO |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, according to communication with investigator |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind; pills packaged identically |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up: unclear due to inconsistent reporting across and within reports COC: 9% at 9 weeks, 22% at 16 weeks, 34% at 24 weeks POP: 2% at 9 weeks, 14% at 16 weeks, 32% at 24 weeks |
COC: combined oral contraceptive
IUD: intrauterine device
LNG‐IUS: levonorgestrel‐releasing intrauterine system
OC: oral contraceptive
POP: progestin‐only contraceptive
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aleem 1996 | Not a randomized controlled trial |
| Barsivala 1973 | Unclear whether participant allocation was random |
| Bassol 2002 | Outcomes not applicable for this review |
| Betrabet 1987 | Per correspondence with the investigator (Betrabet), study participants received personal choice of intervention, not random allocation. |
| Bhatia 1987 | Not a randomized controlled trial |
| Borglin 1971 | Study described as a "carefully controlled trial." No evidence of randomization |
| Chen 2011 | Secondary analysis; trial of immediate versus delayed insertion of levonorgestrel‐releasing intrauterine system (LNG‐IUS) Interim contraception varied for delayed‐insertion group until LNG‐IUS was inserted at 6 to 8 weeks postpartum; included condoms, depot medroxyprogesterone acetate (DMPA), abstinence, and none. Women were not randomized to specific method during those first few weeks. |
| Croxatto 1982 | Per correspondence with the investigator, study participants received personal choice of intervention, not random allocation. |
| Croxatto 1983 | Per correspondence with the investigator, study participants received personal choice of intervention, not random allocation. |
| Diaz 1983 | Per correspondence with the investigator, study participants received personal choice of intervention, not random allocation. |
| Diaz 1985 | Per correspondence with the investigator, study participants received personal choice of intervention, not random allocation. |
| Diaz 1997 | Not a randomized controlled trial |
| Drury 1986 | Subgroup analysis of WHO 1984 |
| Gellen 1984 | Subgroup analysis of WHO 1984 |
| Gupta 1974 | Not a randomized controlled trial |
| Hefnawi 1970 | Unclear whether participant allocation was random |
| Kader 1969 | Unclear whether participant allocation was random |
| Kader 1975 | Unclear whether participant allocation was random |
| Kaern 1967 | Unclear whether participant allocation was random |
| Kamal 1969a | Unclear whether participant allocation was random |
| Kamal 1969b | Unclear whether participant allocation was random |
| Kamal 1970 | Unclear whether participant allocation was random |
| Karim 1971 | Not a randomized controlled trial |
| Koetsawang 1972a | Unclear whether participant allocation was random |
| Koetsawang 1972b | Unclear whether participant allocation was random |
| Peralta 1983 | Per correspondence with the investigator, all trials in the series were not randomized. Participants chose their intervention. |
| Rodrigues da Cunha 2001 | Not an RCT; choice of contraceptive |
| Seth 1977 | Unclear whether participant allocation was random |
| Shaaban 2013 | Does not have any of our primary outcomes. |
| Sinchai 1995 | Not a randomized controlled trial |
| Toaff 1969 | Trial does not include regularly marketed combined oral contraceptive. Intervention on postpartum days 1 to 5 further limited its usefulness. |
| Were 1997 | No relevant outcome when outcomes were divided into primary and secondary in 2014; trial did not address lactation. Further, losses were > 85% in each arm. |
| Zanartu 1976 | Not a randomized controlled trial |
Characteristics of ongoing studies [ordered by study ID]
Turok 2014.
| Trial name or title | BLIS ‐ Breastfeeding Levonorgestrel IUD Study |
| Methods | Time frame and location: January 2014 to December 2015 in Albuquerque, NM, and Salt Lake City, UT (USA) Randomized controlled trial, non‐inferiority, phase 4; open label Sample size calculation and outcome of focus: no mention |
| Participants | General with N: 317 pregnant women Inclusion criteria: healthy; 18 to 40 years old; pregnant; intention to breastfeed; desire for LNG‐IUD (levonorgestrel‐releasing intrauterine device) as method of contraception; delivered healthy term infant (37 weeks gestation) Exclusion criteria: chorioamnionitis; obstetric complications including transfusion, severe pregnancy‐induced hypertension, prolonged hospitalization, coagulopathy, liver disease, undiagnosed genital bleeding, or other relative contraindication to LNG‐IUD insertion (known or suspected pregnancy, uterine cavity; abnormality, known, suspected, or history of breast cancer; or hypersensitivity to any of the components in the LNG‐IUD) |
| Interventions | Levonorgestrel‐releasing IUD Treatment: immediately after delivery of baby and placenta (within 10 minutes) Comparison: 4 to 6 weeks after delivery Timing: as above |
| Outcomes | Measures: breastfeeding continuation and exclusivity; lactogenesis stage 2 (first 5 days after birth) Assessments: 5 days (lactogenesis stage 2); 8 and 26 weeks (breastfeeding) |
| Starting date | January 2014; estimated primary completion December 2015 |
| Contact information | Contact: Maria Masters; maria.masters@hsc.utah.edu; 801‐213‐2286 PI: David K Turok, MD; University of Utah, Department of Obstetrics and Gynecology |
| Notes | ‐‐‐ |
Contributions of authors
2014 revision LM Lopez conducted the searches and primary data extraction, incorporated the six trials, and revised the review. TW Grey conducted the secondary data extraction for study characteristics and outcomes. M Chen contributed to the methodology and to statistical interpretation, and examined the quality assessment data. AM Stuebe contributed research expertise in lactation to the background and discussion sections. ST Truitt contributed clinical expertise in lactation to the interpretation of results. MF Gallo commented on the overall presentation and interpretation of results.
2005, 2008, and 2010 updates LM Lopez wrote the plain language summary, reviewed the search results, and edited the text for Cochrane style.
2003 initial review Sarah T Truitt conceived and designed the review, performed initial database searches and retrieval of papers, wrote the protocol, and wrote to study authors for additional information. Anna B Fraser (formerly of St John's Clinic, Springfield, Montana) screened search results and retrieved papers, edited the protocol, and wrote the review. Maria F Gallo designed the database searches, screened search results and retrieved papers, and edited the protocol and the review. David A Grimes (formerly of FHI 360) supervised the review, provided advice, edited the review, screened retrieved papers, and wrote to study authors for additional information. Kenneth F Schulz (from FHI 360) provided advice, interpreted data, and contributed statistical expertise.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
2002 through 2014: Support for conducting the review and updates at FHI 360
-
US Agency for International Development, USA.
2002 through 2010: Support for conducting the review and updates at FHI 360 2014: This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of The Evidence Project, cooperative agreement no. AID‐OAA‐A‐13‐00087. The findings and conclusions are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Declarations of interest
Lopez LM, Grey TW, Stuebe AM, Chen M, Truitt ST, Gallo MF: none.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brito 2009 {published and unpublished data}
- Brito MB, Ferriani RA, Meijers JC, Garcia AA, Quintana SM, Silva de Sa MF, et al. Effects of the etonogestrel‐releasing contraceptive implant inserted immediately postpartum on maternal hemostasis: a randomized controlled trial. Thrombosis Research 2012;130(3):355‐60. [DOI] [PubMed] [Google Scholar]
- Brito MB, Ferriani RA, Quintana SM, Yazlle ME, Silva de Sa MF, Vieira CS. Safety of the etonogestrel‐releasing implant during the immediate postpartum period: a pilot study. Contraception 2009;80(6):519‐26. [NCT00828542] [DOI] [PubMed] [Google Scholar]
Dutta 2013 {published and unpublished data}
- Dutta DK, Dutta I. Desogestrel mini pill: is this safe in lactating mother?. Journal of the Indian Medical Association 2013; Vol. 111, issue 8:553‐5. [PubMed]
Espey 2012 {published and unpublished data}
- Espey E, Ogburn T, Leeman L, Singh R, Ostrom K, Schrader R. Effect of progestin compared with combined oral contraceptive pills on lactation: a randomized controlled trial. Obstetrics and Gynecology 2012;119(1):5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giner Velazquez 1976 {published data only}
- Giner Velázquez J, Córtes Gallegos V, Sotelo López A, Bondani A. Effects of the daily administration of 0.350 mg of norethindrone on breastfeeding and milk composition [Efecto de la administracion oral diaria de 0.350 mg de noretindrona en la lactancia y en la composicion de la leche]. Ginecologia y Obstetricia de Mexico 1976;40(237):31‐9. [PubMed] [Google Scholar]
Gurtcheff 2011 {published and unpublished data}
- Gurtcheff SE, Turok DK, Stoddard G, Murphy PA, Gibson M, Jones KP. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstetrics and Gynecology 2011;117(5):1114‐21. [DOI] [PubMed] [Google Scholar]
Heikkilä 1982 {published data only}
- Heikkilä M. Puerperal insertion of a copper‐releasing and a levonorgestrel‐releasing intrauterine contraceptive device. Contraception 1982;25(6):561‐72. [DOI] [PubMed] [Google Scholar]
- Heikkilä M, Luukkainen T. Duration of breast‐feeding and development of children after insertion of a levonorgestrel‐releasing intrauterine contraceptive device. Contraception 1982;25(3):279‐92. [DOI] [PubMed] [Google Scholar]
Miller 1970 {published data only}
- Miller GH, Hughes LR. Lactation and genital involution effects of a new low‐dose oral contraceptive on breast‐feeding mothers and their infants. Obstetrics and Gynecology 1970;35(1):44‐50. [PubMed] [Google Scholar]
Semm 1966 {published data only}
- Semm K, Dittmar FW. Post partum ovulation: inhibition and milk yield. Current Therapeutic Research 1966;8(2):48‐51. [PubMed] [Google Scholar]
Shaamash 2005 {published data only}
- Shaamash AH, Sayed GH, Hussien MM, Shaaban MM. A comparative study of the levonorgestrel‐releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast‐feeding performance, infant growth and infant development. Contraception 2005;72(5):346‐51. [DOI] [PubMed] [Google Scholar]
Stuart 2014 {published and unpublished data}
- Stuart G. Postpartum levonorgestrel‐releasing intrauterine system and breastfeeding (PPIUD1). http://clinicaltrials.gov/show/NCT01555931 (accessed 13 August 2014).
- Stuart GS, Lesko C, Stuebe A, Bryant A. Breastfeeding and postpartum insertion of the levonorgestrel intrauterine system: a randomized trial. Obstetrics and Gynecology 2014;123(Suppl 1):15S. [Google Scholar]
WHO 1984 {published and unpublished data}
- Sas M, Gellen JJ, Dusitsin N, Tankeyoon M, Chalapati S, Crawford MA, et al. An investigation on the influence of steroidal contraceptives on milk lipid and fatty acids in Hungary and Thailand. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Oral Contraceptives. Contraception 1986;33(2):159‐78. [DOI] [PubMed] [Google Scholar]
- Tankeyoon M, Dusitain N, Chalapati S, Koetsawang S, Saibiang S, Sas M, et al. Effects of hormonal contraceptives on milk volume and infant growth. WHO Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Oral Contraceptives. Contraception 1984;30(6):505‐22. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Task Force on Oral Contraceptives. Effects of hormonal contraceptives on breast milk composition and infant growth. Studies in Family Planning 1988;19(6):361‐9. [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Aleem 1996 {published data only}
- Abdel‐Aleem H, Abol‐Oyoun EM, Shaaban MM, el‐Saeed M, Shoukry M, Makhlouf A, et al. The use of nomegestrol acetate subdermal contraceptive implant, Uniplant, during lactation. Contraception 1996;54:281‐6. [DOI] [PubMed] [Google Scholar]
Barsivala 1973 {published data only}
- Barsivala VM, Virkar KD. The effect of oral contraceptives on concentrations of various components of human milk. Contraception 1973;7:307‐12. [Google Scholar]
Bassol 2002 {published data only}
- Bassol S, Nava‐Hernandez MP, Hernandez‐Morales C, Trujillo‐Macias AM, Lopez‐Lozano MR, Recio R. Effects of levonorgestrel implant upon TSH and LH levels in male infants during lactation. International Journal of Gynaecology and Obstetrics 2002;76:273‐7. [DOI] [PubMed] [Google Scholar]
Betrabet 1987 {published and unpublished data}
- Betrabet SS, Shikary ZK, Toddywalla VS, Toddywalla SP, Patel D, Saxena BN. ICMR Task Force Study on Hormonal Contraception: transfer of norethisterone (NET) and levonorgestrel (LNG) from a single tablet into the infant's circulation through the mother's milk. Contraception 1987;35:517‐23. [DOI] [PubMed] [Google Scholar]
Bhatia 1987 {published data only}
- Bhatia S, Becker S, Kim YJ. The effect of oral contraceptive acceptance on fertility in the postpartum period. International Journal of Gynaecology and Obstetrics 1987;25(Suppl):1‐11. [DOI] [PubMed] [Google Scholar]
Borglin 1971 {published data only}
- Borglin Nils‐B, Sandholm Lars‐E. Effect of oral contraceptives on lactation. Fertility and Sterility 1971;22:39‐41. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen BA, Hayes JL, Hohmann HL, Perriera LK, Reeves MF, Creinin MD. A randomized trial of postplacental compared to delayed insertion of the levonorgestrel‐releasing intrauterine device after vaginal delivery (abstract). Contraception 2009;80(2):205. [NCT00476021] [Google Scholar]
- Chen BA, Reeves MF, Creinin MD, Schwarz EB. Postplacental or delayed levonorgestrel intrauterine device insertion and breast‐feeding duration. Contraception 2011;84(5):499‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BA, Reeves MF, Hayes JL, Hohmann HL, Perriera LK, Creinin MD. Postplacental or delayed insertion of the levonorgestrel intrauterine device after vaginal delivery. Obstetrics and Gynecology 2010;116(5):1079‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Croxatto 1982 {published and unpublished data}
- Croxatto HB, Diaz S, Peralta O, Juez G, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: II. Comparative performance of progesterone implants versus placebo and copper T. American Journal of Obstetrics and Gynecology 1982;144:201‐8. [PubMed] [Google Scholar]
Croxatto 1983 {published and unpublished data}
- Croxatto HB, Diaz S, Peralta O, Juez G, Herreros C, Casado ME, et al. Fertility regulation in nursing women: IV. Long‐term influence of a low‐dose combined oral contraceptive initiated at day 30 postpartum upon lactation and infant growth. Contraception 1983;27:13‐25. [DOI] [PubMed] [Google Scholar]
Diaz 1983 {published and unpublished data}
- Diaz S, Peralta O, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: III. Short‐term influence of low‐dose combined oral contraceptive upon lactation and infant growth. Contraception 1983;27:1‐10. [DOI] [PubMed] [Google Scholar]
Diaz 1985 {published and unpublished data}
- Diaz S, Herreros C, Juez G, Casado ME, Salvatierra AM, Miranda P, et al. Fertility regulation in nursing women: VII. Influence of Norplant levonorgestrel implants upon lactation and infant growth. Contraception 1985;32:53‐74. [DOI] [PubMed] [Google Scholar]
Diaz 1997 {published and unpublished data}
- Diaz S, Zepeda A, Maturana X, Reyes MV, Miranda P, Casado ME, et al. Fertility regulation in nursing women: IX. Contraceptive performance, duration of lactation, infant growth, and bleeding patterns during use of progesterone vaginal rings, progestin‐only pills, Norplant implants, and Copper T 380‐A intrauterine devices. Contraception 1997;56:223‐32. [DOI] [PubMed] [Google Scholar]
Drury 1986 {published data only}
- Drury PJ, Crawford MA, Ayeni O, Pinol A. Fatty acids in human milk and steroidal contraceptives in Hungary and Thailand. Progress in Lipid Research 1986;25:229‐33. [DOI] [PubMed] [Google Scholar]
Gellen 1984 {published data only}
- Gellen JJ, Sas M. Lactation and contraception: effect of low dose oral contraceptives on infant's growth during lactation. Orvosi Hetilap 1984;125:193‐6. [PubMed] [Google Scholar]
Gupta 1974 {published data only}
- Gupta AN, Mathur VS, Garg SK. Effect of oral contraceptives on quantity and quality of milk secretion in human beings. Indian Journal of Medical Research 1974;62:964‐70. [PubMed] [Google Scholar]
Hefnawi 1970 {published data only}
- Hefnawi F, Fawzi G, Badrawi MH, Kader MM, Bahgat MH. Attempts to select a suitable hormonal contraceptive during lactation. The VI World Congress of Ob‐Gyn; New York (NY). 1970.
Kader 1969 {published data only}
- Kader MM, Hay AA, El‐Safouri S, Aziz MT, El‐Din JS, Kamal I, et al. Clinical, biochemical, and experimental studies on lactation: III. Biochemical changes induced in human milk by gestagens. American Journal of Obstetrics and Gynecology 1969;105:978‐85. [DOI] [PubMed] [Google Scholar]
Kader 1975 {published data only}
- Kader M, Aziz MT, Bahgat MR, Talaat K, Osman A, Osman M. Effect of two long acting injectable progestogens on lactation in the human. Acta Biologica et Medica Germanica 1975;34:1199‐204. [PubMed] [Google Scholar]
Kaern 1967 {published data only}
- Kaern T. Effect of an oral contraceptive immediately post partum on initiation of lactation. British Medical Journal 1967;3:644‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamal 1969a {published data only}
- Kamal I, Hefnawi F, Ghoneim M, Talaat M, Younis N, Tagui A, et al. Clinical, biochemical, and experimental studies on lactation: II. Clinical effects of gestagens on lactation. American Journal of Obstetrics and Gynecology 1969;105:324‐34. [DOI] [PubMed] [Google Scholar]
Kamal 1969b {published data only}
- Kamal I, Hefnawi F, Ghoneim M, Talaat M, Younis N, Tagui A, et al. Clinical, biochemical, and experimental studies on lactation: I. Lactation pattern in Egyptian women. American Journal of Obstetrics and Gynecology 1969;105:314‐6. [DOI] [PubMed] [Google Scholar]
Kamal 1970 {published data only}
- Kamal I, Hefnawi F, Ghoneim M, Abdallah M, Razek SA. Clinical, biochemical, and experimental studies on lactation: V. Clinical effects of steroids on the initiation of lactation. American Journal of Obstetrics and Gynecology 1970;108:655‐8. [DOI] [PubMed] [Google Scholar]
Karim 1971 {published data only}
- Karim M, Ammar R, Mahgoub SE, Ganzoury BE, Fikri F, Abdou I. Injected progestogen and lactation. British Medical Journal 1971;1:200‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koetsawang 1972a {published data only}
- Koetsawang S, Bhiraleus P, Chiemprajert T. Effects of oral contraceptives on lactation. Fertility and Sterility 1972;23:24‐8. [DOI] [PubMed] [Google Scholar]
Koetsawang 1972b {published data only}
- Koetsawang S, Chiemprajert T, Kochananda P. The effects of injectable contraceptives on lactation. Clinical Proceedings I.P.P.F. Southeast Asia and Oceania Medical and Scientific Congress; Sydney (Australia). 1972;1:84‐9.
Peralta 1983 {published and unpublished data}
- Peralta O, Diaz S, Juez G, Herreros C, Casado ME, Salvatierra AM, et al. Fertility regulation in nursing women: V. Long‐term influence of a low‐dose combined oral contraceptive initiated at day 90 postpartum upon lactation and infant growth. Contraception 1983;27:27‐38. [DOI] [PubMed] [Google Scholar]
Rodrigues da Cunha 2001 {published data only}
- Rodrigues da Cunha AC, Dorea JG, Cantuaria AA. Intrauterine device and maternal copper metabolism during lactation. Contraception 2001;63(1):37‐9. [DOI] [PubMed] [Google Scholar]
Seth 1977 {published data only}
- Seth U, Yadava HS, Agarwal N, Laumas KR, Hingorani V. Effect of a subdermal silastic implant containing norethindrone acetate on human lactation. Contraception 1977;16:383‐98. [DOI] [PubMed] [Google Scholar]
Shaaban 2013 {published data only}
- Shaaban OM, Hassen SG, Nour SA, Kames MA, Yones EM. Emergency contraceptive pills as a backup for lactational amenorrhea method (LAM) of contraception: a randomized controlled trial. Contraception 2013;87(3):363‐9. [DOI] [PubMed] [Google Scholar]
Sinchai 1995 {published data only}
- Sinchai W, Sethavanich S, Asavpiriyanont S, Sittipiyasakul V, Sirikanchanakul R, Udomkiatsakul P, et al. Effects of a progestogen‐only pill (Exluton) and an intrauterine device (Multiload Cu250) on breastfeeding. Advances in Contraception 1995;2:143‐55. [DOI] [PubMed] [Google Scholar]
Toaff 1969 {published data only}
- Toaff R, Ashkenazi H, Schwartz A, Herzberg M. Effects of oestrogen and progestagen on the composition of human milk. Journal of Reproduction and Fertility 1969;19:475‐82. [DOI] [PubMed] [Google Scholar]
Were 1997 {published and unpublished data}
- Were EO, Nyongesa K, Nyongesa P. Randomized clinical trial to determine optimum initiation time of norgestrel‐progestin only contraception in Eldoret teaching hospital, Kenya. East African Medical Journal 1997;74:103‐7. [PubMed] [Google Scholar]
Zanartu 1976 {published data only}
- Zanartu J, Aguilera E, Munoz G, Peliowsky H. Effect of a long‐acting contraceptive progestogen on lactation. Obstetrics and Gynecology 1976;47:174‐6. [PubMed] [Google Scholar]
References to ongoing studies
Turok 2014 {published data only}
- Turok D. BLIS ‐ Breastfeeding Levonorgestrel IUD Study. http://clinicaltrials.gov/show/NCT01990703 (accessed 13 August 2014).
Additional references
AAP 2012
- American Academy of Pediatrics. Section on Breastfeeding. Policy Statement. Breastfeeding and the use of human milk. Pediatrics 2012;129(3):e827‐41. [DOI: 10.1542/peds.2011-3552] [DOI] [PubMed] [Google Scholar]
Bahamondes 2013
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bohler 1996
- Bohler E, Bergstrom S. Child growth during weaning depends on whether mother is pregnant again. Journal of Tropical Pediatrics 1996;42(2):104‐9. [DOI] [PubMed] [Google Scholar]
Brownell 2012
- Brownell EA, Fernandez ID, Howard CR, Fisher SG, Ternullo SR, Buckley RJ, et al. A systematic review of early postpartum medroxyprogesterone receipt and early breastfeeding cessation: evaluating the methodological rigor of the evidence. Breastfeeding Medicine 2012;7(1):10‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2010
- Centers for Disease Control and Prevention. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. Appendix A. Summary of Changes to the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th Edition, to Create the U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. Morbidity and Mortality Weekly Report (MMWR) 2010;59(RR04):7‐10. [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. Update to CDC's U.S. Medical Eligibility Criteria for Contraceptive Use, 2010: revised recommendations for the use of contraceptive methods during the postpartum period. Morbidity and Mortality Weekly Report (MMWR) 2011;60(26):878‐83. [PubMed] [Google Scholar]
Conde‐Agudelo 2000
- Conde‐Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. British Medical Journal 2000;321:1255‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erwin 1994
- Erwin PC. To use or not use combined hormonal oral contraceptives during lactation. Family Planning Perspectives 1994;26:26‐30,33. [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hall 2012
- Hall KS, Trussell J, Schwarz EB. Progestin‐only contraceptive pill use among women in the United States. Contraception 2012;86(6):653‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd.
Ip 2007
- Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Evidence Report/Technology Assessment 2007;Apr(153):1‐186. [PMC free article] [PubMed] [Google Scholar]
K4 Health 2014
- K4 Health. Lactational Amenorrhea Method Toolkit. https://www.k4health.org/toolkits/lam (accessed 20 August 2014).
Kapp 2010a
- Kapp N, Curtis K, Nanda K. Progestogen‐only contraceptive use among breastfeeding women: a systematic review. Contraception 2010;82(1):17‐37. [DOI] [PubMed] [Google Scholar]
Kapp 2010b
- Kapp N, Curtis KM. Combined oral contraceptive use among breastfeeding women: a systematic review. Contraception 2010;82(1):10‐6. [DOI] [PubMed] [Google Scholar]
Kennedy 2011
- Kennedy KI, Trussell J. Postpartum contraception and lactation. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, et al. editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:483‐511. [Google Scholar]
Knijff 2000
- Knijff SCM, Goorissen EM, Velthuis‐te Wierik EJM, Korver T, Grimes DA. Summary of Contraindications to Oral Contraceptives. New York: Parthenon Publishing Group, Inc., 2000. [Google Scholar]
Kramer 2012
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD003517.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 2007
- Pang WW, Hartmann PE. Initiation of human lactation: secretory differentiation and secretory activation. Journal of Mammary Gland Biology and Neoplasia 2007;12(4):211‐21. [DOI] [PubMed] [Google Scholar]
RamaRao 2013
- RamaRao S, Clark H, Merkatz R, Sussman H, Sitruk‐Ware R. Progesterone vaginal ring: introducing a contraceptive to meet the needs of breastfeeding women. Contraception 2013;88(5):591‐8. [DOI] [PubMed] [Google Scholar]
Raymond 2011
- Raymond EG. Progestin‐only pills. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Kowal D, Policar MS, et al. editor(s). Contraceptive Technology. 20th Edition. New York: Ardent Media, Inc., 2011:237‐47. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; Vol. 340:c332. [DOI] [PMC free article] [PubMed]
Speroff 2004
- Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th Edition. Baltimore: Lippincott Williams and Wilkins, 2004. [Google Scholar]
Strauss 2005
- Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence‐based Medicine: How to Practice and Teach EBM. Third Edition. New York: Churchill Livingstone, 2005. [Google Scholar]
Tsui 1997
- Tsui AO, Wasserheit JN, Haaga J, National Research Council (U.S.) Panel on Reproductive Health. Reproductive Health in Developing Countries: Expanding Dimensions, Building Solutions. Washington: National Academy Press, 1997. [PubMed] [Google Scholar]
USAID 2009
- USAID, ACCESS‐FP. The Lactational Amenorrhea Method METHOD (LAM): A Postpartum Contraceptive Choice for Women Who Breastfeed; 2009. https://www.k4health.org/toolkits/lam/essential‐knowledge (accessed 20 August 2014).
Van der Wijden 2003
- Wijden C, Brown J, Kleijnen J. Lactational amenorrhea for family planning. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001329] [DOI] [PubMed] [Google Scholar]
Westhoff 2012
- Westhoff CF. Unmet Need for Modern Contraceptive Methods. DHS Analytical Studies No. 28. Princeton, NJ: Office of Population Research, Princeton University, 2012 September.
WHO 1986
- World Health Organization. Protocols for the development and field testing of techniques for monitoring physical growth and psychosocial development. World Health Organization Division of Family Health and Division of Mental Health; 1986. WHO document: WHO/MCH/MNH.
WHO 1994
- World Health Organization. Progestogen‐only contraceptives during lactation: II. Infant development. World Health Organization, Task Force for Epidemiological Research on Reproductive Health; Special Programme of Research, Development, and Research Training in Human Reproduction. Contraception 1994;50(1):55‐68. [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Report of a WHO Technical Consultation on Birth Spacing; 2005 Jun 13‐15; Geneva. http://www.who.int/maternal_child_adolescent/documents/birth_spacing.pdf (accessed 26 August 2014).
WHO 2009
- World Health Organization. Medical Eligibility Criteria for Contraceptive Use. Fourth Edition, 2009. http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/. Geneva: World Health Organization, (accessed 23 July 2014):16.
WHO 2010
- World Health Organization. Combined hormonal contraceptive use during the postpartum period: 2010. http://www.who.int/reproductivehealth/publications/family_planning/rhr_10_15/en/ (accessed 21 August 2014).
WHO 2013
- World Health Organization. Statement for Collective Action for Postpartum Family Planning. http://www.who.int/reproductivehealth/publications/family_planning/statmt_ppfp/en/ (accessed 10 September 2014).
References to other published versions of this review
Truitt 2003
- Truitt S, Fraser A, Grimes D, Gallo M, Schulz K. Hormonal contraception during lactation. Contraception 2003;68:233‐8. [DOI] [PubMed] [Google Scholar]


