Abstract
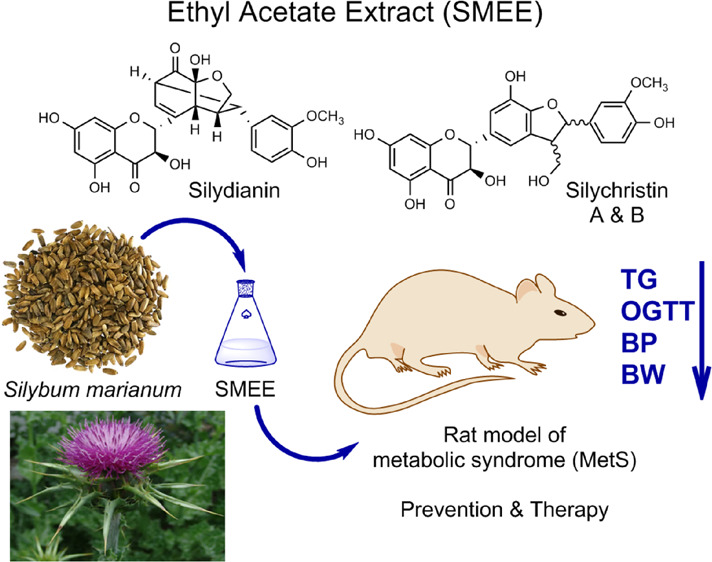
Metabolic syndrome (MetS) has become an increasing global health problem, which leads to cardiovascular diseases and type 2 diabetes. Silybum marianum extracts have been reported to possess several biological activities. In this study, an ethyl acetate extract prepared from S. marianum seeds of the Iraqi Kurdistan region was analyzed to identify its chemical constituents. Subsequently, its potential for the prevention and treatment of MetS was studied in a rat model induced by a high-fat/high-fructose diet (HFD/F). Silydianin and silychristin were the most abundant flavonolignan constituents (39.4%) identified in the S. marianum extract (SMEE). HFD/F-induced rats treated with SMEE exhibited preventive effects including reduced serum triglyceride levels (TG), decreased glucose levels in an oral glucose tolerance test (p < 0.001), attenuated weight gain, and reduced blood pressure compared to the untreated control group. Therapeutic application of SMEE after inducing MetS led to lowering of TG (p < 0.001) and glucose levels, in addition to reducing weight gain and normalizing blood pressure (p < 0.005). Thus, S. marianum extract rich in silydianin and silychristin may be useful for preventing and attenuating MetS, and further research and clinical trials are warranted.
Keywords: flavonolignans, metabolic syndrome, prevention, rat model, Silybum marianum, therapeutic
Metabolic syndrome (MetS) is described by a cluster of symptoms including change in glucose metabolism, insulin dysfunction, high blood pressure, dyslipidemia, and liver steatosis.1−3 The syndrome is often associated with obesity1−3 and with increasing low-grade inflammation and oxidative stress that lead to cardiovascular disorders.4 The prevalence of this and related risk factors is estimated to rise in future decades due to lifestyle factors.3 Globally, 25% of the adult population is suffering from metabolic syndrome as estimated by the International Diabetes Federation.5
According to the World Health Organization (WHO), the fundamental diagnostic criteria for MetS included impaired glucose tolerance, diabetes mellitus, and insulin resistance.6 The National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) introduced updated guidelines, requiring patients to fulfill three out of five criteria (increased waist circumference, elevated triglycerides, reduced high-density lipoprotein (HDL) cholesterol, elevated blood pressure, and elevated fasting glucose) to be officially diagnosed with MetS.7 Subsequently, the International Diabetes Foundation (IDF) outlined the diagnostic criteria for MetS as any two of the following factor: obesity, elevated triglycerides, low HDL cholesterol, elevated blood pressure, and impaired fasting glucose.8
The high-fat, high-fructose diet (HFD/F) has been extensively studied and is implicated as a significant contributor to the epidemic of MetS. It often leads to excessive calorie consumption, which is a primary driver of obesity, a central component of MetS;9 also, diets rich in saturated fats can induce insulin resistance, elevated triglycerides, and low-density lipoprotein (LDL) cholesterol, which are components of MetS.10,11
The current management of metabolic syndrome by therapeutic drugs is insufficient and accompanied by unwanted side effects.12 Therefore, it is of great importance to explore alternative approaches not only for the treatment of metabolic disorders but even more importantly for its prevention. In this regard, phytochemicals isolated from medicinal plants may be effective.13
Medicinal plants are favored in many communities due to their cost-effectiveness and perceived safety.14 Moreover, the WHO has advocated for the integration of natural remedies, including medicinal plants, into healthcare systems, emphasizing their potential in enhancing treatment strategies.15 In addition, nutraceuticals, food-derived natural products, have been reported to have important biological activities with preventive and therapeutic opportunities for a number of diseases.16−20
Naturally occurring bioactive organic molecules isolated from plants, animals, or microorganisms have inspired the development of many drugs.21 In fact, about 50% of all modern clinical drugs are estimated to be directly or indirectly derived from natural products.22
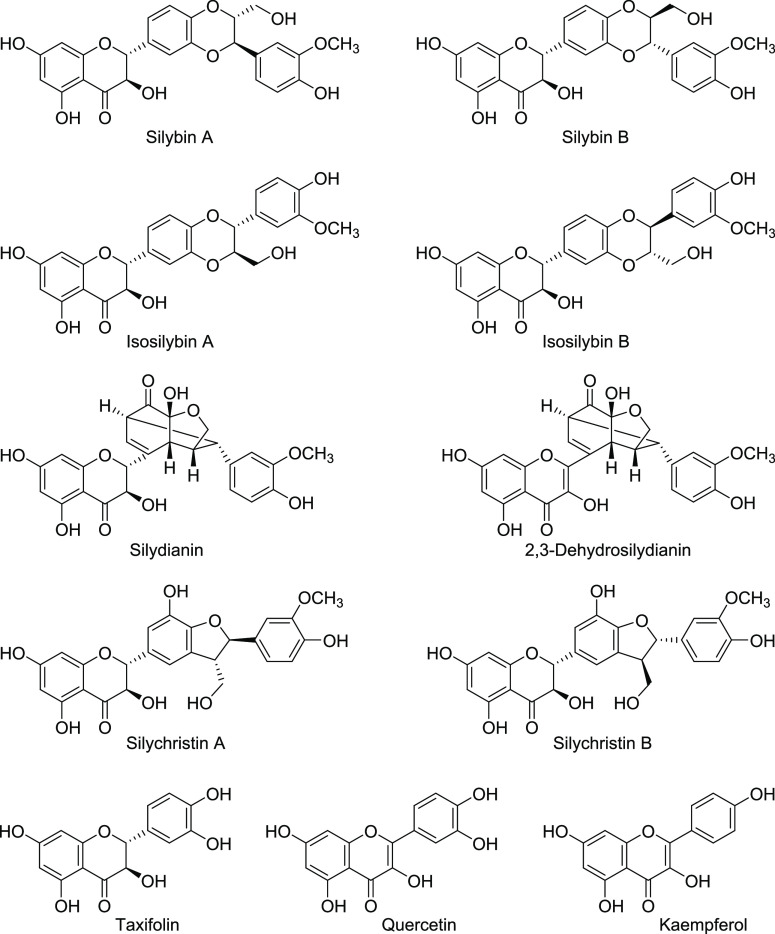
Silybum marianum (L.) Gaertn (Asteraceae), commonly known as milk thistle, is a medicinal plant traditionally used for hepatoprotective and -therapeutic applications, e.g., for treating alcoholic liver disease.23 In addition, it has been reported to exhibit many other activities, including antimicrobial, anticancer, cardioprotective, neuroprotective, and antidiabetic effects.24,25 The main component of the plant is silymarin, a mixture of flavonolignans (70–80%) found in the fruits and seeds of Silybum marianum. The flavonolignans are composed of silybin (50%, silybin (A and B), isosilybin (5%, isosilybin A and B), silydianin (10%), and silychristin (20%); for structures, see Figure 1). In addition, the plant contains flavanols, mainly 2,3-dehydrosilybin and taxifolin, and flavonols, including quercetin and kaempferol (Figure 1).26 Silymarin is a standardized extract of milk thistle fruits with silybin as its major constituent.27
Figure 1.
Polyphenolic compounds identified in silymarin, a commercialized S. marianum extract.
Several accessions of the plant S. marianum have been reported in literature, e.g., from Canada, New Zeeland, Egypt, Europe (Poland, Hungary, Bulgaria, Germany, Italy), Iran, and USA.28 Our study is the first of its kind to be conducted on the purple flower type of the Kurdistan-Iraqi origin of S. marianum.
A novel extract was prepared from S. marianum seeds, and its main chemical constituents were identified and quantified. Subsequently, its pharmacological effects regarding the prevention and therapy of MetS induced in rats were evaluated.
Materials and Methods
Plant Material
The seeds of S. marianum, the purple flower kind, were purchased from a local herbal market in Erbil, Iraq, and the botanical authentication was performed in the College of Agriculture, University of Salahaddin, by Dr. Serwan Taha Al-Dabbagh. The collected fresh S. marianum seeds of the Kurdistan region of Iraq were dried at room temperature in the dark and ground to a fine powder by an electric grinder (Avinas AV-120, Switzerland). A voucher specimen (No. Naza1) was deposited at the herbarium of the Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, for future reference.
Preparation of the S. marianum Ethyl Acetate Extract (SMEE)
Powdered seeds of S. marianum (500 g) were treated with petroleum ether (40–60 °C) at a ratio of 1:10 (w/v) by ultrasonic-assisted extraction (200 kHz, 3h, 40 °C), and the petroleum ether extract was discarded. The remaining plant solid materials were soaked in a mixture of ethanol/water (7:3) at a ratio of 1:10 (w/v) by ultrasonic-assisted extraction (200 kHz, 3h, 40 °C).29 The ethanolic extracts were combined and filtered through a Whatman No.1 filter paper in a Buchner funnel, and the filtrate was evaporated in a rotary evaporator at 40 °C, yielding 33.1 g of gummy materials. The resulting mixture was redissolved in 10% aqueous ethanol (50 mL) followed by liquid–liquid fractionation (10 × 50 mL) with an equal volume of ethyl acetate in a separatory funnel. The obtained extracts in ethyl acetate (SMEE) were concentrated under a vacuum to obtain solid materials (14.7 g). The extracts were kept at 4 °C until used for further investigations.
Liquid Chromatography–Mass Spectrometry (LC–MS)
Liquid chromatography–mass spectrometry (LC–MS) analyses were performed on a 1260 Infinity II instrument with a diode array detector and with an Infinity LC-MSD mass detector with a single quadrupole equipped with electrospray ionization (both Agilent, Germany). The quantification was performed on a Nucleodur Gravity EC 50/23 μm (Macherey and Nagel, Germany) equipped with a precolumn under gradient elution (mobile phase: A = 5% acetonitrile, 0.1% formic acid; B = 80% methanol, 0.1% formic acid; gradient: 0 min 30% B, within 12 min to 60% B, for 12–15 min 100% B, flow rate 0.5 mL/min, t = 25 °C). The diode array detector (DAD) data were acquired in the 190–600 nm range, with sampling at 40 Hz and a response time of 0.013 s, and signals at 220–600 nm were extracted; the injection volume was 4 μL. This method was analogous to a previously described procedure.30
Acute Toxicity Study
The ethyl acetate extract of S. marianum seeds (SMEE) was investigated for acute oral toxicity in accordance with the Organization for Economic Cooperation and Development (OECD) guideline no. 423 for testing chemicals.31 In brief, rats were selected randomly (n = 6) and fasted for 12 h prior to the experiment, and a single dose of 2000 mg/kg of SMEE suspended in carboxymethylcellulose (CMC) 1% was administered. The rats were observed for development of any toxicity and gross behavioral changes after 30 min and then every 2, 4, 8, 24, and 48 h.
In Vivo Assessment of Anti-MetS Potential
Experimental Animals
Twenty-seven male albino rats (8 weeks old, mean weight 150 ± 50 g) were obtained from the experimental animal house, College of Pharmacy, Hawler Medical University. The rats were housed in custom-made polypropylene cages (33 × 46 cm). The animal facility was well-ventilated and maintained under conditions with a standard 12 h light/dark cycle at an environmental temperature of 22 ± 2 °C having 50–60% relative humidity. Rats were acclimatized to the laboratory conditions for 1 week before experimentation and provided with a normal diet (ND) and water ad libitum. All experimental and animal management protocols were approved by the College of Pharmacy, Hawler Medical University ethics committee (code number HMU ECPH-02112021-707) and complied with the guide for the care and use of laboratory animals of the Institutes of Health, Kurdistan region, Iraq. All efforts were made to minimize animal suffering and to reduce the number of animals used to perform this study.
Induction of Metabolic Syndrome
MetS was induced by feeding the animals with a high-fat and high-fructose (HFD/F) diet for 6 weeks ad libitum. The employed model was according to a previously published work with minor modification.32 The HFD/F diet (in weight percent) contained approximately 60% fat, 20% proteins, and 20% carbohydrates, and in addition to this diet, the drinking water contained 10% dissolved fructose. Meanwhile the normal diet contained 18% fat, 24% protein, and 58% carbohydrates. After administration of the HFD/F diet, the induction of MetS was confirmed by significant elevation of three or more of the following parameters with respect to initial values: triglycerides (TG), total cholesterol (TC), body weight (BW), fasting blood sugar (FBS), diastolic blood pressure (DBP), and systolic blood pressure (SBP). The rats showed significantly higher average values for TG, BW, DBP, and SBP after MetS induction compared with initial values (Figure 2).
Figure 2.
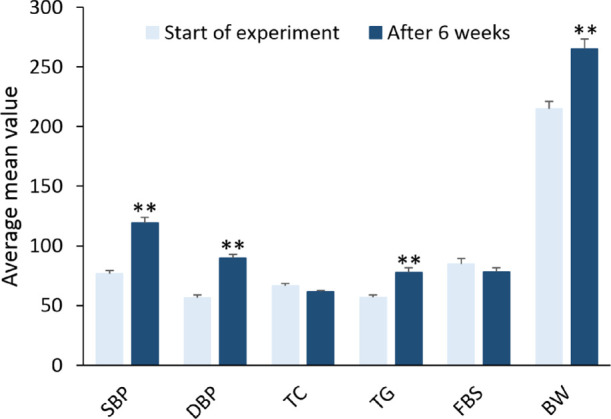
Induction of metabolic syndrome in rats by the HFD/F for 6 weeks. SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); TC, total cholesterol (mg/dL); TG, triglycerides (mg/dL); FBS, fasting blood sugar (mg/dL); BW, body weight (g). Values are represented as mean ± SEM; ** p < 0.001 compared to initial values.
Experimental Protocol
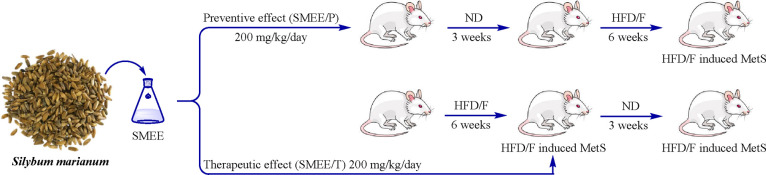
Five experimental groups were established after 1 week of adaptation: (A) metabolic syndrome control group (n = 5), (B and C) positive control groups (n = 5), (D) preventive group (n = 6), and (E) treatment group (n = 6), comprising 27 animals in total (Figure 3). In the MetS control group (A), MetS was induced and no treatment was administered. The preventive group (D) received SMEE (200 mg/kg/day) for 3 weeks before HFD/F was initiated. The treatment group (E) received SMEE (200 mg/kg/day) for 3 weeks after MetS induction, and the positive control groups received metformin 100 mg/kg/day (B) or atorvastatin 10 mg/kg/day (C) for 3 weeks after MetS induction. The final measurements from the preventive groups were compared with those from the control group at week 9, whereas measurements from the treatment groups were compared with those from the positive and negative control groups at week 12.33
Figure 3.
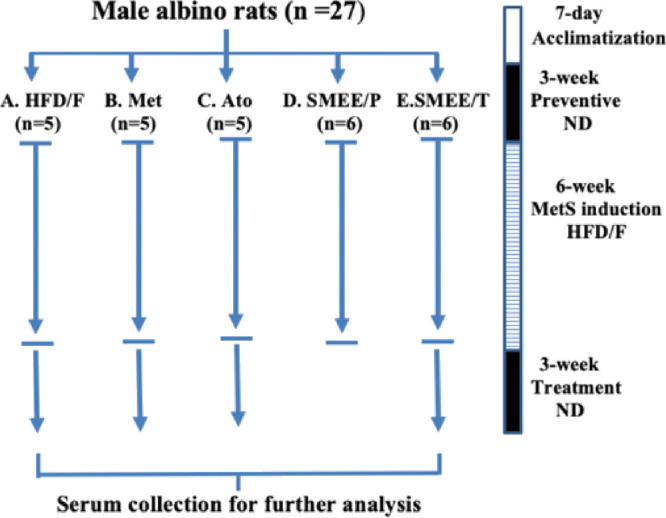
Schematic diagram of the experimental design. (A) HFD/F (negative control): HFD/F was given for 6 weeks with no treatment at any time. (B) Met (positive control): HFD/F was given for 6 weeks followed by metformin (100 mg/kg/day) for 3 weeks. (C) Ato (positive control): HFD/F was given for 6 weeks followed by atorvastatin 10 mg/kg/day for 3 weeks. (D) SMEE/P (preventive): SMEE (200 mg/kg/day) was administered for 3 weeks followed by HFD/F for 6 weeks. (E) SMEE/T (treatment): HFD/F was given for 6 weeks followed by SMEE (200 mg/kg/day) for 3 weeks. Comparisons were made with the negative control group at the end of week 9 for the preventive groups or with the positive and negative control groups at the end of week 12 for the treatment groups.
Treatments of the MetS Induced Rats
The plant seed extract (SMEE), metformin, or atorvastatin (200, 100, and 10 mg/kg/day), respectively, was suspended in 1% carboxymethylcellulose sodium salt (CMC; Awamedica) and administered daily by oral gavage 30 min before the dark phase of the circadian light/dark. The dose of S. marianum extract was determined based on the acute toxicity study, whereas doses for metformin and atorvastatin were chosen on the basis of the literature.34
Biochemical Analysis
Blood samples from all animal groups were analyzed for various biochemical parameters at two time intervals. For the preventive study animal group, the parameters were determined after week 9 of feeding, whereas for the treatment study animal group, the parameters were determined after week 12 of feeding. Blood was collected from the retro-orbital sinus of 12 h fasting rats under mild anesthesia with intraperitoneal injection (5 mg/kg of xylazine hydrochloride and 40 mg/kg of ketamine hydrochloride). Serum was separated from clotted blood by centrifugation at 4000 rpm for 10 min and stored at −20 °C until it was analyzed for biochemical parameters. Samples were analyzed for FBS, TG, and TC levels using a Cobas analyzer (Cobas 6000 Analyzer Roche) as previously reported.35
Oral Glucose Tolerance Test (OGTT)
An oral glucose tolerance test (OGTT) was conducted on week 9 of feeding for the preventive rat group and on week 12 of feeding for the treatment rat group. After 12 h of fasting, all groups were administered glucose (2 g/kg) by gastric gavage. Blood samples were collected from a small cut in the tail vein and measured by a glucometer (STANDARD GlucoNavii/Home Health UK) at 0, 30, 60, and 120 min. OGTT data are expressed as the area under the curve (AUC).
Measurement of Body Weight
Body weight was measured at the starting time, at the end of week 9 of feeding for the preventive rat group, and at the end of week 12 of feeding for the treatment rat group.
Measurement of Blood Pressure (SBP and DBP)
Blood pressure (SBP and DBP) was measured under the conditions intended to minimize stress using the CODA Non-Invasive Blood Pressure system. In a silent room, rats were warmed on a warming platform of 38 °C for 20 min to dilate their veins. The rats were put in an acrylic unrestricted breathing holder with the platform heater on level 3 corresponding to 36–38 °C and time set up for 5–6 h. Blood pressure was measured at the starting time, then on week 9 of feeding for the preventive rat group, and on week 12 of feeding for the treatment rat group using an occlusive tail-cuff plethysmograph (Kent Scientific Corporation model, United States). A sensor-type transducer and cuff were placed 2 cm from the proximal end of the rat tail; the potentiometer was then adjusted to stabilize the signal. The SBP and DBP were measured as the average of 25 consecutive measurements following a published procedure.36
Statistical Analysis
Statistical analysis of the results was carried out using IBM SPSS software, Version 26. The outcomes are presented as means ± SEM (standard error of the mean) from the experiments. Post hoc comparisons were performed using the Lilliefors and Shapiro–Wilk procedures, with the significance determined by a p value less than 0.05. The data were analyzed utilizing one-way ANOVA, paired sample, and independent sample tests.
Results
Preparation of the S. marianum Ethyl Acetate Extract (SMEE)
The resulting dried solid material (SMEE, 14.7 g) corresponded to 2.94% of the employed S. marianum seed material (500 g). The SMEE contains a mixture of compounds expected to be enriched in polyphenols of the plant S. marianum, which are flavonolignans and flavonoids derived from the taxifolin scaffold.
Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
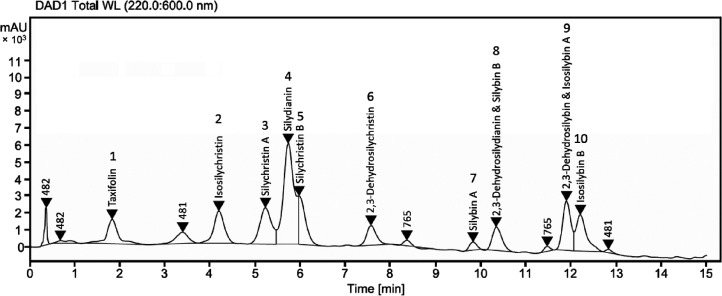
Qualitative and quantitative phytochemical analysis of the S. marianum ethyl acetate extract (SMEE) was conducted by liquid chromatography coupled to mass spectrometry (LC–MS, Figure 4). SMEE was confirmed to be rich in polyphenolic compounds, including flavonolignans and flavonols (Table 1 and Figure 1). The polyphenol constituents at the particular retention times (Rt's) were identified using a previously developed protocol for the separation of silymarin mixtures, and their molecular weights were determined by mass spectrometry.30 The quantity of the flavonolignans was estimated based on the area under the curve (area %) using high-performance liquid chromatography coupled to a diode array ultraviolet detector (HPLC-DAD-UV). Among the identified constituents, silydianin and silychristin B showed the highest concentration (39.4%) followed by 2,3-dehydrosilybin and isosilybin A (12.3%) and silychristin A (11.8%), whereas, surprisingly, silybin (A and B) was found to amount to only 7.3%.
Figure 4.
HPLC-DAD-UV chromatograms of the S. marianum seed ethyl acetate extract (SMEE). For assignment, see Figure 1 and Table 1. AU, arbitrary units.
Table 1. Phytochemical Constituents of S. marianum Ethyl Acetate Extract (SMEE) Determined by HPLC-(UV)-MS.
| peak no. | component | Rt[min] | [M – H]– | area % |
|---|---|---|---|---|
| 1 | taxifolin | 1.84 | 303 | 2.3 |
| 2 | isosilychristin | 4.18 | 481 | 7.0 |
| 3 | silychristin A | 5.27 | 481 | 11.8 |
| 4 and 5 | silydianin and silychristin B | 5.76 and 6.00 | 481 | 39.4 |
| 6 | 2,3-dehydrosilychristin | 7.64 | 479 | 2.8 |
| 7 | silybin A | 9.82 | 481 | 1.6 |
| 8 | 2,3-dehydrosilydianin & silybin B | 10.36 | 479 and 481 | 5.7 |
| 9 | 2,3-dehydrosilybin & isosilybin A | 11.91 | 479 and 481 | 12.3 |
| 10 | isosilybin B | 12.26 | 481 | 10.4 |
Acute Toxicity Study of the S. marianum Ethyl Acetate Extract (SMEE)
The animals showed normal behavioral, neurological, and autonomic profiles at the high dose of tested SMEE (2000 mg/kg). No mortality or sign of physiological impairment was detected during the 48 h period of observation; therefore, a 10-fold lower dose (200 mg/kg) was selected for subsequent pharmacological studies.
Preventive Effect of the S. marianum Ethyl Acetate Extract (SMEE)
A potential preventive effect of the plant extract was studied in healthy rats fed with healthy food for 3 weeks followed by feeding the rats with an HFD/F diet for 6 weeks. Only during the initial 3 weeks did the rats receive SMEE (200 mg/kg/day) (Figure 5). The results indicated a significantly lower triglyceride (TG) value (p < 0.05) in the preventive SMEE/P group compared to the HFD/F control group. At the same time, the oral glucose tolerance test (OGTT) in the preventive treatment group showed significantly lower blood glucose levels (p < 0.001) compared to the control animals (HFD/F). The preventive group did not significantly differ from the controls with regard to the total cholesterol (TC) and fasting blood sugar (FBS) levels (Table 2).
Figure 5.
Experimental design: preventive and therapeutic rat groups, SMEE/P and SMEE/T, respectively. ND, normal diet.
Table 2. Preventive Effects of the S. marianum Ethyl Acetate Extract (SMEE) on MetS in Rats Measured at Week 9a.
| parameters | HFD/F | SMEE/P |
|---|---|---|
| OGTT (AUC mg/dL/120 min) | 7880.00 ± 1349.86 | 3109.67 ± 141.39** |
| TC (mg/dL) | 68.37 ± 4.01 | 63.78 ± 2.89 |
| TG (mg/dL) | 100.04 ± 9.73 | 68.16 ± 4.05* |
| FBS (mg/dL) | 86.60 ± 6.78 | 75.21 ± 3.6 |
Values are represented as means ± SEM. OGTT, oral glucose tolerance test; AUC, area under the curve; TC, total cholesterol; TG, triglycerides; FBS, fasting blood sugar. **p < 0.001 vs negative control group (HFD/F). *p < 0.05 vs negative control group (HFD/F).
Therapeutic Effect of the S. marianum Ethyl Acetate Extract (SMEE)
The therapeutic effect of the plant extract (SMEE/T) was studied after 6 weeks in HFD/F fed rats. Treatment was performed for 3 weeks (Figure 5). SMEE significantly reduced the TG level (p < 0.001) in comparison to the untreated control (HFD/F). Rats in the SMEE/T group showed reduced glucose levels (OGTT) in comparison to the untreated control (HFD/F) and also compared to the metformin-treated group (p < 0.05 and p < 0.005, respectively) (Table 3).
Table 3. Treatment Effects of the S. marianum Extract on MetS in Rats Measured at Week 12a.
| parameters | HFD/F | metformin (Met) | atorvastatin (Ato) | SMEE/T |
|---|---|---|---|---|
| OGTT (AUC mg/dL/120 min) | 2690 ± 61.14 | 2825 ± 124.73 | 3048 ± 87.06 | 2236.33 ± 205.34*,# |
| TC (mg/dL) | 76.14 ± 3.78 | 67.56 ± 4.14 | 72.48 ± 4.18 | 82.08 ± 5.41 |
| TG (mg/dL) | 63.18 ± 4.53 | 59.42 ± 8.17 | 40.06 ± 5.52 | 43.23 ± 3.34** |
| FBS (mg/dL) | 60.40 ± 6.17 | 77.54 ± 8.14 | 79.82 ± 4.79 | 76.05 ± 3.65 |
Values are represented as means ± SEM. OGTT, oral glucose tolerance test; AUC, area under the curve; TC, total cholesterol; TG, triglycerides; FBS, fasting blood sugar. *p < 0.05 vs negative control group (HFD/F). **p < 0.001 vs negative control group (HFD/F). #p < 0.005 vs positive control group (Met or Ato).
Effect of the S. marianum Ethyl Acetate Extracts on Body Weight
The effect of the S. marianum extract on the body weight is summarized in Figure 6. Daily food intake per cage did not differ among groups. The percentage weight gain in the preventive group SMEE/P was 11.1%, which is significantly lower (p < 0.001) compared to the untreated HFD/F control (24.2%). Rats in the treatment group SMEE/T showed a significant decrease in weight gain in comparison with the untreated control (p < 0.05) and both positive controls (Met and Ato) (p < 0.005).
Figure 6.
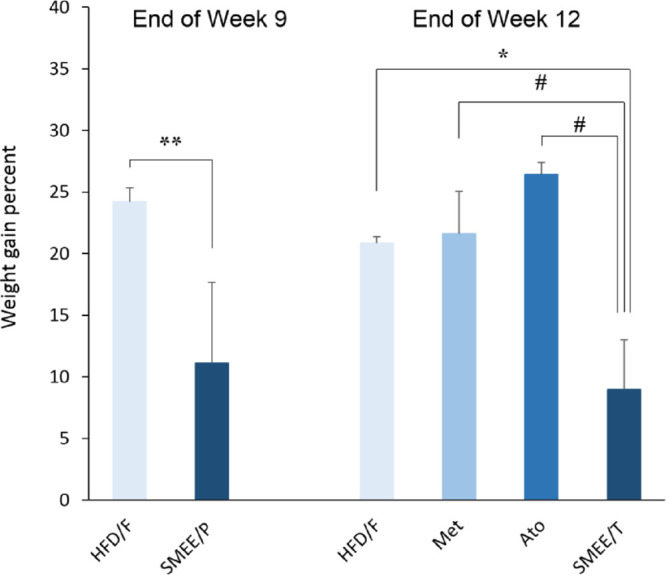
Effect of S. marianum extracts on the percent weight gain in rats with induced metabolic syndrome. Values are expressed as mean ± SEM; * p < 0.05 and ** p < 0.001 vs untreated HFD/F control group; # p < 0.005 vs positive (Met and Ato) control groups.
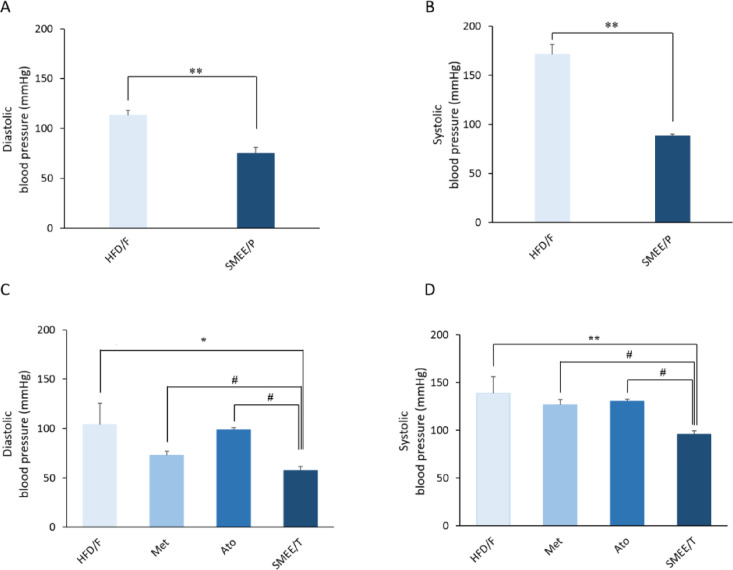
Effect of S. marianum Ethyl Acetate Extracts on Blood Pressure
Blood pressure (SBP and DBP) was significantly decreased (p < 0.001) in the preventive group SMEE/P compared to the HFD/F control as shown in Figure 7A,B. Rats in the treatment group SMEE/T also showed a significant decrease in SBP and DBP compared to both positive controls (Met and Ato) and to untreated HFD/F control groups (Figure 7C,D).
Figure 7.
Effect of S. marianum seed extracts on blood pressure in rats with induced metabolic syndrome. Values are expressed as mean ± SEM. (A) Preventive effect (DBP); (B) preventive effect (SBP); (C) therapeutic effect (DBP); and (D) therapeutic effect (SBP). * p < 0.05 and ** p < 0.001 vs untreated HFD/F control group; # p < 0.005 vs positive (Met and Ato) control groups.
Discussion
MetS, a common disease with an increasing incidence worldwide, involves hyperglycemia, insulin resistance, dyslipidemia, hepatic steatosis, obesity, and atherogenic events. Genetic and environmental factors work together to induce the onset of MetS.37 Long-term high-fat consumption is the environmental component that has been reported to be the main leading cause of MetS.38,39 Moreover, a diet rich in fructose can lead to insulin resistance, hyperinsulinemia, hypertriglyceridemia, and other symptoms of MetS in both humans and experimental rats resulting in the development of type 2 diabetes and cardiovascular diseases.40
In this study, we have prepared and characterized a novel S. marianum seed ethyl acetate extract (SMEE). The preventive and therapeutic effects of SMEE were investigated in vivo in a MetS induced rat model. SMEE administration led to a significant reduction in TG levels in rats with established MetS compared to untreated MetS-induced rats. This outcome aligns with the potential lipid-lowering effects of silymarin, as reported in published studies.41,42 However, it is important to note that the extent of TG reduction and the underlying mechanisms may vary depending on the specific composition of the extract.
In alloxan-induced diabetic rats, with a 5 week regimen of silybin (200 mg/kg), a significant reduction in TG levels near the level of those of healthy controls had been observed.43 The mechanism behind the effects of silybin on TG is probably through induction of the fatty acid β-oxidation pathway. Additionally,24 it has been hypothesized that silymarin may reduce the liver’s capacity to produce TG, and silymarin-treated rats had lower plasma levels of TG compared to hyperlipidemia and negative control animals.34,44
S. marianum extracts had previously shown antioxidant activities by increasing the activity of antioxidant enzymes like superoxide dismutase and catalase and increasing intracellular and liver glutathione levels and scavenging free radicals, thereby exerting hepatoprotective effects.45 In another study, silymarin was found to improve renal function, and it protected the liver and pancreas against protein damage without affecting hyperglycemia in diabetic animals.46 Also, it was demonstrated that this herbal plant can improve lipid profiles by decreasing the oxidative stress parameters that are elevated in hypercholesterolemia and by inhibiting lipid-peroxidation enzymes through flavonolignan constituents.47
SMEE demonstrated preventive and therapeutic effects on glucose levels, as evidenced by lower FBS levels and improved results in OGTT. These findings are consistent with the antidiabetic potential of S. marianum extracts observed in previous studies.48,49 It is noteworthy that SMEE performed well even in comparison to metformin, a well-established antidiabetic drug. In addition, SMEE exhibited preventive effects against weight gain in the preventive group and therapeutic effects on lowering weight in the therapeutic group. Weight management is a crucial aspect of MetS, and similar effects have been reported for silymarin in preventing obesity-related issues.50 Furthermore, the blood pressure-lowering effects of SMEE align with the potential cardiovascular benefits of silymarin constituents, which have been reported in studies investigating its antioxidant and hepatoprotective properties.50
Silymarin, the main extract of S. marianum, has been extensively investigated in the literature as documented in recent reviews.51−54 However, these studies mainly focused on silybin, the main component of silymarin, ignoring the effect of other constituents.43 Additionally, the composition of silymarin is often unknown and can vary to a certain extent depending on the chemovariety of the plant, climatic conditions during plant growth, and processing conditions.
Using an established LC–MS analysis protocol,30 the SMEE prepared and investigated in the present study was found to be rich in silydianin and silychristin (51.2%), whereas silybin was found to be present in a much lower amount (<10%). It is worth mentioning that all published studies on S. marianum extracts have been focusing on silymarin that is rich in silybin (50%).23−28,30,33,43−47,51−57 Our findings may hint at yet unexplored effects of silydianin and silychristin, and therefore, more research should be concentrated on these understudied flavonolignans.
There is limited research specifically on silydianin and silychristin in the context of MetS. However, these compounds may share the same mechanisms of action of silybin, as they belong to the same class of flavonolignans. Probably, their expected antioxidant properties help to neutralize harmful free radicals, reduce oxidative stress, enhance insulin sensitivity, and protect against metabolic abnormalities.58 On the other hand, they may exhibit anti-inflammatory properties by suppressing the production of proinflammatory cytokines and enzymes that help mitigate inflammation in MetS;59,60 at the same time, they may affect lipid metabolism and help reduce LDL cholesterol and TG levels.50
However, this study highlights the multifaceted therapeutic potential of S. marianum, and more research and clinical trials are needed to confirm the safety and efficacy of SMEE. Additionally, it confirms that not only silybin but also other silymarin constituents such as silydianin and silychristin should be further investigated and could be key players in the pharmacological activities of silymarin and other S. marianum extracts.
Conclusions
In conclusion, an ethyl acetate extract of S. marianum seeds (SMEE) rich in silydianin and silychristin (51.2%) showed triglyceride-lowering effects, antihypertensive activity, positive effects on body weight (reduction compared to controls), and improved blood glucose levels in OGTT in both preventive and therapeutic groups in MetS-induced rats. This study highlights the effect of silydianin and silychristin among other flavonolignans found in S. marianum and validates its traditional use not only for the treatment but also for the prevention of MeS. In future studies, silydianin and silychristin deserve further investigation, as single molecules and in combination, for the prevention and treatment of MetS-associated disorders.
Glossary
ABBREVIATIONS
- Ato
atorvastatin
- AUC
area under the curve
- BW
body weight
- CMC
carboxymethylcellulose
- DAD
diode array detector
- DBP
diastolic blood pressure
- FBS
fasting blood sugar
- HDL
high-density lipoprotein
- HFD
high-fat diet
- HFD/F
high-fat, high-fructose diet
- HPLC-DAD
high-performance liquid chromatography with diode array detection
- IDF
International Diabetes Foundation
- LC–MS
liquid chromatography–mass spectrometry
- LC-MSD
liquid chromatography–mass detector
- LDL
low-density lipoprotein
- Met
metformin
- MetS
metabolic syndrome
- NCEP ATP III
The National Cholesterol Education Program Adult Treatment Panel
- ND
normal diet
- OECD
Organization for Economic Cooperation and Development
- OGTT
oral glucose tolerance test
- Rt
retention time
- SBP
systolic blood pressure
- SEM
standard error of mean
- SMEE
S. marianum ethyl acetate extract
- SMEE/P
S. marianum ethyl acetate extract/preventive
- SMEE/T
S. marianum ethyl acetate extract/treatment
- TC
total cholesterol
- TG
triglycerides
- WHO
World Health Organization
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This research was funded by the Arab–German Young Academy of Sciences and Humanities (AGYA) grants (01DL16002 and 01DL20003).
The authors declare no competing financial interest.
References
- Després J. P.; Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- González-Muniesa P.; Mártinez-González M. A.; Hu F. B.; Després J. P.; Matsuzawa Y.; Loos R. J. F.; Moreno L. A.; Bray G. A.; Martinez J. A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. 10.1038/nrdp.2017.34. [DOI] [PubMed] [Google Scholar]
- Hayden M. R. Overview and new insights into the metabolic syndrome: Risk factors and emerging variables in the development of type 2 diabetes and cerebrocardiovascular disease. Medicina 2023, 59, 561. 10.3390/medicina59030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świątkiewicz I.; Wróblewski M.; Nuszkiewicz J.; Sutkowy P.; Wróblewska J.; Woźniak A. The role of oxidative stress enhanced by adiposity in cardiometabolic diseases. Int. J. Mol. Sci. 2023, 24, 6382. 10.3390/ijms24076382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill S.; O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- Alberti K. G.; Zimmet P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. . [DOI] [PubMed] [Google Scholar]
- Janus E. D.; Watt N. M.; Lam K. S.; Cockram C. S.; Siu S. T.; Liu L. J.; Lam T. H. The prevalence of diabetes, association with cardiovascular risk factors and implications of diagnostic criteria (ADA 1997 and WHO 1998) in a 1996 community-based population study in Hong Kong Chinese. Hong Kong Cardiovascular Risk Factor Steering Committee. American Diabetes Association. Diabet. Med. 2000, 17, 741–745. 10.1046/j.1464-5491.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- Zimmet P. M. M.; Alberti K. G.; Serrano Ríos M.. A new international diabetes federation worldwide definition of the metabolic syndrome: the rationale and the results. Rev. Esp. Cardiol. 2005, 58, 1371–1376. Rev. Esp. Cardiol. 2005, (58), , 1371–1375. doi: 10.1016/S1885-5857(06)60742-1. [DOI] [PubMed] [Google Scholar]
- Bray G. A.; Popkin B. M. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: health be damned!. Pour on the sugar. Diabetes Care. 2014, 37, 950–956. 10.2337/dc13-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V. T.; Shulman G. I. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012, 148, 852–871. 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G.; Cassader M.; Rosina F.; Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012, 55, 885–904. 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- Taylor S. I. The high cost of diabetes drugs: Disparate impact on the most vulnerable patients. Diabetes Care 2020, 43, 2330–2332. 10.2337/dci20-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe P.; Mathangasinghe Y.; Jayawardena R.; Hills A. P.; Misra A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: a systematic review. BMC Public Health 2017, 17, 1–9. 10.1186/s12889-017-4041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri S.; Sahoo J.; Roy A.; Kamalanathan S.; Naik D. Treatment on Nature’s lap: Use of herbal products in the management of hyperglycemia. World J. Diabetes. 2023, 14, 412–423. 10.4239/wjd.v14.i4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W. H.WHO traditional medicine strategy: 2014–2023. World Health Organization, 2022, https://apps.who.int/gb/ebwha/pdf_files/EB152/B152_37-en.pdf, accessed on 19/09/2023. [Google Scholar]
- Zhang Z.; Li X.; Sang S.; McClements D. J.; Chen L.; Long J.; Jiao A.; Jin Z.; Qiu C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. 10.3390/foods11152189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola S.; Scotto d’Abusco A. Nutraceuticals and the Network of Obesity Modulators. Nutrients 2022, 14, 5099. 10.3390/nu14235099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alawi R. A.; Al-Mashiqri J. H.; Al-Nadabi J. S. M.; Al-Shihi B. I.; Baqi Y. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Front. Plant Sci. 2017, 8, 845. 10.3389/fpls.2017.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Castaneda C. R.; Altamirano-Lamarque F.; Ortega-Macías A. G.; Santa Cruz-Pavlovich F. J.; Gonzalez-De la Rosa A.; Armendariz-Borunda J.; Santos A.; Navarro-Partida J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. 10.3390/nu14235014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Alawi R.; Alhamdani M. S. S.; Hoheisel J. D.; Baqi Y. Antifibrotic and tumor microenvironment modulating effect of date palm fruit (Phoenix dactylifera L.) extracts in pancreatic cancer. Biomed. Pharmacother. 2020, 121, 109522. 10.1016/j.biopha.2019.109522. [DOI] [PubMed] [Google Scholar]
- Atanasov A. G.; Zotchev S. B.; Dirsch V. M.; Supuran C. T. International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discovery 2021, 20, 200–216. 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Pradhan S. C.; Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006, 124, 491–504. [PubMed] [Google Scholar]
- Tajmohammadi A.; Razavi B. M.; Hosseinzadeh H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phyther. Res. 2018, 32, 1933–1949. 10.1002/ptr.6153. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang Z.; Wu S. C. Health benefits of Silybum marianum: Phytochemistry, pharmacology, and applications. J. Agric. Food Chem. 2020, 68, 11644–11664. 10.1021/acs.jafc.0c04791. [DOI] [PubMed] [Google Scholar]
- Abenavoli L.; Capasso R.; Milic N.; Capasso F. Milk thistle in liver diseases: past, present, future. Phyther. Res. 2010, 24, 1423–1432. 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- European Union herbal monograph on Silybum marianum (L.) Gaertn., fructus. EMA/HMPC/294187/2012. Accessed 02.08.2023 https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-silybum-marianum-l-gaertn-fructus_en-0.pdf.
- Chambers C. S.; Holečková V.; Petrásková L.; Biedermann D.; Valentová K.; Buchta M.; Křen V. The silymarin composition··· and why does it matter???. Food Res. Int. 2017, 100, 339–353. 10.1016/j.foodres.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Juee L. Y. M.; Naqishbandi A. M. Calabash (Lagenaria siceraria) potency to ameliorate hyperglycemia and oxidative stress in diabetes. J. Funct. Foods 2020, 66, 103821. 10.1016/j.jff.2020.103821. [DOI] [Google Scholar]
- Petrásková L.; Káňová K.; Biedermann D.; Křen V.; Valentová K. Simple and rapid HPLC separation and quantification of flavonoid, flavonolignans, and 2, 3-dehydroflavonolignans in silymarin. Foods 2020, 9, 116. 10.3390/foods9020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. S. A.; Lakshman K.; Jayaveera K. N.; Shekar D. S.; Kumar A. A.; Manoj B. Antioxidant and antipyretic properties of methanolic extract of Amaranthus spinosus leaves. Asian Pac. J. Trop. Med. 2010, 3, 702–706. 10.1016/S1995-7645(10)60169-1. [DOI] [Google Scholar]
- Gancheva S.; Zhelyazkova-Savova M.; Galunska B.; Chervenkov T. Experimental models of metabolic syndrome in rats. Scr. Sci. Medica 2015, 47, 14–21. 10.14748/ssm.v47i2.1145. [DOI] [Google Scholar]
- Chen I.; Chen Y.; Chou C.; Chuang R.; Sheen L.; Chiu C. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J. Sci. Food Agric. 2012, 92, 1441–1447. 10.1002/jsfa.4723. [DOI] [PubMed] [Google Scholar]
- Wang J. H.; Bose S.; Shin N. R.; Chin Y. W.; Choi Y. H.; Kim H. Pharmaceutical impact of Houttuynia Cordata and metformin combination on high-fat-diet-induced metabolic disorders: Link to intestinal microbiota and metabolic endotoxemia. Front. Endocrinol. 2018, 9, 620. 10.3389/fendo.2018.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli E. L. B.; Diniz Y. S.; Galhardi C. M.; Ebaid G. M. X.; Rodrigues H. G.; Mani F.; Fernandes A. A. H.; Cicogna A. C.; Novelli Filho J. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- Bhandari U.; Kumar V.; Khanna N.; Panda B. P. The effect of high-fat diet-induced obesity on cardiovascular toxicity in Wistar albino rats. Hum. Exp. Toxicol. 2011, 30, 1313–1321. 10.1177/0960327110389499. [DOI] [PubMed] [Google Scholar]
- Fahed G.; Aoun L.; Bou; Zerdan M.; Allam S.; Bou Zerdan M.; Bouferraa Y.; Assi H. I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani P.; Mirzaei S.; Asadi A.; Akhlaghi M.; Saneei P. Nutrient patterns in relation to metabolic health status in overweight and obese adolescents. Sci Rep 2023, 13, 119. 10.1038/s41598-023-27510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi H.; Farrokhi F. R.; Butler A. E.; Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- Kourek C.; Karatzanos E.; Raidou V.; Papazachou O.; Philippou A.; Nanas S.; Dimopoulos S. Effectiveness of high intensity interval training on cardiorespiratory fitness and endothelial function in type 2 diabetes: A systematic review. World J. Cardiol. 2023, 15, 184–199. 10.4330/wjc.v15.i4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formisano E.; Pasta A.; Cremonini A. L.; Favari E.; Ronca A.; Carbone F.; Semino T.; Di Pierro F.; Sukkar S. G.; Pisciotta L. Efficacy of nutraceutical combination of monacolin K, berberine, and silymarin on lipid profile and PCSK9 plasma level in a cohort of hypercholesterolemic patients. J. Med. Food. 2020, 23, 658–666. 10.1089/jmf.2019.0168. [DOI] [PubMed] [Google Scholar]
- Baldini F.; Portincasa P.; Grasselli E.; Damonte G.; Salis A.; Bonomo M.; Florio M.; Serale N.; Voci A.; Gena P.; Vergani L.; Calamita G. Aquaporin-9 is involved in the lipid-lowering activity of the nutraceutical silybin on hepatocytes through modulation of autophagy and lipid droplets composition. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids. 2020, 1865, 158586. 10.1016/j.bbalip.2019.158586. [DOI] [PubMed] [Google Scholar]
- Kazazis C. E.; Evangelopoulos A. A.; Kollas A.; Vallianou N. G. The therapeutic potential of milk thistle in diabetes. Rev. Diabet. Stud. 2014, 11, 167. 10.1900/RDS.2014.11.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F.; Gao F.; Zhou S.; Wang L. The therapeutic effects of silymarin for patients with glucose/lipid metabolic dysfunction: A meta-analysis. Medicine 2020, 99, e22249 10.1097/MD.0000000000022249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghonaim A. H.; Hopo M. G.; Ismail A. K.; AboElnaga T. R.; Elgawish R. A.; Abdou R. H.; Elhady K. A. Hepatoprotective and renoprotective effects of silymarin against salinomycin-induced toxicity in adult rabbits. Vet. World. 2022, 15, 2244–2252. 10.14202/vetworld.2022.2244-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda L. M. O.; Agostini L. D. C.; Lima W. G.; Camini F. C.; Costa D. C. Silymarin attenuates hepatic and pancreatic redox imbalance independent of glycemic regulation in the alloxan-induced diabetic rat model. Biomed. Environ. Sci. 2020, 33, 690–700. 10.3967/bes2020.090. [DOI] [PubMed] [Google Scholar]
- Doostkam A.; Fathalipour M.; Anbardar M. H.; Purkhosrow A.; Mirkhani H.; Caspa Gokulan R. Therapeutic effects of milk thistle (Silybum marianum L.) and artichoke (Cynara scolymus L.) on nonalcoholic fatty liver disease in type 2 diabetic rats. Can. J. Gastroenterol. Hepatol. 2022, 2022, 1. 10.1155/2022/2868904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Avelar C. R.; Nunes B. V. C.; da Silva Sassaki B.; Santos Dos; Vasconcelos M.; de Oliveira L. P. M.; Lyra A. C.; Bueno A. A.; de Jesus R. P. Efficacy of silymarin in patients with non-alcoholic fatty liver disease - the Siliver trial: a study protocol for a randomized controlled clinical trial. Trials. 2023, 24, 177. 10.1186/s13063-023-07210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengesha T.; Gnanasekaran N.; Mehare T. Hepatoprotective effect of silymarin on fructose induced nonalcoholic fatty liver disease in male albino wistar rats. BMC Complement Med. Ther. 2021, 21, 104. 10.1186/s12906-021-03275-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilagavathi R.; Begum S. S.; Varatharaj S. D.; Balasubramaniam A. K.; George J. S.; Selvam C. Recent insights into the hepatoprotective potential of medicinal plants and plant-derived compounds. Phytother. Res. 2023, 37, 2102–2118. 10.1002/ptr.7821. [DOI] [PubMed] [Google Scholar]
- Singh M.; Kadhim M. M.; Turki Jalil A.; Oudah S. K.; Aminov Z.; Alsaikhan F.; Jawhar Z. H.; Ramírez-Coronel A. A.; Farhood B. A systematic review of the protective effects of silymarin/silibinin against doxorubicin-induced cardiotoxicity. Cancer Cell Int. 2023, 23, 88. 10.1186/s12935-023-02936-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latacela G. A.; Ramaiah P.; Patra I.; Jalil A. T.; Gupta R.; Madaminov F. A.; Shaker Shafik S.; Al-Gazally M. E.; Ansari M. J.; Kandeel M.; Mustafa Y. F.; Farhood B. The radioprotective potentials of silymarin/silibinin against radiotherapy-induced toxicities: A systematic review of clinical and experimental studies. Curr. Med. Chem. 2023, 30, 3775–3797. 10.2174/0929867330666221124155339. [DOI] [PubMed] [Google Scholar]
- Khazaei R.; Seidavi A.; Bouyeh M. A review on the mechanisms of the effect of silymarin in milk thistle (Silybum marianum) on some laboratory animals. Vet. Med. Sci. 2022, 8, 289–301. 10.1002/vms3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleymani S.; Ayati M. H.; Mansourzadeh M. J.; Namazi N.; Zargaran A. The effects of Silymarin on the features of cardiometabolic syndrome in adults: A systematic review and meta-analysis. Phytother. Res. 2022, 36, 842–856. 10.1002/ptr.7364. [DOI] [PubMed] [Google Scholar]
- Feng B.; Meng R.; Huang B.; Shen S.; Bi Y.; Zhu D. Silymarin alleviates hepatic oxidative stress and protects against metabolic disorders in high-fat diet-fed mice. Free Radic. Res. 2016, 50, 314–327. 10.3109/10715762.2015.1116689. [DOI] [PubMed] [Google Scholar]
- Orolin J.; Večeřa R.; Jung D.; Meyer U. A.; Škottová N.; Anzenbacher P. Hypolipidemic effects of silymarin are not mediated by the peroxisome proliferator-activated receptor alpha. Xenobiotica 2007, 37, 725–735. 10.1080/00498250701463333. [DOI] [PubMed] [Google Scholar]
- Cacciapuoti F.; Scognamiglio A.; Palumbo R.; Forte R.; Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J. Hepatol. 2013, 5, 109–113. 10.4254/wjh.v5.i3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos J. E.; Kalogerinis P. T.; Milanov V.; Kalogerinis C. T.; Poulos E. J. The effects of vitamin E, silymarin and carnitine on the metabolic abnormalities associated with nonalcoholic liver disease. J. Diet. Suppl. 2022, 19, 287–302. 10.1080/19390211.2021.1874587. [DOI] [PubMed] [Google Scholar]
- DuBreuil D. M.; Lai X.; Zhu K.; Chahyadinata G.; Perner C.; Chiang B. M.; Battenberg A.; Sokol C. L.; Wainger B. J. Phenotypic screen identifies the natural product silymarin as a novel anti-inflammatory analgesic. Mol. Pain. 2023, 19, 17448069221148351. 10.1177/17448069221148351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. Y.; Liu S.; Yang M. Antioxidant and anti-inflammatory agents in chronic liver diseases: Molecular mechanisms and therapy. World J. Hepatol. 2023, 15, 180–200. 10.4254/wjh.v15.i2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]