Abstract
An estimated 1.2 million persons in the USA are infected with HIV, of whom approximately 20% are unaware they are infected. HIV testing and knowledge of HIV serostatus have important individual and public health benefits, including reduction of morbidity, mortality and HIV transmission. Although testing is the necessary first step to prevention, more than half of the US adult population has never been tested for HIV. However, this proportion is increasing due to revised national recommendations to make HIV testing a routine part of healthcare, expansion of testing efforts at local, state and national levels, and progress in the development and adoption of new testing technologies. In this article, we describe the essential role of HIV testing as a public health prevention strategy, examine recent advances in HIV testing technologies and testing implementation, and identify future directions for HIV testing in the USA.
Keywords: acute HIV infection, HIV diagnostics, HIV prevention, HIV testing, linkage to care, retention in care
Approximately 1.2 million persons are living with HIV infection in the USA [1] and it is estimated that there are approximately 50,000 new HIV infections nationwide each year [2]. Although HIV incidence has been stable since the late 1990s, the prevalence of HIV in the USA is increasing, in large part due to reduced mortality from combination antiretroviral therapy (ART) [3–6], and HIV prevalence in the USA is estimated to increase by up to 38% in the next 10 years [7]. An estimated 20% of HIV-infected persons are unaware they are infected [1], and this group contributes disproportionately to HIV incidence [8]. HIV testing is a key factor in HIV prevention. Individuals must be diagnosed to have the opportunity to access care, treatment and prevention services. Although testing is the necessary first step to prevention, more than half of the US adult population has never been tested for HIV. Only 45% of persons aged 18–64 years who responded to the 2009 US National Health Interview Survey reported ever having been tested for HIV [9,10]. Recommendations to make HIV testing a routine part of healthcare are expected to increase this proportion [11]. Substantial progress has also been made in recent years in the development and adoption of new testing technologies that allow same-day delivery of results and diagnoses earlier in the course of infection.
In addition to improvements in testing technologies that make earlier HIV diagnosis possible, the advent and widespread use of potent combination ART has dramatically reduced HIV-related morbidity and mortality and HIV transmission [12,13]. However, of US adults diagnosed with HIV in 2007, approximately 30% were diagnosed late during the course of infection (i.e., received an AIDS diagnosis within 12 months of their initial HIV diagnosis) [10]. To benefit fully from ART, people must be diagnosed and linked to care in a timely manner, receive regular HIV medical care, and be prescribed and adhere to ART [14]. To quantify the spectrum of engagement in care in the USA, Gardner and coworkers assessed the proportion of persons linked to and retained in care among those diagnosed with HIV and the proportion receiving and adhering to ART; they estimated that only 19% of HIV-infected individuals in the USA have undetectable viral loads [14].
In an effort to advance HIV prevention in the USA, President Obama released the National HIV/AIDS Strategy in 2010. Recent advances in testing and treatment should support efforts to achieve two important outcomes for HIV-infected persons outlined in the National Strategy: increase the proportion of HIV-infected persons aware of their diagnosis from 80 to 90% and the proportion of newly diagnosed patients linked to care within 3 months of HIV diagnosis from 65 to 85% by 2015 [201]. Policies and interventions must be developed and implemented to optimize and ensure testing early in the course of infection and linkage to and retention in care to reduce the incidence and, ultimately, the prevalence of HIV infection. In this article, we describe the essential role of HIV testing as a public health prevention strategy. Furthermore, we examine recent advances in HIV testing technologies and HIV testing implementation, and identify future directions for HIV testing in the USA.
HIV testing as public health prevention
HIV testing and knowledge of HIV serostatus have individual and public health benefits. Individual benefits arise primarily from the opportunity to access care and treatment that can reduce HIV-related morbidity and mortality. HIV medical care, which includes primary medical care, prevention and treatment of opportunistic infections, and treatment with antiretroviral medications, has led to substantial reductions in adverse health outcomes, including reduction in the incidence of opportunistic illnesses, and increased life expectancy [4,15–21]. The primary public health benefit of testing is reduced onward transmission of HIV, resulting from decreased risk behaviors in those aware of their infection, exposure to prevention interventions and reduction of viral concentrations in plasma and genital secretions by the use of and adherence to ART.
Approximately 80% of HIV-infected persons in the USA are infected through sexual exposure [202], and persons aware they are infected modify their transmission risk. In a meta-analysis, Marks and coworkers found that high-risk sexual behavior is substantially reduced in HIV-infected persons who are aware of their infection as compared with their counterparts who are unaware of their infection [22]. In a separate analysis building on findings from their meta-analysis, Marks and coworkers estimated that HIV-infected persons who are unaware of their serostatus account for up to 70% of sexually transmitted HIV infections and have a transmission rate more than three times that of HIV-positive persons who are aware of their infection [8].
Early diagnosis of HIV is critical because it allows earlier access to care, which can reduce immunologic compromise and reduce the time individuals are able to transmit HIV. Diagnosis during acute HIV infection (AHI) is particularly important because of the high risk of transmission during this stage [23,24]. AHI is the stage during the first 10–12 weeks following HIV infection when levels of infectious virus in plasma and genital secretions are very high [25–28], and persons with AHI often engage in sexual intercourse more frequently than those with later stages of infection [29]. Persons with AHI may be up to ten times as likely to transmit HIV per sex act [30] and more than 25 times as likely to transmit HIV as those with established infection [25,31]. A study by Brenner and coworkers found that persons with recent infections (i.e., those infected <6 months following seroconversion) accounted for almost half of onward HIV transmission [32].
Current recommendations call for annual testing of sexually active men who have sex with men (MSM), injection drug users and other persons at risk for HIV infection [11]. However the optimal frequency of testing for particular groups still needs to be determined. For instance, among MSM who participated in the 2008 National HIV Behavioral Surveillance System (NHBS) cycle in 2008, 45% of those who were found to be HIV infected and unaware of their infection had been tested within the previous year, indicating that they had acquired HIV recently [33] and that more frequent testing of MSM may be warranted [34].
Plasma HIV RNA concentrations are directly correlated with HIV transmission [35,36]; higher genital HIV RNA concentrations appear to be associated with increased transmission as well [37]. ART is associated with a substantial reduction in HIV RNA and has been shown to be associated with decreased transmission [12,13]. There has been increasing interest in using ART as an HIV prevention intervention for its public health benefits. In 2006, Montaner and coworkers proposed using ART to prevent HIV transmission [38]. Starting ART at an earlier stage of infection may increase the impact of expanded therapy in reducing transmission [39–41]. In 2009, the Panel on Antiretroviral Guidelines for Adults and Adolescents recommended initiating ART for patients with CD4 count ≤500 cells/μl, and 50% favored starting ART at higher CD4 cell counts [203]; although the panel considered the effect of ART for its benefits to individuals in terms of HIV-related morbidity and mortality, these treatment recommendations will also impact HIV transmission in the USA. In May 2011, the HIV Prevention Trials Network (HPTN) Study 052, which evaluated the effect of immediate versus delayed initiation of ART on heterosexual transmission from HIV-infected persons to their HIV-uninfected partners, was halted because it found that immediate initiation of ART resulted in a 96% reduction in sexual transmission of HIV as well a 41% reduction in the number of HIV-related clinical events [42].
In 2009, Granich and coworkers proposed using a ‘test and treat’ model in South Africa to reduce HIV transmission and incidence [43]. Using mathematical models, they estimated that a strategy of universal annual voluntary testing and immediate ART treatment could reduce HIV incidence to less than one case per 1000 people within 10 years, and reduce HIV prevalence to less than 1% in 50 years [43]. Walensky and coworkers found that, compared with current practice, testing and treating with optimized ART could lead to a 27% reduction in time spent with transmissible infection within 5 years [44]. In 2010, the NIH and partners initiated HPTN Study 065 (also referred to as the Expanded Test, Link to Care, Plus Treat Approach for HIV Prevention in the USA, or TLC-Plus), a 3-year study, to assess the feasibility of enhanced testing and linkage to care plus treatment in Bronx (NY, USA) and Washington, DC (USA) [204].
Testing methodologies & diagnostic applications
The current algorithm for diagnosing HIV infection in the USA, in use for more than 20 years, consists of confirming a repeatedly reactive HIV immunoassay with a western blot test or immunofluorescence assay [45]. However, over the past two decades, there have been major improvements in HIV diagnostics technology. Identifying the approximately 240,000 HIV-infected US adults and adolescents who are unaware of their serostatus will require faster, cheaper HIV tests that detect HIV infection earlier during infection [25]. The first HIV immunoassay was approved by the US FDA in 1985. First-generation immunoassays detect IgG antibodies to HIV using whole viral lysate as the antigen in a standard indirect immunoassay format. These assays detect HIV infection in the same time frame as the Western blot, approximately 45–60 days following infection [46,47]. Second-generation assays also detect IgG in an indirect format, but were designed to increase specificity by incorporating recombinant proteins or peptides as the antigens for detection. Second-generation immunoassays detect IgG to HIV approximately 5–7 days sooner than first-generation assays [47]. Third-generation immunoassays detect IgG to HIV, but also detect IgM to HIV by using peptides and recombinant proteins in an antigen sandwich format and improve detection of recent HIV infection by about 7–10 days as compared with second-generation assays [47,48]. Third-generation assays are often positive before any bands appear on western blot assays [25,47,48]. Most third-generation assays detect HIV-2 in addition to HIV-1. Fourth-generation assays, which detect p24 antigen in addition to detecting IgM and IgG to HIV, can detect HIV infection approximately 15 days following infection [48]. Although fourth-generation (or combination antigen/antibody) assays have been approved and used in other countries and have continued to be improved since their introduction in the late 1990s [49–52], only two such assays have been approved for use in the USA [53,205,206]. Figure 1 shows laboratory markers of HIV infection and times from infection to detection of HIV infection for first-, second-, third- and fourth-generation immunoassays as well as for the HIV-1 western blot.
Figure 1.
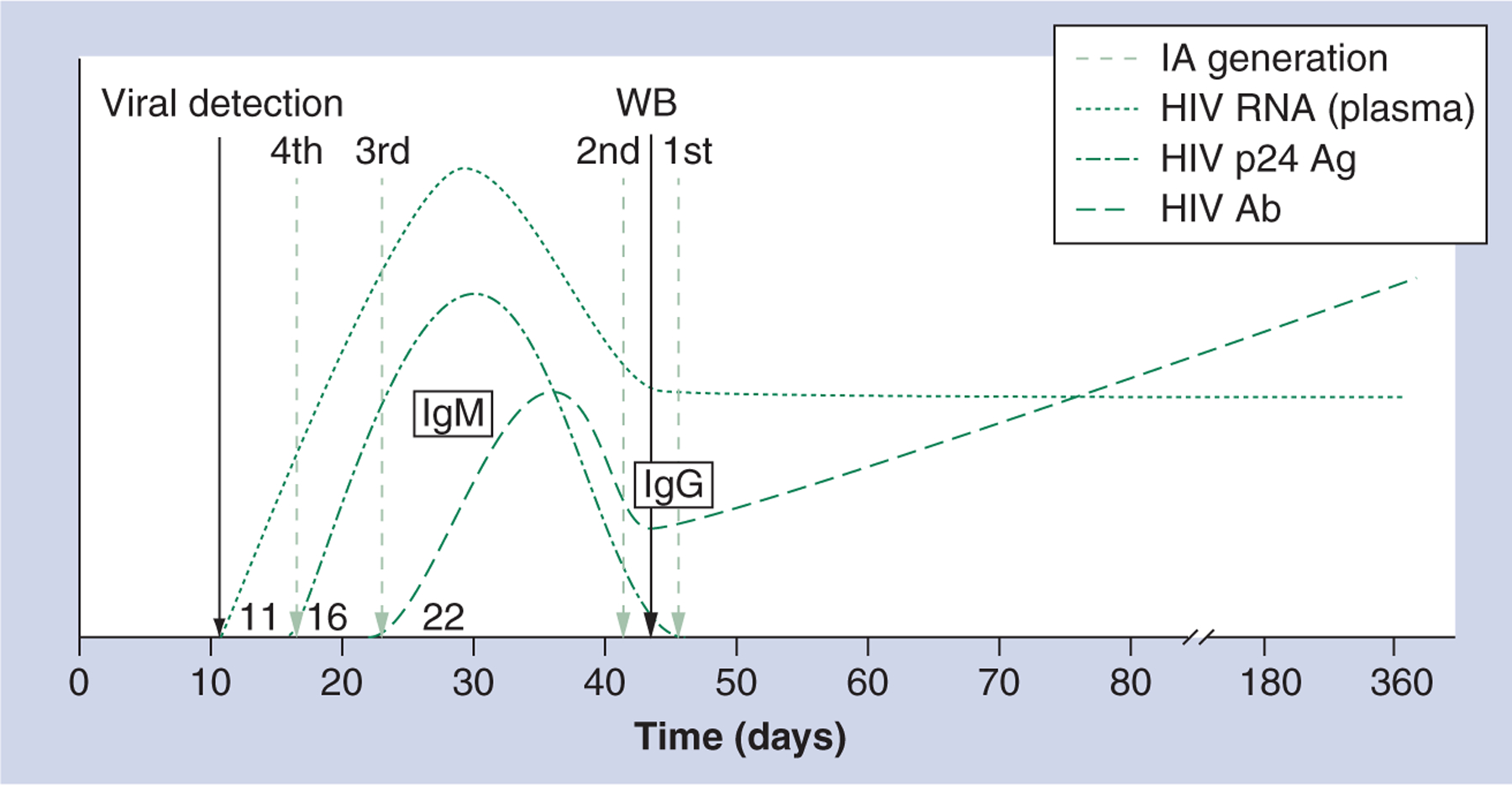
Laboratory markers of HIV infection and times from infection to detection of HIV infection for first-, second-, third- and fourth-generation HIV immunoassays, and the HIV-1 western blot assay.
Ab: Antibody; Ag: Antigen; Gen: Generation; IA: Immunoassay; Ig: Immunoglobulin; WB: Western blot.
Adapted with permission from [26].
Seven rapid HIV tests have been approved by the FDA for use in the USA. These tests are relatively inexpensive and have sensitivities and specificities comparable to first- and second-generation immunoassays [25,54]. Several rapid HIV tests detect both HIV-1 and HIV-2, and one can differentiate HIV-1 from HIV-2 infection [55]. Since rapid test results are available in minutes and require less technical skill to perform, rapid HIV testing can increase the reach of testing, allowing point-of-contact testing in a variety of settings, including those without on-site laboratories [56]. Like laboratory-based tests, negative rapid test results are considered to be definitive for absence of HIV antibody. However, these tests do not rule out the possibility of AHI. Reactive rapid test results are considered to be preliminary positive and require supplemental laboratory testing for a definitive diagnosis [57]. Rapid testing may be more acceptable to clients because of the advantage of receiving results on the same day as testing and collection of specimens by finger stick or oral mucosa swab rather than blood draw [58]. Rapid tests have been integrated into many clinical settings, and they have been widely adopted as the standard method of HIV testing in many developing countries [56]. However, the window period for rapid tests can be substantially longer than immunoassays used by many reference laboratories. Currently, the confirmation of reactive rapid tests can take up to a week or longer [25].
Quantitative nucleic acid amplification tests (NAAT) detect HIV RNA within approximately 10–12 days of infection [46,59,60] and have typically been used to determine viral burden and monitor response to therapy, but are not approved by the FDA for diagnosis of HIV infection. In 2006, a qualitative NAAT was approved by the FDA as a supplemental assay for HIV infection [207]. This assay allows the detection of HIV infection prior to the appearance of HIV-specific antibody [47,61]. NAAT has several limitations, however, including the need to draw blood, its cost and its processing time, which can delay delivery of results by several days to over a week. Furthermore, it has been demonstrated that there is a risk of false-negative NAAT results in individuals with established HIV infection, and this can be up to 3.7% [47,59,61].
The US CDC and the Association of Public Health Laboratories issued preliminary algorithms for HIV diagnosis in 2009 and recommend that individuals with clinical manifestations suggesting acute retroviral infection (e.g., combination of some or all of the following: unexplained fever, sore throat, lymphadenopathy and rash) or reporting recent high-risk exposure undergo supplementary testing using an assay capable of detecting either HIV RNA or HIV p24 antigen to rule out AHI [62,208]. NAAT and fourth-generation HIV tests can efficiently detect AHI. To identify AHI, NAAT is conducted on single or pooled specimens collected from persons who have negative HIV antibody screening tests. The addition of NAAT to an HIV testing algorithm increases case detection, although the magnitude of increase depends on the type of initial screening test that is used and the HIV incidence in the population being tested. Pilcher and coworkers found that pooled NAAT increased HIV case detection by 3.9% over standard antibody testing using a first-generation assay [63], Truong and coworkers found that it increased detection of HIV by 8.1% over standard antibody testing using a first-generation immunoassay [64], and Patel and coworkers found that it increased HIV case detection over standard antibody testing overall by 2.2% (24.1% increase over second-generation assays and 1.5% over third-generation assays) [61]. Studies demonstrate that NAAT detects HIV RNA in 0.02–0.4% of persons who have negative HIV antibody results on HIV screening [23,61,64–66]. Fourth-generation testing was shown in one study to increase HIV case detection by 5.3% over testing using a third-generation immunoassay [67]. Several studies have shown that combination antigen/antibody assays can identify 80% or more of HIV infections that would only be detectable otherwise by NAAT [25,53,68].
Although HIV-2 infection is uncommon in the USA, it should be considered in persons of West African origin and those who have sex or drug-using partners of West African origin [69,70]. It is clinically important to identify HIV-2 infections because diagnosis, treatment, and monitoring of response to treatment differ from HIV-1 infection. Compared with HIV-1 infection, infection with HIV-2 typically results in a longer asymptomatic stage, lower plasma viral load and reduced mortality [71,72,203], and assays that measure HIV-1 RNA do not detect HIV-2. In addition, some antiretroviral medications that are effective against HIV-1, in particular non-nucleoside reverse transcriptase inhibitors, are not active against HIV-2 [73,203]. Most HIV tests on the market detect, but do not differentiate between, HIV-1 and HIV-2. Differentiating HIV-1 from HIV-2 is a challenge because of cross-reactivity between HIV-1 and HIV-2 antigens, and the fact that HIV-1 western blots from specimens from persons with HIV-2 infection may be interpreted as being positive for HIV-1 [25,74,75].
The tremendous progress that has been made in the field of HIV diagnostics in the past 20 years provides opportunities to develop new strategies for diagnosing HIV infection more quickly and with greater accuracy. Two-test algorithms are used in the USA and elsewhere to diagnose HIV infection. Optimally, tests with high sensitivity should be used to screen for HIV, whereas tests with high specificity should be used to diagnose infection when screening tests are positive [47]. The two-test algorithm used in the USA to diagnose HIV since 1989 is complex, expensive, and may take up to a week or longer to return results. Rapid HIV tests and random access assays (assays run on automated platforms for a variety of tests, including HIV, and which can be run on individual and batched specimens [25]) can provide test results in less than 1 h, and newer generations of immunoassays and several rapid tests can detect HIV earlier in the course of infection than the western blot assay, the current gold standard for confirming HIV infection in the USA. A new algorithm for diagnosing HIV was proposed at the 2010 HIV Diagnostics Conference [76] to take advantage of progress in diagnostic technology, address challenges posed by AHI and HIV-2, and mitigate the shortcomings of the HIV-1 western blot [25]. The proposed algorithm calls for using the most sensitive serologic HIV test possible (preferably a combination antigen/antibody assay) to screen for HIV followed, if repeatedly reactive, by an HIV-1/HIV-2 antibody differentiation test; specimens with positive results on the antigen/antibody assay but negative results on the antibody assay are tested for HIV-1 RNA to detect AHI [76,77]. Existing data suggest that false-positive results will not be significantly higher with the proposed algorithm since the specificity of the new combination antigen/antibody assays is not significantly different from the specificity of the third-generation assays [53,78].
In the USA, HIV diagnostic testing devices are regulated by the FDA. Historically, HIV diagnostic tests have been categorized as Class III devices, which are devices the FDA considers to be of highest risk. This classification results in a submission process that requires a premarket approval. The premarket approval process is the most stringent and costly mechanism for submitting products to the FDA and the initial application alone can cost as much as US$235,000 [209]. In addition to the filing fee, there are substantial costs associated with conducting clinical trials to fulfill the regulatory requirements. The class III classification for HIV diagnostics is substantially different from classification of most other diagnostic devices, which are typically categorized as class II devices. Class II devices are not required to undergo large clinical trials and are submitted under a much less costly review process (the standard filing fee for this application ranges from $2000 to $4,200) [209]. The regulatory requirements and increased cost for approval of HIV diagnostic tests may be factors in preventing or delaying some companies from introducing new technologies, such as combination antigen/antibody assays, to the US market. Discussions about downgrading the classification of HIV test devices to Class II were held between the CDC, the Association of Public Health Laboratories, and the FDA at the 2010 HIV Diagnostics Conference. Balancing the advantages (better technology reaching the market sooner) with the disadvantages (reduced pre-and post-approval quality oversight of devices by the FDA) will inform the decision about whether to designate HIV test devices as Class II.
Testing & HIV surveillance
HIV surveillance activities in the USA have been designed to monitor trends in HIV prevalence and incidence, patterns of HIV drug resistance, behaviors associated with acquisition and transmission of HIV, and HIV-related morbidity and mortality. Estimation of HIV prevalence and incidence require obtaining accurate information on the circumstances of HIV testing, including the type of HIV test used, confirmation of preliminary positive test results, and reporting of confirmed diagnoses to public health officials. The approval of new testing technologies and the formulation of new testing algorithms have a substantial impact on HIV surveillance. Currently, HIV cases are reported based on various criteria, including HIV laboratory diagnostic test results such as an HIV-1 western blot, immunofluorescence assay, or qualitative NAAT [79]. Therefore, changes in diagnostic testing technologies (e.g., development and implementation of new two-test algorithms) will require a revision of the HIV case surveillance definition. These types of changes also have implications for surveillance practices and completeness of case reporting. A considerable proportion of new case reports are generated based on electronic laboratory reporting. Algorithms that can be performed solely with point-of-contact tests will require that case reports be initiated by programs doing point-of-contact testing, rather than relying on electronic reporting by laboratories. These changes in reporting practices will be important not only for monitoring the epidemic, they will also have implications at the state and local levels as federal funds for prevention and care services in the USA are allocated based on HIV surveillance data. The CDC funding for HIV testing programs stipulates that positive test results must be reported to the appropriate local or state surveillance programs, in accordance with applicable laws and regulations. Therefore, effective and efficient methods for reporting test results will need to be developed in advance of widespread implementation of point-of-contact testing using new algorithms.
Currently, the HIV case definition used in the USA does include criteria that allow HIV-1 infection to be differentiated from HIV-2. infection. However, because the clinical implications of HIV-1 differ from those of HIV-2, and since the introduction of a new laboratory testing algorithm will allow more accurate diagnosis of HIV-2, efforts are underway to develop an HIV-2 case definition [70].
In addition to its importance for HIV case surveillance, HIV testing is an integral component of the CDC’s HIV behavioral surveillance. NHBS, which monitors HIV prevalence, behaviors and exposure to HIV prevention interventions among groups at risk for HIV infection (MSM, injection drug users and heterosexuals at high risk for HIV infection), conducts HIV testing and surveys among more than 10,000 persons annually. These data have been instrumental in providing HIV prevalence estimates among populations at highest risk for HIV infection (e.g., 19% among MSM [33], 9% among injection drug users [80] and 2% among heterosexuals at risk for HIV infection [81]).
Distinguishing recent from established HIV infection is important for monitoring behaviors that lead to HIV acquisition and identifying partners who have potentially been exposed. The Serologic Testing Algorithm for Recent HIV Seroconversion, also referred to as the Recent Infection Testing Algorithm, has been used to identify recent HIV infection [82–86]. Two Serologic Testing Algorithm for Recent HIV Seroconversion assays, the BED capture enzyme immunoassay (an assay that detects levels of anti-HIV IgG to immunodominant sequences from HIV-1 subtypes B, E and D relative to total IgG) [87] and the avidity index assay (which measures tightness of binding of anti-HIV antibodies to antigen) [82,88], have been used to determine HIV incidence at the population level (e.g., for monitoring trends), but have inadequate specificity due to misclassification of persons with longstanding infection as having acquired HIV recently [82,87,89,90]. The development of new assays and combinations of existing assays promises to improve detection of recent infection and enhance efforts to monitor patterns of HIV acquisition and transmission at local, state, and national levels.
Expanding HIV testing in the USA
HIV testing has been promoted as a key public health approach for preventing HIV in the USA for the past decade. In 2001, the CDC published the Serostatus Approach to Fighting the HIV Epidemic (SAFE), which outlined steps for diagnosing HIV infection in all HIV-infected people and linking them to care [91], and published revised guidelines for HIV counseling, testing and referral, which provided guidance on HIV testing to all providers and highlighted the need for early knowledge of HIV status and making testing more accessible and available [92]. In 2003, the CDC announced the Advancing HIV Prevention (AHP) initiative, which aimed to make HIV testing a routine part of medical care and to implement new models for diagnosing HIV infections outside medical settings, building on advances in rapid HIV tests [93]. SAFE, the 2001 revised testing guidelines, and the AHP initiative laid the groundwork for a series of demonstration projects conducted during 2003–2007 to assess the feasibility and acceptability of using a variety of approaches to perform HIV testing in clinical and nonclinical settings. Over 100,000 rapid HIV tests were performed in six demonstration projects and more than 1,100 people (1% of those tested) were newly diagnosed with HIV [94]. The demonstration projects also collected data on barriers to and facilitators of providing HIV testing.
Findings from the AHP demonstration projects informed the CDC’s 2006 revised recommendations for testing in healthcare settings, which called for voluntary, opt-out HIV testing (e.g., letting patients know that HIV testing will be conducted unless they specifically decline to be tested) of all persons 18–64 years of age in all healthcare settings in which the prevalence of undiagnosed HIV infection is at least 0.1% [11]. The CDC has worked to increase uptake of the recommendations to make HIV testing a routine part of medical care, without regard to risk or clinical manifestations of HIV infection, and has also sought to expand HIV testing and linkage to care in nonclinical settings, particularly in those most likely to reach individuals with undiagnosed infections. The CDC is currently revising the guidelines for HIV counseling, testing and referral in nonclinical settings in collaboration with other US federal agencies and nongovernmental partners. The goal of the revised guidelines is to increase HIV testing in nonclinical settings and to increase the number of HIV-infected people who are both aware of their infection and linked to care. The revised guidelines are expected to be available in 2012.
In 2007, the CDC launched the Expanded Testing Initiative (ETI) to increase the availability and accessibility of HIV testing, promote the adoption of the revised recommendations for testing in healthcare settings (including using an opt-out approach to screening), and increase identification of unrecognized HIV infection among persons disproportionately affected by HIV [210]. During the ETI’s first 3 years, the CDC provided $102 million to 25 public health jurisdictions (cities, counties and states) and 2.8 million HIV tests were performed, 18,432 (0.7%) new HIV diagnoses were made, and, of persons with new diagnoses for whom follow-up data were available, 75% were successfully linked to HIV medical care [95].
The Ryan White Comprehensive AIDS Resources Emergency (CARE) Act was enacted in 1990 to improve the quality and availability of care for medically underserved individuals and families affected by HIV [211] and it has been reauthorized periodically between 1996 and 2009. When it was reauthorized in 2009, it established a national goal of conducting 5,000,000 HIV tests annually through federally supported HIV/AIDS prevention, treatment, and care programs, including programs administered by the CDC and other federal agencies such as the Veterans Health Administration (which is responsible for providing medical care to US veterans), the Indian Health Service, and the Substance Abuse and Mental Health Services Administration [212]. The CDC continues to support HIV testing in clinical and nonclinical settings through the ETI and, in 2011, it announced a 5-year ‘high-impact’ HIV prevention funding opportunity for health departments in states, territories, and select cities focusing on prevention strategies with the highest impact [213]. First-year awards will total $360 million, of which at least 75% will support health departments’ core prevention programs, including: HIV testing (routine opt-out testing in clinical settings; targeted testing in nonclinical settings; routine, early HIV screening for all pregnant women; and screening for sexually transmitted diseases, viral hepatitis and tuberculosis in conjunction with HIV testing); approximately $55 million will support expanded HIV testing among disproportionately affected populations; and $20 million will support demonstration projects, including ones that focus on HIV testing approaches that increase the identification of undiagnosed infections and enhanced linkage and retention in medical care for HIV-infected individuals [213].
In 2010, the Veterans Health Administration announced a campaign to increase HIV testing in Veterans Affairs facilities. The Veterans Health Administration is the largest single provider of HIV care in the USA, and its Public Health Strategic Healthcare Group requires all providers in the system to offer routine HIV testing to all patients in order to diagnose HIV infection at the earliest possible stage [214]. In December 2010, the Veterans Health Administration’s Office of Clinical Public Health awarded eight grants to fund projects to increase testing in the Veterans Affairs health system [215]. The Substance Abuse and Mental Health Services Administration implemented a Rapid HIV Testing Initiative in 2005 to facilitate early diagnosis of HIV among at-risk minority populations involved in substance abuse or living with mental health disorders [216] and continues to support this effort through supplemental grants to provide rapid HIV testing, counseling, and referral to care. The Indian Health Service has developed recommendations for HIV testing that support the CDC revised recommendations for HIV testing in healthcare facilities, has begun to provide support for expanded HIV testing at Indian Health Service facilities in some locations, is assisting healthcare facilities raise awareness of this health maintenance testing [217] and is providing media materials to a nationwide community campaign to promote routine HIV testing for American Indians and Alaska natives [218].
During the past 5 years, several jurisdictions have scaled up their HIV testing activities, and multiple jurisdictions, facilities and groups have worked to make HIV testing a routine part of healthcare. Between 2005 and 2008, the number of tests funded by the New York City Health and Hospital Corporation increased by 76%, from 62,000 to 107,000 [96]. In April 2008, the New York City Department of Health and Mental Hygiene implemented a 3-year campaign to test all adults living in Bronx. To date, the Bronx-wide testing initiative has conducted more than 607,000 tests and identified over 4,800 confirmed HIV-positive individuals [219]. In June 2006, the Washington, DC, Department of Health (DC DOH) launched a city-wide program to increase the availability of routine screening, raise awareness of HIV, reduce stigma associated with testing, assist persons with new diagnoses receive care and reduce transmission [97]. To implement the recommendations for HIV testing in healthcare settings, DC DOH worked with providers to perform rapid HIV screening, initiated a social marketing campaign to educate the public and providers, and trained providers to link people to care immediately after receipt of a preliminary positive test result [98]. In 2006, Howard University Hospital initiated routine HIV testing as part of DC DOH’s program and, in the first year, more than 7,500 patients were tested and 2.3% had preliminary positive test results [99]. An analysis of surveillance data from Washington, DC, indicates that during 2004–2008, there was a 335% increase in the number of publicly funded HIV tests conducted by community providers (from 16,748 tests in 2004 to 72,864 in 2008) and a 353% increase in the number of persons testing positive (from 246 in 2004 to 1115 in 2008) [98].
HIV testing campaigns in Bronx and Washington, DC, preceded the TLC-Plus study and were part of the basis for choosing these cities to be the intervention cities in the study. The TLC-Plus study, which began data collection in February 2011, will evaluate the feasibility of an enhanced community-level HIV testing, linkage to care and treatment adherence intervention in the two cities and use surveillance data from these communities to monitor and improve these outcomes [204]. The expanded HIV testing component of the study involves social mobilization and universal offer of HIV testing in emergency departments and to persons admitted to hospitals; the linkage-to-care and viral suppression components involve site randomization to test the effectiveness of using financial incentives as compared with standard practices for linking persons with positive test results to care and achieving and maintaining viral suppression [204].
Emergency departments have been on the leading edge of expanded HIV testing in health-care settings. Studies and testing programs in a number of emergency departments have found a prevalence of undiagnosed HIV infection of 0.6–6.4% [56,100–103]. Emergency departments have used a variety of models to provide HIV testing to populations they serve, such as offering diagnostic testing (testing patients based on clinical manifestations suggesting HIV infection), targeted testing (testing patients selected on the basis of perceived or actual risk of HIV infection), and nontargeted screening (testing patients without regard to clinical manifestations or risk); most testing in US emergency departments has been conducted by existing emergency department staff or by supplemental staff hired specifically to perform testing [104]. In November 2007, the inaugural conference of the National Emergency Department HIV Testing Consortium was held in Baltimore (MD, USA) with the goal of developing consensus definitions and reporting guidelines for HIV screening in emergency departments [105]. Since that meeting, the Consortium has worked to develop policies and practices for HIV screening in emergency departments and encourage providers in emergency departments to perform HIV screening [106]. During 2007–2010, studies were initiated to evaluate opt-out testing in two large urban emergency departments. A study conducted by Haukoos and coworkers compared non-targeted opt-out screening with physician-directed targeted and diagnostic testing in an emergency department in Denver (CO, USA); they found that opt-out screening was associated with a modest increase in new HIV diagnoses [107]. White and coworkers found that rates of HIV testing in an emergency department in Oakland (CA, USA) were nearly identical using opt-out screening compared with opt-in screening (e.g., letting patients know that HIV screening is available and asking if they would like to participate); they found that while a significantly higher proportion of patients were offered screening using the opt-out approach, this was counterbalanced by lower acceptance and completion of testing in this group [108].
Linkage to & retention in care
The benefits of HIV testing can be realized only if those who test positive are linked to and retained in HIV care. In a meta-analysis, Marks and coworkers found that, averaged over time, 69% of persons diagnosed with HIV in the USA entered medical care and 72% of these entered care within 4 months of diagnosis [109]. Using routine surveillance data collected in New York City and defining time to initiation of care as the interval between first positive western blot test result and first reported viral load and/or CD4 cell count, Torian and coworkers found that 64% of persons diagnosed with HIV in 2003 initiated care within 3 months of diagnosis, 19% initiated care more than 3 months after diagnosis and 17% never initiated care [110]. Strategies that have been proposed to increase linkage to care include immediately referring persons with preliminary positive rapid test results to care and using a rapid HIV testing algorithm and immediately referring those with repeatedly reactive rapid test results to care. Delaney and coworkers evaluated the use of a rapid HIV testing algorithm, in which persons with two or more reactive sequential rapid test results were immediately referred to care, as compared with standard rapid testing, in which persons with preliminary positive rapid test results had confirmatory testing and were asked to return for their results before referral to care [111]. They found that using the algorithm plus immediate referral to care resulted in 67% being linked to care within 90 days; however, almost half (49%) of persons who had preliminary positive rapid test results on standard rapid testing and who did not return for their confirmatory test results and referrals were linked to care within 90 days.
Challenges in linking persons diagnosed with HIV infection to care begin with impediments to delivering HIV test results. Using conventional testing, approximately two-thirds of HIV-infected persons return to receive their test results, although this varies by the setting in which testing is conducted [102,103,112–114]. When rapid testing is conducted, most HIV-infected persons receive preliminary positive test results, however some refuse confirmatory testing. Data from six rapid testing studies showed that an average of 75% (range: 0–100%) of HIV-infected participants received their confirmatory test results [102,103,112–116].
In their meta-analysis evaluating entry and retention in care, Marks and coworkers found that the percentage of persons diagnosed with HIV who entered care varied by site of testing: 76% of those testing positive in emergency departments and urgent care clinics entered care compared with 67% of those testing positive in community settings [109]. Torian and coworkers found that predictors of delayed care included diagnosis at a community testing site, in a correctional system, or at a sexually transmitted disease or tuberculosis clinic compared with diagnosis at a testing site co-located with a primary medical care clinic; they concluded that because most diagnoses occur in clinical settings, improving linkage to care in these settings would have the greatest impact on timely initiation of care [110].
Use of linkage case management has been shown to increase linkage to care following diagnosis. In the Antiretroviral Treatment Access Study (ARTAS), persons who received case management for linkage to care were 36% more likely to be successfully linked to HIV medical care within 6 months of diagnosis than persons who received passive referrals to care following diagnosis [117]. In a follow-up project, ARTAS-II, Craw and coworkers found that testing sites with co-located HIV clinics were more successful than those without such clinics at linking patients to HIV medical care within 6 months of diagnosis (87% vs 73%) [118]. Use of financial incentives may increase linkage to care; the TLC-Plus study will randomly assign participating test sites to use a financial incentive intervention or standard of care to test this concept [201].
Retention in care after linkage is essential. Patients on ART who see providers regularly are more likely to have suppressed viral loads [119]. The HIV Medical Association’s primary care guidelines include recommendations to emphasize the importance of adherence to care rather than just focusing on adherence to medications [120,121]. Adherence to medications and medical care are necessary for long-term survival [122], and regular monitoring provides opportunities to optimize viral suppression and immune function [16,123], maximize survival [119,124] and reduce transmission [13,35,124]. Marks and coworkers found that 59% of HIV-infected persons had multiple HIV medical care visits averaged across time intervals of 6 months to 5 years [109]. Torian and coworkers, defining regular care as at least one visit every 6 months, found that among New York City residents diagnosed with HIV in 2005 who initiated care within 3 months of diagnosis, 94% made at least one subsequent visit and 45% were in regular care [124]. Testing programs should foster ties between public health agencies and providers, as this would likely increase linkage and encourage retention in care. These studies illustrate how laboratory data that is reported to HIV surveillance programs can be used to monitor linkage and retention in care. Ensuring complete case reporting from new testing initiatives is also critical to better evaluate the impact of such programs on trends in HIV infections.
Cost–effectiveness of testing & linkage to care
Several studies demonstrate that HIV screening is cost effective, except when HIV prevalence is very low [125], and that the cost–effectiveness of screening programs varies with type and frequency of testing. Although the cost–effectiveness of HIV screening in clinical settings is greatest when conducted in populations in which the undiagnosed HIV seroprevalence is ≥0.1% [126,127], testing in nonclinical settings is often also cost effective and is particularly important for reaching populations with limited access to and use of healthcare services. Published reports reveal that the cost per new HIV diagnosis for screening in a variety of clinical settings using various approaches has ranged from approximately $2,000 to $12,000 [128–132], whereas the cost per new HIV diagnosis for screening in nonclinical settings using a variety of approaches has ranged from approximately $3,000 to $30,000 [132–135]. Costs also vary widely by the clinical setting in which testing is performed, although this is due in part to differences in the underlying prevalence in populations served and the testing strategies used (e.g., routine screening versus using a targeted or diagnostic approach to testing). A study assessing the cost–effectiveness of HIV testing in three clinical settings found that HIV screening in sexually transmitted disease clinics and in emergency departments was cost effective when compared with testing persons admitted to the hospital with clinical manifestations suggesting HIV [125].
Cost–effectiveness ratios for HIV testing cluster around $33,000 per quality-adjusted life year (QALY) gained when the prevalence of undiagnosed HIV infection is at 1% [136]. Paltiel and coworkers determined that in populations with an HIV prevalence of 1%, screening once, every 5 years, and every 3 years were associated with incremental cost–effectiveness ratios of $38,000, $71,000 and $85,000 per QALY gained, respectively [126]. Although testing is cost effective when considering individual benefits to those tested (e.g., reduced morbidity and mortality arising from diagnosis and access to treatment earlier in the course of infection), it is frequently cost-saving when the reduced transmission associated with awareness of serostatus and with low or undetectable viral load following initiation of ART are taken into account [125].
Proposed costs of intensifying prevention efforts to reduce transmission further in the USA are high, but potential cost savings are greater. Schackman and coworkers estimate that each new HIV infection averted saves approximately $367,000 in lifetime medical costs [10,137], and Walensky and coworkers found that targeting HIV screening resources to ensure that individuals who test positive receive their results and are linked to care is more cost–effective than offering testing to additional people [136]. Long and coworkers estimate that one-time screening of low-risk persons (i.e., persons other than MSM or injection drug users) coupled with annual screening of high-risk persons (MSM and injection drug users) would prevent almost 7% of projected new infections and cost $22,000 per QALY gained; expanding ART utilization to 75% of those eligible would prevent 10% of infections and cost $20,000 per QALY gained; and combining the two approaches would prevent 17% of infections and cost $22,000 per QALY gained [138].
Policy issues related to HIV testing
Policies guiding program implementation and cost reimbursement can restrict the potential reach of HIV testing programs in both clinical and nonclinical settings. Such policies include those related to obtaining consent for testing, providing pre- and post-test counseling and third-party reimbursement for HIV testing that is performed for reasons other than clinical manifestations of HIV infection [139]. When the CDC recommended providing voluntary, opt-out HIV testing to all persons aged 13–64 years in all healthcare settings, it was anticipated that requirements for specific consent for HIV testing and pre-test counseling would prove to be barriers to increasing HIV testing in clinical settings, so the recommendations stated that:
Specific consent for HIV testing should not be required because general consent for medical care should cover consent for HIV testing;
Prevention counseling should not be required for HIV diagnostic testing or as part of HIV screening programs in healthcare settings, although oral or written information explaining HIV infection and the meaning of positive and negative test results should be provided [11].
Laws regarding HIV testing are under the jurisdiction of states, and at the time the revised recommendations were published, many states had laws that prevented their implementation [140]. In 2006, 21 states had laws and regulations compatible with the consent and counseling parts of the revised recommendations [140]. Between 2006 and 2011, 23 states and Washington, DC, changed their statutes and/or administrative codes so they would be more compatible with the CDC’s testing recommendations; as of January 2011, the HIV testing laws and administrative codes of 90% of US jurisdictions were compatible with the CDC’s revised recommendations for HIV testing in healthcare settings [140,220].
Removal of the requirement for written informed consent may promote routine screening. After adjusting for individual (demographic, socioeconomic and HIV risk characteristics) and state (AIDS prevalence and amount of federal funds received for HIV prevention activities) factors, persons residing in states with statutes requiring informed consent were less likely to report a recent HIV test [141]. Increases have been observed in the number of rapid tests performed and new case detection at a large emergency department [56,142] and in the number of HIV tests performed statewide [143] following simplification of consent requirements at a San Francisco hospital and in New York, respectively. Halpern and coworkers argue for uniform standards for nonconsented testing [144], but Neff and Goldschmidt believe that lack of national conformity will persist, because states write their own laws [145]. The National HIV/AIDS Clinician’s Consultation Center of the University of California at San Francisco has developed the ‘Compendium of State HIV/AIDS Testing Laws’, which describes state HIV testing laws and policies and was designed to help providers understand HIV testing laws and to implement sound HIV testing [220].
The Veterans Health Administration has been leading the effort to simplify testing procedures at federally funded healthcare facilities. To help promote early diagnosis of HIV and simplify the process, the Veterans Health Administration revised its policies on HIV testing and informed consent. Written informed consent has not been required for HIV testing at Veterans Affairs facilities since August 2009. Instead, veterans are able to provide verbal consent for voluntary HIV testing. Also, lengthy pre- and post-test counseling are no longer required; rather, providers must give patients written materials about HIV and HIV testing. Another important change is that HIV testing is no longer limited to only those veterans who report risk behaviors. In line with current federal public health recommendations, voluntary HIV testing is now recommended as a part of routine medical care for all veterans. These policy changes have a common goal: to diagnose infection as soon as possible so that veterans can receive state-of-the-art care and remain healthy [214].
Inadequate reimbursement for testing has been cited as a barrier to implementing the CDC’s revised recommendations outside of public healthcare settings [146–148]. California enacted legislation requiring health plans to cover the cost of HIV testing in 2008 – including screening and diagnostic testing [146] – and other states may follow California’s lead in coming years.
Conclusion
During the past decade, dramatic progress in HIV diagnostic technologies, extensive experience conducting testing in clinical and nonclinical settings, improvements in care and treatment for HIV-infected persons and efforts to couple HIV testing with early ART have led to improved conditions for scaling up HIV testing in the USA and reducing HIV transmission. Scaling up HIV testing is a key objective of the 2009 reauthorization of the Ryan White CARE Act, and the CDC’s high-impact prevention funding opportunity, the Veterans Health Administration’s HIV testing campaign, the Indian Health Service’s and the Substance Abuse and Mental Health Services Administration’s HIV testing activities support this objective. The supply and distribution of available antiretroviral medications, and the infrastructure to support these, will be important issues to consider as HIV testing programs are scaled up. Likewise, expanding HIV testing brings into question issues related to sustainability and equitable access for chronic treatment [39,149]. The HPTN 052 Study showed that early HIV treatment results in substantial reduction in HIV transmission and clinical events [42]. Although earlier treatment has individual and public health benefits, expanded testing coupled with earlier initiation of ART will result in an increase in the number of people recognized who need ART and will likely lengthen the waiting lists for financial assistance to obtain ART that currently exist in the USA. The implications that delays in receipt of ART might have on HIV testing and care-seeking behaviors at the individual level are unknown, but inability to access ART may reduce a person’s motivation to seek testing and care [120]. The 2010 Affordable Care Act, which was developed to enhance health security and the quality of healthcare in the USA by implementing comprehensive health insurance reforms [221], may obviate some barriers to scaling up HIV testing as HIV testing becomes an accepted and integral component of routine healthcare, and it may facilitate earlier initiation of ART by facilitating access to healthcare and antiretroviral medications. Efforts to expand HIV testing and early treatment will be critical for achieving the goals of the US National HIV/AIDS Strategy to reduce HIV incidence, improve access to care and health outcomes among persons with HIV infection, and reduce disparities in HIV infection.
Future perspective
Identifying effective, efficient & sustainable models for HIV testing & linkage to care
Key objectives of HIV testing programs include increasing the number of people who know their HIV serostatus and diagnosing HIV infection early. To achieve these objectives, it is imperative to determine and implement optimal models for HIV testing in clinical and nonclinical settings [150]. There have been substantial efforts to increase HIV testing in clinical settings since the revised recommendations for HIV testing in healthcare settings were published in 2006, but substantial barriers remain. Current testing programs will help inform feasible, acceptable, and sustainable strategies that can be used to expand HIV testing in healthcare settings and promote the expectation that HIV testing is a routine part of healthcare. Efforts to increase HIV testing will not meet essential objectives, however, unless they are coupled with efforts to ensure that everyone who is diagnosed with HIV is linked to and retained in HIV medical care. Efforts to increase linkage to and retention in care following diagnosis may be more cost effective than efforts to increase HIV testing [136], and more resources will undoubtedly need to be allocated for increasing linkage and retention in coming years, whether in the form of financial incentives, increased linkage case management or a combination of the two.
Expanding HIV testing in nonclinical settings is critical for persons who have limited access to or use of healthcare services. Current testing programs that are being conducted in nonclinical settings will be important for identifying effective, efficient and sustainable models for reaching hard-to-reach populations and groups at high risk for HIV infection who should be tested frequently. Determining and making the best use of current diagnostic technologies and those on the horizon and ensuring that persons identified with positive HIV test results are immediately linked to care will be crucial. Use of email, text messaging and online resources may facilitate ongoing contact with persons who test positive and ensure that they are successfully linked to and engaged in HIV medical care.
New technologies: assays, algorithms & biomarkers for incidence
New technologies are in development that will continue to change the field of HIV diagnostics inside and outside the USA. While the availability and use of combination antigen/antibody assays in the USA has lagged behind the rest of the world, such tests are now available. In 2010, the FDA approved a fourth-generation assay [205] and in 2011, it approved a second fourth-generation assay for use in the USA [206]. Additionally, two diagnostics companies are preparing to introduce other combination antigen/antibody assays to the US market, including another laboratory-based assay [151] and a point-of-contact assay [222].
In addition to the introduction of combination antigen/antibody assays to the US market, there is considerable work being done to generate rapid nucleic acid assays that would allow easier detection of AHI. Many of these technologies rely on isothermal amplification techniques and include loop-mediated isothermal amplification [152], helicase-dependent amplification [153], and a simplified amplification-based assay that incorporates a visual dip-stick detection device [154]. There is also considerable work being done to decrease the assay time for real-time PCR assays and package them so they can be used in point-of-contact settings [155–157]. All of these rapid tests have high reported sensitivities and specificities, provide potentially cost effective alternatives to tests currently in use, and would be useful as supplemental tests for AHI in point-of-contact and other settings where rapid and definitive diagnosis is crucial. However, until large-scale clinical trials and the FDA review of these types of assays are completed, it will be difficult to ascertain their true value in the diagnosis of HIV infection.
Besides advances in technology, there are ongoing, concerted efforts to develop new algorithms to diagnose HIV infection in the USA. An algorithm that consists of a sensitive third- or fourth-generation screening assay followed by an assay that can differentiate HIV-1 from HIV-2 was proposed at the 2010 HIV Diagnostics Conference [76]. The CDC and the Association of Public Health Laboratories are currently working to update guidelines on HIV testing algorithms that will likely include this or similar algorithms for diagnosing HIV infection in the USA [76,77].
Along with advances in the area of diagnosis, there is considerable work to be done on identifying recent infection that can be used for incidence surveillance. This research is focused on new assays, many of which measure antibody avidity maturation as it has been demonstrated that antibodies to HIV become more avid (bind more tightly to HIV antigens) as the duration of infection increases [158–160]. There is also considerable attention being focused on combining assays in an algorithm [161] or combining multiple analytes within a single assay format to improve identification of recent infection [162]. There are also groups working to use molecular techniques such as deep sequence analysis to distinguish between recent and long-term infection [163]. In addition to development of assays, there is an effort by several organizations to collaborate to move the field of recent infection testing forward in a uniform manner [164]. Having a standardized approach to identifying recent infections will likely lead to more rapid progress in identifying HIV early in the course of infection, monitoring recent infections, starting treatment and care as early as possible, and assessing the effectiveness of preventive interventions.
Executive summary.
HIV testing is a key factor in HIV prevention in the USA. Individuals must be diagnosed to have the opportunity to access care, treatment and prevention services.
HIV testing and knowledge of HIV serostatus have important public health benefits. The primary public health benefit of testing is reduced onward transmission of HIV.
The tremendous progress in the field of HIV diagnostics during the past 20 years has led to new strategies for diagnosing HIV infection more quickly and with greater accuracy.
Rapid HIV testing, the development of new assays and use of combinations of assays have improved detection of HIV infection but present challenges for HIV case surveillance.
Expansion of HIV testing efforts in the USA have followed the CDC’s 2006 revised recommendations for HIV testing in healthcare settings and subsequent implementation of HIV testing programs at the national, state, and local levels.
The benefits of HIV testing can only be realized if those who test positive are linked to and retained in HIV care.
Although the costs of increasing HIV testing and other prevention efforts to reduce transmission in the USA are high, the potential cost savings are likely to be greater.
Changes in policies guiding program implementation and reimbursement of HIV testing promise to facilitate HIV testing in clinical and nonclinical settings.
Implementation of effective, efficient and sustainable models for HIV testing and linkage to care and continued development of novel HIV diagnostic technologies are necessary to reduce HIV transmission, incidence and prevalence.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as: ■ of interest
- 1.Centers for Disease Control and Prevention. HIV Surveillance–United States, 1981–2008. MMWR Morb. Mortal. Wkly Rep 60(21), 689–693 (2011). [PubMed] [Google Scholar]
- 2.Prejean J, Song R, Hernandez A et al. Estimated HIV Incidence in the United States, 2006–2009. PLoS One 6(8), E17502 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleming PL, Ward JW, Karon JM, Hanson DL, De Cock KM. Declines in AIDS incidence and deaths in the USA: a signal change in the epidemic. AIDS 12(Suppl. A), S55–S61 (1998). [PubMed] [Google Scholar]
- 4.McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS 13(13), 1687–1695 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Cain LE, Cole SR, Chmiel JS, Margolick JB, Rinaldo CR Jr, Detels R. Effect of highly active antiretroviral therapy on multiple AIDS-defining illnesses among male HIV seroconverters. Am. J. Epidemiol 163(4), 310–315 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Bhaskaran K, Hamouda O, Sannes M et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 300(1), 51–59 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Hall HI, Green TA, Wolitski RJ et al. Estimated future HIV prevalence, incidence, and potential infections averted in the United States: a multiple scenario analysis. J. Acquir. Immune Defic. Syndr 55(2), 271–276 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 20(10), 1447–1450 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Lansky A, Brooks JT, DiNenno E, Heffelfinger J, Hall HI, Mermin J. Epidemiology of HIV in the United States. J. Acquir. Immune Defic. Syndr 55(Suppl. 2), S64–S68 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Vital signs: HIV testing and diagnosis among adults – United States, 2001–2009. MMWR Morb. Mortal. Wkly Rep 59(47), 1550–1555 (2010). [PubMed] [Google Scholar]
- 11.Branson BM, Handsfield HH, Lampe MA et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm. Rep 55(RR-14), 1–17; quiz CE11–CE14 (2006). [PubMed] [Google Scholar]; ■ The CDC’s revised recommendations for HIV testing in healthcare settings. These recommendations updated previous recommendations for HIV testing in healthcare settings and for screening of pregnant women. They were developed to promote routine HIV testing in healthcare settings.
- 12.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J. Acquir. Immune Defic. Syndr 40(1), 96–101 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Donnell D, Baeten JM, Kiarie J et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 375(9731), 2092–2098 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin. Infect. Dis 52(6), 793–800 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med 338(13), 853–860 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Walensky RP, Paltiel AD, Losina E et al. The survival benefits of AIDS treatment in the United States. J. Infect. Dis 194(1), 11–19 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Detels R, Munoz A, McFarlane G et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA 280(17), 1497–1503 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Jones JL, Hanson DL, Dworkin MS et al. Surveillance for AIDS-defining opportunistic illnesses, 1992–1997. MMWR CDC Surveill. Summ 48(2), 1–22 (1999). [PubMed] [Google Scholar]
- 19.Mocroft A, Vella S, Benfield TL et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 352(9142), 1725–1730 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Miller V, Mocroft A, Reiss P et al. Relations among CD4 lymphocyte count nadir, antiretroviral therapy, and HIV-1 disease progression: results from the EuroSIDA study. Ann. Intern. Med 130(7), 570–577 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Dore GJ, Li Y, McDonald A, Ree H, Kaldor JM. Impact of highly active antiretroviral therapy on individual AIDS-defining illness incidence and survival in Australia. J. Acquir. Immune Defic. Syndr 29(4), 388–395 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J. Acquir. Immune Defic. Syndr 39(4), 446–453 (2005). [DOI] [PubMed] [Google Scholar]; ■ This meta-analysis found that the prevalence of unprotected anal or vaginal intercourse is substantially reduced (by more than 50%, on average) among HIV-infected persons who are aware they are infected compared to HIV-infected persons who are unaware they are infected. The findings from this study have led to efforts to expand HIV testing in an attempt to increase knowledge of HIV serostatus in the USA.
- 23.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N. Engl. J. Med 364(20), 1943–1954 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Describes recent important advances in the understanding of acute HIV infection, including the detection, management and prevention of acute HIV infection.
- 24.Powers KA, Ghani AC, Miller WC et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 378(9787), 256–268 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branson BM. The future of HIV testing. J. Acquir. Immune Defic. Syndr 55(Suppl. 2), S102–S105 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Morrison CS, Demers K, Kwok C et al. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS 24(4), 573–582 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N. Engl. J. Med 324(14), 961–964 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Pilcher CD, Joaki G, Hoffman IF et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS 21(13), 1723–1730 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koopman JS, Simon CP. Response to Rapatski BL, Suppe F, Yorke JA. HIV epidemics driven by late disease stage transmission. J. Acquir. Immune Defic. Syndr 41(5), 677 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Wawer MJ, Gray RH, Sewankambo NK et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis 191(9), 1403–1409 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J. Infect. Dis 198(5), 687–693 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Brenner BG, Roger M, Routy JP et al. High rates of forward transmission events after acute/early HIV-1 infection. J. Infect. Dis 195(7), 951–959 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Prevalence and awareness of HIV infection among men who have sex with men–21 cities, United States, 2008. MMWR Morb. Mortal. Wkly Rep 59(37), 1201–1207 (2010). [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. HIV testing among men who have sex with men – 21 cities, United States, 2008. MMWR Morb. Mortal. Wkly Rep 60(21), 694–699 (2011). [PubMed] [Google Scholar]
- 35.Quinn TC, Wawer MJ, Sewankambo N et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med 342(13), 921–929 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Tovanabutra S, Robison V, Wongtrakul J et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J. Acquir. Immune Defic. Syndr 29(3), 275–283 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Baeten JM, Kahle E, Lingappa JR et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci. Transl. Med 3(77), 77RA29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montaner JS, Hogg R, Wood E et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368(9534), 531–536 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Venkatesh KK, Lurie MN, Mayer KH. How HIV treatment could result in effective prevention. Future Virol. 5(4), 405–415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J. Acquir. Immune Defic. Syndr 41(5), 632–641 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Garnett GP, Gazzard B. Risk of HIV transmission in discordant couples. Lancet 372(9635), 270–271 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Cohen MS, Chen YQ, McCauley M et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ HIV Prevention Trials Network 052 study conducted in nine countries, which evaluated the effect of early antiretroviral therapy on HIV-1 transmission to HIV-1-negative heterosexual partners. The study demonstrated that early treatment of HIV-infected individuals resulted in a substantial decrease in sexual transmission of HIV-1 to uninfected partners.
- 43.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet, 373(9657), 48–57 (2009). [DOI] [PubMed] [Google Scholar]; ■ Used mathematical models to evaluate whether a strategy of universal voluntary HIV testing coupled with provision of immediate antiretroviral therapy to HIV-infected persons would reduce HIV incidence and transmission. The authors found that this strategy combined with current prevention approaches has the potential to mitigate severe generalized HIV epidemics substantially.
- 44.Walensky RP, Paltiel AD, Losina E et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin. Infect. Dis 51(4), 392–400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. Interpretation and use of the western blot assay for serodiagnosis of human immunodeficiency virus type 1 infections. MMWR Morb. Mortal. Wkly Rep 38(Suppl. 7), 1–7 (1989). [PubMed] [Google Scholar]
- 46.Fiebig EW, Wright DJ, Rawal BD et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17(13), 1871–1879 (2003). [DOI] [PubMed] [Google Scholar]; ■ Describes the sequential emergence of reactivity in HIV assays. This study helped classify the stages of acute HIV infection, facilitating the diagnosis of recent infection.
- 47.Owen SM, Yang C, Spira T et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J. Clin. Microbiol 46(5), 1588–1595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Describes the use of currently licensed HIV tests in alternative algorithms to detect and confirm established HIV infection. It showed that alternative algorithms compare favorably with the conventional algorithm that has been used to diagnose HIV infection for more than 20 years and improve detection of early HIV infection.
- 48.Parry JV, Mortimer PP, Perry KR, Pillay D, Zuckerman M. Towards error-free HIV diagnosis: guidelines on laboratory practice. Commun. Dis. Public Health 6(4), 334–350 (2003). [PubMed] [Google Scholar]
- 49.Weber B, Gurtler L, Thorstensson R et al. Multicenter evaluation of a new automated fourth-generation human immunodeficiency virus screening assay with a sensitive antigen detection module and high specificity. J. Clin. Microbiol 40(6), 1938–1946 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ly TD, Martin L, Daghfal D et al. Seven human immunodeficiency virus (HIV) antigen-antibody combination assays: evaluation of HIV seroconversion sensitivity and subtype detection. J. Clin. Microbiol 39(9), 3122–3128 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ly TD, Laperche S, Brennan C et al. Evaluation of the sensitivity and specificity of six HIV combined p24 antigen and antibody assays. J. Virol. Methods 122(2), 185–194 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Weber B Screening of HIV infection: role of molecular and immunological assays. Expert Rev. Mol. Diagn 6(3), 399–411 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Chavez P, Wesolowski L, Patel P, Delaney K, Owen SM. Evaluation of the performance of the Abbott ARCHITECT HIV Ag/Ab combo assay. J. Clin. Virol (2011) (In Press). [DOI] [PubMed] [Google Scholar]
- 54.Delaney KP, Branson BM, Uniyal A et al. Evaluation of the performance characteristics of 6 rapid HIV antibody tests. Clin. Infect. Dis 52(2), 257–263 (2011). [DOI] [PubMed] [Google Scholar]
- 55.O’Conell RJ, Peel SA. Multispot HIV-1/HIV-2 Rapid Test: advantages over other rapid HIV tests. Expert Rev. Mol. Diagn 7(5), 499–505 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Pilcher CD, Christopoulos KA, Golden M. Public health rationale for rapid nucleic acid or p24 antigen tests for HIV. J. Infect. Dis 201(Suppl. 1), S7–S15 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Notice to readers: protocols for confirmation of reactive rapid HIV tests. MMWR Morb. Mortal. Wkly Rep 53(10), 221–222 (2004). [Google Scholar]
- 58.Greensides DR, Berkelman R, Lansky A, Sullivan PS. Alternative HIV testing methods among populations at high risk for HIV infection. Public Health Rep. 118(6), 531–539 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Busch MP, Satten GA. Time course of viremia and antibody seroconversion following human immunodeficiency virus exposure. Am. J. Med 102(5B), 117–124; discussion 125–116 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Lindback S, Karlsson AC, Mittler J et al. Viral dynamics in primary HIV-1 infection. Karolinska Institutet Primary HIV Infection Study Group. AIDS 14(15), 2283–2291 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Patel P, Mackellar D, Simmons P et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Arch Intern. Med 170(1), 66–74 (2010). [DOI] [PubMed] [Google Scholar]; ■ Assessed the feasibility and yield of pooled nucleic acid amplification testing relative to HIV antibody screening assays. Although pooled nucleic acid amplification testing increased HIV case detection following testing with third-generation immunoassays, it increased HIV testing most when used with less sensitive immunoassays.
- 62.Facente SN, Pilcher CD, Hartogensis WE et al. Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One 6(7), E21813 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pilcher CD, Fiscus SA, Nguyen TQ et al. Detection of acute infections during HIV testing in North Carolina. N. Engl. J. Med 352(18), 1873–1883 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Truong HM, Grant RM, McFarland W et al. Routine surveillance for the detection of acute and recent HIV infections and transmission of antiretroviral resistance. AIDS 20(17), 2193–2197 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Priddy FH, Pilcher CD, Moore RH et al. Detection of acute HIV infections in an urban HIV counseling and testing population in the United States. J. Acquir. Immune Defic. Syndr 44(2), 196–202 (2007). [DOI] [PubMed] [Google Scholar]
- 66.Stekler JD, Swenson PD, Coombs RW et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin. Infect. Dis 49(3), 444–453 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark JL, Segura ER, Montano SM et al. Routine laboratory screening for acute and recent HIV infection in Lima, Peru. Sex. Transm. Infect 86(7), 545–547 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandori MW, Hackett J Jr, Louie B et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J. Clin. Microbiol 47(8), 2639–2642 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ One of the first studies to evaluate a fourth-generation (or combination antigen/antibody) HIV immunoassay in the USA. It found that the immunoassay was able to detect most HIV infections and 80% of infections considered to be acute HIV infections.
- 69.O’Brien TR, George JR, Holmberg SD. Human immunodeficiency virus type 2 infection in the United States. Epidemiology, diagnosis, and public health implications. JAMA 267(20), 2775–2779 (1992). [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. HIV-2 Infection Surveillance–United States, 1987–2009. MMWR Morb. Mortal. Wkly Rep 60, 985–988 (2011). [PubMed] [Google Scholar]
- 71.Matheron S, Pueyo S, Damond F et al. Factors associated with clinical progression in HIV-2 infected-patients: the French ANRS cohort. AIDS 17(18), 2593–2601 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Marlink R, Kanki P, Thior I et al. Reduced rate of disease development after HIV-2 infection as compared with HIV-1. Science 265(5178), 1587–1590 (1994). [DOI] [PubMed] [Google Scholar]
- 73.Ntemgwa ML, d’Aquin Toni T, Brenner BG, Camacho RJ, Wainberg MA. Antiretroviral drug resistance in human immunodeficiency virus type 2. Antimicrob. Agents Chemother 53(9), 3611–3619 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torian LV, Eavey JJ, Punsalang AP et al. HIV type 2 in New York City, 2000–2008. Clin. Infect. Dis 51(11), 1334–1342 (2010). [DOI] [PubMed] [Google Scholar]
- 75.Nasrullah M, Ethridge SF, Delaney KP et al. Comparison of alternative interpretive criteria for the HIV-1 western blot and results of the Multispot HIV-1/HIV-2 rapid test for classifying HIV-1 and HIV-2 infections. J. Clin. Virol (2011) (In Press). [DOI] [PubMed] [Google Scholar]
- 76.Branson B Conference wrap-up: unified laboratory algorithm. Presented at: 2010 HIV Diagnostics Conference. Orlando, FL, USA, 24–26 March 2010. [Google Scholar]
- 77.Pandori MW, Branson BM. 2010 HIV Diagnostics Conference. Expert Rev. Anti. Infect. Ther 8(6), 631–633 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of alternative diagnostic algorithms using specimens from early and established HIV infections in the United States. J. Clin. Virol (2011) (In Press). [DOI] [PubMed] [Google Scholar]
- 79.Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years – United States, 2008. MMWR Recomm. Rep 57(RR-10), 1–12 (2008). [PubMed] [Google Scholar]
- 80.Centers for Disease Control and Prevention. HIV infection and HIV-associated behaviors among injecting-drug users–20 Cities, United States, 2009. MMWR Morb. Mortal. Wkly Rep 58(13), 329–332 (2010). [Google Scholar]
- 81.Centers for Disease Control and Prevention. Characteristics Associated with HIV Infection Among Heterosexuals in Urban Areas with High AIDS Prevalence–24 Cities, United States, 2006–2007. MMWR Morb. Mortal. Wkly Rep 60, 1045–1049 (2011). [PubMed] [Google Scholar]
- 82.Braunstein SL, Nash D, Kim AA et al. Dual Testing algorithm of BED-CEIA and AxSYM avidity index assays performs best in identifying recent HIV infection in a sample of Rwandan sex workers. PLoS One 6(4), E18402 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDougal JS, Pilcher CD, Parekh BS et al. Surveillance for HIV-1 incidence using tests for recent infection in resource-constrained countries. AIDS 19 (Suppl. 2), S25–S30 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Parekh BS, Hu DJ, Vanichseni S et al. Evaluation of a sensitive/less-sensitive testing algorithm using the 3A11-LS assay for detecting recent HIV seroconversion among individuals with HIV-1 subtype B or E infection in Thailand. AIDS Res. Hum. Retroviruses 17(5), 453–458 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Rutherford GW, Schwarcz SK, McFarland W. Surveillance for incident HIV infection: new technology and new opportunities. J. Acquir. Immune Defic. Syndr 25(Suppl. 2), S115–S119 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Janssen RS, Satten GA, Stramer SL et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280(1), 42–48 (1998). [DOI] [PubMed] [Google Scholar]
- 87.Parekh BS, Kennedy MS, Dobbs T et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res. Hum. Retroviruses 18(4), 295–307 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Suligoi B, Massi M, Galli C et al. Identifying recent HIV infections using the avidity index and an automated enzyme immunoassay. J. Acquir. Immune Defic. Syndr 32(4), 424–428 (2003). [DOI] [PubMed] [Google Scholar]
- 89.McDougal JS, Parekh BS, Peterson ML et al. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res. Hum. Retroviruses, 22(10), 945–952 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Barnighausen T, McWalter TA, Rosner Z, Newell ML, Welte A. HIV incidence estimation using the BED capture enzyme immunoassay: systematic review and sensitivity analysis. Epidemiology 21(5), 685–697 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Janssen RS, Holtgrave DR, Valdiserri RO, Shepherd M, Gayle HD, De Cock KM. The Serostatus Approach to Fighting the HIV Epidemic: prevention strategies for infected individuals. Am. J. Public Health 91(7), 1019–1024 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Centers for Disease Control and Prevention. Revised guidelines for HIV counseling, testing, and referral. MMWR Recomm. Rep 50(RR-19), 1–57; quiz CE51-19a51-CE56-19a51 (2001). [PubMed] [Google Scholar]
- 93.Centers for Disease Control and Prevention. Advancing HIV Prevenion: new strategies for a changing epidemic – United States, 2003. Morb. Mortal. Wkly Rep 52(15), 329–332 (2003). [PubMed] [Google Scholar]
- 94.Heffelfinger JD, Sullivan PS, Branson BM et al. Advancing HIV prevention demonstration projects: new strategies for a changing epidemic. Public Health Rep. 123(Suppl. 3), 5–15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Centers for Disease Control and Prevention. Results of Expanded HIV Testing Initiative – 25 Jurisdictions, 2007–2010. MMWR Morb. Mortal. Wkly Rep 60(24), 805–810 (2011). [PubMed] [Google Scholar]
- 96.Daskalakis D HIV diagnostic testing: Evolving technology and testing strategies. Topics Antiviral. Med 19(1), 18–22 (2011). [PMC free article] [PubMed] [Google Scholar]
- 97.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Aff. (Millwood), 28(6), 1677–1687 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Centers for Disease Control and Prevention. Expanded HIV testing and trends in diagnoses of HIV infection - District of Columbia, 2004–2008. MMWR Morb. Mortal. Wkly Rep 59(24), 737–741 (2010). [PubMed] [Google Scholar]
- 99.Maxwell CJ, Sitapati AM, Abdus-Salaam SS et al. A model for routine hospital-wide HIV screening: lessons learned and public health implications. J. Natl Med. Assoc 102(12), 1165–1172 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Goggin MA, Davidson AJ, Cantril SV, O’Keefe LK, Douglas JM. The extent of undiagnosed HIV infection among emergency department patients: results of a blinded seroprevalence survey and a pilot HIV testing program. J. Emerg. Med 19(1), 13–19 (2000). [DOI] [PubMed] [Google Scholar]
- 101.Kelen GD, Hexter DA, Hansen KN et al. Feasibility of an emergency department-based, risk-targeted voluntary HIV screening program. Ann. Emerg. Med 27(6), 687–692 (1996). [DOI] [PubMed] [Google Scholar]
- 102.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann. Emerg. Med 33(2), 147–155 (1999). [DOI] [PubMed] [Google Scholar]
- 103.Centers for Disease Control and Prevention. Routinely recommended HIV testing at an urban urgent-care clinic – Atlanta, Georgia, 2000. MMWR Morb. Mortal. Wkly Rep 50(25), 538–541 (2001). [PubMed] [Google Scholar]
- 104.Torres GW, Heffelfinger JD, Pollack HA, Barrera SG, Rothman RE. HIV screening programs in US emergency departments: a cross-site comparison of structure, process, and outcomes. Ann. Emerg. Med 58(Suppl. 1), S104–S113 (2011). [DOI] [PubMed] [Google Scholar]
- 105.Lyons MS, Lindsell CJ, Haukoos JS et al. Nomenclature and definitions for emergency department human immunodeficiency virus (HIV) testing: report from the 2007 conference of the National Emergency Department HIV Testing Consortium. Acad. Emerg. Med 16(2), 168–177 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kecojevic A, Lindsell CJ, Lyons MS et al. Public health and clinical impact of increasing emergency department-based HIV testing: perspectives from the 2007 conference of the National Emergency Department HIV Testing Consortium. Ann. Emerg. Med 58(Suppl. 1), S151–S159 E151 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Haukoos JS, Hopkins E, Conroy AA et al. Routine opt-out rapid HIV screening and detection of HIV infection in emergency department patients. JAMA 304(3), 284–292 (2010). [DOI] [PubMed] [Google Scholar]
- 108.White DA, Scribner AN, Vahidnia F et al. HIV screening in an urban emergency department: comparison of screening using an opt-in versus an opt-out approach. Ann. Emerg. Med 58(Suppl. 1), S89–S95 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Marks G, Gardner LI, Craw J, Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS 24(17), 2665–2678 (2010). [DOI] [PubMed] [Google Scholar]
- 110.Torian LV, Wiewel EW, Liu KL, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch. Intern. Med 168(11), 1181–1187 (2008). [DOI] [PubMed] [Google Scholar]
- 111.Delaney K, Knoble T, J. R et al. Using a rapid HIV testing algorithm to improve the accuracy of HIV testing, receipt of test results, and linkage to care: Results of a demonstration project in 2 US cities. Presented at: 18th Conference on Retroviruses and Opportunistic Diseases. Boston, MA, USA, 27 February–2 March 2011. [Google Scholar]
- 112.Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS 20(12), 1597–1604 (2006). [DOI] [PubMed] [Google Scholar]
- 113.Kassler WJ, Dillon BA, Haley C, Jones WK, Goldman A. On-site, rapid HIV testing with same-day results and counseling. AIDS 11(8), 1045–1051 (1997). [DOI] [PubMed] [Google Scholar]
- 114.Kendrick SR, Kroc KA, Withum D, Rydman RJ, Branson BM, Weinstein RA. Outcomes of offering rapid point-of-care HIV testing in a sexually transmitted disease clinic. J. Acquir. Immune Defic. Syndr 38(2), 142–146 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Lyss SB, Branson BM, Kroc KA, Couture EF, Newman DR, Weinstein RA. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J. Acquir. Immune Defic. Syndr 44(4), 435–442 (2007). [DOI] [PubMed] [Google Scholar]
- 116.Liang TS, Erbelding E, Jacob CA et al. Rapid HIV testing of clients of a mobile STD/HIV clinic. AIDS Patient Care STDS 19(4), 253–257 (2005). [DOI] [PubMed] [Google Scholar]
- 117.Gardner LI, Metsch LR, Anderson-Mahoney P et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 19(4), 423–431 (2005). [DOI] [PubMed] [Google Scholar]
- 118.Craw J, Gardner L, Rossman A et al. Structural factors and best practices in implementing a linkage to HIV care program using the ARTAS model. BMC Health Serv. Res 10, 246 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giordano TP, Gifford AL, White AC Jr et al. Retention in care: a challenge to survival with HIV infection. Clin. Infect. Dis 44(11), 1493–1499 (2007). [DOI] [PubMed] [Google Scholar]
- 120.Mugavero MJ, Norton WE, Saag MS. Health care system and policy factors influencing engagement in HIV medical care: piecing together the fragments of a fractured health care delivery system. Clin. Infect. Dis 52(Suppl. 2), S238–S246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aberg JA, Kaplan JE, Libman H et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin. Infect. Dis 49(5), 651–681 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Buscher AL, Giordano TP. Gaps in knowledge in caring for HIV survivors long-term. JAMA 304(3), 340–341 (2010). [DOI] [PubMed] [Google Scholar]
- 123.Mellors JW, Munoz A, Giorgi JV et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med 126(12), 946–954 (1997). [DOI] [PubMed] [Google Scholar]
- 124.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005–2009: Do patients who initiate care stay in care? AIDS Patient Care STDS, 25(2), 79–88 (2011). [DOI] [PubMed] [Google Scholar]
- 125.Prabhu VS, Farnham PG, Hutchinson AB et al. Cost-effectiveness of HIV screening in STD clinics, emergency departments, and inpatient units: a model-based analysis. PLoS One 6(5), E19936 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Paltiel AD, Weinstein MC, Kimmel AD et al. Expanded screening for HIV in the United States – an analysis of cost–effectiveness. N. Engl. J. Med 352(6), 586–595 (2005). [DOI] [PubMed] [Google Scholar]; ■ Describes the findings from a computer simulation model of HIV screening and teatment comparing routine, voluntary HIV counseling, testing and referral with screening in clinical settings based on actual or perceived risk factors or clinical manifestations of HIV infection. The authors found that conducting routine, voluntary HIV screening once every 3–5 years was cost effective, except in populations at the lowest risk for infection.
- 127.Sanders GD, Bayoumi AM, Sundaram V et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N. Engl. J. Med, 352(6), 570–585 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Walensky RP, Losina E, Malatesta L et al. Effective HIV case identification through routine HIV screening at urgent care centers in Massachusetts. Am. J. Public Health, 95(1), 71–73 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Silva A, Glick NR, Lyss SB et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann. Emerg. Med 49(5), 564–572 (2007). [DOI] [PubMed] [Google Scholar]
- 130.Mehta SD, Hall J, Greenwald JL, Cranston K, Skolnik PR. Patient risks, outcomes, and costs of voluntary HIV testing at five testing sites within a medical center. Public Health Rep. 123(5), 608–617 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Farnham PG, Hutchinson AB, Sansom SL, Branson BM. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 123(Suppl. 3), 51–62 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shrestha RK, Clark HA, Sansom SL et al. Cost-effectiveness of finding new HIV diagnoses using rapid HIV testing in community-based organizations. Public Health Rep, 123(Suppl. 3), 94–100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Varghese B, Peterman TA. Cost-effectiveness of HIV counseling and testing in US prisons. J. Urban Health 78(2), 304–312 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Golden MR, Gift TL, Brewer DD et al. Peer referral for HIV case-finding among men who have sex with men. AIDS 20(15), 1961–1968 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Shrestha RK, Sansom SL, Richardson-Moore A et al. Costs of voluntary rapid HIV testing and counseling in jails in 4 states–advancing HIV Prevention Demonstration Project, 2003–2006. Sex. Transm. Dis 36(Suppl. 2), S5–S8 (2009). [DOI] [PubMed] [Google Scholar]
- 136.Walensky RP, Weinstein MC, Smith HE, Freedberg KA, Paltiel AD. Optimal allocation of testing dollars: the example of HIV counseling, testing, and referral. Med. Decis. Making, 25(3), 321–329 (2005). [DOI] [PubMed] [Google Scholar]
- 137.Schackman BR, Gebo KA, Walensky RP et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med. Care 44(11), 990–997 (2006). [DOI] [PubMed] [Google Scholar]
- 138.Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann. Intern. Med 153(12), 778–789 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mimiaga MJ, Johnson CV, Reisner SL, Vanderwarker R, Mayer KH. Barriers to routine HIV testing among Massachusetts community health center personnel. Public Health Rep. 126(5), 643–652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neff S, Goldschmidt R. Centers for Disease Control and Prevention 2006 human immunodeficiency virus testing recommendations and state testing laws. JAMA 305(17), 1767–1768 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Ehrenkranz PD, Pagan JA, Begier EM, Linas BP, Madison K, Armstrong K. Written informed-consent statutes and HIV testing. Am. J. Prev. Med 37(1), 57–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zetola NM, Grijalva CG, Gertler S et al. Simplifying consent for HIV testing is associated with an increase in HIV testing and case detection in highest risk groups, San Francisco January 2003-June 2007. PLoS One 3(7), E2591 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wing C Effects of written informed consent requirements on HIV testing rates: evidence from a natural experiment. Am. J. Public Health, 99(6), 1087–1092 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Halpern SD, Metkus TS, Fuchs BD et al. Nonconsented human immunodeficiency virus testing among critically ill patients: intensivists’ practices and the influence of state laws. Arch Intern. Med 167(21), 2323–2328 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Neff S, Goldschmidt RH. State human immunodeficiency virus testing laws. Arch Intern. Med 168(15), 1717–1718 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Meng YY, Coffman JM, Ripps JC, Lee C, Kominski GF. Financial impact of California’s new law to increase HIV screening by mandating insurance coverage. AIDS Care 23(2), 206–212 (2011). [DOI] [PubMed] [Google Scholar]
- 147.Burke RC, Sepkowitz KA, Bernstein KT et al. Why don’t physicians test for HIV? A review of the US literature. AIDS 21(12), 1617–1624 (2007). [DOI] [PubMed] [Google Scholar]
- 148.Rodnick JE. The CDC and USPSTF recommendations for HIV testing. Am. Fam. Physician 76(10), 1456, 1459 (2007). [PubMed] [Google Scholar]
- 149.Harries AD, Schouten EJ, Libamba E. Scaling up antiretroviral treatment in resource-poor settings. Lancet 367(9525), 1870–1872 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Merchant RC, Waxman MJ. HIV screening in health care settings: some progress, even more questions. JAMA 304(3), 348–349 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Kilmartin P Development of the VITROS HIV combo assay. Presented at: 2010 HIV Diagnostics Conference. Orlando, FL, USA, 24–26 March 2010. [Google Scholar]
- 152.Curtis KA, Rudolph DL, Owen SM. Rapid detection of HIV-1 by reverse-transcription, loop-mediated isothermal amplification (RT-LAMP). J. Virol. Methods 151(2), 264–270 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Tang W, Chow WH, Li Y, Kong H, Tang YW, Lemieux B. Nucleic acid assay system for tier II laboratories and moderately complex clinics to detect HIV in low-resource settings. J. Infect. Dis 201(Suppl. 1), S46–S51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee HH, Dineva MA, Chua YL, Ritchie AV, Ushiro-Lumb I, Wisniewski CA. Simple amplification-based assay: a nucleic acid-based point-of-care platform for HIV-1 testing. J. Infect. Dis 201(Suppl. 1), S65–S72 (2010). [DOI] [PubMed] [Google Scholar]
- 155.Tanriverdi S, Chen L, Chen S. A rapid and automated sample-to-result HIV load test for near-patient application. J. Infect. Dis 201(Suppl. 1), S52–S58 (2010). [DOI] [PubMed] [Google Scholar]
- 156.Pau C-P, Wells SK, Owen SM, Granade TC. Development of a simple, rapid and inexpensive method for the qualitative detection of HIV-1 RNA. Presented at: 2010 HIV Diagnostics Conference. Orlando, FL, USA, 24–26 March 2010. [Google Scholar]
- 157.Mazzola LT, Arsham AM, Pederson AM, Bhatia R, Stern D, Laser DJ. Innovative point-of-care HIV viral load detection in RLS. Presented at: 2010 HIV Diagnostics Conference. Orlando, FL, USA, 24–26 March 2010. [Google Scholar]
- 158.Wei X, Liu X, Dobbs T et al. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res. Hum. Retroviruses 26(1), 61–71 (2010). [DOI] [PubMed] [Google Scholar]
- 159.Masciotra S, Dobbs T, Candal D et al. Antibody avidity-based assay for identifying recent HIV-1 infections based on Genetic SystemsTM 1/2 Plus O EIA. Presented at: Conference on Retroviruses and Opportunistic Infections. San Francisco, CA, USA, 3–5 March 2010. [Google Scholar]
- 160.Keating SM, Hanson D, Lebedeva L et al. Optimization and calibration of less sensitive and avidity modified protocols for the Vitros immunodiagnostic products anti-HIV-1+2 assay for detection of early HIV infections. Presented at: 2010 HIV Diagnostics Conference. Orlando, FL, USA, 24–26 March 2010. [Google Scholar]
- 161.Laeyendecker O, Oliver A, Astemborski J et al. Improved precision of cross-sectional HIV incidence testing using a multi-assay algorithm that includes BED and an avidity assay with modified assay cut-offs. Presented at: Conference on Retroviruses and Opportunistic Infections. San Francisco, CA, USA, 5–8 March 2010. [Google Scholar]
- 162.Curtis KA, Kennedy MS, Charurat M et al. Development and characterization of a bead-based, multiplex assay for estimation of recent HIV type 1 infection. AIDS Res. Hum. Retroviruses doi: 10.1089/aid.2011.0037 (2011) (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 163.Park SY, Love TM, Nelson J, Thurston SW, Perelson AS, Lee HY. Designing a genome-based HIV incidence assay with high sensitivity and specificity. AIDS 25(16), F13–F19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Incidence Assay Critical Path Working Group. More and better information to tackle HIV epidemics: towards improved HIV incidence assays. PLoS Med. 8(6), E1001045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.Office of National AIDS Policy, National HIV/AIDS Strategy, Office of National AIDS Policy, Editor 2010: Washington, DC, USA: www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf [Google Scholar]
- 202.CDC. HIV/AIDS Surveillance Report, 2000. (U.S. Department of Health and Human Services, Centers for Disease Control and Prevention., Atlanta, 2005) 1–46. Department of Health and Human Services www.cdc.gov/hiv/topics/surveillance/resources/reports/pdf/hasr1202.pdf [Google Scholar]
- 203.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 10 January 2011. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf [Google Scholar]
- 204.HIV Prevention Trials Network. HPTN 065. TLC-Plus: a study to evaluate the feasibility of an enhanced test, link to care, plus treat approach for HIV prevention in the United States. What is HPTN 065? www.hptn.org/research_studies/hptn065.asp (Accessed June 17 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Food and Drug Administration. Approval Letter - ARCHITECT HIV Ag/Ab Combo. www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/BloodDonorScreening/InfectiousDisease/ucm216300.htm(Accessed 13 June 2011)
- 206.Bio-Rad. Bio-Rad receives FDA premarket application approval for its fourth-generation HIV assay. 2011. www.bio-rad.com/evportal/en/US/evolutionPortal.portal?_nfpb=true&_pageLabel=news_article_detail_page&type=Corporate+News&artid=Bio-Rad-Receives-FDA_1311380729&country=US&lang=en&javascriptDisabled=true (Accessed 3 August 2011)
- 207.US FDA. FDA Approves Test to Help Diagnose Main Virus that Causes AIDS. News Release 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108754.htm [Google Scholar]
- 208.Association of Public Health Laboratories (APHL), CDC (2009). HIV Testing Algorithms: A Status Report. 2009 www.aphl.org/aphlprograms/infectious/hiv/Documents/StatusReportFINAL.pdf (Accessed 17 June 2011)
- 209.US FDA. Overview of Device Regulation. 2009. www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/default.htm (Accessed 13 June 2011)
- 210.CDC. Funding announcement for CDC program “Expanded and Integrated Human Immunodeficiency Virus (HIV) Testing for Populations Disproportionately Affected by HIV, Primarily African Americans. 2007. www.federalgrants.com/Expanded-and-Integrated-Human-Immunodeficiency-Virus-HIV-Testing-for-Populations-Disproportionately-Affected-by-HIV-Primarily-African-Americans-9965.html
- 211.AIDS United. Ryan White Care Act. 2011. www.aidsunited.org/policy-advocacy/issues/ryan-white-care-act (Accessed 4 August 2011)
- 212.United States Congress. PUBLIC LAW 111–87—OCT. 30, 2009. 2009 www.caear.org/downloads/RW_2009.pdf (Accessed 4 August 2011)
- 213.CDC. CDC’S New High-Impact Approach to HIV Prevention Funding for Health Departments: Advancing the National HIV/AIDS Strategy. 2011. www.cdc.gov/hiv/strategy/hihp/healthDepartments/index.htm (Accessed 5 August 2011)
- 214.Veterans Health Administration. VA National HIV Testing Day: June 27, 2011: A national campaign to increase HIV testing in VA facilities. 2010. www.hiv.va.gov/provider/campaigns-HIVtesting.asp#S1X (Accessed 17 June 2011)
- 215.Veterans Health Administration. Grant program aims to increase HIV testing rate in VA. 2010. www.va.gov/health/NewsFeatures/20101201a.asp (Accessed 17 June 2010)
- 216.Substance Abuse and Mental Health Administration, SAMHSA’s Rapid HIV Testing Initiative (RHTI). 2007. www.samhsa.gov/hivhep/rhti_factsheet02.aspx
- 217.Indian Health Service. HIV Testing and Guidance www.ihs.gov/medicalprograms/hivaids/index.cfm?module=testing&option=ihsGuidance (Accessed 4 August 2011)
- 218.Indian Health Service, HIV testing promotional materials www.ihs.gov/medicalprograms/hivaids/index.cfm?module=promoMat
- 219.New York City Department of Health and Mental Hygiene. Health Department Reports More than 600,000 HIV Tests Conducted through the Bronx Knows Initiative. 27 June 2011. http://m.usablenet.com/mt/www.nyc.gov/html/doh/html/pr2011/pr014-11.shtml (Accessed 30 August 2011)
- 220.National HIV/AIDS Clinicians’ Consultation Center. 2011 Compendium of state HIV testing laws. 2011. www.nccc.ucsf.edu/consultation_library/state_hiv_testing_laws (Accessed 6 June 2011)
- 221.White House. The Affordable Care Act. 2010. www.whitehouse.gov/healthreform/healthcare-overview (Accessed 4 August 2011)
- 222.Alere International. Alere re-launches Determine portfolio for rapid diagnosis of infectious diseases. 2011. www.prohealthservicezone.com/index.php?option=com_zone&task=detail&newsId=8047#axzz1Wj463h8V (Accessed 4 August 2011)


