Abstract
Background
Head and neck cancer (HNSCC) is a heterogeneous group of cancers, affecting multiple regions such as oral cavity, pharynx, larynx, and nasal region, each showing a distinct molecular profile. HNSCC accounts for more than 6 million cases worldwide, soaring mainly in the developing countries.
Recent findings
The aetiology of HNSCC is complex and multifactorial, involving both genetic and environmental factors. The critical role of microbiome, which includes bacteria, viruses, and fungi, is under spotlight due to the recent reports on their contribution in the development and progression of HNSCC. This review focuses on the effect of opportunistic pathogens on the host genome and epigenome, which contributes to the disease progression. Drawing parallels from the host‐pathogen interactions observed in other tumour types arising from the epithelial tissue such as colorectal cancer, the review also calls attention to the potential explorations of the role of pathogens in HNSCC biology and discusses the clinical implications of microbiome research in detection and treatment of HNSCC.
Conclusion
Our understanding of the genomic effects of the microbes on the disease progression and the mechanistic insights of the host‐pathogen interaction will pave way to novel treatment and preventive approaches in HNSCC.
Keywords: cancer associated pathogens, head and neck cancer, host‐pathogen interaction
1. INTRODUCTION
Head and neck cancer (HNSCC) is a complex set of diseases, mainly caused due to exposure to etiological factors, including the carcinogens derived from the metabolism of tobacco, smoking, and alcohol. The disease accounts for new 660 000 cases and 325 000 deaths annually. 1 Occult node metastases and poor overall 5‐year survival rate after advanced treatment with chemo‐ and radiotherapy modalities remain the major challenges in the HNSCC. Over 65% of the HNSCC cases are attributable to the tobacco use, smoking and alcohol consumption. 2 The HNSCC genome undergoes several insults due to the exposure to diverse endogenous and exogenous etiological factors. Comprehensive genomic analysis has identified several molecular subtypes based on mutation, copy number alteration, gene expression and change in methylation at cancer gene loci. 3 The analysis identified a distinct genomic subtype associated with human papilloma virus (HPV) infection, possessing specific mutation profile (detailed later). Smoking‐related HNSCCs demonstrate near universal loss‐of‐function TP53 mutations, inactivation of CDKN2A by copy loss and frequent copy number alterations including amplification of 3q26/28 and 11q13/22. The peculiar genomic characteristics also include loss‐of‐function alterations of the chromatin modifier NSD1, WNT pathway genes AJUBA and FAT1, and activation of oxidative stress factor NFE2L2, mainly in laryngeal tumours. 3 Ethnic and site‐specific HNSCC cancer genome profiles and variability in the frequency of the common cancer genes is also described in other studies. 4 , 5 Genomic characterisation has resulted in discovery many therapeutically relevant alterations, however additional risk‐factors remain to be discovered as the HNSCC cases are on the rise in the developing countries, which do not associate with the known etiological factors. In recent years, additional risk factors have emerged in the form of pathogenic microbes, which influence the genesis and progression of many different cancers. Global estimates suggest an approximate cancer attribution of 15%–20% cases to microbes, 6 with a burden of nearly 2.2 million infection associated cases in the 2018 alone. 7 Majority of the pathogens associated with cancer are oncogenic viruses including human papilloma virus (HPV), Epstein–Barr virus (EBV), Human T‐lymphotropic virus 1 (HTLV1), Kaposi sarcoma‐associated herpesvirus (KSHV), hepatitis B (HBV) and hepatitis C virus (HCV). 8 Among bacteria, oncogenicity in animal model systems has been established for Helicobacter pylori. 7 , 8 Other reported cancer‐pathogen associations include, Chlamydia pneumonia with lung cancer, 9 Salmonella typhi infection with gallbladder cancer 10 , 11 and Streptococcus bovis, genotoxic Escherichia coli, enterotoxigenic Bacteroides fragilis and Fusobacterium nucleatum with colon cancer. 12 , 13 , 14 , 15 , 16 Head and neck cancer is no exception to this. In fact, oral cavity being the most exposed sites in the human body, acts a gateway constantly interfacing with the microbes. The constant exposure of the microbial species is through the fluids we drink, the food we ingest and in the nasopharyngeal area via the air we breathe. Hence, microbes colonising any part of oral cavity tend to spread to the neighbouring epithelial surfaces in the head and neck region, passively helped by the fluid phase of saliva in the subgingival and gingival region. Opportunistic pathogens among the colonisers, causing dysbiosis in the oral microbiota, cause several diseases including periodontal disease, dental caries, candidiasis, and others. 17 Systemic and chronic inflammation associated diseases such as rheumatoid arthritis, 18 Alzheimer's, 19 hypertension, 20 systemic lupus erythematous (SLE) 21 and cancers, including colorectal, 22 pancreatic, 23 oesophageal, 24 lung 25 , 26 and head and neck, 27 have been associated with oral microbes. The overall genetic material of the microbes in the oral cavity is termed as oral microbiome, which consist of wide range of species of viruses, archaea, fungi and over 700 species of bacteria. The Human microbiome project 28 aimed to provide a comprehensive catalogue of microbes across different human tissues and establishment of a ‘core microbiome’ for every niche in the healthy humans tissue, if any. To assess the possibility of establishing a core microbiome for the oral cavity (a subset of head and neck), Caselli E., et al. 29 studied the microbiome variation among 20 healthy individual using whole genome sequencing (WGS). This study concluded that the composition of oral microbiome remained largely conserved, with no statistically different microbial ratios, providing a ‘eubiosis’ reference. Diverging from the normalcy, a dysbiosis state specifically caused by enrichment of some pathogenic strains may result into chronic inflammation state, assisting the tumorigenesis process. Different sites of head and neck region are no exception to this.
2. MICROBES ASSOCIATED WITH HEAD AND NECK SQUAMOUS CELL CARCINOMA
Many cancer‐associated pathogens are involved in development of HNSCC. The most well studied and established association is the HPV infection in oropharyngeal cancer. Systematic genomic analysis of the HNSCC tumours have identified the HPV‐positive tumours to be having a distinct pattern, with lower frequency alterations observed in the tumour suppressor genes, as compared to the HPV negative sub‐type. 3 The HPV positive tumours form a distinct clinical and molecular sub‐type, with having better prognosis and higher overall survival. 30 The detailed molecular mechanism of the HPV‐induced carcinogenesis, via inactivation of p53 and pRb, has been detailed in the following review. 31 Clinically relevant stratification of the HNSCC tumours based on pathogens, like HPV provide an avenue to design therapeutic and preventive strategies. Other oncogenic viruses, such as HCV, EBV and HBV, have also been reported to cause site specific HNSCC. 32 , 33 , 34 EBV also forms a distinct sub‐type with site specific infection in nasopharynx, discussed later in the review. Expression of latent EBV genes such as Epstein–Barr nuclear antigen 1 (EBNA1) and Latent Membrane Protein 1 (LIMP1), interfere with core cellular machinery and cell cycle genes in nasopharyngeal carcinoma (NPC). 35 As with many different cancer types, such as cervical and liver cancer, viral carcinogenesis has been studied at molecular and pathway resolution in HNSCC.
In recent years, application of genome and metagenome (16S RNA) sequencing has revealed the complex composition of the microbiome across various sites in the head and neck region and their association with cancer. 27 Several bacterial species found in different sites of head and neck region are potentially flagged as cancer causing. Abundance of bacteria like, Porphyromonas gingivalis, Fusobacterium nucleatum, Streptococcus, Prevotella, Veillonella, Actinomycetes, Bacteroides, Clostridium, Klebsiella, Citrobacter, Streptococcus, Enterobacter and Serratia, have been found to be abundant or enriched in head and neck cancer 36 , 37 , 38 Each of these bacteria are independently known to induce inflammation in the state of dysbiosis. Site specific cancer‐pathogen associations have also been identified for the above‐mentioned bacteria, including Fusobacterium, Streptococcus, Prevotella with laryngeal, 39 Fusobacterium with oral cavity and tongue tumours, 40 , 41 , 42 Porphyromonas gingivalis with oral cancer, 43 are some of the examples. Among the mentioned bacteria, Porphyromonas gingivalis and Fusobacterium nucleatum are reported to promote tumour progression in‐vivo, in the oral‐specific chemical carcinogenesis mode. 44 Mechanistically, the lipopolysaccharide in the outer membrane of these Gram‐negative bacteria interacts with Toll‐like receptor 4 (TLR4), which leads to downstream activation of Myeloid differentiation primary response 88 (MyD88) dependent and MyD88‐independent pathways, which then trigger the nuclear factor κB pathway leading to the release of pro‐inflammatory cytokines. 45 , 46 Action of such bacteria in the tumour microenvironment are primarily driven by heightening of the innate immunity against them. 47 Streptococcus and Prevotella have also been postulated to have similar effect on the change in cytokine profile and to activate of innate immunity. 36 Porphyromonas gingivalis is also reported to increase the invasiveness of the oral cancer cells, via induction and activation of pro‐MMP9. 48 Similar correlation is also observed in abundance of Fusobacterium and expression of MMP10 in tongue cancer, 40 a biomarker of lymph node metastases mechanistically shown to be driving invasion and migration in tongue cancer. 49 Details of the mechanistic involvement of the 2 gram‐negative opportunistic pathogens with oral cancer has been reviewed elsewhere. 50 , 51 As bacteria remain the dominant species of the metagenome, the association analysis warrants a careful inspection and requires supporting biological assessment of the role of cancer associated pathogens in the cancer microenvironment.
The field of microbiome analysis in cancer research is presented with multiple challenges. Including biases due to sensitivities of different detection methods, microbial contamination in the process of tumour sample processing, such as clinical acquisition of sample, collection and processing, quality control and quantification. Also, oral cavity and its sub‐sites being the most exposed regions of the human body, environment, food, and habits may also alter the composition greatly, adding another layer of microbial composition dynamics, which may be only identified and corrected in a large scale study of patient samples. 52 Moreover, the variation observed in the microbial makeup in the HNSCC sub‐sites may yield conflicting outcomes regarding potential involvement of an opportunistic pathogen in cancer prognosis. 51 However, detailed mechanism‐based studies involving the bacterial species in other cancer types, including colorectal and oesophageal, provide clues about the involvement of these microbes in other epithelial squamous cell carcinomas such as oral cancer. Although, primary focus of investigation largely remains to be association of viral and bacterial pathogens with HNSCC, fungal species of genus Candida, have also been associated with treatment induced complications as well as carcinogenesis. 53 A recent pan‐cancer analysis has revealed a direct or indirect (via interactions with bacterial colonies) role of fungal species in cancer progression across several different cancer types. 54 In overall, a complex conjunction of the microbial abundances and presence of specific pathogenic species in the tumour microenvironment determine the fate of the disease progression. A compilation of studies characterising the microbiome, its function and implications across various tumour types including the HNSCC has been provided in a review. 55 Another recent review provides an exhaustive compilation the clinical studies investigating HNSCC associated microbes, their tumour stage specific presence and their relation to prognostic outcomes. 56 The measurable molecular effects of the dysbiosis of these pathogenic microbes can be seen in form of genomic, epigenomic or immune cell composition derived signatures. These are further discussed in the following sections and summarised in Figure 1.
FIGURE 1.
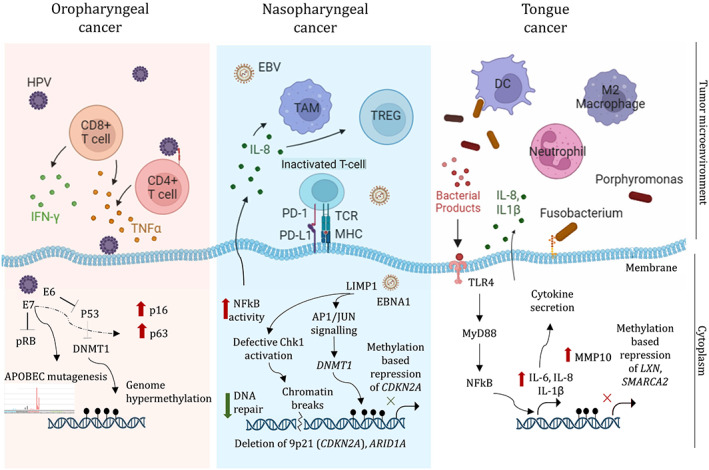
Genomic and epigenomic influence of the pathogens in distinct anatomical sub‐sites of HNSCC. Illustration depicts the immune cell profile in the tumour micro‐environment consisting of predominant cell types such as T cells (CD4+, CD8+ in HPV positive subtype), tumour associated macrophages (TAM), regulatory T cells (TREG) (in EBV +ve subtype) and innate immune cell enrichment – dendritic cells (DCs), neutrophils and M2 macrophages (in Fusobacterium enriched tongue tumours). Inside the cytoplasm, pathogenic particles are mentioned which directly or indirectly interact with the host cellular machinery causing different types of genomic aberrations. The up‐ red arrows indicate increase in activity or expression and the down‐ green arrows indicate the decrease in activity. Black circular flags on the genome represent methylation.
3. DIRECT IMPACT OF MICROBES ON THE HNSCC GENOME
Mutational profile of the HNSCC tumours show differential alterations in the genome, which correlate to the etiological factors and anatomical subsites. Analysis of landscape of genomic alterations provided a molecular classification for HNSCC. 3 Among the broad class of tumours related to smoking, loss of function mutations in the tumour suppressor gene TP53 and deep deletion of CDKN2A gene copy, are the commonly observed alterations. A distinct sub‐class of oral tumours harbour activating mutations of HRAS or PIK3CA, and inactivating mutations in CASP8, NOTCH1 genes. On the other hand, majority of the laryngeal tumours harboured alterations in Wnt pathway genes, a chromatin modifier (NSD1) and activation of NFE2L2, an oxidative stress factor. As with the exposure to environmental factors, genomic alterations are also defined by pathogenic infection in HSNC. 57 The incidence of HPV associated with HNSCC varies across the anatomical sites and geographical locations (populations), which creates a difference in the global genomic alteration profile of the HNSCC tumours. HPV proteins E6 and E7, inactivate P53 and RB protein, respectively, which is causally linked to malignancy. 58 Because the deactivation of the tumour suppressor genes in the HPV positive tumours takes place due to viral activity, this sub‐group of tumours harbour lower mutations in TP53 and CDKN2A genes at early stages and instead a higher mutation abundance is found in the PIK3CA, E2F1, and TRAF3 genes. 3 Hence, the overall progression of genome evolution of the HPV positive tumours follows a different trajectory. Tumours positive for HPV are also known to undergo apolipoprotein B mRNA‐editing catalytic polypeptide‐like (APOBEC) mediated mutations, 59 , 60 which causes well‐known characteristic endogenous mutational signature. Further, integration of HPV in human genome is commonly observed in both cervical and HNSCC tumours. Integration of HPV into the human genome, has been found to be enriched in the HNSCC associated genes, as well as genomic loci (such as CD274, official gene name for PD‐L1) important for immune function, ultimately correlating with survival outcomes of the patients. 61 Additional molecular features altered due to HPV prevalence in HNSCC are reviewed elsewhere. 33
In contradiction to the HPV infected HNSCC patient, recurrent somatic copy number changes are observed in EBV infected nasopharyngeal carcinoma (NPC) patients. Most of the EBV infected NPC patients harbour frequent deletion of 9p21 chromosomal region containing CDKN2A gene. 62 In the EBV enriched sub‐group of NPC patients, additional copy number variations were also observed in well‐known cancer genes including, CCND1, AKT2, MYC, TP53, and frequent deletions in an important component of the SWI/SNF complex, ARID1A. EBV infected tumours are also reported to have a distinct mutational signature not contributed by endogenous APOBEC3‐induced mutagenesis, unlike the HPV positive sub‐group of patients. 62 Furthermore, EBV viral gene activity has been reported to results in chromosomal instability in NPC. The LIMP1 viral gene impairs the G2 checkpoint in human NPC cells through defective Chk1 activation, leading to unrepaired chromatin breaks. 63 EBV infection also causes reduced DNA damage repair responsiveness, by controlling expression of mismatch repair (MMR) and base excision repair (BER) pathway members, including MLH1. 64 Although the genome alteration landscape of EBV and HPV positive HNSCC patients vary greatly, immunotherapy and therapeutic vaccination is a proposed treatment option due to similarity in the immune activity profiles of the two sub‐groups. 65 , 66
Sparse experimental evidence currently exists for activity of bacterial strains in altering HNSCC genomes. However, effect of bacterial interaction with epithelium is reported to have direct implications on the DNA stability and mutation distributions. The classical example is of Helicobacter pylori which is known to induce genomic instability in gastric cancer cells. The association of H. pylori with the decrease in the DNA repair ability, generation of mutator phenotype and mutation induction in mitochondrial genes in gastric cancer, provides a framework to study genome damaging effects of a bacteria (reviewed here 67 ). Multiple studies in recent years have identified role of other bacterial metabolites in the genomic instability, increase in mutation burden and its distribution across the colon cancer genome. Escherichia coli is known to induce DNA double strand breaks in the eukaryotic cells. 68 Building on this study, Pleguezuelos‐Manzano C., et al., identified a mutation signature induced by colibactin – a protein encoded by PKS genes, produced by the pks + E. coli. 68 The study also highlights the effect of this mutagenesis at specific gene loci, including genes like APC, which are the founder mutations for the colon tumorigenesis. Also, Bacteroides fragilis toxin (BFT) has been reported to up‐regulate Spermine oxidase (SMO), a polyamine catabolic enzyme, which increases the DNA damage via reactive oxygen species (ROS). 69 Bacteroides fragilis along with Fusobacterium nucleatum – a known colorectal cancer associated oncobacterium, 13 show significant enrichment in the tumours deficient for DNA mismatch repair in colon tumours. 70 Another study suggests correlation of microsatellite alterations and DNA damage with Fusobacterium infection in colon cancer. 71 Since gastric and colon tumours are extensively characterised with respect to the microbiome, we find pronounced evidence of effect of bacterium and their metabolites on the genomes of these tissue types. However, the studies also highlight characteristics of the individual microbes in disease biology and details of their interaction with the human genome. Among the mentioned colon associated bacterium, Fusobacterium nucleatum has been reported to form a distinct subgroup of HNSC, exclusive to HPV. 40 The Fusobacterium enriched tumours behave clinically different, having increased extracapsular spread and higher inflammation. In vitro and in‐vivo, studies also provide strong validation for the Fusobacterium induced inflammation. 44 , 72 Extensive evidence of direct effect of Fusobacterium on the DNA in HNSCC may be lacking, but an indirect inflammation induced host‐genome damaging role of the opportunistic pathogen cannot be ruled out. 73 Also, preliminary experimental evidence of Fusobacterium induced DNA damage in oral cancer exists, 74 and it needs further validation across different experimental systems in‐vitro and in‐vivo.
4. MODULATION OF EPIGENOME BY MICROBES IN HNSCC
The behaviour of a cancer cell cannot be explained entirely based on its genetic makeup. Epigenome plays an equally important role in cancer progression. Epigenome includes the control of gene expression via DNA methylation on the CpG island regions near the gene promoter and the chromatin architecture. DNA methylation (both hyper‐ and hypo‐) affects HNSCC tumours differently at different stages of the disease. In the human cell, DNA methylation is catalysed by the DNA methyltransferase (DNMT) family, consisting of 3 members: DNMT1, DNMT3a and DNMT3a. In cancer, alteration of expression of DNMT members is well characterised. 75 In the scheme of events leading to carcinogenesis, DNA methylation is one of the early events observed in the pre‐malignant tissue even before the onset of cancer. This hypothesis has also been validated in the HNSCC cell line model systems. 76 An overview of the role of methylation in HNSCC disease progression is reviewed elsewhere. 77 Since the methylation status of specific gene loci in a cell is robustly maintained, some of the initial studies also used marker gene–based status as a predictive marker for HNSCC. 78 In a general scenario, a global hypomethylation prevails at the initiation of the tumour formation leading to the expression of the repetitive elements, which constitute ~50% of the genome. The global hypomethylation has been associated with smoking, alcohol consumption and disease stage, 79 whereas HPV–associated HNSCC tumours are known to be hypermethylated. As DNA methylation is a technique used by the host cells to suppress expression of the invading viral particles, its aberrant execution leads to carcinogenesis. 80 As with HPV in HNSCC, 3 these virus–induced global methylation changes have also been captured for other viruses involved in several different cancer types, such as EBV in gastric cancer, 81 HBV and HCV with hepatocellular carcinoma 82 and HPV for cervical cancer. 83 A strong CpG methylator phenotype (CIMP) is also a common feature of the EBV‐associated nasopharyngeal cancer as EBV may alter the activity of host DNA methyltransferase and demethylase to establish viral methylome. 35 A specific example is loss of expression of p16 due to promoter hypermethylation, in EBV infected nasopharyngeal carcinoma samples. 84 The latent EBV protein LIMP1 is also reported to upregulated DNMT1 expression, via activation of AP‐1/JUN signalling. Promoter hypermethylation–based silencing of crucial cell regulatory proteins, such as PTEN, by LIMP1 has been reported in gastric carcinoma. 85 Since the viral proteins target important hub proteins in human cells, like p53 in case of HPV infection or p16 in EBV infected squamous cells, their inactivation leads to a global change. For example, p53 negatively regulates DNMT1 by repressing its expression. However, p53 inactivation due to mutation or viral activity leads to over–expression and hypermethylation in HNSCC tumours. 86
Recent studies suggest that the microbiome may modulate the host epigenome through production of metabolites, immune system modulation, and direct interaction with host cells. Preliminary assessment of the possible involvement of microbiota in methylation events has been assessed using animal model studies and they conclude that use of antibiotics against majority of primary gut colonisers affects the host methylation pattern. 87 Gut microbiome, along with diet also influences the epigenome in cancer and other diseases. The changes in the epigenome are found to be specific to the species in question, such as Lactobacillus acidophilus, Bifidobacterium infantis, and Klebsiella species. 88 There are multiple ways in which this effect takes place. One prominent effector molecule which creates a differential methylation effect is the bacterial metabolites, such as short‐chain fatty acids (SCFAs). Gut microbiome has been shown to produce SCFAs that can modulate histone acetylation and gene expression. SCFAs have also been shown to regulate immune cell function, which may indirectly affect the epigenome. 89 , 90 As a model system, colon tissue provides examples of pathogen–associated methylation changes which also act as a risk factor for cancer. 91 An integrated analysis of metagenomic sequencing and DNA methylation has revealed that Hungatella hathewayi and F. nucleatum enrichment was associated with hypermethylation of tumour suppressor genes in colon cancer tissue. 92 Specifically, H. hathewayi caused promoter hypermethylation of SOX11, THBD, SFRP2, GATA5, and ESR1, whereas F. nucleatum associated with methylation of MTSS1, RBM38, PKD1, and PTPRT. Promoterspecific methylation activity of microbes may assist the cancer cell at specific stages of development, also providing an explanation for the association of stage–specific dynamics of the bacterial population in tumour microenvironment. Interestingly, a recent study provides evidence that Fusobacterium nucleatum has the ability to regulate epitranscriptome. KIF26B, a member of the kinesin (KIF) family, is a known oncogene which promotes metastasis in gastric cancer and colorectal cancer. 93 , 94 METTL3 is an m6A methyltransferase suppressing KIF26B via degradation. However, F. nucleatum via activation of YAP signalling and subsequent transcriptional regulation of METTL3, results in KIF26B reactivation and ultimately metastases of colorectal cancer cells. 95 Although methylation plays a major role in HNSCC development, very little is known about effect of specific pathogen in modulating the same. A recent study by Chen Z., et al. identified association of enrichment of Fusobacterium nucleatum with the gene promoter methylation within host, particularly causing hypermethylation of tumour suppressor genes LXN and SMARCA2. 96 Although this review does not detail effect of microbes on other epigenetic interactions in head and neck cancer, there exist sparse reports which point in that direction. In addition to affecting the methylation status on at specific loci in human genome, microbial interactions may also act via histone acetylation (chromatic states), as well as non‐coding RNA (ncRNA) expression modulation. The periodontal pathogens have direct impact on the histone acetylation status in oral mucosa. 97 Several reported associations are catalogued in form of a database between the microbes and acetylation or regulatory ncRNA expression in different cancer types, including head and neck cancer. 98 In overall, literature suggests that microbes greatly influence the epigenome of the host epithelial cancer cells. However, more investigations are required in understanding the breadth of effect of pathogens on epigenetics of HNSCC. Also, mechanistic studies and crossmodel system validations are need of hour in this area of research.
5. CLINICAL AND THERAPEUTIC RELEVANCE OF MICROBIOME IN HNSCC
The genomic and epigenomic characteristics imparted by various pathogens lead to phenotypic variability in the tumours. The precise mechanisms connecting molecular influences and clinical outcomes may require further systematic investigation. Nevertheless, microbiome research is on the verge of entering into mainstream clinical evaluation with several leads arising from the associations and impact of microbes on HNSCC. Microbial dysbiosis of the oral cavity has been previously proposed to be a primary screening tool in not only for oral cancer, 99 but other cancer types including colorectal 100 and pancreatic cancer. 101 Moreover, the microbial dysbiosis is correlated with survival differences in HNSCC. Specifically, an abundance of Fusobacterium nucleatum and Porphyromonas gingivilis is clinically correlated with poor survival in HNSCC in a site‐specific manner. 40 , 102 Poor survival outcomes with Fusobacterium abundance has been also previously reported in colorectal cancer, wherein the authors proposed development of preventive and treatment strategies targeting intestinal microflora by probiotics and antibiotics. 103 Use of antibiotics in colorectal adenocarcinoma in‐vivo models have shown to have positive impact on disease outcomes and in preventing metastases. 104 Survival benefit with antibiotic use has been shown in patient cohort receiving chemotherapy for metastatic pancreatic ductal adenocarcinoma. 105 Currently, literature is unavailable for such clinical trials in HNSCC, however similar treatment strategies need to be tested in in‐vivo models, followed by its implementation in patient cohorts, in a tumour subsite‐specific manner. On the contrary, HPV‐positive oropharyngeal (OPSCC) and EBV‐positive nasopharyngeal tumours are proposed to show better prognosis, higher survival and respond to immunotherapeutic interventions due to their intra‐tumour immune composition. 106 , 107 The molecular profile of the HPV positive OPSCC also are anticipated to demand lower intensity adjuvant treatment, which is currently under scrutiny in a clinical trial (NCT02215265). Also, for EBV positive nasopharyngeal cancers therapeutic recombinant vaccine has been tested in a phase II clinical trial to treat patients with residual EBV DNA load after conventional therapy (NCT01094405).
Inversely, the microbiome affects the outcome of the therapy regimens in HNSCC patients. A detailed review cataloguing the clinical studies investigating associations between the HNSCC associated microbiome and their therapeutic implications has been presented elsewhere. 56 Use of broad range antibiotics in patients with locally advanced head and neck cancer treated with chemo‐radiotherapy had negative impact on overall survival, progression‐free and disease‐specific survival. 108 Another study reported enrichment of Fusobacterium nucleatum and Mycoplasma in patients with reduced sensitivity to induction chemotherapy, functionally through platinum drug resistance pathway in oral squamous cell carcinoma. 109 Similarly, chemotherapy resistance due to abundance of opportunistic microbes is clearly demonstrated in colorectal carcinoma. 110 Large HNSCC cohort studies screening for levels of the inflammatory bacterium and intervention with targeted antibiotics for site specific tumours are indispensable for development of novel treatment strategies to improve disease outcomes.
Large scale genomic studies have broadly classified the HNSCC tumours into distinct sub‐types mentioned above, including HPV positive, EBV positive and Fusobacterium (or infectious gram negative oncobacterium) positive tumours. Due to the influence of the specific microbes, these tumours also show a distinct immune profile which present novel targeting opportunities in some, whereas additional treatment challenges in others. Immune infiltration is closely linked to the therapy response in HNSCC, as well as other solid tumours. 111 , 112 , 113 The immune profiles also have a very close association with the landscape of infectious pathogens present in the tumour microenvironment. Hence, its summary is equally important in this context. However, it is found to be out of scope for the current review and has been cursorily depicted in Figure 1 based on the following literature. 114 , 115 , 116 In depth associations and interactions of genomic, microbial, and immune landscape of the tumours, will provide several additional answers on the way tumours respond to specific therapies, as well as point towards novel therapeutic approaches.
6. CONCLUSION
Microbial dysbiosis or pathogenic infection of specific microbes may greatly influence host genome and epigenome, leading to changes in gene expression and cellular processes that contribute to HNSCC pathogenesis. However, more research is needed to understand the complex interactions between host–microbe interactions in HNSCC pathogenesis, similar to colon cancer. Systematic analyses bridging gap between the pathogen induced molecular portraits of HNSCC and the clinical disease outcomes due to microbiome interference will pave way to novel treatment and preventive approaches.
AUTHOR CONTRIBUTIONS
Sanket Desai: Conceptualization (lead); investigation (lead); writing – original draft (lead); writing – review and editing (lead).
FUNDING INFORMATION
No funding was obtained for this work.
CONFLICT OF INTEREST STATEMENT
The author declare that they have no competing interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGEMENT
None.
Desai S. Influence of pathogens on host genome and epigenome in development of head and neck cancer. Cancer Reports. 2023;6(11):e1846. doi: 10.1002/cnr2.1846
[Correction added on 05 September 2023, after first online publication: Affiliation details have been updated.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee Y‐CA. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head Neck. 2013;35(7):914‐922. [DOI] [PubMed] [Google Scholar]
- 3. Network TCGA . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Upadhyay P, Gardi N, Desai S, et al. Genomic characterization of tobacco/nut chewing HPV‐negative early stage tongue tumors identify MMP10 as a candidate to predict metastases. Oral Oncol. 2017;73:56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ju Y, Wu X, Wang H, et al. Genomic landscape of head and neck squamous cell carcinoma across different anatomic sites in Chinese population. Front Genet. 2021;12:680699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parkin DM. The global health burden of infection‐associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030‐3044. [DOI] [PubMed] [Google Scholar]
- 7. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180‐e190. [DOI] [PubMed] [Google Scholar]
- 8. Krump NA, You J. Molecular mechanisms of viral oncogenesis in humans. Nat Rev Microbiol. 2018;16(11):684‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Littman AJ, Jackson LA, Vaughan TL. Chlamydia pneumoniae and Lung cancer: epidemiologic evidence. Cancer Epidemiol Biomarkers Prev. 2005;14(4):773‐778. [DOI] [PubMed] [Google Scholar]
- 10. Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iyer P, Barreto SG, Sahoo B, et al. Non‐typhoidal Salmonella DNA traces in gallbladder cancer. Infect Agents Cancer. 2016;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellmerich S, Scholler M, et al. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis. 2000;21(4):753‐756. [DOI] [PubMed] [Google Scholar]
- 13. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulger Toprak N, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12(8):782‐786. [DOI] [PubMed] [Google Scholar]
- 15. Swidsinski A, Khilkin M, Kerjaschki D, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115(2):281‐286. [DOI] [PubMed] [Google Scholar]
- 16. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodríguez‐Lozano B, González‐Febles J, Garnier‐Rodríguez JL, et al. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: a case–control study. Arthritis Res Ther. 2019;21(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X‐X, Jiao B, Liao X‐X, et al. Analysis of salivary microbiome in patients with Alzheimer's disease. J Alzheimers Dis. 2019;72(2):633‐640. [DOI] [PubMed] [Google Scholar]
- 20. Czesnikiewicz‐Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non‐surgical periodontal therapy. Eur Heart J. 2019;40(42):3459‐3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pessoa L, Aleti G, Choudhury S, et al. Host‐microbial interactions in systemic lupus erythematosus and periodontitis. Front Immunol. 2019;10:2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Flemer B, Warren RD, Barrett MP, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut. 2018;67(8):1454‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan X, Alekseyenko AV, Wu J, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population‐based nested case‐control study. Gut. 2018;67(1):120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawasaki M, Ikeda Y, Ikeda E, et al. Oral infectious bacteria in dental plaque and saliva as risk factors in patients with esophageal cancer. Cancer. 2020;127(4):512‐519. [DOI] [PubMed] [Google Scholar]
- 25. Shi J, Yang Y, Xie H, et al. Association of oral microbiota with lung cancer risk in a low‐income population in the southeastern USA. Cancer Causes Control. 2021;32(12):1423‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogtmann E, Hua X, Yu G, et al. The Oral microbiome and Lung cancer risk: an analysis of 3 prospective cohort studies. J Natl Cancer Inst. 2022;114(11):1501‐1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hayes RB, Ahn J, Fan X, et al. Association of Oral Microbiome with Risk for incident head and neck squamous cell cancer. JAMA Oncol. 2018;4(3):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caselli E, Fabbri C, D'Accolti M, et al. Defining the oral microbiome by whole‐genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol. 2020;20(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bratman SV, Bruce JP, O'Sullivan B, et al. Human papillomavirus genotype association with survival in head and neck squamous cell carcinoma. JAMA Oncol. 2016;2(6):823. [DOI] [PubMed] [Google Scholar]
- 31. Narisawa‐Saito M, Kiyono T. Basic mechanisms of high‐risk human papillomavirus‐induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koppad R, Suresh G, Prakash BV, Sabitha KS, Dhara PS. Prognostic indicators of oral squamous cell carcinoma. Ann Maxillofac Surg. 2019;9(2):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leemans CR, Snijders PJF, Brakenhoff RH. The molecular landscape of head and neck cancer. Nat Rev Cancer. 2018;18(5):269‐282. [DOI] [PubMed] [Google Scholar]
- 34. Fernandes Q, Merhi M, Raza A, et al. Role of Epstein–Barr virus in the pathogenesis of head and neck cancers and its potential as an immunotherapeutic target. Front Oncol. 2018;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsang CM, Lui VWY, Bruce JP, Pugh TJ, Lo KW. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol. 2020;61:84‐100. [DOI] [PubMed] [Google Scholar]
- 36. Vasquez AA, Ram JL, Qazazi MS, Sun J, Kato I. Oral microbiome: potential link to systemic diseases and Oral cancer. Mechanisms underlying host‐microbiome interactions in pathophysiology of human diseases. Springer, Boston, MA, 2018:195‐246. [Google Scholar]
- 37. Hooper SJ, Crean S‐J, Fardy MJ, et al. A molecular analysis of the bacteria present within oral squamous cell carcinoma. J Med Microbiol. 2007;56(12):1651‐1659. [DOI] [PubMed] [Google Scholar]
- 38. Frank DN, Qiu Y, Cao Y, et al. A dysbiotic microbiome promotes head and neck squamous cell carcinoma. Oncogene. 2022;41(9):1269‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong H, Shi Y, Xiao X, et al. Alterations of microbiota structure in the larynx relevant to laryngeal carcinoma. Sci Rep. 2017;7(1):5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Desai S, Dharavath B, Manavalan S, et al. Fusobacterium nucleatum is associated with inflammation and poor survival in early‐stage HPV‐negative tongue cancer. NAR Cancer. 2022;4(1):zcac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ganly I, Yang L, Giese RA, et al. Periodontal pathogens are a risk factor of oral cavity squamous cell carcinoma, independent of tobacco and alcohol and human papillomavirus. Int J Cancer. 2019;145(3):775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagy KN, Sonkodi I, Szoke I, Nagy E, Newman HN. The microflora associated with human oral carcinomas. Oral Oncol. 1998;34(4):304‐308. [PubMed] [Google Scholar]
- 43. Wen L, Mu W, Lu H, et al. Porphyromonas gingivalis promotes Oral squamous cell carcinoma progression in an immune microenvironment. J Dent Res. 2020;99(6):666‐675. [DOI] [PubMed] [Google Scholar]
- 44. Gallimidi AB, Fischman S, Revach B, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral‐specific chemical carcinogenesis model. Oncotarget. 2015;6(26):22613‐22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshimura A, Kaneko T, Kato Y, Golenbock DT, Hara Y. Lipopolysaccharides from Periodontopathic BacteriaPorphyromonas gingivalisandCapnocytophaga ochraceaAre antagonists for human toll‐like receptor 4. Infect Immun. 2002;70(1):218‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsolis RM, Kamarajan P, Ateia I, et al. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020;16(10):e1008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. 2021;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalispromotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dharavath B, Butle A, Pal A, et al. Role of miR‐944/MMP10/AXL‐ axis in lymph node metastasis in tongue cancer. Commun Biol. 2023;6(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Singh S, Singh AK. Porphyromonas gingivalis in oral squamous cell carcinoma: a review. Microbes Infect. 2022;24(3):104925. [DOI] [PubMed] [Google Scholar]
- 51. McIlvanna E, Linden GJ, Craig SG, Lundy FT, James JA. Fusobacterium nucleatum and oral cancer: a critical review. BMC Cancer. 2021;21(1):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dohlman AB, Arguijo Mendoza D, Ding S, et al. The cancer microbiome atlas: a pan‐cancer comparative analysis to distinguish tissue‐resident microbiota from contaminants. Cell Host Microbe. 2021;29(2):281‐98.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung L‐M, Liang J‐A, Lin C‐L, Sun L‐M, Kao C‐H. Cancer risk in patients with candidiasis: a nationwide population‐based cohort study. Oncotarget. 2017;8(38):63562‐63573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Narunsky‐Haziza L, Sepich‐Poore GD, Livyatan I, et al. Pan‐cancer analyses reveal cancer‐type‐specific fungal ecologies and bacteriome interactions. Cell. 2022;185(20):3789‐806.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xue C, Chu Q, Zheng Q, et al. Current understanding of the intratumoral microbiome in various tumors. Cell Rep Med. 2023;4(1):100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reis Ferreira M, Pasto A, Ng T, et al. The microbiota and radiotherapy for head and neck cancer: what should clinical oncologists know? Cancer Treat Rev. 2022;109:102442. [DOI] [PubMed] [Google Scholar]
- 57. Hayes DN, Van Waes C, Seiwert TY. Genetic landscape of human papillomavirus–associated head and neck cancer and comparison to tobacco‐related tumors. J Clin Oncol. 2015;33(29):3227‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and E7 gene silencing and transformed phenotype of human papillomavirus 16‐positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101(6):412‐423. [DOI] [PubMed] [Google Scholar]
- 59. Henderson S, Chakravarthy A, Su X, Boshoff C, Fenton TR. APOBEC‐mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus‐driven tumor development. Cell Rep. 2014;7(6):1833‐1841. [DOI] [PubMed] [Google Scholar]
- 60. Kondo S, Wakae K, Wakisaka N, et al. APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene. 2016;36(12):1687‐1697. [DOI] [PubMed] [Google Scholar]
- 61. Koneva LA, Zhang Y, Virani S, et al. HPV integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol Cancer Res. 2018;16(1):90‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lin D‐C, Meng X, Hazawa M, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46(8):866‐871. [DOI] [PubMed] [Google Scholar]
- 63. Ernberg IT, Deng W, Pang PS, et al. Epstein‐Barr virus‐encoded latent membrane protein 1 impairs G2 checkpoint in human nasopharyngeal epithelial cells through defective Chk1 activation. PLoS One. 2012;7(6):e39095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Leong MML, Cheung AKL, Dai W, et al. EBV infection is associated with histone bivalent switch modifications in squamous epithelial cells. Proc Natl Acad Sci. 2019;116(28):14144‐14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beyaert S, Machiels J‐P, Schmitz S. Vaccine‐based immunotherapy for head and neck cancers. Cancer. 2021;13(23):6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schneider K, Grønhøj C, Hahn CH, von Buchwald C. Therapeutic human papillomavirus vaccines in head and neck cancer: a systematic review of current clinical trials. Vaccine. 2018;36(45):6594‐6605. [DOI] [PubMed] [Google Scholar]
- 67. Machado AMD, Figueiredo C, Seruca R, Rasmussen LJ. Helicobacter pylori infection generates genetic instability in gastric cells. Biochim Biophys Acta. 2010;1806(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 68. Nougayrède J‐P, Homburg S, Fdr T, et al. Escherichia coli induces DNA double‐Strand breaks in eukaryotic cells. Science. 2006;313(5788):848‐851. [DOI] [PubMed] [Google Scholar]
- 69. Goodwin AC, Shields CED, Wu S, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis‐induced colon tumorigenesis. Proc Natl Acad Sci. 2011;108(37):15354‐15359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hale VL, Jeraldo P, Chen J, et al. Distinct microbes, metabolites, and ecologies define the microbiome in deficient and proficient mismatch repair colorectal cancers. Genome Med. 2018;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Okita Y, Koi M, Takeda K, et al. Fusobacterium nucleatum infection correlates with two types of microsatellite alterations in colorectal cancer and triggers DNA damage. Gut Pathog. 2020;12(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harrandah AM, Chukkapalli SS, Bhattacharyya I, Progulske‐Fox A, Chan EKL. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol. 2020;13(1):1849493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kay J, Thadhani E, Samson L, Engelward B. Inflammation‐induced DNA damage, mutations and cancer. DNA Repair. 2019;83:102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Geng F, Zhang Y, Lu Z, Zhang S, Pan Y. Fusobacterium nucleatumCaused DNA damage and promoted cell proliferation by theKu70/p53Pathway in Oral cancer cells. DNA Cell Biol. 2020;39(1):144‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jin B, Robertson KD. DNA Methyltransferases, DNA damage repair, and cancer. Epigenetic alterations in Oncogenesis. Adv Exp Med Biol. 2013;754:3‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Suzuki T, Yamazaki H, Honda K, et al. Altered DNA methylation is associated with aberrant stemness gene expression in early‐stage HNSCC. Int J Oncol. 2019;55:915‐924. [DOI] [PubMed] [Google Scholar]
- 77. Demokan S, Dalay N. Role of DNA methylation in head and neck cancer. Clin Epigenetics. 2011;2(2):123‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen D, Wang M, Guo Y, et al. An aberrant DNA methylation signature for predicting the prognosis of head and neck squamous cell carcinoma. Cancer Med. 2021;10(17):5936‐5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith IM, Mydlarz WK, Mithani SK, Califano JA. DNA global hypomethylation in squamous cell head and neck cancer associated with smoking, alcohol consumption and stage. Int J Cancer. 2007;121(8):1724‐1728. [DOI] [PubMed] [Google Scholar]
- 80. Li HP, Leu YW, Chang YS. Epigenetic changes in virus‐associated human cancers. Cell Res. 2005;15(4):262‐271. [DOI] [PubMed] [Google Scholar]
- 81. Matsusaka K, Kaneda A, Nagae G, et al. Classification of Epstein–Barr virus–positive gastric cancers by definition of DNA methylation Epigenotypes. Cancer Res. 2011;71(23):7187‐7197. [DOI] [PubMed] [Google Scholar]
- 82. Ally A, Balasundaram M, Carlsen R, et al. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169(7):1327‐41.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vellano CP, Wentzensen N, Ojesina AI. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543(7645):378‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lo KW, Cheung ST, Leung SF, et al. Hypermethylation of the p16 gene in nasopharyngeal carcinoma. Cancer Res. 1996;56(12):2721‐2725. [PubMed] [Google Scholar]
- 85. Hino R, Uozaki H, Murakami N, et al. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69(7):2766‐2774. [DOI] [PubMed] [Google Scholar]
- 86. Yeung CLA, Tsang WP, Tsang TY, Co NN, Yau PL, Kwok TT. HPV‐16 E6 upregulation of DNMT1 through repression of tumor suppressor p53. Oncol Rep. 2010;24(6):1599–604. [DOI] [PubMed] [Google Scholar]
- 87. Pan X, Gong D, Nguyen DN, et al. Early microbial colonization affects DNA methylation of genes related to intestinal immunity and metabolism in preterm pigs. DNA Res. 2018;25(3):287‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Paul B, Barnes S, Demark‐Wahnefried W, et al. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin Epigenetics. 2015;7(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miro‐Blanch J, Yanes O. Epigenetic regulation at the interplay between gut microbiota and host metabolism. Front Genet. 2019;10:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mirzaei R, Afaghi A, Babakhani S, et al. Role of microbiota‐derived short‐chain fatty acids in cancer development and prevention. Biomed Pharmacother. 2021;139:111619. [DOI] [PubMed] [Google Scholar]
- 91. Sobhani I, Rotkopf H, Khazaie K. Bacteria‐related changes in host DNA methylation and the risk for CRC. Gut Microbes. 2020;12(1):1800898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Xia X, Wu WKK, Wong SH, et al. Bacteria pathogens drive host colonic epithelial cell promoter hypermethylation of tumor suppressor genes in colorectal cancer. Microbiome. 2020;8(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang H, Ma RR, Wang XJ, et al. KIF26B, a novel oncogene, promotes proliferation and metastasis by activating the VEGF pathway in gastric cancer. Oncogene. 2017;36(40):5609‐5619. [DOI] [PubMed] [Google Scholar]
- 94. Wang J, Cui F, Wang X, et al. Elevated kinesin family member 26B is a prognostic biomarker and a potential therapeutic target for colorectal cancer. J Exp Clin Cancer Res. 2015;34(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen S, Zhang L, Li M, et al. Fusobacterium nucleatum reduces METTL3‐mediated m6A modification and contributes to colorectal cancer metastasis. Nat Commun. 2022;13(1):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen Z, Wong PY, Ng CWK, et al. The intersection between Oral microbiota, host gene methylation and patient outcomes in head and neck squamous cell carcinoma. Cancer. 2020;12(11):3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Martins MD, Jiao Y, Larsson L, et al. Epigenetic modifications of histones in periodontal disease. J Dent Res. 2015;95(2):215‐222. [DOI] [PubMed] [Google Scholar]
- 98. Wang L, Zhang W, Wu X, et al. MIAOME: human microbiome affect the host epigenome. Comput Struct Biotechnol J. 2022;20:2455‐2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhou X, Hao Y, Peng X, et al. The clinical potential of Oral microbiota as a screening tool for Oral squamous cell carcinomas. Front Cell Infect Microbiol. 2021;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kong C. Human oral microbiome dysbiosis as a novel tool for detecting noninvasive biomarkers for colorectal cancer. J Clin Oncol. 2021;39(3_suppl):23. [Google Scholar]
- 101. Sun H, Zhao X, Zhou Y, et al. Characterization of Oral microbiome and exploration of potential biomarkers in patients with pancreatic cancer. Biomed Res Int. 2020;2020:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33(5):1055‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatumin colorectal carcinoma tissue and patient prognosis. Gut. 2016;65(12):1973‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bullman S, Pedamallu CS, Sicinska E, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fulop DJ, Zylberberg HM, Wu YL, et al. Association of Antibiotic Receipt with Survival among Patients with Metastatic Pancreatic Ductal Adenocarcinoma Receiving Chemotherapy. JAMA Netw Open. 2023;6(3):e234254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Julian R, Savani M, Bauman JE. Immunotherapy approaches in HPV‐associated head and neck cancer. Cancer. 2021;13(23):5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hong M, Tang K, Qian J, et al. Immunotherapy for EBV‐associated nasopharyngeal carcinoma. Crit Rev Oncog. 2018;23(3–4):219‐234. [DOI] [PubMed] [Google Scholar]
- 108. Nenclares P, Bhide SA, Sandoval‐Insausti H, et al. Impact of antibiotic use during curative treatment of locally advanced head and neck cancers with chemotherapy and radiotherapy. Eur J Cancer. 2020;131:9‐15. [DOI] [PubMed] [Google Scholar]
- 109. Rui M, Zhang X, Huang J, et al. The baseline oral microbiota predicts the response of locally advanced oral squamous cell carcinoma patients to induction chemotherapy: a prospective longitudinal study. Radiother Oncol. 2021;164:83‐91. [DOI] [PubMed] [Google Scholar]
- 110. Pandey K, Umar S. Microbiome in drug resistance to colon cancer. Curr Opin Phys Ther. 2021;23:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17):e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Economopoulou P, Perisanidis C, Giotakis EI, Psyrri A. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): anti‐tumor immunity and clinical applications. Ann Transl Med. 2016;4(9):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Datta M, Coussens LM, Nishikawa H, Hodi FS, Jain RK. Reprogramming the tumor microenvironment to improve immunotherapy: emerging strategies and combination therapies. Am Soc Clin Oncol Educ Book. 2019;39:165‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chen YP, Wang YQ, Lv JW, et al. Identification and validation of novel microenvironment‐based immune molecular subgroups of head and neck squamous cell carcinoma: implications for immunotherapy. Ann Oncol. 2019;30(1):68‐75. [DOI] [PubMed] [Google Scholar]
- 115. Varn FS, Schaafsma E, Wang Y, Cheng C. Genomic characterization of six virus‐associated cancers identifies changes in the tumor immune microenvironment and altered genetic programs. Cancer Res. 2018;78(22):6413‐6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


