This post hoc analysis of the Torsemide Comparison With Furosemide for Management of Heart Failure (TRANSFORM-HF) randomized clinical trial investigates if torsemide improves mortality and hospitalization outcomes at 12 months compared with furosemide in either de novo heart failure or worsening chronic heart failure.
Key Points
Question
Does torsemide improve mortality and hospitalization outcomes at 12 months compared with furosemide in either de novo heart failure (HF) or worsening chronic HF (WHF)?
Findings
In this post hoc analysis of the Torsemide Comparison With Furosemide for Management of Heart Failure (TRANSFORM-HF) randomized clinical trial including 2858 patients, patients with de novo HF had a significantly lower all-cause mortality at 12 months when compared with WHF. No significant association of discharge diuretic type with outcomes was noted in either de novo HF or WHF.
Meaning
Among patients discharged after hospitalization for HF, de novo HF was associated with better outcomes when compared with WHF. Regardless of HF type, there was no significant difference in outcomes with a loop diuretic strategy of torsemide vs furosemide.
Abstract
Importance
Differences in clinical profiles, outcomes, and diuretic treatment effects may exist between patients with de novo heart failure (HF) and worsening chronic HF (WHF).
Objectives
To compare clinical characteristics and treatment outcomes of torsemide vs furosemide in patients hospitalized with de novo HF vs WHF.
Design, Setting, and Participants
All patients with a documented ejection fraction who were randomized in the Torsemide Comparison With Furosemide for Management of Heart Failure (TRANSFORM-HF) trial, conducted from June 18 through March 2022, were included in this post hoc analysis. Study data were analyzed March to May 2023.
Exposure
Patients were categorized by HF type and further divided by loop diuretic strategy.
Main Outcomes and Measures
End points included all-cause mortality and hospitalization outcomes over 12 months, as well as change from baseline in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS).
Results
Among 2858 patients (mean [SD] age, 64.5 [14.0] years; 1803 male [63.1%]), 838 patients (29.3%) had de novo HF, and 2020 patients (70.7%) had WHF. Patients with de novo HF were younger (mean [SD] age, 60.6 [14.5] years vs 66.1 [13.5] years), had a higher glomerular filtration rate (mean [SD], 68.6 [24.9] vs 57.0 [24.0]), lower levels of natriuretic peptides (median [IQR], brain-type natriuretic peptide, 855.0 [423.0-1555.0] pg/mL vs 1022.0 [500.0-1927.0] pg/mL), and tended to be discharged on lower doses of loop diuretic (mean [SD], 50.3 [46.2] mg vs 63.8 [52.4] mg). De novo HF was associated with lower all-cause mortality at 12 months (de novo, 65 of 838 [9.1%] vs WHF, 408 of 2020 [25.4%]; adjusted hazard ratio [aHR], 0.50; 95% CI, 0.38-0.66; P < .001). Similarly, lower all-cause first rehospitalization at 12 months and greater improvement from baseline in KCCQ-CSS at 12 months were noted among patients with de novo HF (median [IQR]: de novo, 29.94 [27.35-32.54] vs WHF, 23.68 [21.62-25.74]; adjusted estimated difference in means: 6.26; 95% CI, 3.72-8.81; P < .001). There was no significant difference in mortality with torsemide vs furosemide in either de novo (No. of events [rate per 100 patient-years]: torsemide, 27 [7.4%] vs furosemide, 38 [10.9%]; aHR, 0.70; 95% CI, 0.40-1.14; P = .15) or WHF (torsemide 212 [26.8%] vs furosemide, 196 [24.0%]; aHR, 1.08; 95% CI, 0.89-1.32; P = .42; P for interaction = .10), In addition, no significant differences in hospitalizations, first all-cause hospitalization, or total hospitalizations at 12 months were noted with a strategy of torsemide vs furosemide in either de novo HF or WHF.
Conclusions and Relevance
Among patients discharged after hospitalization for HF, de novo HF was associated with better clinical and patient-reported outcomes when compared with WHF. Regardless of HF type, there was no significant difference between torsemide and furosemide with respect to 12-month clinical or patient-reported outcomes.
Introduction
Although decongestion with intravenous diuretics remains a mainstay of therapy for patients hospitalized for heart failure (HF),1,2 significant differences in clinical profiles and outcomes exist between patients with de novo HF and worsening chronic HF (WHF). Although furosemide remains the dominant loop diuretic used in clinical practice, some patients are subsequently switched to torsemide as HF disease severity progresses, including patients who exhibit diuretic resistance with comorbid kidney disease. However, it remains unclear if an approach of early up-front use of torsemide starting at the time of HF diagnosis may improve outcomes. In this context, our study aimed to describe differences in patient characteristics and assess for a differential treatment outcome between torsemide and furosemide in de novo HF vs WHF.
Methods
Study Design
The trial design and results of the Torsemide Comparison With Furosemide for Management of Heart Failure (TRANSFORM-HF) study have been previously published.3,4 Briefly, this open-label, pragmatic, randomized clinical trial, which was conducted from June 2018 through March 2022 with follow-up through 30 months for death and 12 months for hospitalizations, recruited 2859 participants hospitalized with HF (irrespective of ejection fraction [EF]) at 60 hospitals in the US and assessed the effect of 2 diuretic strategies (torsemide vs furosemide) on outcomes at 12 months.3,4 The present post hoc analysis aimed to (1) characterize the patient profile and outcomes of patients with de novo HF vs WHF and (2) estimate the randomized treatment outcome (torsemide vs furosemide) associated with de novo HF vs WHF status, overall and stratified by EF category (EF ≤40%, EF >40%). The trial was approved by the Duke University institutional review board (IRB), as well as a central IRB or local site IRBs, and all patients provided written, informed consent before enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Study Population and End Points
All patients enrolled and successfully randomized in the TRANSFORM-HF trial4 were included in this analysis with the exception of patients with undocumented HF type (de novo vs WHF) and unknown EF. Patients self-identified with the following race and ethnicity categories: American Indian or Alaska Native, Asian, Black or African American, Hispanic or Latino, multiracial, Native Hawaiian or Other Pacific Islander, White, or other race and ethnicity. The inclusion of race and ethnicity data was aligned with National Institutes of Health guidance. The selected primary end point was all-cause mortality over 12 months. Secondary end points included all-cause mortality and/or first rehospitalization over 12 months, time to first all-cause rehospitalization over 12 months, total hospitalizations over 12 months, and Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) change from baseline over 12 months.
Statistical Analysis
Patient characteristics were compared by HF type (de novo HF vs WHF). Differences between groups were assessed using the χ2/Fisher exact test for categorical comparisons as appropriate, and the Wilcoxon/Kruskal-Wallis test for comparison of continuous variables.
For the time-to-event analysis on the primary end point (all-cause mortality) and the secondary end points of all-cause death/hospitalization, Cox proportional hazards regression models were used to compare de novo vs WHF. For the secondary end point of first rehospitalization over 12 months, a Cox proportional hazards regression model was used with censoring for death. The proportional hazards assumption was confirmed using weighted Schoenfeld residuals. For the secondary end point of total hospitalizations over 12 months, relative risk was determined from a negative binomial regression of the frequency of rehospitalizations.
To compare de novo HF vs WHF, models were adjusted for prespecified covariates including HF type (de novo, WHF), age, sex, race, EF category (≤40%, 41%-49%, ≥50%, unknown), systolic blood pressure, estimated glomerular filtration rate (eGFR), hemoglobin level, body mass index, diabetes, atrial fibrillation/flutter, ischemic etiology, and chronic lung disease. For adjusted model in analyses stratified by EF levels (≤40%, >40%), the same covariates were used, with continuous baseline EF as an additional covariate. To test the interaction effect between HF types and EF category among patients with known EF level, an interaction term between baseline EF level (≤40%, >40%) and HF type was tested separately for each end point, and the model included HF type (de novo, WHF), age, sex, race, baseline EF level (≤40%, >40%), systolic blood pressure, eGFR, hemoglobin level, body mass index, diabetes, atrial fibrillation/flutter, ischemic etiology, chronic lung disease, and the interaction term.
In addition, unadjusted Kaplan-Meier curves for all-cause mortality comparing de novo and WHF were performed for the overall cohort and separately stratified by EF levels (≤40%, >40%). Unadjusted cumulative incidence curves of all-cause hospitalization comparing de novo and worsening HF, accounting for death as competing risk, were presented for the overall cohort, and separately stratified by EF levels (≤40%, >40%).
The KCCQ-CSS end point was analyzed using a linear mixed model with an unstructured covariance matrix. Repeated measures were the KCCQ-CSS score change from baseline by visit.
To estimate the randomized diuretic treatment association according to HF type with all outcomes, a model was produced for each outcome first for the overall patients, then separately for each EF category (≤40% and >40%). For the overall patient model, covariates included diuretic treatment (torsemide, furosemide), HF type, age, sex, and baseline EF categories (≤40%, 41%-49%, ≥50%, unknown). For each stratified analysis by EF level, the covariate was diuretic treatment, HF type, age, sex, and continuous baseline EF. For both overall and stratified analysis, the interaction P value between diuretic treatment and HF type was estimated separately. For the primary outcome, a 2-sided P value < .05 was considered statistically significant. For all other analyses, including secondary analyses and subgroup analyses, a P value < .005 was considered statistically significant to improve the reproducibility of study results. Study data were analyzed March to May 2023. All analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
Results
Patient Characteristics
Among 2858 patients (mean [SD] age, 64.5 [14.0] years; 1803 male [63.1%]; 1055 female [36.9%]), 838 patients (29.3%) had de novo HF, and 2020 patients (70.7%) had WHF (Table 1). Patients self-identified with the following race and ethnicity categories: 12 American Indian or Alaska Native (0.4%), 63 Asian (2.2%), 968 Black or African American (33.9%), 154 Hispanic or Latino (5.4%), 43 multiracial (1.5%), 20 Native Hawaiian or Other Pacific Islander (0.7%), 1668 White (58.5%), and 79 other race or ethnicity (2.8%). Patients with de novo HF were younger (mean [SD] age, 60.6 [14.5] years vs 66.1 [13.5] years), had a higher glomerular filtration rate (mean [SD], 68.6 [24.9] vs 57.0 [24.0]), lower levels of natriuretic peptides (median [IQR], brain-type natriuretic peptide, 855.0 [423.0-1555.0] pg/mL vs 1022.0 [500.0-1927.0] pg/mL; to convert brain-type natriuretic peptide to nanograms per liter, multiply by 1), and tended to be discharged on lower doses of loop diuretic (mean [SD], 50.3 [46.2] mg vs 63.8 [52.4] mg). No significant differences in sex, race, or blood pressure were found between the 2 groups. Additionally, both groups showed a similar distribution across EF categories. Patients with WHF were more likely than patients with de novo HF to be taking an oral diuretic before the index hospital admission (torsemide: 249 of 2020 [14.4%] vs 9 of 838 [4.7%]; bumetanide: 123 of 2020 [7.1%] vs 6 of 838 [3.1%]), with furosemide being the most commonly prescribed diuretic (furosemide: 1532 of 2858 [79.8%] vs torsemide: 258 of 2858 [13.4%] and bumetanide: 129 of 2858 [6.7%]). Moreover, higher discharge diuretic dose was noted among patients with WHF compared with patients with de novo HF (mean [SD] total daily dose, 63.8 [52.4] mg vs 50.3 [46.2] mg) (Table 1 and eTables 1 and 2 in Supplement 1).
Table 1. Baseline Characteristics of Study Participants (Demographics, Laboratory, and Vital Signs, Heart Failure Characteristics and Medical History) by Heart Failure (HF) Type.
| Characteristic | All patients (N = 2858) | De novo (n = 838) | WHF (n = 2020) | P value |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 64.5 (14.0) | 60.6 (14.5) | 66.1 (13.5) | <.001 |
| Median (IQR) | 65.0 (56.0-75.0) | 61.0 (51.0-71.0) | 67.0 (58.0-76.0) | |
| Sex, No. (%) | ||||
| Female | 1055 (36.9) | 309 (36.9) | 746 (36.9) | .98 |
| Male | 1803 (63.1) | 529 (63.1) | 1274 (63.1) | |
| Race, No. (%) | ||||
| American Indian or Alaska Native | 12 (0.4) | 3 (0.4) | 9 (0.4) | <.001 |
| Asian | 63 (2.2) | 27 (3.2) | 36 (1.8) | |
| Black or African American | 968 (33.9) | 240 (28.7) | 728 (36.1) | |
| Multiracial | 43 (1.5) | 10 (1.2) | 33 (1.6) | |
| Native Hawaiian or Other Pacific Islander | 20 (0.7) | 6 (0.7) | 14 (0.7) | |
| White | 1668 (58.5) | 516 (61.6) | 1152 (57.1) | |
| Other | 79 (2.8) | 35 (4.2) | 44 (2.2) | |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 154 (5.4) | 48 (5.7) | 106 (5.3) | .60 |
| Laboratory values, mean (SD) | ||||
| Sodium, mEq/L | 137.8 (3.7) | 137.8 (3.6) | 137.8 (3.7) | .80 |
| Potassium, mEq/L | 4.04 (0.46) | 4.01 (0.45) | 4.05 (0.46) | .03 |
| Blood urea nitrogen, mg/dL | 33.1 (40.1) | 29.2 (46.0) | 34.8 (37.3) | <.001 |
| Creatinine, mg/dL | 1.403 (0.641) | 1.263 (0.627) | 1.461 (0.638) | <.001 |
| Estimated glomerular filtration ratea | 60.4 (24.9) | 68.6 (24.9) | 57.0 (24.0) | <.001 |
| N-terminal pro–brain-type natriuretic peptide, pg/mL | ||||
| No. | 1376 | 393 | 983 | .01 |
| Median (IQR) | 3912.5 (1937.0-8353.5) | 3569.0 (1855.0-6746.0) | 4136.0 (1954.0-8808.0) | |
| Brain-type natriuretic peptide, pg/mL | ||||
| No. | 1381 | 411 | 970 | .004 |
| Median (IQR) | 964.7 (474.0-1809.0) | 855.0 (423.0-1555.0) | 1022.0 (500.0-1927.0) | |
| Hemoglobin, mean (SD), g/L | 12.10 (4.72) | 12.58 (2.48) | 11.90 (5.37) | <.001 |
| Vital signs | ||||
| Systolic blood pressure, mean (SD), mm Hg | 118.8 (19.8) | 119.6 (19.8) | 118.4 (19.9) | .18 |
| Diastolic blood pressure, mean (SD), mm Hg | 69.7 (12.9) | 71.7 (12.9) | 68.9 (12.7) | <.001 |
| Heart rate, mean (SD), bpm | 80.6 (15.8) | 83.8 (16.1) | 79.3 (15.5) | <.001 |
| BMI, mean (SD) | 32.18 (9.49) | 32.45 (9.21) | 32.07 (9.60) | .18 |
| Heart failure characteristics | ||||
| Ejection fraction rate, No. (%) | ||||
| ≤40 | 1835 (64.2) | 558 (66.6) | 1277 (63.2) | .09 |
| >40 | 799 (28.0) | 227 (27.1) | 572 (28.3) | |
| Unknown | 224 (7.8) | 53 (6.3) | 171 (8.5) | |
| Duration of diagnosis prior to hospitalization, No. (%) | ||||
| ≤ 30 d | 119 (5.9) | 0 | 119 (5.9) | |
| > 30 d and ≤12 mo | 345 (17.1) | 0 | 345 (17.1) | |
| > 12 mo | 1365 (67.6) | 0 | 1365 (67.6) | |
| Unknown | 191 (9.5) | 0 | 191 (9.5) | |
| NYHA class at hospital admission, No. (%) | ||||
| I | 35 (1.2) | 7 (0.8) | 28 (1.4) | .001 |
| II | 284 (10.0) | 88 (10.5) | 196 (9.7) | |
| III | 1190 (41.7) | 320 (38.3) | 870 (43.2) | |
| IV | 404 (14.2) | 102 (12.2) | 302 (15.0) | |
| Unknown | 939 (32.9) | 319 (38.2) | 620 (30.8) | |
| NYHA class at randomization, No. (%) | ||||
| I | 70 (2.4) | 15 (1.8) | 55 (2.7) | <.001 |
| II | 651 (22.8) | 206 (24.6) | 445 (22.0) | |
| III | 977 (34.2) | 246 (29.4) | 731 (36.2) | |
| IV | 196 (6.9) | 53 (6.3) | 143 (7.1) | |
| Unknown | 964 (33.7) | 318 (37.9) | 646 (32.0) | |
| KCCQ Baseline Clinical Summary Score | ||||
| Mean (SD) | 43.18 (23.23) | 46.27 (23.43) | 41.91 (23.03) | <.001 |
| Median (IQR) | 41.1 (25.0-59.8) | 45.3 (28.1-62.8) | 39.6 (24.0-58.3) | |
| Ischemic etiology, No. (%) | 808 (28.3) | 162 (19.3) | 646 (32.0) | <.001 |
| Chronic kidney disease, No. (%) | 1008 (35.3) | 144 (17.2) | 864 (42.8) | <.001 |
| Diabetes, No. (%) | 1363 (47.7) | 319 (38.1) | 1044 (51.7) | <.001 |
| Hypertension, No. (%) | 2329 (81.7) | 609 (72.9) | 1720 (85.3) | <.001 |
| Atrial fibrillation or atrial flutter, No. (%) | 1274 (44.9) | 254 (30.5) | 1020 (50.8) | <.001 |
| Chronic lung disease (including COPD), No. (%) | 675 (23.8) | 112 (13.5) | 563 (28.0) | <.001 |
| Loop diuretics | ||||
| Oral loop diuretic before index hospital admission (most recent), No. (%) | ||||
| No | 939 (32.9) | 645 (77.0) | 294 (14.6) | <.001 |
| Yes | 1919 (67.1) | 193 (23.0) | 1726 (85.4) | |
| Total daily dose prescribed (furosemide equivalents), mg | ||||
| No. | 1901 | 190 | 1711 | <.001 |
| Mean (SD) | 66.1 (61.5) | 43.8 (28.8) | 68.6 (63.6) | |
| Specific loop diuretic before index admission (among those on a loop diuretic), No. (%)b | ||||
| Furosemide | 1532 (79.8) | 178 (92.2) | 1354 (78.4) | |
| Torsemide | 258 (13.4) | 9 (4.7) | 249 (14.4) | |
| Bumetanide | 129 (6.7) | 6 (3.1) | 123 (7.1) | |
| Ethacrynic acid | 0 | 0 | 0 | |
| Discharge, No. (%) | ||||
| Prescribed assigned loop diuretica | 2490 (90.4) | 735 (91.3) | 1755 (90.0) | .05 |
| Total daily dose prescribed (furosemide equivalents), mean (SD), mgb | 79.3 (63.3) | 64.9 (51.7) | 85.4 (66.6) | <.001 |
| Total daily dose prescribed (reported), mean (SD), mg | 59.8 (51.0) | 50.3 (46.2) | 63.8 (52.4) | <.001 |
| Baseline medications taken at the time of randomization, No. (%) | ||||
| ACE or ARB | 1243 (43.5) | 411 (49.0) | 832 (41.2) | <.001 |
| ARNic | 536 (18.8) | 142 (16.9) | 394 (19.5) | .11 |
| ACE or ARB or ARNi | 1717 (60.1) | 533 (63.6) | 1184 (58.6) | .01 |
| Aldosterone antagonist/MRA | 1021 (35.7) | 280 (33.4) | 741 (36.7) | .10 |
| β-Blocker | 2245 (78.6) | 613 (73.2) | 1632 (80.8) | <.001 |
| SGLT-2 inhibitor | 170 (6.2) | 45 (5.5) | 125 (6.4) | .35 |
| Long-acting nitrate | 391 (13.7) | 78 (9.3) | 313 (15.6) | <.001 |
| Thiazide diuretic | 126 (4.4) | 35 (4.2) | 91 (4.5) | .69 |
| Digoxin | 168 (5.9) | 33 (3.9) | 135 (6.7) | .005 |
| Hydralazine | 432 (15.1) | 104 (12.4) | 328 (16.2) | .01 |
| Ivabradine | 8 (0.3) | 1 (0.1) | 7 (0.3) | .45 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor-neprilysin inhibitor; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; KCCQ, Kansas City Cardiomyopathy Questionnaire; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SGLT-2, sodium-glucose transport protein 2.
SI conversion factor: To convert brain-type natriuretic peptide to nanograms per liter, multiply by 1; to convert creatinine to micromoles per liter, multiply by 88.4; to convert hemoglobin to grams per deciliter, divide by 10; to convert potassium to millimoles per liter, multiply by 1; to convert sodium to millimoles per liter, multiply by 1.
Denominator is the patients with known prescription status of the assigned loop diuretic at each visit (ie, excludes patients who died by the time of the visit or who had unknown prescription status at the visit).
Loop diuretic doses were converted to furosemide equivalents with 1-mg bumetanide = 20-mg torsemide = 50-mg ethacrynic acid = 40-mg furosemide for oral diuretics; and 1-mg bumetanide = 50-mg ethacrynic acid = 20-mg furosemide for intravenous diuretics.
ARNi was reported as being taken (yes) or missing. For calculating the percentage of patients taking this medication at baseline, if it was not reported as yes, it was assumed to be no.
Outcomes for Patients With De Novo HF vs WHF
De novo HF was associated with lower all-cause 12-month mortality (de novo, 65 of 838 [9.1%] vs WHF, 408 of 2020 [25.4%]; adjusted hazard ratio [aHR], 0.50; 95% CI, 0.38-0.66; P < .001) (eTable 3 and eFigure 1 in Supplement 1). After multivariate adjustments and stratification by EF, this finding persisted among patients with EF of 40% or less but not in patients with EF greater than 40% (eTable 3, eFigures 2 and 3 in Supplement 1).
Findings were generally similar in analyses of composite all-cause mortality or rehospitalization over 12 months, all-cause hospitalization over 12 months, and total hospitalizations over 12 months (eTables 3 and 4 and eFigures 4, 5, and 6 in Supplement 1).
Similarly, lower all-cause first rehospitalization at 12 months and greater improvement from baseline in KCCQ-CSS at 12 months were noted among patients with de novo HF (median [IQR]: de novo, 29.94 [27.35-32.54] vs WHF, 23.68 [21.62-25.74]; adjusted estimated difference in means: 6.26; 95% CI, 3.72-8.81; P < .001). After adjustment and EF stratification, this finding remained present only among patients with EF of 40% or less (eTable 5 in Supplement 1).
Outcome of Torsemide vs Furosemide in De Novo HF vs WHF
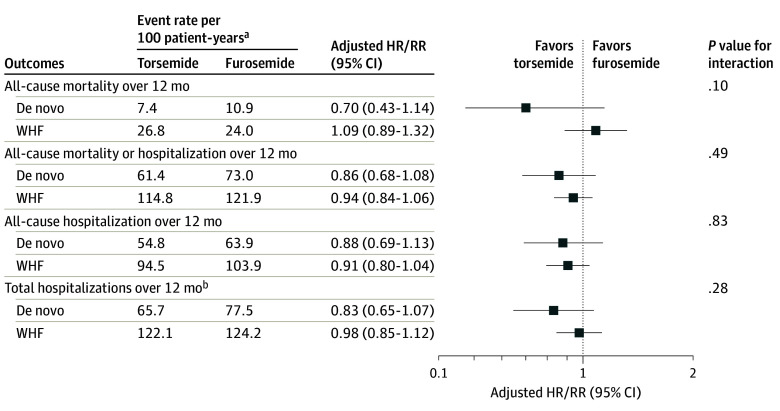
In comparing a treatment strategy of torsemide vs furosemide, there was no statistically significant difference in 12-month mortality among de novo HF (No. of events [rate per 100 patient-years]: torsemide, 27 [7.4%] vs furosemide, 38 [10.9%]; aHR, 0.70; 95% CI, 0.40-1.14; P = .15) or WHF (torsemide 212 [26.8%] vs furosemide, 196 [24.0%]; aHR, 1.08; 95% CI, 0.89-1.32; P = .42; P for interaction = .10) (Table 2 and Figure). Moreover, when stratified by EF, there was an associated trend in reduced mortality at 12 months among the torsemide group in patients with de novo HF with EF of 40% or less, but this finding was not seen among patients with WHF (Table 2 and eFigures 7 and 8 in Supplement 1).
Table 2. Time-to-Event Outcomes Comparison Between Randomized Diuretic Treatment Groups Stratified by De Novo vs Worsening Chronic Heart Failure (WHF).
| Outcomes | De novo | WHF | P for interactionc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Torsemide events (rate per 100 PY)a | Furosemide events (rate per 100 PY)a | HR/RR (T vs F)b | P value | Torsemide rate (rate per 100 PY)a | Furosemide rate (rate per 100 PY)a | HR/RR (T vs F)b | P value | ||
| All-cause mortality over 12 mo a , b | |||||||||
| Overall | 27 (7.4) | 38 (10.9) | 0.70 (0.4-1.14) | .15 | 212 (26.8) | 196 (24.0) | 1.08 (0.89-1.32) | .42 | .10 |
| EF level, % | |||||||||
| ≤40 | 13 (5.4) | 25 (10.9) | 0.50 (0.26-0.99) | .04 | 140 (27.7) | 122 (24.4) | 1.10 (0.86-1.41) | .45 | .03 |
| >40 | 11 (10.7) | 8 (8.2) | 1.22 (0.49-3.07) | .67 | 53 (23.0) | 56 (23.6) | 0.93 (0.63-1.36) | .71 | .59 |
| All-cause mortality or rehospitalization over 12 mo | |||||||||
| Overall | 137 (61.4) | 156 (73) | 0.86 (0.68-1.08) | .20 | 535 (114.8) | 545 (121.9) | 0.94 (0.83-1.06) | .33 | .49 |
| EF level, % | |||||||||
| ≤40 | 84 (55.2) | 89 (60.9) | 0.93 (0.69-1.25) | .63 | 345 (115.7) | 333(121.9) | 0.94 (0.81-1.10) | .47 | .92 |
| >40 | 45 (76.6) | 50 (93.1) | 0.87 (0.58-1.30) | .49 | 151 (110.9) | 160 (124.2) | 0.90 (0.72-1.13) | .35 | .88 |
| All-cause first rehospitalization over 12 mo | |||||||||
| Overall | 121 (54.8) | 132 (63.9) | 0.88 (0.69-1.13) | .32 | 411 (94.5) | 442 (103.9) | 0.91 (0.79-1.04) | .17 | .83 |
| EF level, % | |||||||||
| ≤40 | 74 (49.0) | 72 (51.0) | 0.99 (0.72-1.8) | .97 | 264 (95.0) | 267 (102.1) | 0.93 (0.78-1.10) | .38 | .71 |
| >40 | 39 (67.6) | 46 (88.4) | 0.81 (0.53-1.25) | .35 | 118 (92.0) | 134 (110.2) | 0.85 (0.66-1.09) | .21 | .85 |
Abbreviations: EF, ejection fraction; F, furosemide; HR, hazard ratio; PY, patient-years; RR, rate ratio; T, torsemide.
For each cell, for all-cause mortality and all-cause mortality or rehospitalization over 12 months, the numbers displayed are number of events (Kaplan-Meier failure rate %) event rate per 100 PY of follow-up. For all-cause first rehospitalization over 12 months, the numbers displayed are number of events (cumulative incidence rate %) event rate per 100 PY of follow-up.
For all-cause mortality and all-cause mortality/re-hospitalization over 12 months, HR, 95% CI, and P value are based on a Cox proportional hazards regression model. For all-cause first primary rehospitalization over 12 months, HR, 95% CI, and P value is based on a Cox proportional hazards model where death is considered as competing risk and is censored at time of death. For the overall patient model, the covariates are diuretic treatment (T, F), HF type, age, sex, baseline EF categories (≤40%, 41%-49%, ≥50%, unknown). For each stratified analysis by EF level, the covariates are diuretic treatment, HF type, age, sex, and continuous baseline EF.
The interaction P values are between diuretic treatment and HF type for overall patients and each EF level.
Figure. Treatment Effect of Torsemide vs Furosemide Among Patients With De Novo vs Worsening Chronic Heart Failure.
Forest plot of hazard ratio (HR)/rate ratio (RR) comparing randomized diuretic treatments (torsemide vs furosemide) for all end points among patients with de novo heart failure and patients with worsening chronic heart failure (WHF).
aFirst event rates per 100 patient-years are reported for all-cause mortality, all-cause mortality or hospitalization, and all-cause hospitalization.
bFor total hospitalizations, the total event rates per 100 patient-years are reported.
Additionally, irrespective of randomized loop diuretic, there were no significant differences in all-cause mortality or rehospitalization, first all-cause hospitalization, or total hospitalizations at 12 months, in either de novo HF or WHF (Table 2 and eTable 6 in Supplement 1). No significant difference in change in KCCQ-CSS was noted for either de novo HF or WHF (eTable 7 and eFigures 7 and 8 in Supplement 1).
Study Drug Adherence
Diuretic adherence at discharge and month 1, 6, and 12, respectively, is displayed in eTable 8 in Supplement 1. At the time of discharge, overall rates of diuretic discontinuation were low (76 of 2786 [2.8%]) with no significant differences among those with de novo HF or WHF. However, a steady increase in discontinuation of loop diuretics was noted among patients with de novo HF at month 1 (123 of 2786 [6.5%]), month 6 (142 of 2786 [9.1%]), and month 12 (164 of 2786 [12.9%]).
Discussion
In the TRANSFORM-HF trial, de novo HF status was associated with better outcomes as compared with WHF. However, irrespective of de novo vs WHF status, results of this post hoc analysis suggest that there was no significant difference between torsemide and furosemide with respect to 12-month clinical or patient-reported outcomes.
Consistent with previous data,5,6,7 patients with de novo HF were slightly younger and more likely to have a nonischemic etiology when compared with those with WHF. Yet, to our knowledge, no prior studies have explored the differential association of discharge diuretic type with outcome in de novo HF vs WHF. Although torsemide has been shown to have better absorption and bioavailability when compared with furosemide,8 we found no significant association between diuretic type and selected primary and secondary end points in either de novo HF or WHF. Despite the observed nominally significant reduction in mortality at 12 months among the torsemide group in patients with de novo HF and an EF of 40% or less (with significant interaction P value of .03), the low event rates among patients with de novo HF and absence of a similar trend with other clinical end points makes this isolated finding likely related to chance and, therefore, not clinically relevant.
Limitations
Limitations of this study should be noted. Sample size, crossover rates, and diuretic discontinuation during the follow-up period may all have impacted our results. Despite statistical adjustment, residual or unmeasured confounding may have influenced our results.
Conclusions
In this post hoc analysis of the TRANSFORM-HF randomized clinical trial, among patients discharged with acute HF, de novo HF was associated with better outcomes when compared with WHF. Regardless of de novo HF vs WHF status, there was no significant difference between torsemide and furosemide with respect to 12-month clinical or patient-reported outcomes. Importantly, the observed high mortality among the WHF population and persistence of congestion at the time of randomization merits further investigation.
eTable 1. Baseline Characteristics of Study Participants (Demographics, Laboratory, and Vital Signs, Heart Failure Characteristics, and Medical History) by HF Type, for Patients With ≤40% EF
eTable 2. Baseline Characteristics of Study Participants (Demographics, Laboratory, and Vital Signs, Heart Failure Characteristics, and Medical History) by HF Type, for Patients With >40% EF
eTable 3. Time-to-Event Outcome Comparison Between De Novo and WHF for Overall Patients and Stratified by Ejection Fraction
eTable 4. Effect of HF Type on Total Rehospitalizations Through Month 12 for Overall Patients and stratified by ejection fraction
eTable 5. Comparison of KCCQ Clinical Summary Score by HF Type for overall patients and by EF Levels–Least Square Mean by Visit
eTable 6. Treatment Effect on Total Rehospitalizations Through Month 12 by HF Type for Overall Patients and Stratified by EF Levels
eTable 7. Comparison of KCCQ Clinical Summary Score Between Treatment Diuretics Groups by HF Types–Least Square Mean by Visit for Overall Patients and Stratified by EF Levels
eTable 8. Diuretic Dose Adherence at Discharge, Month 1, 6, and 12 by Heart Failure Type and Diuretic Treatment
eFigure 1. Unadjusted Kaplan-Meier curves for time to all-cause mortality comparing De novo and worsening HF for overall patients over 12 months
eFigure 2. Unadjusted Kaplan-Meier Curves for Time to All-Cause Mortality Comparing De Novo and WHF for Patients With EF Level ≤40% Over 12 Months
eFigure 3. Unadjusted Kaplan-Meier Curves for Time to All-Cause Mortality Comparing De Novo and WHF for Patients With EF Level >40% Over 12 Months
eFigure 4. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Overall Patients
eFigure 5. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Patients With EF Level ≤40%
eFigure 6. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Patients With EF Level >40%
eFigure 7. Forest Plot of HR/RR Comparing Randomized Diuretic Treatments (Torsemide vs Furosemide) for Overall Patients and Patients for All End Points Among Patient With EF Level ≤40%
eFigure 8. Forest Plot of HR/RR Comparing Randomized Diuretic Treatments (Torsemide vs Furosemide) for Overall Patients and Patients for All End Points Among Patient With EF Level >40%
Data Sharing Statement.
References
- 1.Sinnenberg L, Givertz MM. Acute heart failure. Trends Cardiovasc Med. 2020;30(2):104-112. doi: 10.1016/j.tcm.2019.03.007 [DOI] [PubMed] [Google Scholar]
- 2.Mebazaa A, Yilmaz MB, Levy P, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur Heart J. 2015;36(30):1958-1966. doi: 10.1093/eurheartj/ehv066 [DOI] [PubMed] [Google Scholar]
- 3.Greene SJ, Velazquez EJ, Anstrom KJ, et al. ; TRANSFORM-HF Investigators . Pragmatic design of randomized clinical trials for heart failure: rationale and design of the TRANSFORM-HF Trial. JACC Heart Fail. 2021;9(5):325-335. doi: 10.1016/j.jchf.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mentz RJ, Anstrom KJ, Eisenstein EL, et al. ; TRANSFORM-HF Investigators . Effect of torsemide vs furosemide after discharge on all-cause mortality in patients hospitalized with heart failure: the TRANSFORM-HF randomized clinical trial. JAMA. 2023;329(3):214-223. doi: 10.1001/jama.2022.23924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene SJ, Hernandez AF, Dunning A, et al. Hospitalization for recently diagnosed vs worsening chronic heart failure: from the ASCEND-HF Trial. J Am Coll Cardiol. 2017;69(25):3029-3039. doi: 10.1016/j.jacc.2017.04.043 [DOI] [PubMed] [Google Scholar]
- 6.Butt JH, Fosbøl EL, Gerds TA, et al. Readmission and death in patients admitted with new-onset vs worsening of chronic heart failure: insights from a nationwide cohort. Eur J Heart Fail. 2020;22(10):1777-1785. doi: 10.1002/ejhf.1800 [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, et al. ; OPTIMIZE-HF Investigators and Hospitals . Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008;168(8):847-854. doi: 10.1001/archinte.168.8.847 [DOI] [PubMed] [Google Scholar]
- 8.Buggey J, Mentz RJ, Pitt B, et al. A reappraisal of loop diuretic choice in heart failure patients. Am Heart J. 2015;169(3):323-333. doi: 10.1016/j.ahj.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Study Participants (Demographics, Laboratory, and Vital Signs, Heart Failure Characteristics, and Medical History) by HF Type, for Patients With ≤40% EF
eTable 2. Baseline Characteristics of Study Participants (Demographics, Laboratory, and Vital Signs, Heart Failure Characteristics, and Medical History) by HF Type, for Patients With >40% EF
eTable 3. Time-to-Event Outcome Comparison Between De Novo and WHF for Overall Patients and Stratified by Ejection Fraction
eTable 4. Effect of HF Type on Total Rehospitalizations Through Month 12 for Overall Patients and stratified by ejection fraction
eTable 5. Comparison of KCCQ Clinical Summary Score by HF Type for overall patients and by EF Levels–Least Square Mean by Visit
eTable 6. Treatment Effect on Total Rehospitalizations Through Month 12 by HF Type for Overall Patients and Stratified by EF Levels
eTable 7. Comparison of KCCQ Clinical Summary Score Between Treatment Diuretics Groups by HF Types–Least Square Mean by Visit for Overall Patients and Stratified by EF Levels
eTable 8. Diuretic Dose Adherence at Discharge, Month 1, 6, and 12 by Heart Failure Type and Diuretic Treatment
eFigure 1. Unadjusted Kaplan-Meier curves for time to all-cause mortality comparing De novo and worsening HF for overall patients over 12 months
eFigure 2. Unadjusted Kaplan-Meier Curves for Time to All-Cause Mortality Comparing De Novo and WHF for Patients With EF Level ≤40% Over 12 Months
eFigure 3. Unadjusted Kaplan-Meier Curves for Time to All-Cause Mortality Comparing De Novo and WHF for Patients With EF Level >40% Over 12 Months
eFigure 4. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Overall Patients
eFigure 5. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Patients With EF Level ≤40%
eFigure 6. Unadjusted Cumulative Incidence Curves for All-Cause Hospitalization Over 12 Months Comparing De Novo and WHF for Patients With EF Level >40%
eFigure 7. Forest Plot of HR/RR Comparing Randomized Diuretic Treatments (Torsemide vs Furosemide) for Overall Patients and Patients for All End Points Among Patient With EF Level ≤40%
eFigure 8. Forest Plot of HR/RR Comparing Randomized Diuretic Treatments (Torsemide vs Furosemide) for Overall Patients and Patients for All End Points Among Patient With EF Level >40%
Data Sharing Statement.