Abstract
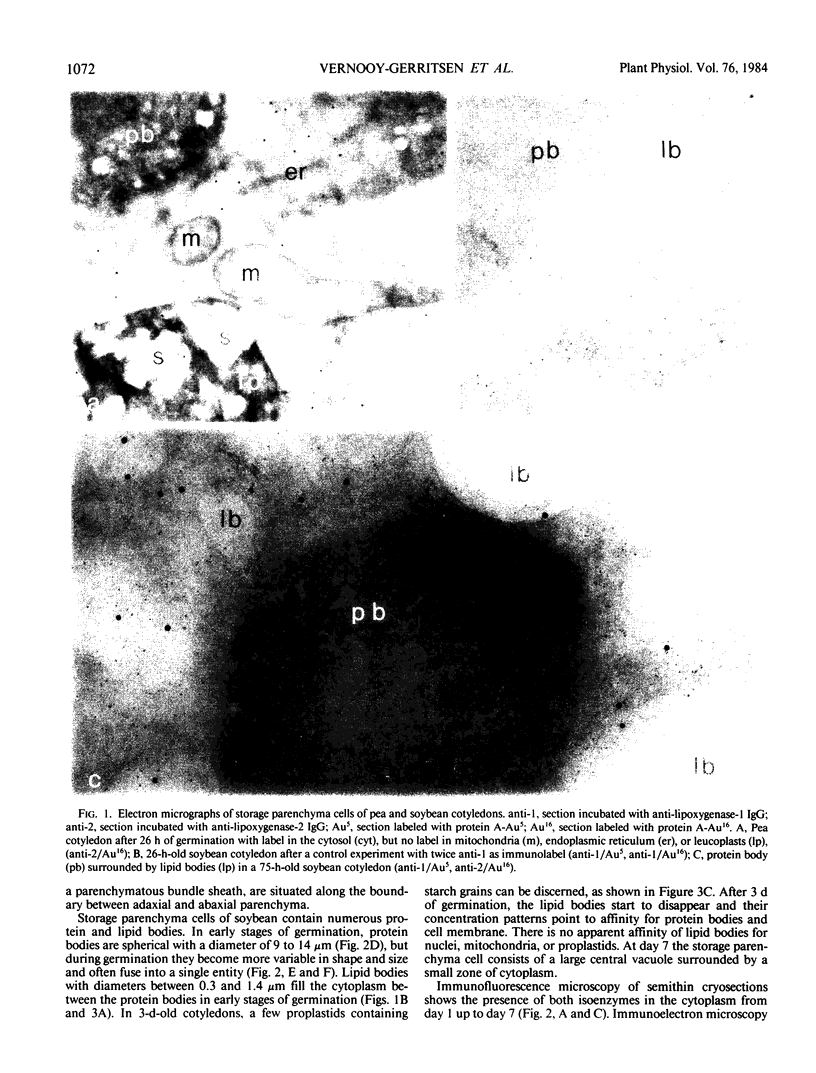
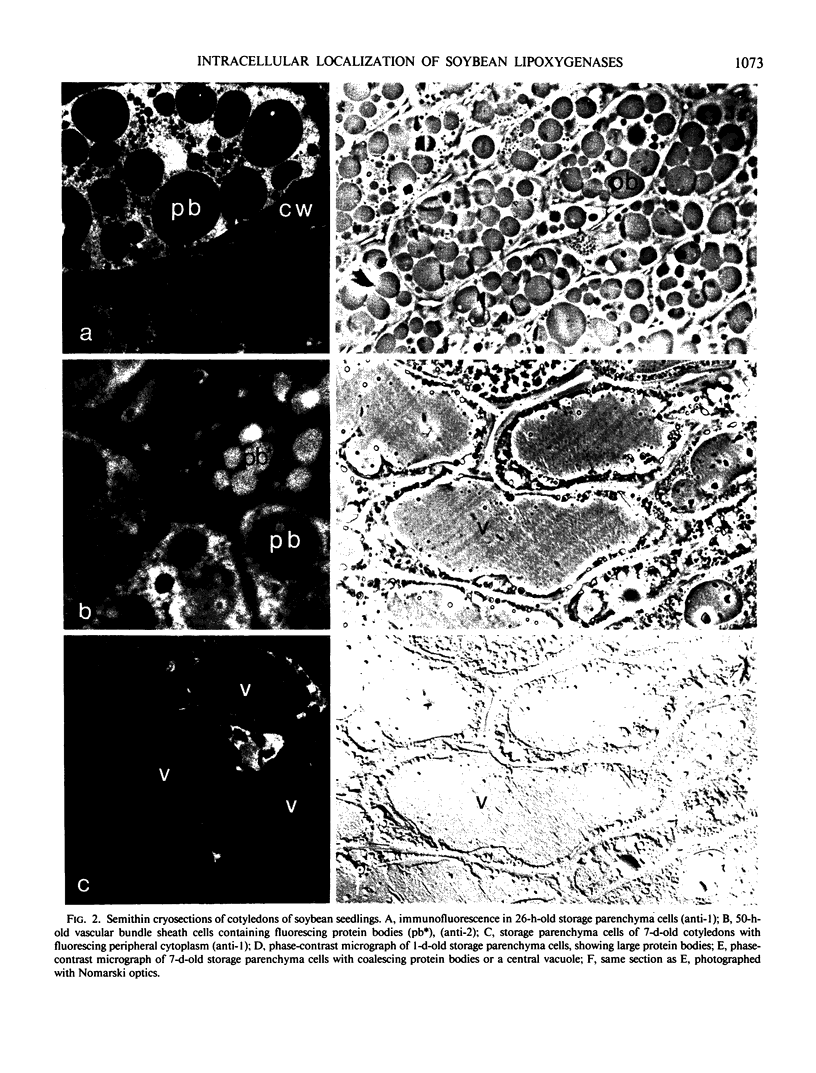
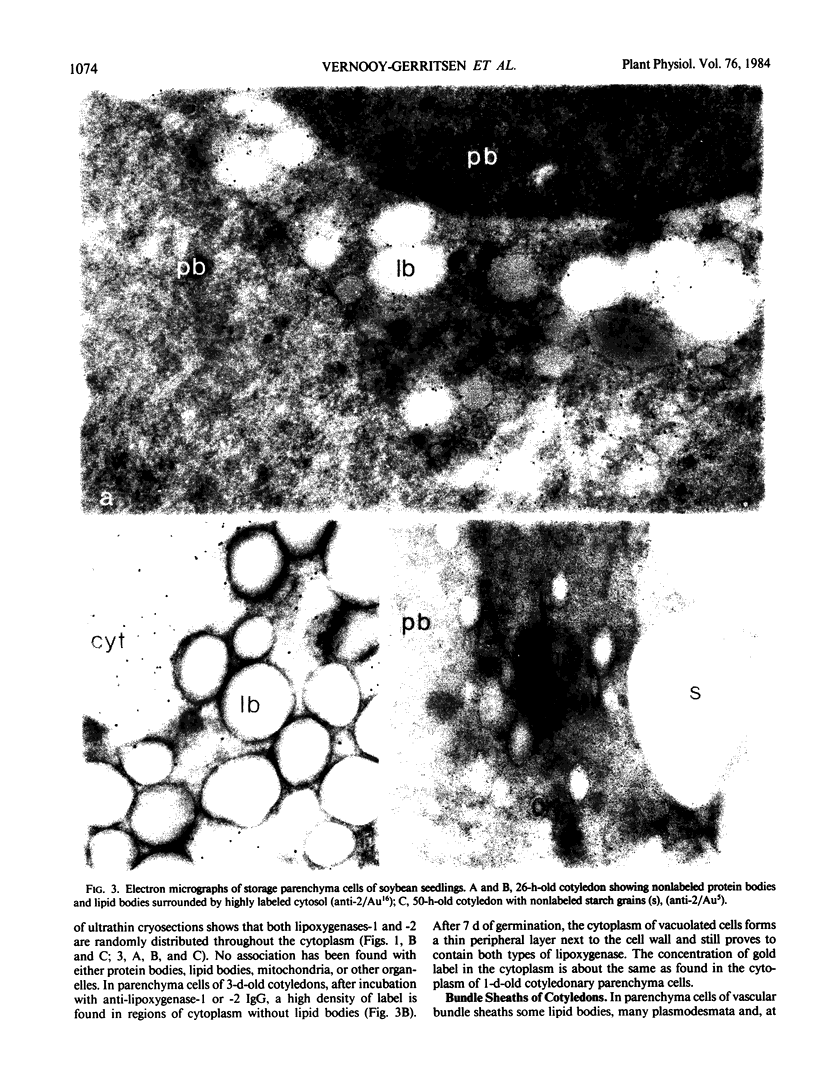
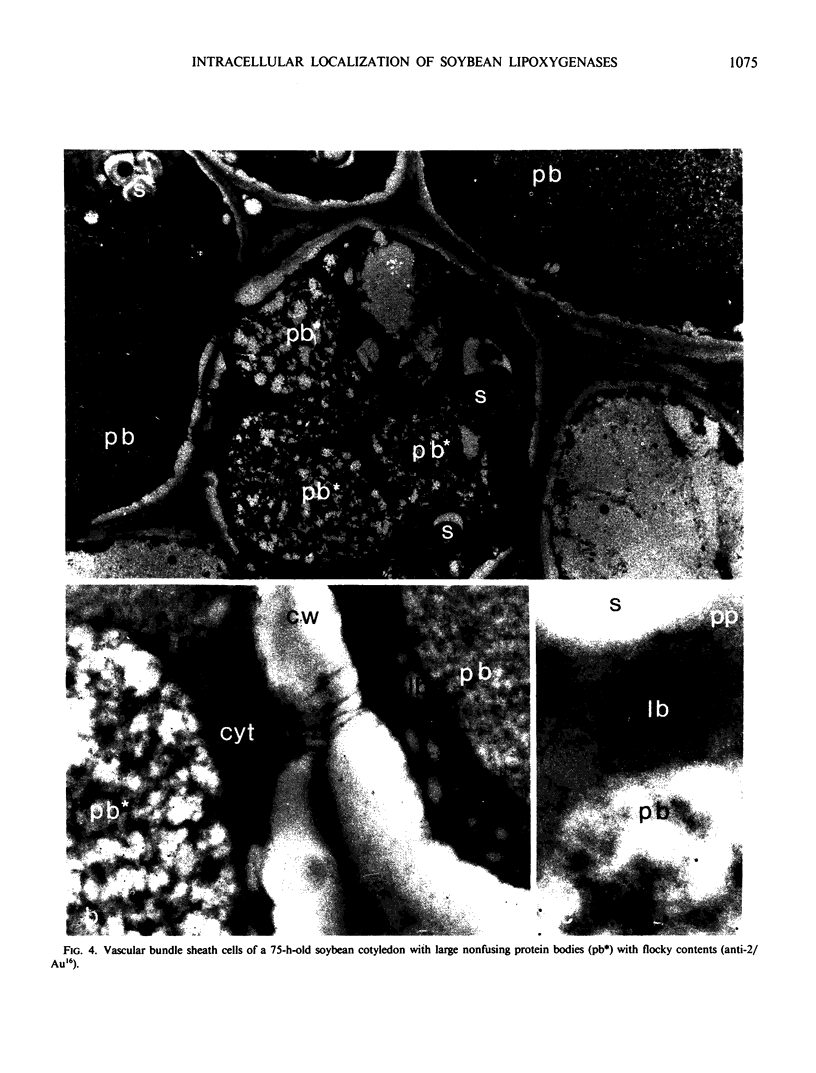
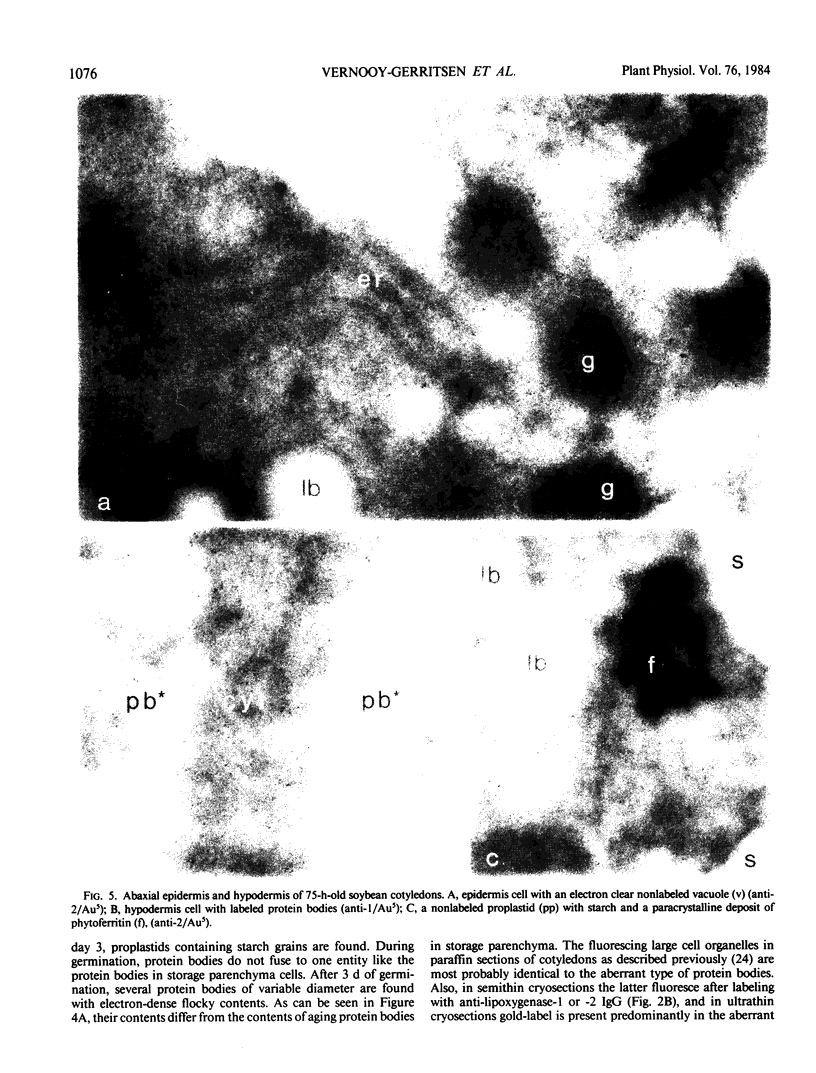
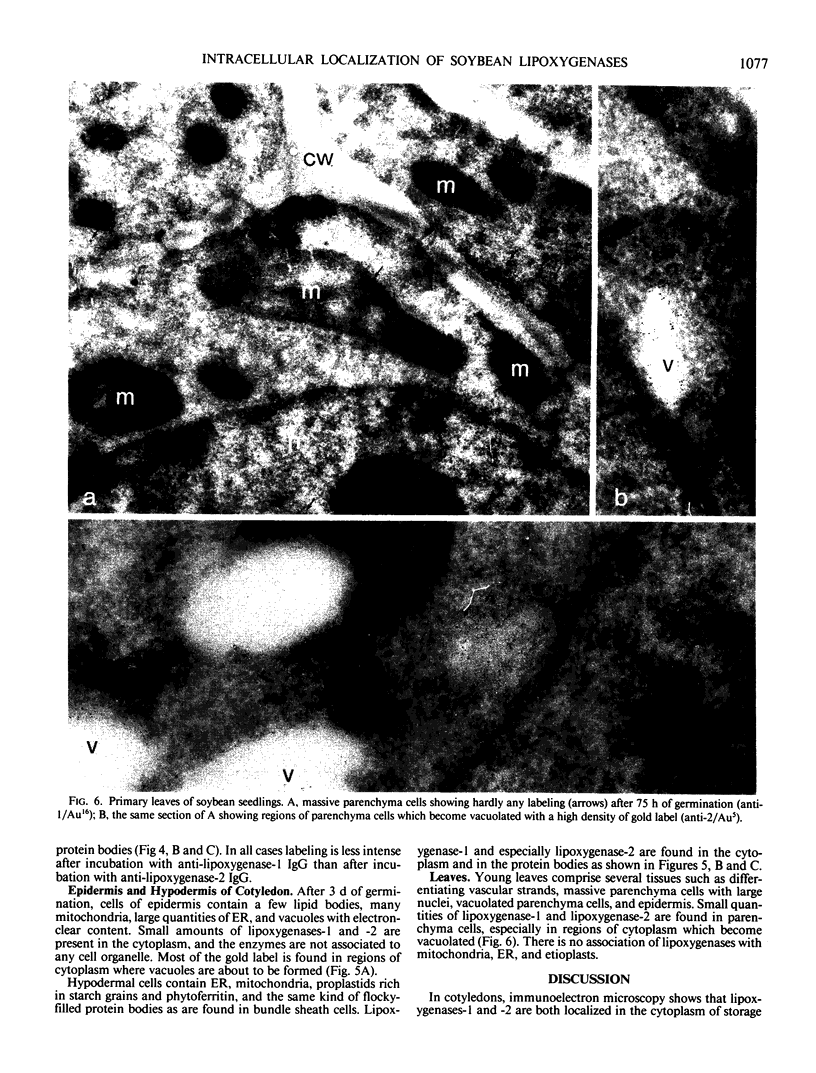
Soybean lipoxygenases-1 and -2 were localized intracellularly in seeds at various stages of germination by indirect labeling of cryosections with protein A-colloidal gold complexes. Two sizes of gold particles (Au5 and Au16) were used in single- and double-labeling experiments. In primary leaves, lipoxygenases are demonstrated to occur in vacuolating parenchyma cells but not in massive, nondifferentiated cells. In cotyledons, both isoenzymes are localized in the cytoplasm of storage parenchyma cells and in an aberrant type of protein bodies, occurring in hypodermis and vascular bundle sheath cells. No association has been found with either protein bodies in storage parenchyma cells or lipid bodies, mitochondria, and other organelles in any type of cell. The possible significance of lipoxygenase in the metabolism of storage lipids and its possible function as a regulatory enzyme are discussed on the basis of the random distribution throughout the cytoplasm of storage parenchyma cells and the course of biochemical processes during seed germination.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christopher J., Pistorius E., Axelrod B. Isolation of an isozyme of soybean lipoxygenase. Biochim Biophys Acta. 1970 Jan 14;198(1):12–19. doi: 10.1016/0005-2744(70)90028-8. [DOI] [PubMed] [Google Scholar]
- Dupont J., Rustin P., Lance C. Interaction between Mitochondrial Cytochromes and Linoleic Acid Hydroperoxide: POSSIBLE CONFUSION WITH LIPOXYGENASE AND ALTERNATIVE PATHWAY. Plant Physiol. 1982 Jun;69(6):1308–1314. doi: 10.1104/pp.69.6.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C. E., Svensson S. G. Lipoxygenase from peas, purification and properties of the enzyme. Biochim Biophys Acta. 1970 Mar 18;198(3):449–459. doi: 10.1016/0005-2744(70)90123-3. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agrò A., Avigliano L., Veldink G. A., Vliegenthart J. F., Boldingh J. The influence of oxygen on the fluorescence of lipoxygenase. Biochim Biophys Acta. 1973 Dec 20;326(3):462–470. doi: 10.1016/0005-2760(73)90146-x. [DOI] [PubMed] [Google Scholar]
- Galpin J. R., Tielens L. G., Veldink G. A., Vliegenthart J. F., Boldingh J. On the interaction of some catechol derivatives with the iron atom of soybean lipoxygenase. FEBS Lett. 1976 Oct 15;69(1):179–182. doi: 10.1016/0014-5793(76)80681-3. [DOI] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. M., Baumgartner B., Chrispeels M. J. Uptake and apparent digestion of cytoplasmic organelles by protein bodies (protein storage vacuoles) in mung bean cotyledons. Eur J Cell Biol. 1981 Jun;24(2):226–235. [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- KOCH R. B., STERN B., FERRARI C. G. Linoleic acid and trilinolein as substrates for soybean lipoxidase (s). Arch Biochem Biophys. 1958 Nov;78(1):165–179. doi: 10.1016/0003-9861(58)90325-4. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Immunochemistry on ultrathin frozen sections. Histochem J. 1980 Jul;12(4):381–403. doi: 10.1007/BF01011956. [DOI] [PubMed] [Google Scholar]
- Tombs M. P. Protein bodies of the soybean. Plant Physiol. 1967 Jun;42(6):797–813. doi: 10.1104/pp.42.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Bos A. L., Veldink G. A., Vliegenthart J. F. Affinity chromatography of antibodies directed against soybean lipoxygenase-1 and -2 and an enzyme-linked immunosorbent assay (ELISA) for antibodies and lipoxygenases. Biochim Biophys Acta. 1983 Oct 17;748(1):148–152. doi: 10.1016/0167-4838(83)90038-9. [DOI] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Bos A. L., Veldink G. A., Vliegenthart J. F. Localization of lipoxygenases 1 and 2 in germinating soybean seeds by an indirect immunofluorescence technique. Plant Physiol. 1983 Oct;73(2):262–267. doi: 10.1104/pp.73.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M., Veldink G. A., Vliegenthart J. F. Specificities of antisera directed against soybean lipoxygenases-1 and -2 and purification of lipoxygenase-2 by affinity chromatography. Biochim Biophys Acta. 1982 Nov 19;708(3):330–334. doi: 10.1016/0167-4838(82)90445-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. L., Snyder H. E. Role of calcium in activating soybean lipoxygenase 2. J Agric Food Chem. 1974 Sep-Oct;22(5):802–805. doi: 10.1021/jf60195a006. [DOI] [PubMed] [Google Scholar]
- van Os C. P., Rijke-Schilder G. P., Vliegenthart J. F. 9-LR-linoleyl hydroperoxide, a novel product from the oxygenation of linoleic acid by type-2 lipoxygenases from soybeans and peas. Biochim Biophys Acta. 1979 Dec 18;575(3):479–484. doi: 10.1016/0005-2760(79)90120-6. [DOI] [PubMed] [Google Scholar]