Abstract
Background
Anticoagulants (AC) were introduced in March 2020 as standard of care in nursing home (NH) residents affected with COVID‐19 in the Stockholm region, Sweden. ACs are proven to reduce the risk of complications and mortality from COVID‐19 among patients of other ages and settings, but there is limited scientific evidence underpinning this practice in the NH setting.
Methods
This matched cohort study included 182 NH residents in the Stockholm Region diagnosed with COVID‐19 in March–May 2020. The main exposure was any AC treatment. Exposed (n = 91), 49% prevalent (pre‐COVID‐19 diagnosis) AC and 51% incident AC were compared with unexposed controls (n = 91). The outcome was 28‐days all‐cause mortality after COVID‐19 infection. The mortality odds ratios (OR) were assessed using logistic regression, adjusted for age, sex, multimorbidity, and mobility, also stratified by incident or prevalent AC‐type, age group, and sex.
Results
Of the 182 individuals diagnosed with COVID‐19 (median age 88 years, 68% women), 39% died within 28 days after diagnosis. Use of either incident or prevalent AC was associated with a reduced, adjusted 28‐day mortality (OR[95% CI]: 0.31[0.16–0.62]). In stratified analyses, the association was significant in both age groups: 70–89 (OR: 0.37 [0.15–0.89]) and 90–99 years of age (OR: 0.22 [0.07–0.65]. In sex‐stratified analysis, the AC‐lowering effect was significant in women only (OR: 0.28[0.11–0.67]). In the analyses stratified by AC type, the mortality‐lowering effect was observed for both prevalent AC (OR: 0.35[0.12–0.99]) and incident AC (OR: 0.29[0.11–0.76]).
Conclusions
Both prevalent and incident use of ACs in prophylactic dosing was associated with reduced 28‐day mortality among older individuals with COVID‐19 in a NH setting. The effect was seen across age‐strata and in women. The findings present new insight in best practice for individuals diagnosed with COVID‐19 in the NH setting.
Keywords: anticoagulants, cohort studies, COVID‐19, mortality, nursing home, treatment outcome
Key points
Both prevalent use of DOAC and newly initiated LMWH at the time of diagnosis of COVID‐19 was associated with lowered mortality among older individuals living in nursing homes.
This effect was significant among females but not males, which may be explained by limited power.
The study did not detect any adverse events, but the number of exposed individuals in our study was not sufficient to explore the risk of serious adverse events, such as bleeding.
Why does this paper matter?
Our findings provide strong real‐world evidence to support the practice of keeping prevalent DOAC or initiating LMWH treatment to reduce mortality from COVID‐19 in the nursing home setting during the prevaccination phase of the pandemic.
1. INTRODUCTION
The force of the coronavirus pandemic is waning, but COVID‐19 still proposes a significant global health concern. 1 Advanced age has emerged as the most important risk factor for severe illness and death from COVID‐19, and older adults residing in nursing homes (NHs) may have been disproportionally affected. 2 , 3 , 4 , 5 It has become clear that coagulopathy, including micro‐ and macroembolic events, significantly contribute to COVID‐19 morbidity and mortality. Meta‐analyses indicate that venous thromboembolism (VTE) occurs in between 15% and 30% of individuals hospitalized with COVID‐19. 6 , 7 The benefits of initiating anticoagulants (AC) in individuals affected with COVID‐19 was recognized early in the pandemic 8 and the protective effect has since been confirmed in systematic reviews of both observational and randomized clinical trials. 9 , 10 , 11 Published studies to date include mainly middle‐aged individuals in inpatient or intensive care, 8 , 9 , 10 , 12 , 13 and scientific evidence on AC treatment in COVID‐19 among older individuals residing in NHs is scarce.
Guidance protocols promoting AC treatment in COVID‐19 was swiftly implemented early in the pandemic to support such use in critical care, and later in general inpatient care. 14 , 15 , 16 , 17 Following the first indication in the Stockholm region, ACs became standard of care also for the older population living in NHs. Our study aims to evaluate the use of ACs in COVID‐19 in the NH setting, which was mainly based on evidence from younger populations.
In the Stockholm Region, 14,500 (3,7%) individuals 65 years or older were residing in NHs for older individuals in 2020. 18 The median age of the population residing in NHs in Sweden is 87 years, 2 and a large proportion are defined as frail, which entails an accumulation of several comorbidities and medications and loss of functional capacity. 19
2. METHODS
2.1. Study design
This study is a retrospective matched cohort study of old NH residents with confirmed COVID‐19. Individuals treated with ACs were compared with age‐ and sex‐matched controls who were not receiving such treatment.
2.2. Study sample
Data were collated for 182 individuals diagnosed with COVID‐19 between March 17 and May 30, 2020, from 23 randomly selected NH units in Stockholm, Sweden. First, we randomly selected individuals with the main exposure (i.e., any use of ACs, n = 91). Then, we selected 91 matched controls for the 91 exposed individuals. We excluded individuals under the age of 70 at the time of COVID‐19 diagnosis and those with restricted journal access (Figure 1).
Figure 1.
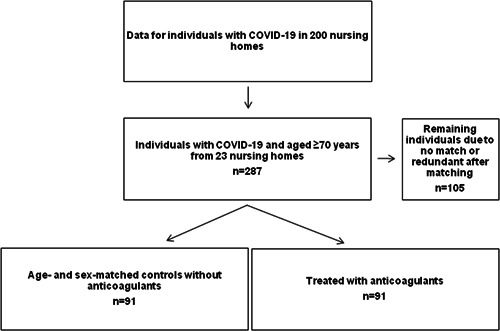
Selection of patients for the analysis.
NHs in Sweden are governed by the local municipalities, funded by tax, and available to the citizens regardless of income or insurance status. Our study was conducted within the administration of one publicly funded, health care provider, which provides physician services on primary care level to around 200 NH units with more than 7500 residents. NHs were included consecutively based on the research group's knowledge of incident cases. Hence, during the data collection period (September 2020–December 2020), 23 NH units were included. The facilities were located throughout the Stockholm Region; 11 units were located within the city of Stockholm, 8 in southern suburban municipalities, and 4 in northern suburban municipalities. The NHs differed in size between 42 and 158 residents (median 74 residents).
2.3. Medical and other information used in the analysis
Demographic characteristics and medical information such as comorbidity, kidney function, mobility, date of diagnosis of COVID‐19, medical interventions including drug treatments, and mortality were extracted from the electronic medical records.
2.4. COVID‐19 diagnosis
COVID‐19 was defined as a documented diagnosis according to the ICD‐10 diagnostic code U07.1 or U07.2 in the medical chart. The diagnosis was based on laboratory‐verified detection of SARS‐CoV‐2 specific nucleic acid by real‐time reverse transcription polymerase chain reaction (rRT‐PCR), serum SARS‐CoV‐2 antibody detection, or a strong clinical suspicion based on clinical course and epidemiological circumstances.
2.5. Definition of exposure
Our main exposure of interest was treatment with any AC during a verified episode of COVID‐19. ACs were defined as any substance within the therapeutic classes low molecular weight heparins (LMWH), direct acting oral anticoagulants (DOAC), or vitamin K‐antagonists.
Exposure to ACs was defined as (i) documented incident use of ACs in prophylactic dose prescribed at the NH, in primary care or at inpatient care, at the time of COVID‐19 diagnosis, or (ii) documented prevalent use of ACs in prophylactic dose for other indications at the time of COVID‐19 diagnosis. Indication for treatment with DOAC was either stroke prevention in atrial fibrillation or secondary prevention of VTE.
2.6. Definition of outcome
The primary outcome was mortality within 28 days after being diagnosed with COVID‐19.
Patients treated with ACs were compared with patients not receiving such treatment.
2.7. Covariates
Level of mobility was categorized into “walks with or without aid” or “in wheel chair or bedridden.” Two missing values were replaced with the most frequent value in the data (“walks with or without aid”). Multimorbidity was defined as three or more concomitant chronic and significant health conditions, present in the medical records within the last year from inclusion, in three or more separate diagnostic groups in the ICD‐10 classification system 20 : Common cancer diseases (prostate C61, mammary C50, colorectal C18, bronchial and pulmonary C34), Diabetes mellitus (E10‐11, E13‐14), Hypertension and ischemic heart disease (I20‐22, I24‐25), Chronic obstructive pulmonary disease (J44), Kidney failure (N17‐19), Congestive heart failure (I50), Depressive disorders (F32‐33), Chronic neurodegenerative disorders (Alzheimer dementia, Lewy body dementia, Parkinson's disease, and related disorders) (G20, G23, G30, G31), Cerebrovascular disease (including stroke and transitory ischemic attack [TIA]) (I60‐69 and G45.9), and Heart arrhythmia (AV‐block II and III, Atrial fibrillation and flutter I44.1, I44.2, and I48). Estimated renal glomerular filtration rate (eGFR) was measured using standard clinical methodology (revised equations based on the Lund–Malmö study cohort). 21 Calendar week of COVID‐19‐diagnosis was used as the variable “time of infection.”
2.8. Statistical analysis
2.8.1. Matching of controls
Controls without AC treatment were matched with individuals treated with ACs 1:1 by sex and age in 5‐year strata. If more than one suitable match was available, a randomized number generator was used to decide which one to include. Only matched pairs were included in the analysis.
2.8.2. Primary outcome
Binary logistic regression was used to assess odds ratios (ORs) and corresponding 95% confidence intervals (CI) for the relationship between the exposure and mortality. For the primary outcome, unadjusted ORs (Model 1) and ORs adjusted for age, sex, mobility, and multimorbidity (Model 2) were assessed. Additionally, this analysis was performed stratified by sex and by individuals exposed to either incident or prevalent ACs.
2.8.3. Additional analyses
In our sensitivity analyses, we performed the mortality analysis using logistic regression with the main exposure adjusted for the same variables as in the main analysis but also adjusting for eGFR and time of infection. Finally, we assessed the mortality ORs for the main exposure stratified by age group and sex.
We visualized the 28‐day survival of the exposed and unexposed individuals, also stratified by sex and AC type using Kaplan–Meier curves and performed pair‐wise comparisons using the Log‐Rank test.
2.8.4. Variable coding and statistical software
The following variables were used as dichotomous variables: exposure, sex, multimorbidity (0–2 diseases or 3+ diseases), and mobility. Age, eGFR, and time of infection were used as continuous variables. In the additional mortality analysis stratified by age group, age groups were 79–89 and 90–99 years of age, and additionally, age group 79–89 was split into 10‐year intervals (79–79 and 80–89 years). In the additional mortality analysis adjusting for eGFR, individuals without information on eGFR were excluded.
Threshold for the statistical significance was set to p value of 0.05. All analyses were performed using R software (version 4.0.5) and R‐packages ggplot2, survival, and survminer.
3. RESULTS
Our sample included 182 individuals (median age 88 years, 68% women) with COVID‐19, residing at any of the 23 NHs (Table 1). Of these, 91 individuals had been treated with ACs to the discretion of the medical professionals in the clinical context of COVID‐19 infection. Ninety‐one matched controls had not been treated with ACs during the course of their COVID‐19 infection. Date of COVID‐19 infection in individuals with AC treatment and matched controls is shown in Figure 2. The treated (exposed) and untreated (unexposed) individuals were matched 1:1 according to sex and age group resulting in balanced groups with respect to the matching factors. Among exposed and unexposed, respectively, 13% versus 18% had no walking disabilities, and 35% versus 32% used a wheelchair or were bedridden. The exposed group had a higher percentage of individuals with multimorbidity (Table 1).
Table 1.
Characteristics of the matched cohort of nursing home residents with COVID‐19.
| Variable | Exposed, (n = 91) | Unexposed, (n = 91) |
|---|---|---|
| Exposure: Anticoagulant usage, n (%) | ||
| LMWH, incident or DOAC/Warfarin, prevalent | 91 (100) | 0 (0) |
| LMWH, incident | 45 (49) | 0 (0) |
| DOAC/Warfarin, prevalent | 46 (51) | 0 (0) |
| Age, years | ||
| Median (25th–75th quartile) | 88 (81–93) | 88 (81–93) |
| 70–79, n (%) | 15 (16) | 15 (16) |
| 80–89, n (%) | 38 (42) | 38 (42) |
| 90–99, n (%) | 38 (42) | 38 (42) |
| Male, n (%) | 29 (32) | 29 (32) |
| COVID‐19 diagnosis, calendar week, Median (25th–75th quartile) | 17 (15–20) | 16 (14–17) |
| Level of mobility, n (%) | ||
| No mobility impairment | 12 (13) | 16 (18) |
| Walking aid/rollator | 45 (49) | 47 (52) |
| Wheelchair/bedridden | 32 (35) | 28 (31) |
| Information not available | 0 | 2 (2) |
| Multimorbidity, 3 or more comorbidities, n (%) | 62 (68) | 41 (45) |
| eGFR, mL/min | ||
| Median (25th–75th quartile) | 56 (46–69) | 56 (44–67) |
| Information not available, n (%) | 3 (3) | 9 (10) |
Abbreviations: DOAC, direct acting oral anticoagulant; eGFR, estimated glomerular rate; LMWH, low molecular weight heparin.
Figure 2.
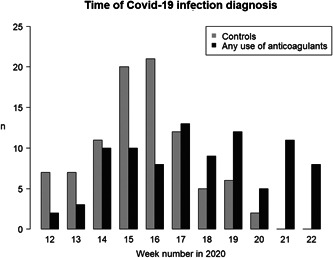
COVID‐19 infections over time in the matched study cohort. The frequencies of COVID‐19 infections by week number of the year in 2020 are shown separately for the anticoagulants exposed (n = 91) and unexposed (n = 91) individuals in the nursing home.
Among the exposed and the unexposed individuals, respectively, 67% (n = 61) versus 75% (n = 69) received standard supporting care at the NH. That is, they received no documented specific therapeutic interventions during their COVID‐19 illness. The specific therapeutic interventions for those exposed to any ACs (and prevalence) were prescribed antibiotics (13%), intravenous fluids (21%), and oxygen treatment on site (5%), and being admitted to hospital (10%). Among the unexposed, the corresponding prevalence rates were 10%, 16%, 3%, and 4%. No patient in the cohort received cortisone treatment. No significant adverse bleeding events were documented during the study period.
Forty‐five individuals (49%) had prevalent (ongoing) AC treatment at the time of COVID‐19 diagnosis; 41 individuals with DOAC (apixaban, dabigatran, or rivaroxaban) and four individuals were treated with warfarin. Forty‐six individuals (51%) were newly initiated on an AC treatment following their COVID‐19 diagnosis, all on LMWH (daltaperin, tinzaparin) in prophylactic low dose regimen with adjustment according to renal clearance. One participant was switched from DOAC to LMWH use on Day 10 due to clinical deterioration and was coded as LMWH exposure. Unfractionated heparin was not used in this study sample and was not relevant for the analysis.
3.1. Primary outcome
Of the 182 NH residents, 39% (n = 71) died within 28 days after COVID‐19 diagnosis (Table 2). The mortality rate was 29% (26/91) among individuals treated with any AC (i.e., the main exposure) and 49% (45/91) among the matched controls. The main exposure was associated with a reduced 28‐day mortality in the unadjusted analysis (model 1) and in the analyses adjusted for age, sex, multimorbidity, and mobility (model 2, OR[95% CI]: 0.31[0.16–0.62]) (Table 2). In the mortality analysis stratified by the type of AC treatment (either prevalent [mainly DOAC] or incident [LMWH]), the mortality‐lowering effect was observed for the incident (model 2, OR[95% CI]: 0.29[0.11–0.76]) and the prevalent AC use (model 2, OR[95% CI]: 0.35[0.12–0.99]) (Table 2).
Table 2.
Anticoagulant treatment and risk of 28‐day COVID‐19 mortality.
| (a) Stratified by anticoagulant type in nursing home residents | ||||||
|---|---|---|---|---|---|---|
| Exposure | Sample | Exposed, n (matched unexposed controls, n) | Deceased, n (%) | OR (95% CI) | ||
| Exposed | Unexposed | Model 1 | Model 2 | |||
| Any use of anticoagulants | All | 91 (91) | 26 (29) | 45 (49) | 0.41 (0.22–0.75) | 0.31 (0.16–0.62) |
| LMWH, incident | Including LMWH, incident users + controls | 46 (46) | 10 (22) | 21 (46) | 0.33 (0.13–0.81) | 0.29 (0.11–0.76) |
| DOAC/Warfarin,a prevalent | Including DOAC/Warfarin, prevalent users +controls | 45 (45) | 16 (36) | 24 (53) | 0.48 (0.20–1.12) | 0.35 (0.12–0.99) |
| (b) Stratified by sex and age group in nursing home residents | |||||
|---|---|---|---|---|---|
| Sample | Exposed, n (matched unexposed controls, n) | Deceased, n (%) | OR (95% CI) | ||
| Exposed | Unexposed | Model 1 | Model 2 | ||
| All | 91 (91) | 26 (29) | 45 (49) | 0.41 (0.22–0.75) | 0.31 (0.16–0.62) |
| Women | 62 (62) | 12 (19) | 25 (40) | 0.36 (0.36–0.78) | 0.28 (0.11–0.67) |
| Men | 29 (29) | 14 (48) | 20 (69) | 0.42 (0.14–1.21) | 0.39 (0.12–1.16) |
| 70–89 years | 53 (53) | 15 (28) | 25 (47) | 0.44 (0.19–0.98) | 0.37 (0.15–0.89) |
| 90–99 years | 38 (38) | 11 (29) | 20 (53) | 0.37 (0.14–0.93) | 0.22 (0.07–0.65) |
Note: The unadjusted (Model 1) and adjusted (Model 2) odds ratios were assessed using binary logistic regression. In model 2, the analysis was adjusted for age, sex, multimorbidity, and mobility. Bold values are significant p < 0.05.
Abbreviations: DOAC, direct acting oral anticoagulant; LMWH, low molecular weight heparin; OR, odds ratio.
Warfarin treatment (n = 4).
3.2. Additional analyses
Survival of the individuals with any AC treatment and their age‐ and sex‐matched controls was visualized using Kaplan–Meier survival estimates (Figure 3A). In addition, similar visualization was performed for individuals with incident or prevalent ACs and the control group (Figure 3B), and stratified by sex (Figure 3C).
Figure 3.
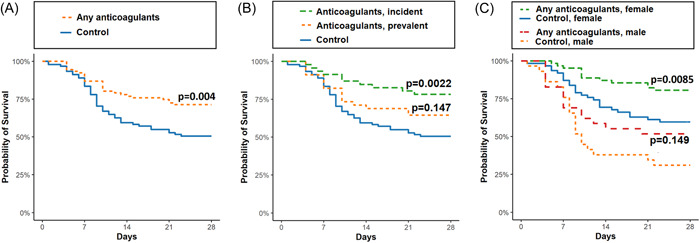
Kaplan–Meier survival estimates in COVID‐19 infected nursing home residents with and without anticoagulant treatment. Survival was compared between individuals with any ACs (n = 91) and their age‐ and sex‐matched controls (n = 91) in (A), and further stratified by incident (n = 46) or prevalent ACs (n = 45) and the control group (n = 91) (B), and stratified by sex (males: with any ACs, n = 29, and controls n = 29; females: with any ACs, n = 62, and controls n = 62) (C). Pairwise comparisons were performed using Log‐Rank test. AC, anticoagulant.
Additionally, adjusting model 2 for eGFR and time of infection did not alter the mortality‐lowering effect (OR[95% CI]: 0.38[0.17–0.82], n = 170). We performed stratified analyses to further assess the effect of ACs within more specific patient groups. In age group‐stratified analysis shown in Table 2, model 2, the association was significant in both age groups: 70–89 (OR[95% CI]: 0.37 [0.15–0.89]) and 90–99 years of age (OR[95% CI]: 0.22 [0.07–0.65]. When stratifying analysis by age groups in 10‐year intervals, the mortality‐lowering effect was not statistically significant in those aged 70–79 (OR[95% CI]: 0.30 [0.05–1.50] or 80–89 years (OR[95% CI]: 0.41 [0.13–1.24]). In the sex‐stratified analysis, the mortality‐lowering effect was statistically significant in females (OR[95% CI]: 0.28[0.11–0.67]), but not in males (OR[95% CI]: 0.39[0.12–1.16]) (Table 2).
4. DISCUSSION
In this matched cohort study, we found that AC treatment reduced 28‐day mortality among older adults diagnosed with COVID‐19 and residing in NHs in the Stockholm region in Sweden. All individuals were diagnosed during the early (prevaccination, preomicron) phase of the pandemic. The reduction in 28‐day mortality pertained both to incident use of LMWH in prophylactic dosing, and to prevalent use of DOAC in prophylactic dosing. The effects were robust and largely unaltered in the fully adjusted model when accounting for age, sex, multimorbidity, mobility, eGFR and calendar time of COVID‐19 infection, and in stratified analyses.
Nearly half of the COVID‐19 deaths in Sweden in 2020 (46%) occurred in older residents (65 to >99 years of age) in NH facilities, a proportion that dropped to 40% during the spring of 2022. 2 , 22 Despite the high case fatality rates of 15%–40% in NHs in the early phase of the pandemic, 2 , 5 , 23 , 24 little research has been performed on best COVID‐19‐practice in older individuals in this setting. AC treatment in COVID‐19 is one pertinent example where scientific evidence is lacking. There is evidence from both observational 9 , 25 and randomized clinical studies 10 , 11 supporting the benefits of heparin‐based ACs in middle‐aged individuals hospitalized with COVID‐19, but such data on older and frail individuals, or individuals residing in NHs is scant. A retrospective cohort study evaluated AC as one of several interventions in COVID‐19 in 133 individuals with a mean age of 87 years, at three NH facilities in France. 24 The calendar period of inclusion, mean age, dependency status, and comorbidity status was seemingly comparable to that of our population. However, the absolute 3‐week mortality rate was around 26%, which was considerably lower than in our unexposed population (49%), indicative of a less frail study population. As in our study, the initiation of prophylactic AC treatment (not stated which) was associated with a lower mortality, OR (95% CI) 0 (0.00–0.24).
Yet another retrospective cohort study included 4297 individuals (mean age 68 years) within the US Veterans Affairs, who were admitted to hospital with COVID‐19. Approximately 1600 of the AC‐treated individuals were older than 70 years of age. Initiation of mainly heparin‐based AC was associated with a 27% relative risk‐reduction for 30‐day mortality (hazard ratio 0.73, 95% CI 0.66–0.81). 25 The study was carefully designed, with high internal validity, and had the same calendar period of inclusion as in our study. The study was not conducted in a NH setting, but individuals within the Veterans Affairs are older and have higher accumulated comorbidity compared with the general US adult population, which makes the comparison to our data relevant. However, although the reduction in relative mortality rate is similar, the absolute mortality rates the absolute mortality rates (19% in the unexposed and 14% in the exposed) are substantially lower compared with our study (49% and 29%, respectively). In part, this reflects the more advanced age and morbidity in the NH residents and underpins the need to perform clinical studies which include this particular population.
A meta‐analysis of relevant randomized clinical trials and cohort studies with younger participants, including 16,185 individuals aged 55–68 years with COVID‐19, reported on a 36% risk reduction associated with incident AC (RR 0.64, 95% CI 0.55–0.74). Of note, and regardless the comprehensive data, the authors stated that the results are “very uncertain” due to serious risk of bias in some of the included studies. 11
In our study, prevalent use of DOAC in prophylactic dose was significantly associated with reduced 28‐day mortality. The notion of DOAC as an effective protection against severe COVID‐19 is enticing, but evidence has not been consistent. One retrospective case study showed that prevalent use of DOAC was associated with lower 35‐day mortality corresponding to a hazard ratio of 0.38 (0.17–0.58), which is a relative risk reduction of the same magnitude as in our study. The sample included 70 patients at a mean age of 79 years with chronic heart disease who were diagnosed with COVID‐19 early in the pandemic. 26
One cohort study investigated initiation of AC treatment, including incident DOAC, while excluding prevalent AC in 3625 individuals (39% were older than 70 years) hospitalized March–May 2020 with moderate to severe COVID‐19 infection. Initiating DOAC (mainly apixaban) in prophylactic or therapeutic dose was associated with reduced mortality compared with no use, particularly among individuals with high d‐dimer values (DD 1– < 3 and DD ≥ 10). 27 On the contrary, several studies could not detect any protective effect of prevalent DOAC. A retrospective multicentre study with partly prospectively collected data included 5883 individuals with a median age of 74 years admitted to hospital with COVID‐19. Oral AC therapy before admission failed to predict both 90‐day mortality and bleeding events in propensity score‐matched analyses. 28 A retrospective analysis of 1612 individuals with a mean age of 67 years consecutively admitted to hospital with COVID‐19 during the early phase of the pandemic (spring of 2020) failed to show any impact on mortality with either oral or parenteral AC. 29 Finally, a large register‐based study did not find any evidence to support that prevalent use of DOAC decreases the risk for severe COVID‐19 infection or mortality rate. 30
The inconclusive evidence of AC effectiveness may, in part, be attributable to interactions with COVID‐19 severity or the extent of coagulopathy, 27 such that any protective effect of DOAC is dependent on the timing of treatment start or disease progression. The failure to demonstrate any favorable effect of preadmission DOAC in hospitalized individuals may also in part be explained by selection bias. A beneficial effect of DOAC may have protected against serious progression of COVID‐19 and prevented patients from being admitted to hospital altogether, selecting a severely ill and potentially treatment resistant cohort at the hospital. Such selection mechanisms are likely less prominent in our study, where all patients were residing in the NH at the time of COVID‐19 infection.
The mortality‐lowering effect of AC treatment was more evident in women in our study, and to the best of our knowledge, no previous study on sex‐specific effects on the effectiveness of AC on COVID‐19 mortality exists. Our findings may be attributed to limited power since males contributed only to one‐third of the study population. However, we cannot rule out that our observation is attributed to sex differences in biological aging, 31 in COVID‐19 outcomes, 32 or both.
Regular use of Vitamin K antagonist (VKA) before COVID‐19 was associated with a lower survival rate in a longitudinal study of 97 frail individuals with a mean age of 89 years, hospitalized for COVID‐19. 33 The authors speculate that the vitamin K cycle may have significance for many aspects of the COVID‐19 pathogenesis which may explain the findings, but also that selection bias cannot be ruled out. In our study, only four individuals were exposed to VKA.
Strengths of our study include the access to all relevant information in the medical records for each study participant, which ensured robust variables for exposure, outcome, matching, and adjustments, and that the study was performed in the NH setting were previous evidence is largely lacking. We also recognize some limitations. As the study was performed during a time period before any vaccine against COVID‐19 existed, it also limits the results from being directly generalizable to the highly vaccinated population living in NHs today. The observational study design has an inherent risk for channeling bias. Frailty substantially increases the risk for COVID‐19 death in older adults in Geriatric inpatient care 34 and NHs, compared with nonfrail individuals. 35 There may be a higher likelihood to initiate LMWH to less frail individuals with a higher perceived chance of surviving, which could falsely drive the OR for mortality below one. For the use of prevalent DOAC, such selection bias is less obvious. DOAC, as stroke prophylaxis in atrial fibrillation, is a well‐established first‐line treatment in Sweden for all ages. Frail individuals are not excluded from such treatment in clinical practice, and therefore, there is little support that individuals with prevalent DOAC would be more fit than those without such treatment. On the contrary, prevalent use of DOAC may indicate a pronounced cardiovascular morbidity and, hence, a higher mortality risk.
In our sample, we did not have access to a comprehensive assessment on frailty according to a validated rating scale. However, we had reliable data on mobility and comorbidities, which are core items of the Clinical Frailty Scale. 36 Moreover, it was not possible to assess adherence to treatment from available medical records, for example, actual intake of DOAC and the number of administrated doses of LMWH. Also, our study did not investigate incident and prolonged use of DOAC, 37 or incident LMWH as add‐on in individuals treated with prevalent DOAC.
No adverse bleeding complications were detected in our study which is reassuring. However, the number of patients may be too limited to make any inference about the risk of major bleedings which could be estimated to occur in 1%–5% of the individuals treated with AC, based on findings in similar studies. 25 , 28 , 38
5. CONCLUSIONS
In this matched cohort study of unvaccinated NH residents with diagnosed COVID‐19, we found that incident use of LMWH and prevalent use of DOAC was significantly associated with reduced mortality in multivariable analyses adjusted for age, sex, multimorbidity, and immobility. The association between prophylactic use of LMWH and reduced mortality was most evident among female patients. Our findings present new insight in best practice for individuals diagnosed with COVID‐19 in the NH setting.
AUTHOR CONTRIBUTIONS
Laura Kananen: Data curation; formal analysis; methodology; visualization; software; data validation and interpretation; writing‐original draft. Christian Molnár: Conceptualization; funding acquisition; data collection and curation; formal analysis; methodology; resources; supervision; investigation; project administration; data validation and interpretation; writing‐original draft. Fredrik Ansker: Data collection and curation; formal analysis; methodology; investigation; writing‐original draft. Daria Julianna Kozlowska: Methodology; investigation. Sara Hägg: Data interpretation; methodology; funding acquisition; supervision; writing‐reviewing and editing. Juulia Jylhävä: Data interpretation; methodology; funding acquisition; supervision; writing‐reviewing and editing. Dorota Religa: Conceptualization; funding acquisition; methodology; resources; supervision; writing‐reviewing and editing; data validation and interpretation. Pauline Raaschou: Conceptualization; funding acquisition; methodology; resources; supervision; writing‐original draft; data validation and interpretation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was approved by the Swedish Ethical Review Authority, Dnr. 2020‐035404.
TRANSPARENCY STATEMENT
The lead author Christian Molnár affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The following funding is acknowledged in enabling this study: Yrjö Jahnsson Foundation (grant no 20217416), Juho Vainio Foundation (grant number 202100335), Päivikki and Sakari Sohlberg Foundation (grant number 220032), Tampere Tuberculosis Foundation and the Academy of Finland through its funding to the Development of electronic frailty index for Finnish healthcare system (FINeFI, 349335) and Centre of Excellence in Research of Ageing and Care (CoEAgeCare, grant numbers 335870, 326567 and 336670). Swedish Research Council grants (2016‐02317, 2018–02077, 2020‐06101[WISER], 2020‐02014, 2022‐01428), the regional agreement on medical training and clinical research between the Stockholm county council and the Karolinska Institutet (ALF), The Strategic Research Area in Epidemiology and Biostatistics grant, King Gustaf V and Queen Victoria's Foundation of Freemasons. The funders had no role in the design of this study, its execution, analyses, interpretation of the data, or decision to submit results.
Kananen L, Molnár C, Ansker F, et al. Anticoagulant treatment and COVID‐19 mortality among older adults living in nursing homes in Sweden. Health Sci Rep. 2023;6:e1692. 10.1002/hsr2.1692
Laura Kananen and Christian Molnár contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Weekly epidemiological update on COVID‐19 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---30-november-2022
- 2. Levin AT, Jylhävä J, Religa D, Shallcross L. COVID‐19 prevalence and mortality in longer‐term care facilities. Eur J Epidemiol. 2022;37(3):227‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Garcia‐Ptacek S, Annetorp M, et al. Decreased mortality over time during the first wave in patients with COVID‐19 in geriatric care: data from the Stockholm GeroCovid study. J Am Med Dir Assoc. 2021;22(8):1565‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID‐19 diagnosis, hospitalization, and subsequent all‐cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36(3):287‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballin M, Bergman J, Kivipelto M, Nordström A, Nordström P. Excess mortality after COVID‐19 in Swedish long‐term care facilities. J Am Med Dir Assoc. 2021;22(8):1574‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID‐19 is high and associated with a higher risk of mortality: a systematic review and meta‐analysis. EClinicalMedicine. 2020;29‐30:100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang C, Shen L, Le KJ, et al. Incidence of venous thromboembolism in hospitalized coronavirus disease 2019 patients: a systematic review and meta‐analysis. Front Cardiovasc Med. 2020;7:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kamel AM, Sobhy M, Magdy N, Sabry N, Farid S. Anticoagulation outcomes in hospitalized Covid‐19 patients: a systematic review and meta‐analysis of case‐control and cohort studies. Rev Med Virol. 2021;31(3):e2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rauniyar R, Kuikel S, Mishra A, et al. Safety and efficacy of prophylactic anticoagulation versus therapeutic anticoagulation in hospital‐admitted COVID‐19 patients: a systematic review and meta‐analysis of randomized controlled trials. Clin Respir J. 2023;17(2):73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flumignan RL, Civile VT, Tinôco JDS, et al. Anticoagulants for people hospitalised with COVID‐19. Cochrane Database Syst Rev. 2022;3(3):013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid‐19. N Engl J Med. 2021;385(9):790‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid‐19. N Engl J Med. 2021;385(9):777‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Living guidance for clinical management of COVID‐19 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2
- 16. Fontana P, Casini A, Robert‐Ebadi H, Glauser F, Righini M, Blondon M. Venous thromboembolism in COVID‐19: systematic review of reported risks and current guidelines. Swiss Med Wkly. 2020;150:w20301. [DOI] [PubMed] [Google Scholar]
- 17. Stockholm Region Drug Committee . Expert group for coagulation disease and plasma products. Guidelines for profylaxis and treatment of thomboembolism in Covid‐19 2023 [Document in swedish]. https://janusinfo.se/behandling/expertgruppsutlatanden/koagulationssjukdomarochplasmaprodukter/koagulationssjukdomarochplasmaprodukter/riktlinjerforprofylaxochbehandlingavvenostromboembolismhospatientermedcovid19.5.735b5f221714e865c978301e.html
- 18.Swedish National Board of Health and Welfare. Statistik om socialtjänstinsatser till äldre och personer med funktionsnedsättning efter regiform. 2022. https://www.socialstyrelsen.se/statistik-och-data/statistik/alla-statistikamnen/socialtjanstinsatser-till-aldre/
- 19. Sund Levander M, Milberg A, Rodhe N, Tingström P, Grodzinsky E. Differences in predictors of 5‐year survival over a 10‐year period in two cohorts of elderly nursing home residents in Sweden. Scand J Caring Sci. 2016;30(4):714‐720. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . ICD‐10: International statistical classification of diseases and related health problems, 10th revision. 2019.
- 21. Bjork J, Grubb A, Sterner G, Nyman U. Revised equations for estimating glomerular filtration rate based on the Lund‐Malmo Study cohort. Scand J Clin Lab Invest. 2011;71(3):232‐239. [DOI] [PubMed] [Google Scholar]
- 22. Swedish National Board of Health and Welfare . Statistics on Covid‐19. 2022. https://www.socialstyrelsen.se/en/statistics-and-data/statistics/statistics-on-covid-19/
- 23. McMichael TM, Currie DW, Clark S, et al. Epidemiology of COVID‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tarteret P, Strazzulla A, Rouyer M, et al. Clinical features and medical care factors associated with mortality in French nursing homes during the COVID‐19 outbreak. Int J Infect Dis. 2021;104:125‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021;372:n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID‐19 era. Eur J Intern Med. 2020;77:158‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Billett HH, Reyes‐Gil M, Szymanski J, et al. Anticoagulation in COVID‐19: effect of enoxaparin, heparin, and apixaban on mortality. Thromb Haemost. 2020;120(12):1691‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arachchillage DJ, Rajakaruna I, Odho Z, et al. Clinical outcomes and the impact of prior oral anticoagulant use in patients with coronavirus disease 2019 admitted to hospitals in the UK—A multicentre observational study. Br J Haematol. 2022;196(1):79‐94. [DOI] [PubMed] [Google Scholar]
- 29. Corrochano M, Acosta‐Isaac R, Mojal S, et al. Impact of pre‐admission antithrombotic therapy on disease severity and mortality in patients hospitalized for COVID‐19. J Thromb Thrombolysis. 2022;53(1):96‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flam B, Wintzell V, Ludvigsson JF, Mårtensson J, Pasternak B. Direct oral anticoagulant use and risk of severe COVID‐19. J Intern Med. 2021;289(3):411‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. eLife. 2021;10:e63425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peckham H, De Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(6317):6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ménager P, Brière O, Gautier J, et al. Regular use of VKA prior to COVID‐19 associated with lower 7‐day survival in hospitalized frail elderly COVID‐19 patients: the GERIA‐COVID cohort study. Nutrients. 2020;13(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hägg S, Jylhävä J, Wang Y, et al. Age, frailty, and comorbidity as prognostic factors for short‐term outcomes in patients with coronavirus disease 2019 in geriatric care. J Am Med Dir Assoc. 2020;21(11):1555‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang XM, Jiao J, Cao J, et al. Frailty as a predictor of mortality among patients with COVID‐19: a systematic review and meta‐analysis. BMC Geriatr. 2021;21(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rockwood K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramacciotti E, Barile Agati L, Calderaro D, et al. Rivaroxaban versus no anticoagulation for post‐discharge thromboprophylaxis after hospitalisation for COVID‐19 (MICHELLE): an open‐label, multicentre, randomised, controlled trial. Lancet. 2022;399(10319):50‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic‐dose heparin vs standard prophylactic or intermediate‐dose heparins for thromboprophylaxis in high‐risk hospitalized patients with COVID‐19: the HEP‐COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612‐1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


