Abstract
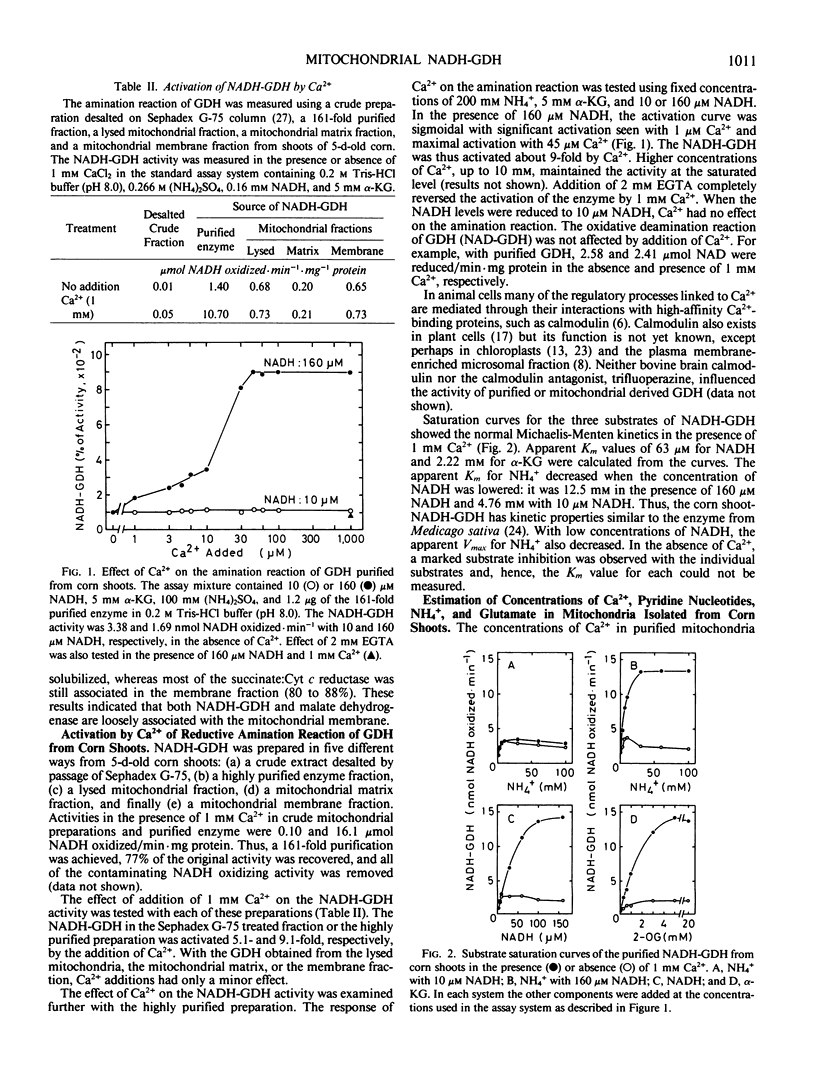
The amination of α-ketoglutarate (α-KG) by NADH-glutamate dehydrogenase (GDH) obtained from Sephadex G-75 treated crude extracts from shoots of 5-day-old seedlings was stimulated by the addition of Ca2+. The NADH-GDH purified 161-fold with ammonium sulfate, DEAE-Toyopearl, and Sephadex G-200 was also activated by Ca2+ in the presence of 160 micromolar NADH. However, with 10 micromolar NADH, Ca2+ had no effect on the NADH-GDH activity. The deamination reaction (NAD-GDH) was not influenced by the addition of Ca2+.
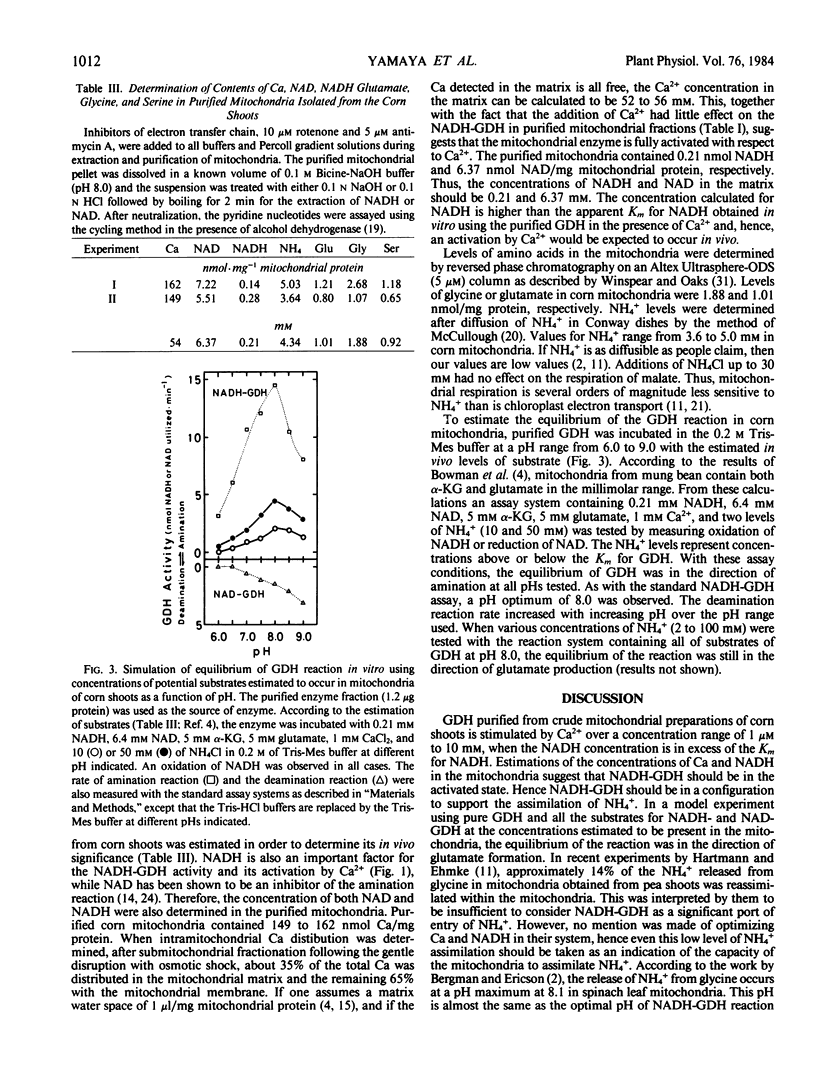
About 25% of the NADH-GDH activity was solubilized from purified mitochondria after a simple osmotic shock treatment, whereas the remaining 75% of the activity was associated with the mitochondrial membrane fraction. When the lysed mitochondria, mitochondrial matrix, or mitochondrial membrane fraction was used as the source of NADH-GDH, Ca2+ had little effect on its activity. The mitochondrial fraction contained about 155 nanomoles Ca per milligram of mitochondrial protein, suggesting that the NADH-GDH in the mitochondria is already in an activated form with regard Ca2+. In a simulated in vitro system using concentrations of 6.4 millimolar NAD, 0.21 millimolar NADH, 5 millimolar α-KG, and 5 millimolar glutamate thought to occur in the mitochondria, together with 1 millimolar Ca2+, 10 and 50 millimolar NH4+, and purified enzyme, the equilibrium of GDH was in the direction of glutamate formation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arron G. P., Spalding M. H., Edwards G. E. Stoichiometry of carbon dioxide release and oxygen uptake during glycine oxidation in mitochondria isolated from spinach (Spinacia oleracea) leaves. Biochem J. 1979 Nov 15;184(2):457–460. doi: 10.1042/bj1840457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E. J., Ikuma H., Stein H. J. Citric Acid cycle activity in mitochondria isolated from mung bean hypocotyls. Plant Physiol. 1976 Sep;58(3):426–432. doi: 10.1104/pp.58.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Chou K. H., Splittstoesser W. E. Glutamate dehydrogenase from pumpkin cotyledons: characterization and isoenzymes. Plant Physiol. 1972 Apr;49(4):550–554. doi: 10.1104/pp.49.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. A simple spectrophotometric assay for fumarate hydratase in crude tissue extracts. Anal Biochem. 1978 Mar;85(1):271–275. doi: 10.1016/0003-2697(78)90299-3. [DOI] [PubMed] [Google Scholar]
- Jarrett H. W., Brown C. J., Black C. C., Cormier M. J. Evidence that calmodulin is in the chloroplast of peas and serves a regulatory role in photosynthesis. J Biol Chem. 1982 Nov 25;257(22):13795–13804. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967 Aug;17(2):297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Nagel M., Hartmann T. Glutamate dehydrogenase from Medicago sativa L., purification and comparative kinetic studies of the organ-specific multiple forms. Z Naturforsch C. 1980 May-Jun;35(5-6):406–415. doi: 10.1515/znc-1980-5-610. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Douce R., Akazawa T. Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol. 1982 Apr;69(4):916–920. doi: 10.1104/pp.69.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya T., Oaks A., Matsumoto H. Stimulation of Mitochondrial Calcium Uptake by Light during Growth of Corn Shoots. Plant Physiol. 1984 Jul;75(3):773–777. doi: 10.1104/pp.75.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]


