Abstract
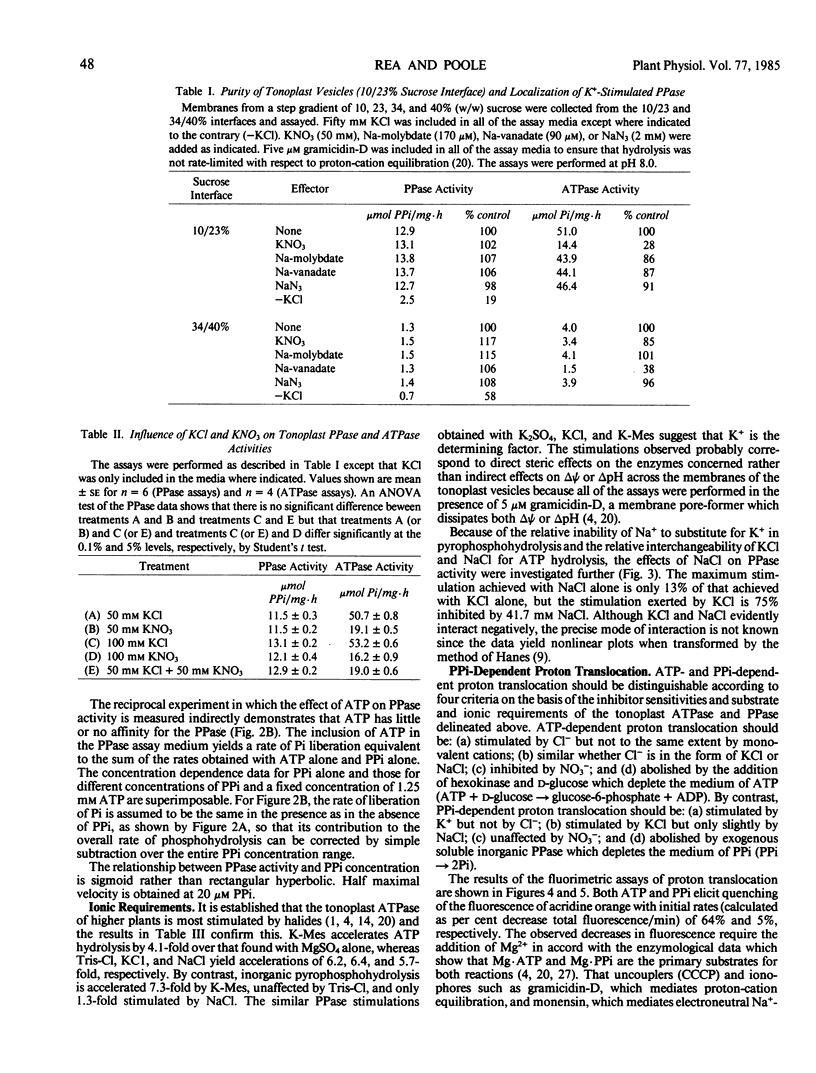
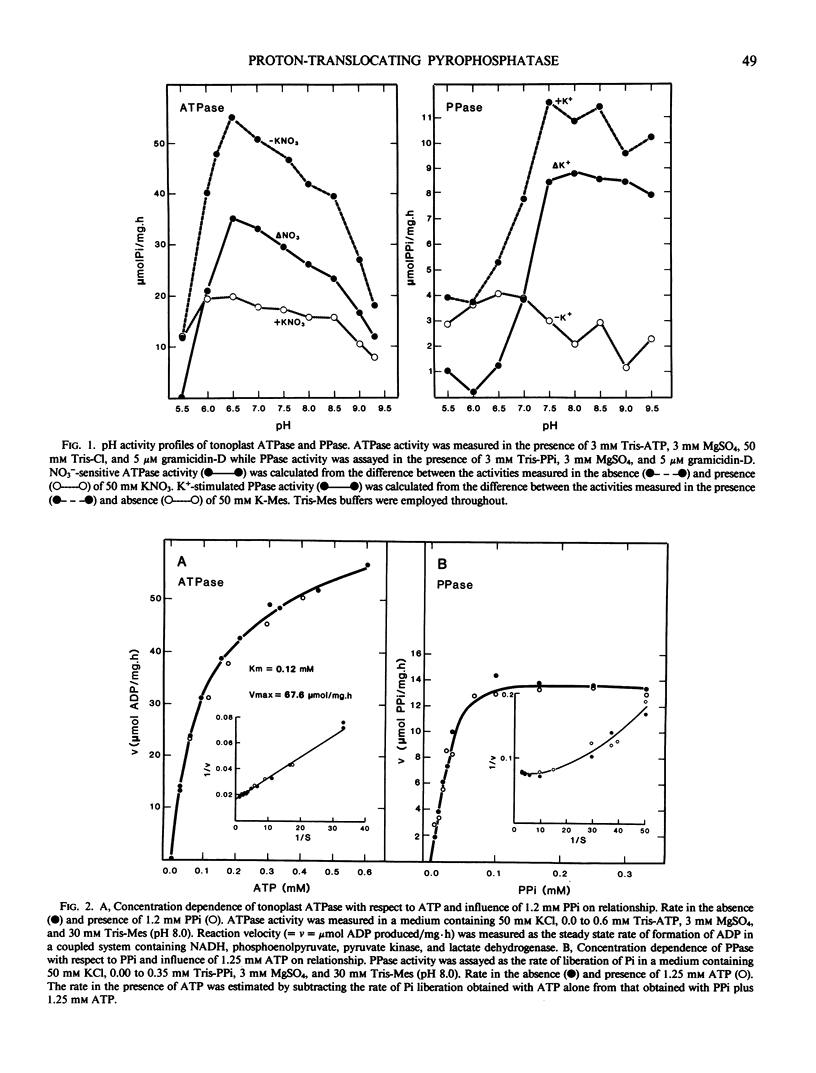
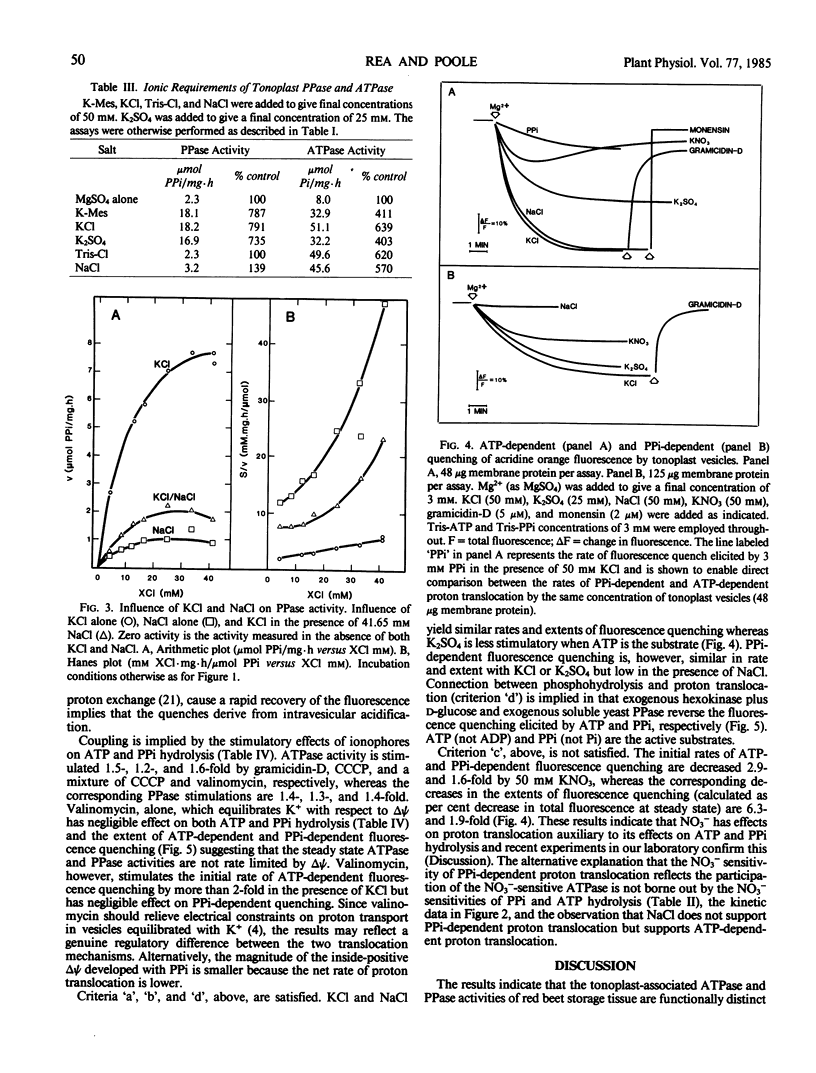
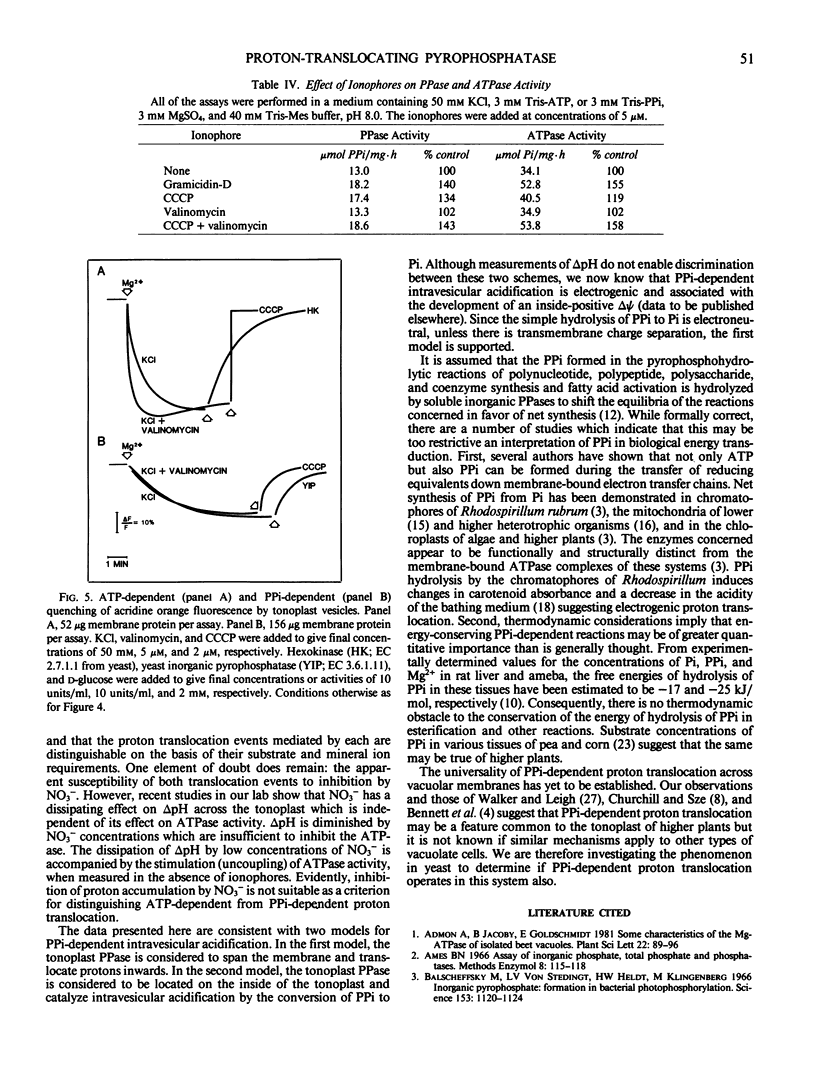
The substrate and ionic requirements of ATP and inorganic pyrophosphate (PPi) hydrolysis by tonoplast vesicles isolated from storage tissue of red beet (Beta vulgaris L.) were compared with the requirements of ATP-and PPi-dependent proton translocation by the same material. Both ATP hydrolysis and ATP-dependent proton translocation are most stimulated by Cl− and inhibited by NO3−. NaCl and KCl support similar rates of ATP hydrolysis and ATP-dependent proton translocation while K2SO4 supports lesser rates for both. PPi hydrolysis and PPi-dependent proton translocation are most stimulated by K+. KCl and K2SO4 support similar rates of PPi hydrolysis and PPi-dependent proton translocation but NaCl has only a small stimulatory effect on both. Since PPi does not inhibit ATP hydrolysis and ATP does not interfere with PPi hydrolysis, it is inferred that the two phosphohydrolase and proton translocation activities are mediated by different tonoplast-associated enzymes. The results indicate the presence of an energy-conserving proton-translocating pyrophosphatase in the tonoplast of red beet.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltscheffsky H., Von Stedingk L. V., Heldt H. W., Klingenberg M. Inorganic pyrophosphate: formation in bacterial photophosphorylation. Science. 1966 Sep 2;153(3740):1120–1122. doi: 10.1126/science.153.3740.1120. [DOI] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H., Fleron P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of (Mg2+), (K+), and ionic strength determined from equilibrium studies of the reaction. J Biol Chem. 1974 Jun 10;249(11):3465–3474. [PubMed] [Google Scholar]
- Karlsson J. Membrane-bound potassium and magnesium ion-stimulated inorganic pyrophosphatase from roots and cotyledons of sugar beet (Beta vulgaris L). Biochim Biophys Acta. 1975 Aug 13;399(2):356–363. doi: 10.1016/0304-4165(75)90264-0. [DOI] [PubMed] [Google Scholar]
- Leigh R. A., Branton D. Isolation of Vacuoles from Root Storage Tissue of Beta vulgaris L. Plant Physiol. 1976 Nov;58(5):656–662. doi: 10.1104/pp.58.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansurova S. E., Shakhov Y. A., Belyakova T. N., Kulaev I. S. Synthesis of inorganic pyrophosphate by animal tissue mitochondria. FEBS Lett. 1975 Jul 15;55(1):94–98. doi: 10.1016/0014-5793(75)80967-7. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell R., Mitchell P. Proton-translocating pyrophosphatase of Rhodospirillum rubrum. FEBS Lett. 1972 Jun 15;23(2):233–236. doi: 10.1016/0014-5793(72)80349-1. [DOI] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. Action and Inhibition of Endogenous Phospholipases during Isolation of Plant Membranes. Plant Physiol. 1978 Dec;62(6):933–937. doi: 10.1104/pp.62.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]


