Abstract
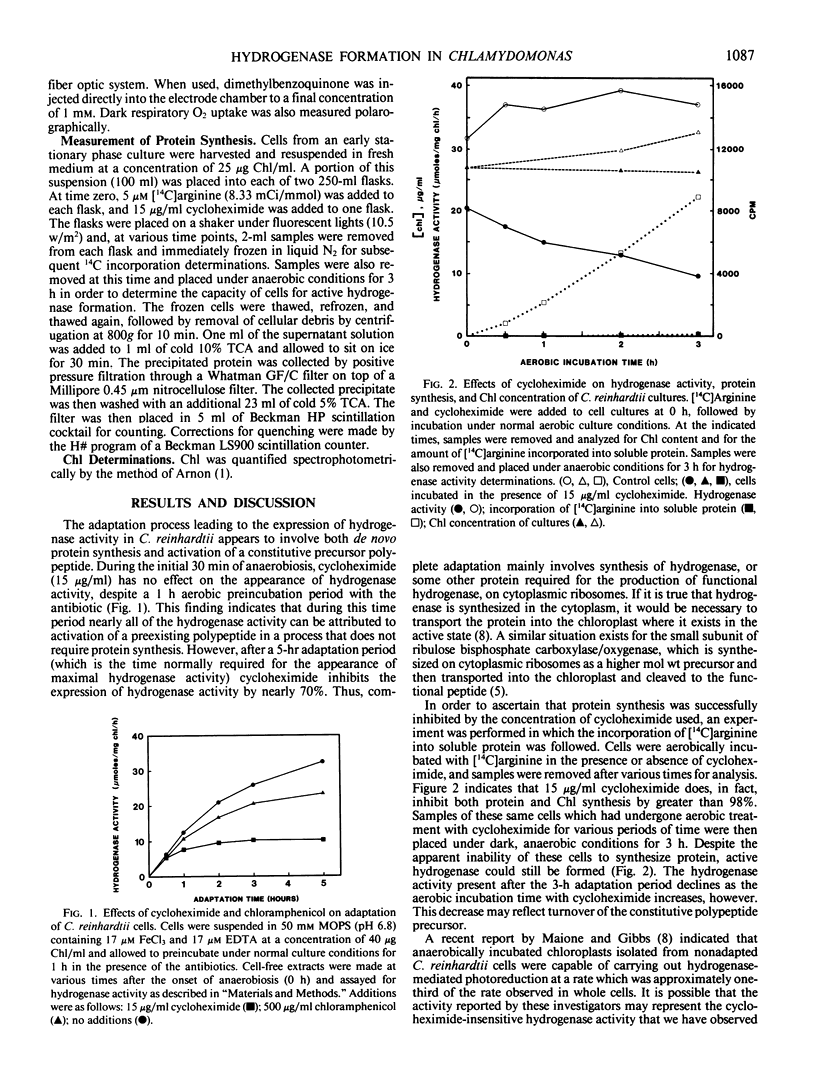
Two distinct processes are involved in the formation of active hydrogenase during anaerobic adaptation of Chlamydomonas reinhardtii cells. In the first 30 minutes of anaerobiosis, nearly all of the hydrogenase activity can be attributed to activation of a constituitive polypeptide precursor, based on the insensitivity of the process to treatment with cycloheximide (15 micrograms per milliliter). This concentration of cycloheximide inhibits protein synthesis by greater than 98%. After the initial activation period, de novo protein synthesis plays a critical role in the adaptation process since cycloheximide inhibits the expression of hydrogense in maximally adapted cells by 70%. Chloramphenicol (500 micrograms per milliliter) has a much lesser effect on the adaptation process.
Incubation of cell-free extracts under anaerobic conditions in the presence of dithionite, dithiothreitol, NADH, NADP, ferredoxin, ATP, Mg2+, Ca2+, and iron does not lead to active hydrogenase formation. Futhermore, in vivo reactivation of oxygen-inactivated hydrogenase does not appear to take place.
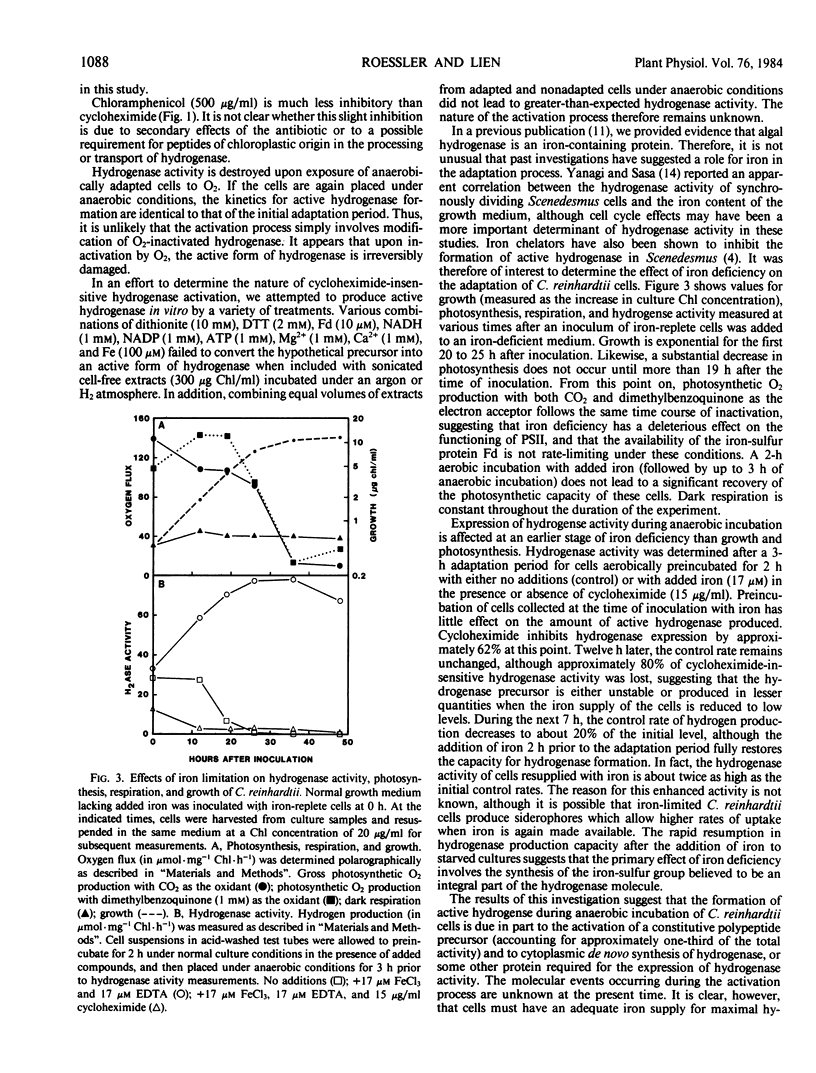
The adaptation process is very sensitive to the availability of iron. Iron-deficient cultures lose the ability to form active hydrogenase before growth, photosynthesis, and respiration are significantly affected. Preincubation of iron-deficient cells with iron 2 hours prior to the adaptation period fully restores the capacity of the cells to synthesize functional hydrogenase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN H., KRASNA A. I. PROPERTIES OF THE HYDROGENASE OF SCENEDESMUS. Biochim Biophys Acta. 1964 Oct 23;92:52–58. doi: 10.1016/0926-6569(64)90268-8. [DOI] [PubMed] [Google Scholar]
- Roessler P. G., Lien S. Purification of Hydrogenase from Chlamydomonas reinhardtii. Plant Physiol. 1984 Jul;75(3):705–709. doi: 10.1104/pp.75.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessler P., Lien S. Anionic modulation of the catalytic activity of hydrogenase from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1982 Jan;213(1):37–44. doi: 10.1016/0003-9861(82)90436-2. [DOI] [PubMed] [Google Scholar]
- STILLER M., LEE J. K. HYDROGENASE ACTIVITY IN CHLORELLA. Biochim Biophys Acta. 1964 Oct 9;93:174–176. doi: 10.1016/0304-4165(64)90274-0. [DOI] [PubMed] [Google Scholar]


