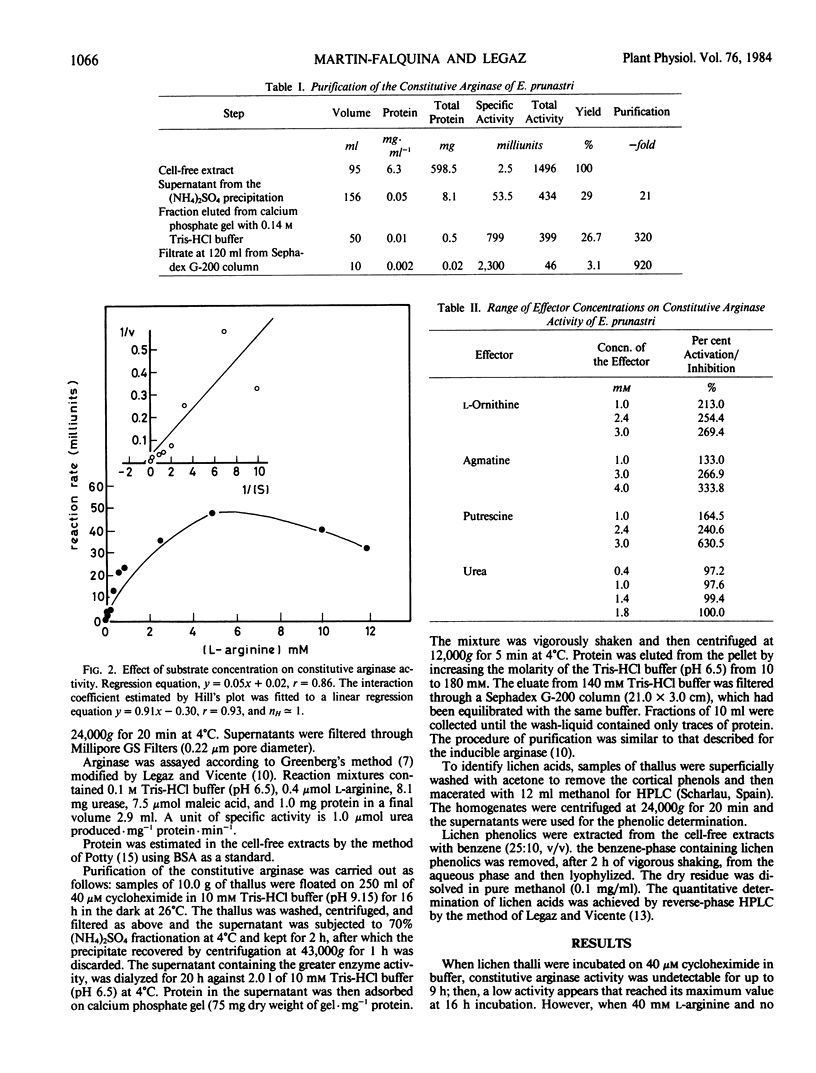
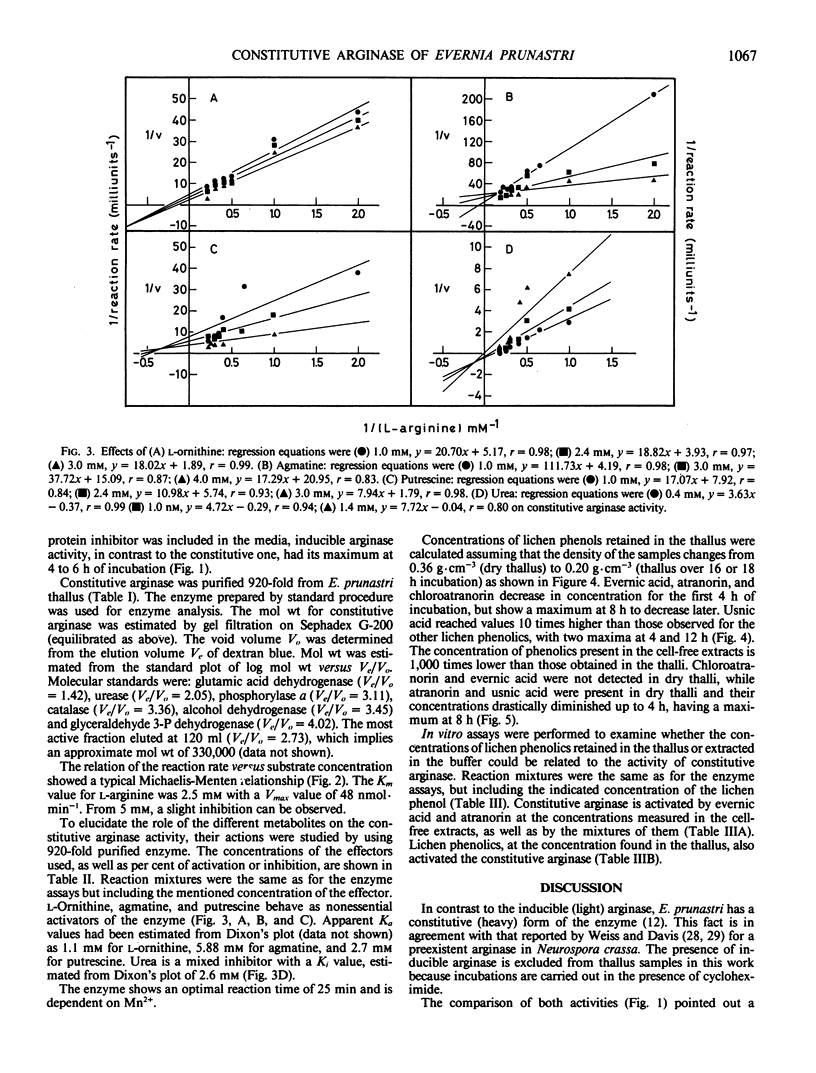
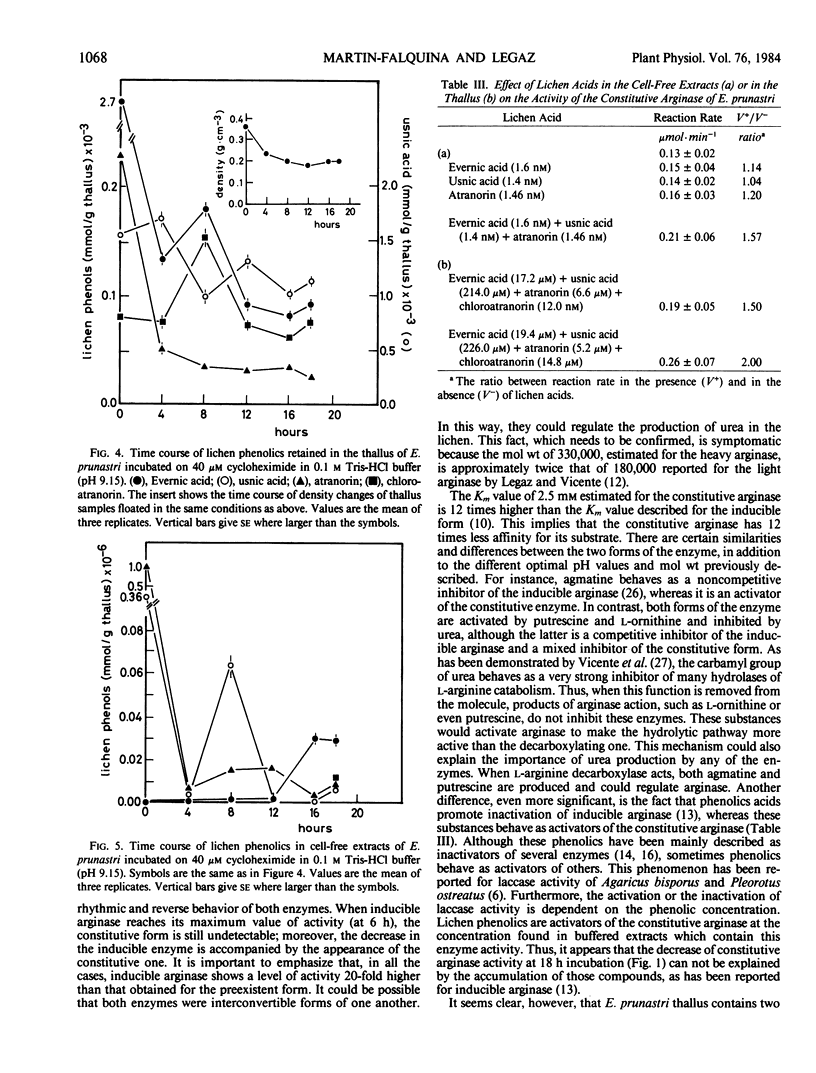
Abstract
Constitutive arginase (molecular weight 330,000) 920-fold purified from Evernia prunastri thallus, is activated by putrescine, l-ornithine, and agmatine with Ka values of 2.7, 1.1, and 5.8 millimolar, respectively. Constitutive arginase is also activated by endogenous l-arginine, reaching its maximum activity at 16 hours of incubation on Tris-HCl (pH 9.15) with a subsequent decrease. Urea behaves as a mixed inhibitor of the enzyme with a Ki value of 2.6 millimolar. Atranorin and evernic acid behave as in vitro activators of the enzyme; usnic acid does not have any significant effect as activator.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carvajal N., Acoria M., Rodríguez J. P., Fernández M., Martínez J. Evidence for cooperative effects in human liver arginase. Biochim Biophys Acta. 1982 Feb 4;701(1):146–148. doi: 10.1016/0167-4838(82)90324-7. [DOI] [PubMed] [Google Scholar]
- Crabeel M., Charlier D., Cunin R., Boyen A., Glansdorff N., Piérard A. Accumulation of arginine precursors in Escherichia coli: effects on growth, enzyme repression, and application to the forward selection of arginine auxotrophs. J Bacteriol. 1975 Sep;123(3):898–904. doi: 10.1128/jb.123.3.898-904.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen G. A., Strecker H. J. Purification and properties of arginase of rat kidney. Biochem J. 1973 Aug;133(4):779–788. doi: 10.1042/bj1330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legaz M. E., Vicente C. Endogenous Inactivators of Arginase, l-Arginine Decarboxylase, and Agmatine Amidinohydrolase in Evernia prunastri Thallus. Plant Physiol. 1983 Feb;71(2):300–302. doi: 10.1104/pp.71.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legaz M. E., Vicente C. Two forms of arginase in Evernia prunastri thallus. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1441–1446. doi: 10.1016/0006-291x(82)91411-5. [DOI] [PubMed] [Google Scholar]
- Potty V. H. Determination of proteins in the presence of phenols and pectins. Anal Biochem. 1969 Jun;29(3):535–539. doi: 10.1016/0003-2697(69)90339-x. [DOI] [PubMed] [Google Scholar]
- Solberg Y. J. Studies on the chemistry of lichens. VII. Chemical investigations of the lichen species Lecanora (Aspicilia) Myrinii (Fr.) Nyl. Z Naturforsch B. 1969 Apr;24(4):447–451. [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Control of arginine utilization in Neurospora. J Bacteriol. 1977 Feb;129(2):866–873. doi: 10.1128/jb.129.2.866-873.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]


