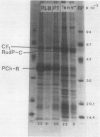
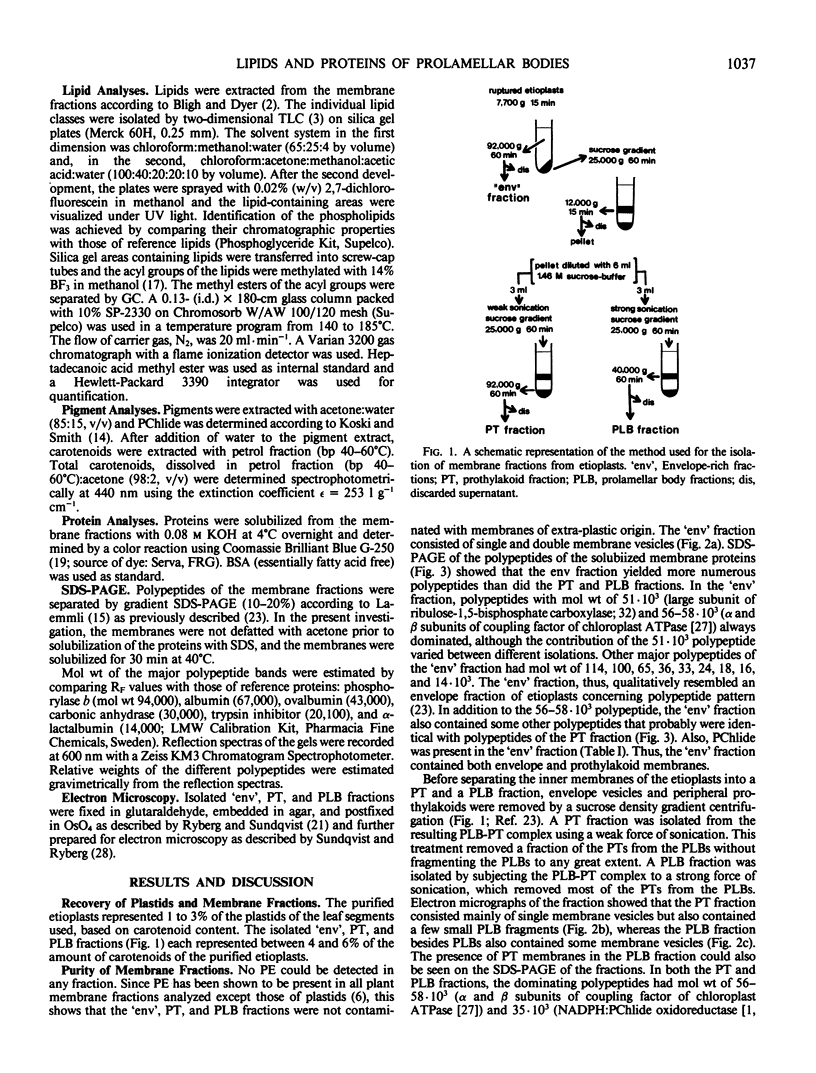
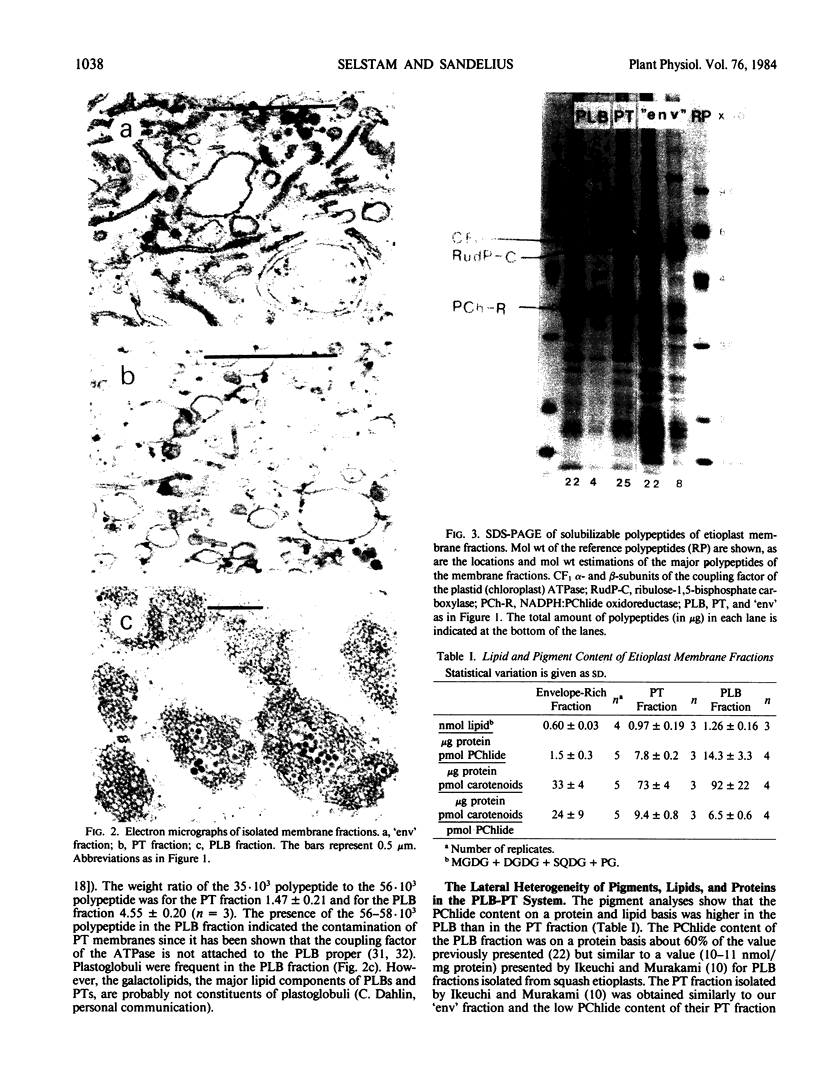
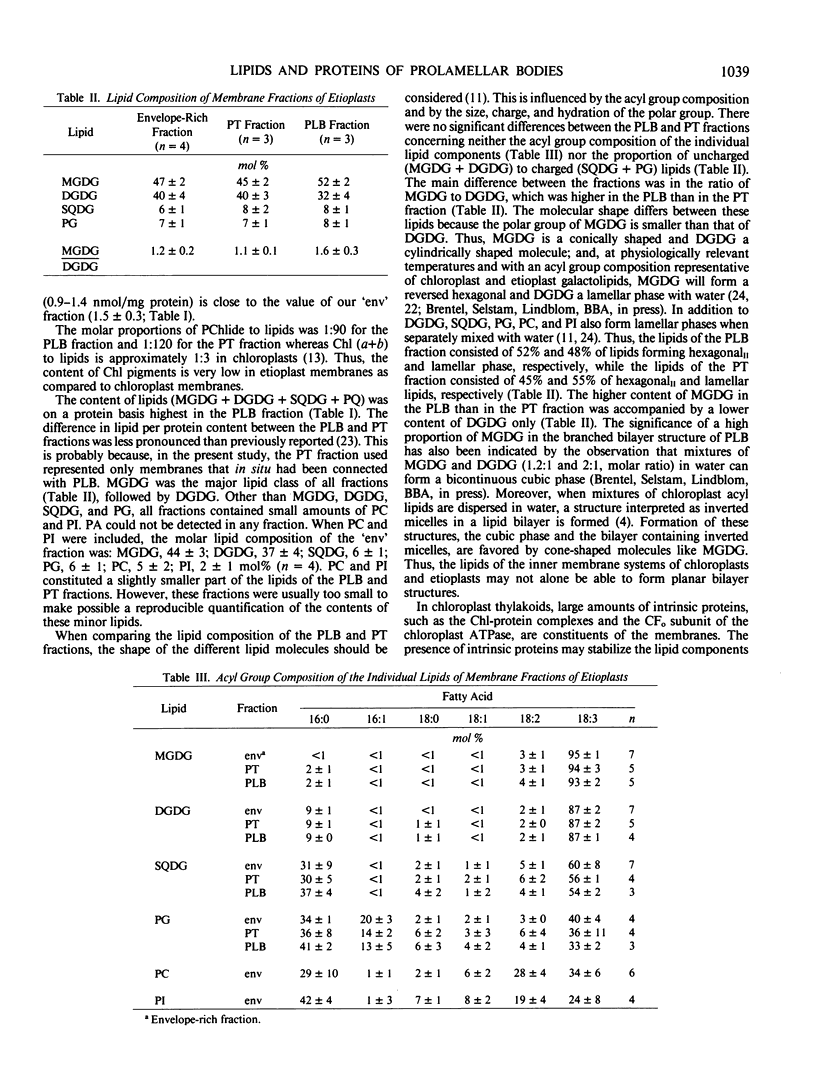
Abstract
The aim of the present investigation was to find factors critical for the co-existence of prolamellar bodies and prothylakoids in etioplasts of wheat (Triticum aestivum L. cv Starke II). The lipid composition of the prolamellar body and prothylakoid fractions was qualitatively similar. However, the molar ratio of monogalactosyl diacylglycerol to digalactosyl diacylglycerol was higher in the prolamellar body fraction (1.6 ± 0.1), as was the lipid content on a protein basis. Protochlorophyllide was present in both fractions. The dominating protein of the prolamellar body fraction was protochlorophyllide oxidoreductase. This protein was present also in prothylakoid fractions. The other major protein of the prothylakoid fraction was the coupling factor 1, subunit of the chloroplast ATPase. From the lipid and protein data, we conclude that prolamellar bodies are formed when monogalactosyl diacylglycerol is present in larger amounts than can be stabilized into planar bilayer prothylakoid membranes by lamellar lipids or proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Santel H. J., Redlinger T. E., Falk H. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Isolation and characterization of the NADPH:protochlorophyllide oxidoreductase. Eur J Biochem. 1980 Oct;111(1):251–258. doi: 10.1111/j.1432-1033.1980.tb06100.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Oliver R. P., Griffiths W. T. Identification of the polypeptides of NADPH--protochlorophyllide oxidoreductase. Biochem J. 1980 Oct 1;191(1):277–280. doi: 10.1042/bj1910277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Sandelius A. S., Selstam E. Localization of Galactolipid Biosynthesis in Etioplasts Isolated from Dark-Grown Wheat (Triticum aestivum L.). Plant Physiol. 1984 Dec;76(4):1041–1046. doi: 10.1104/pp.76.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley G. G., Green J. P., Nichols B. W. The phase behavior of monogalactosyl, digalactosyl, and sulphoquinovosyl diglycerides. Biochim Biophys Acta. 1973 Jul 18;311(4):531–544. doi: 10.1016/0005-2736(73)90128-4. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]