Abstract
This study examines the frequency of drug patent invalidations based on inequitable conduct.
Median brand-name drug launch prices increased from $2115 in 2008 to $180 007 in 2021.1 An important factor allowing drug manufacturers to sustain high prices is market exclusivity, length of which is usually defined by drug patents.2 Therefore, ensuring that patents are obtained legitimately from the US Patent and Trademark Office (USPTO) without misrepresenting or omitting information is important. Inequitable conduct is a legal claim that can be brought to invalidate patents acquired by fraud or deceit, such as intentionally withholding or misrepresenting material information. Fraudulently granted patents could increase patient and health care system costs by enabling extended periods of monopoly pricing.
One way to reduce inequitable conduct, as sought by members of Congress,3 might be for the USPTO to enhance communication with the Food and Drug Administration (FDA), which receives detailed information about drugs and their uses. Effective communication between the FDA and USPTO could help identify inequitable conduct and prevent fraud by encouraging full disclosure to both agencies. We examined frequency of drug patent invalidations based on inequitable conduct.
Methods
We used the Compendium of Federal Circuit Decisions and Lex Machina (a commercial patent litigation database) to identify all inequitable conduct cases from October 2004 to December 2021 at the Court of Appeals for the Federal Circuit, the sole appellate court with jurisdiction over patents.4 These cases covered a wide range of products such as cable modems, computerized navigation systems, magnetic purse fasteners, and drugs and medical devices. When inequitable conduct was found, we extracted the type of product (FDA-regulated vs not) and whether it was a patent covering a small molecule drug listed in the FDA’s Approved Drug Products With Therapeutic Evaluations (Orange Book); for FDA-regulated products, we determined the basis for inequitable conduct. More than 1 patent can be associated with a case.
In 2011, the Court of Appeals for the Federal Circuit raised the bar to prove inequitable conduct, adopting higher standards for demonstrating intent and materiality. We analyzed number of inequitable conduct cases involving FDA-regulated products from 2004-2011 vs 2012-2021. Analyses were descriptive (Excel version 16).
Results
Between 2004 and 2021, the appellate court ruled on 125 (2%) inequitable conduct cases among 5355 total rulings and found inequitable conduct in 36 cases (29%), leading to invalidation of 75 patents. In 24 (67%) of the 36 cases relating to 34 (45%) of the 75 patents, the court found inequitable conduct covering FDA-regulated products (Figure).
Figure. Inequitable Conduct Appellate Cases by Year.
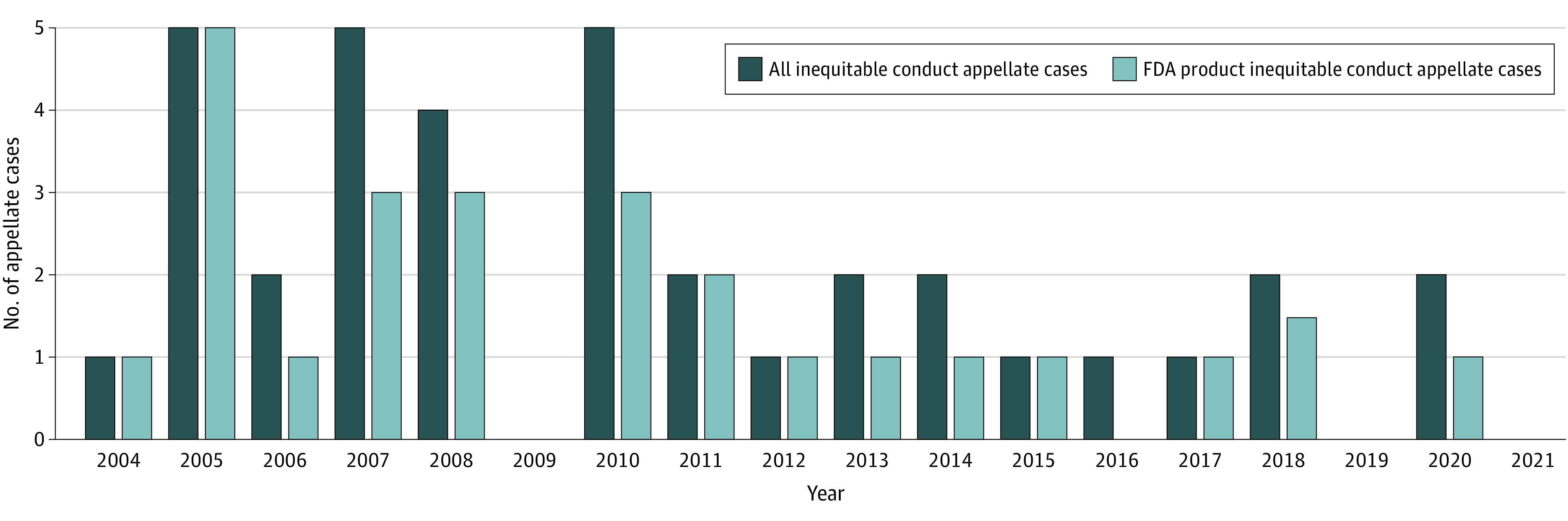
Court of Appeals for the Federal Circuit cases with patents invalidated by inequitable conduct from October 1, 2004, to December 31, 2021. Dark shaded bars represent all inequitable conduct cases (both FDA- and non–FDA-regulated products) that resulted in invalidation of a patent. Lighter shaded bars represent only the FDA-regulated product inequitable conduct cases that resulted in invalidation of a patent. FDA indicates Food and Drug Administration.
Of the 34 invalidated patents related to FDA-regulated products, 15 (44%) were drug related, 10 (29%) device related, and 8 (24%) food related; 1 was a research tool. Eight drug-related patents (53%) were listed in the Orange Book; the remaining 7 related to patents covering methods of manufacture or biologics. The reasons for invalidation of FDA-regulated products by inequitable conduct ranged from active misrepresentations to material omissions (Table). The most common type of invalidation was not revealing key printed publications to the USPTO.
Table. Reasons for Inequitable Conduct Invalidation for Patents Related to FDA-Regulated Products (N = 34).
| Inequitable conduct type | Patents, No. (%) |
|---|---|
| Material omissionsa | 30 (88) |
| Printed publicationsb | 13 (38) |
| Product sales and public usec | 12 (35) |
| Negative experimentsd | 12 (35) |
| US patents and patent applicationse | 2 (6) |
| Non-US patentse | 2 (6) |
| Active misrepresentationsf | 16 (47) |
| Prophetic examples passed off as working examplesg | 5 (15) |
Abbreviation: FDA, Food and Drug Administration.
Material omissions is the failure to disclose material information with the specific intent to deceive the US Patent and Trademark Office (USPTO). A reference is “material” if there is a substantial likelihood that a reasonable patent examiner would consider it important in deciding whether to allow the application to issue as a patent. Printed publications, product sale and public use, negative experiments, US patents and patent applications, and non-US patents represent the type of material information hidden from the USPTO. Numbers sum to more than 100% because multiple omissions can be associated with each patent.
Printed publication, typically journal articles, known to the patentee but not revealed to the USPTO.
Product sales and public use of the invention are required to be disclosed to the USPTO.
Negative experiments are typically those conducted either for the FDA or in preparation to rebut a USPTO rejection; however, the negative experiment was not revealed to the USPTO.
Material prior patents and patent applications (US and non-US) known to the applicant must be disclosed to the USPTO.
Active misrepresentations include affirmative misrepresentations of material fact and submission of false material information with the specific intent to deceive the USPTO.
Courts explicitly permit made-up “paper” experiments, also called prophetic examples. Prophetic examples describe experiments that have not been performed and cannot be represented as work actually conducted. Paper examples should be described using the present or future tense. In contrast, working examples correspond to work performed or experiments conducted that yielded actual results and should be described using the past tense. Inequitable conduct arises when inventors knowingly assert prophetic examples as working examples.
Inequitable conduct was not found in 89 cases (69%) related to 129 patents. Of the 129 noninvalidated patents, 60 (47%) covered FDA-regulated products comprising 41 (46%) of the 89 cases.
Inequitable conduct findings for FDA-regulated products decreased from a mean (SD) of 2.25 (1.6) per year from 2004-2011 to 0.60 (0.52) per year in 2012-2021 (Figure).
Discussion
Inequitable conduct cases involving FDA-regulated products were infrequent but constituted two-thirds of cases in which inequitable conduct was found, and invalidation of affected patents occurred in approximately one-third of these cases.
A study limitation is that it included only appellate court cases and not district court cases where inequitable conduct was pleaded. Although inequitable conduct appellate cases have affected few drug patents during the past 15 years, the prevalence of inequitable conduct is unknown because not all cases of inequitable conduct are identified or litigated.
The USPTO should develop communication pathways with the FDA to prevent improperly issued patents. Alternatively, Congress could require reexamination of patents on FDA-regulated products after FDA approval.5 Such reexamination would allow the USPTO to review and determine whether there were any material references, statements, or relevant experiments disclosed to the FDA that were hidden or misrepresented to the USPTO.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Karen Lasser, MD, and Kristin Walter, MD, Senior Editors.
Data Sharing Statement
References
- 1.Rome BN, Egilman AC, Kesselheim AS. Trends in prescription drug launch prices, 2008-2021. JAMA. 2022;327(21):2145-2147. doi: 10.1001/jama.2022.5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kesselheim AS, Sinha MS, Avorn J. Determinants of market exclusivity for prescription drugs in the United States. JAMA Intern Med. 2017;177(11):1658-1664. doi: 10.1001/jamainternmed.2017.4329 [DOI] [PubMed] [Google Scholar]
- 3.Letter to Andrew Hirshfeld from Senators Patrick Leahy and Thom Tillis, September 9, 2021. Accessed September 17, 2023. https://pink.citeline.com/-/media/supporting-documents/pink-sheet/2021/09/leahy-tillis-letter-to-pto-on-fda-submissions.pdf?rev=1215dcbfd67e4205af4ba3ff47b5f03f
- 4.Federal Circuit Decisions Database . Accessed June 30, 2023. https://fedcircuit.shinyapps.io/federalcompendium
- 5.Tu SS. FDA reexamination: increased communication between the FDA and USPTO to improve patent quality. Houst Law Rev. 2022;60:403-465. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Sharing Statement


