Abstract
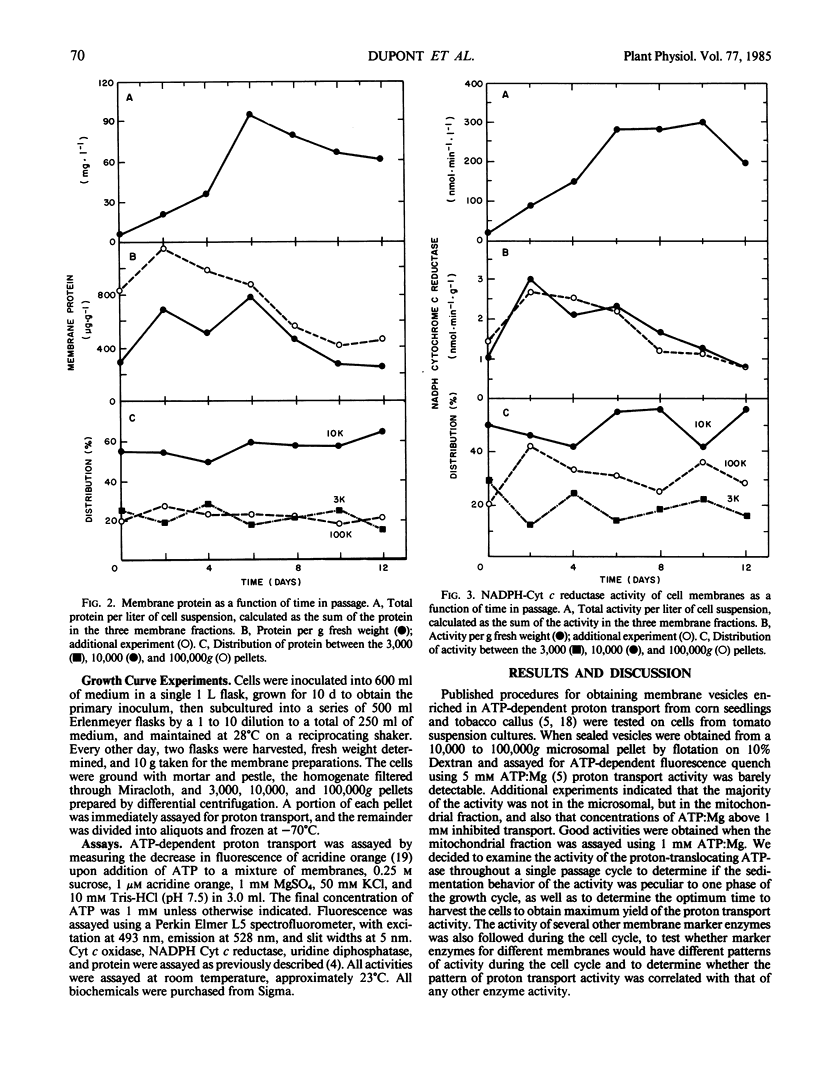
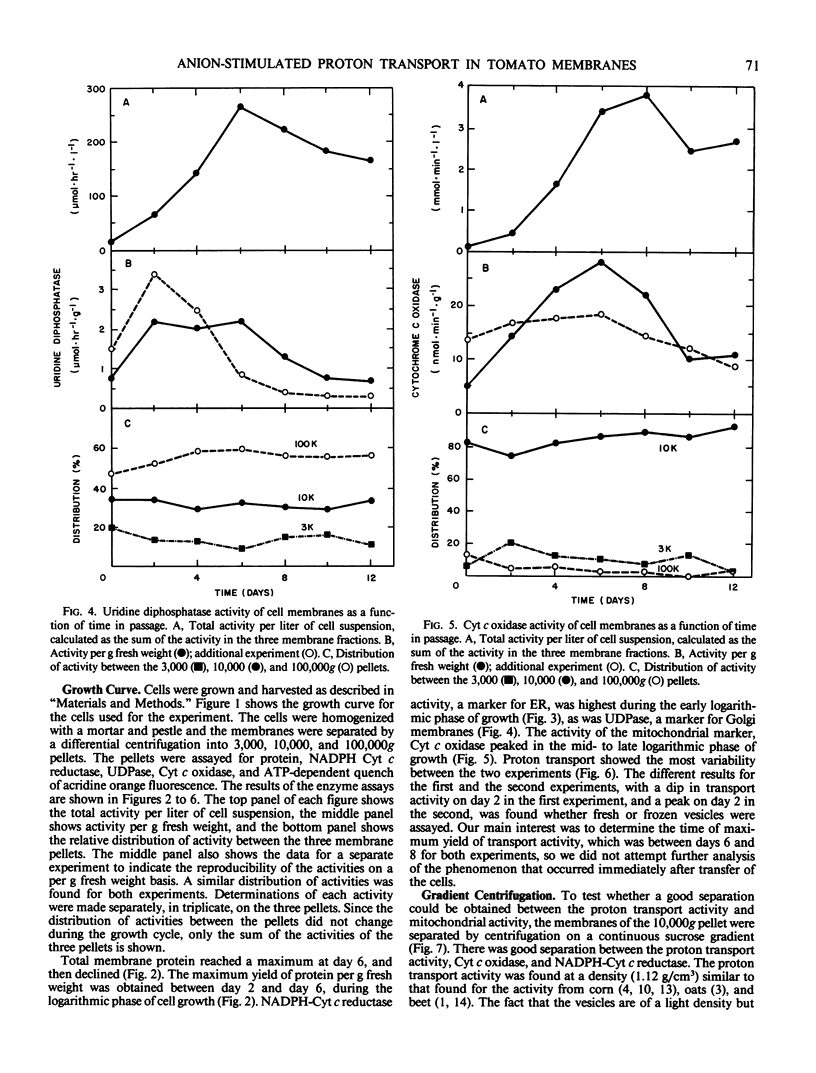
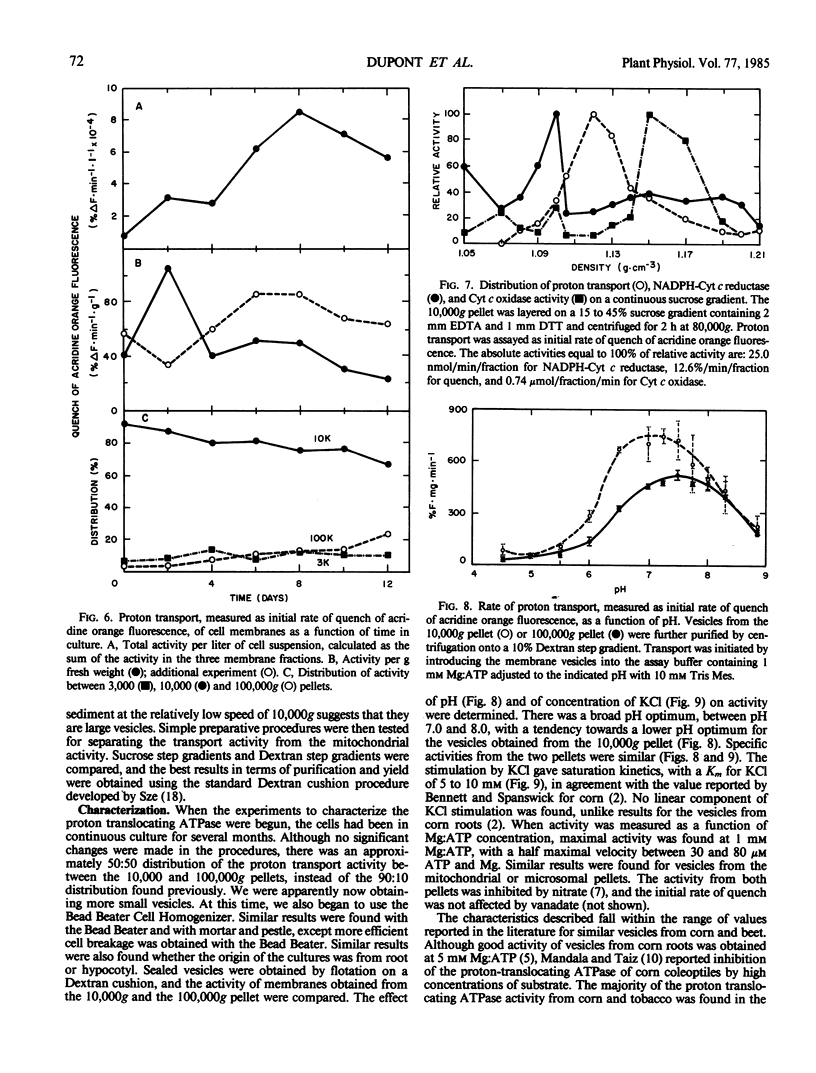
Membranes enriched in ATP-dependent proton transport were prepared from suspension cultures of tomato cells (Lycopersicon esculentum Mill cv VF36). Suspension cultures were a source of large quantities of membranes from rapidly growing, undifferentiated cells. Proton transport activity was assayed as quench of acridine orange fluorescence. The activity of the proton translocating ATPase and of several other membrane enzymes was measured as a function of the cell culture cycle. The relative distribution of the enzymes between the 3,000, 10,000, and 100,000g pellets remained the same throughout the cell culture cycle, but yield of total activity and activity per gram fresh weight with time had a unique profile for each enzyme tested. Maximal yield of the proton translocating ATPase activity was obtained from cells in the middle logarithmic phase of growth, and from 50 to 90% of the activity was found in the 10,000g pellet. The proton translocating ATPase activity was separable from NADPH cytochrome c reductase and cytochrome c oxidase on a sucrose gradient. Proton transport activity had a broad pH optimum (7.0-8.0), was stimulated by KCl with a Km of 5 to 10 millimolar, stimulation being due to the anion, Cl−, and not the cation, K+, and was not inhibited by vanadate, but was inhibited by NO3−. The activity is tentatively identified as the tonoplast ATPase.
Full text
PDF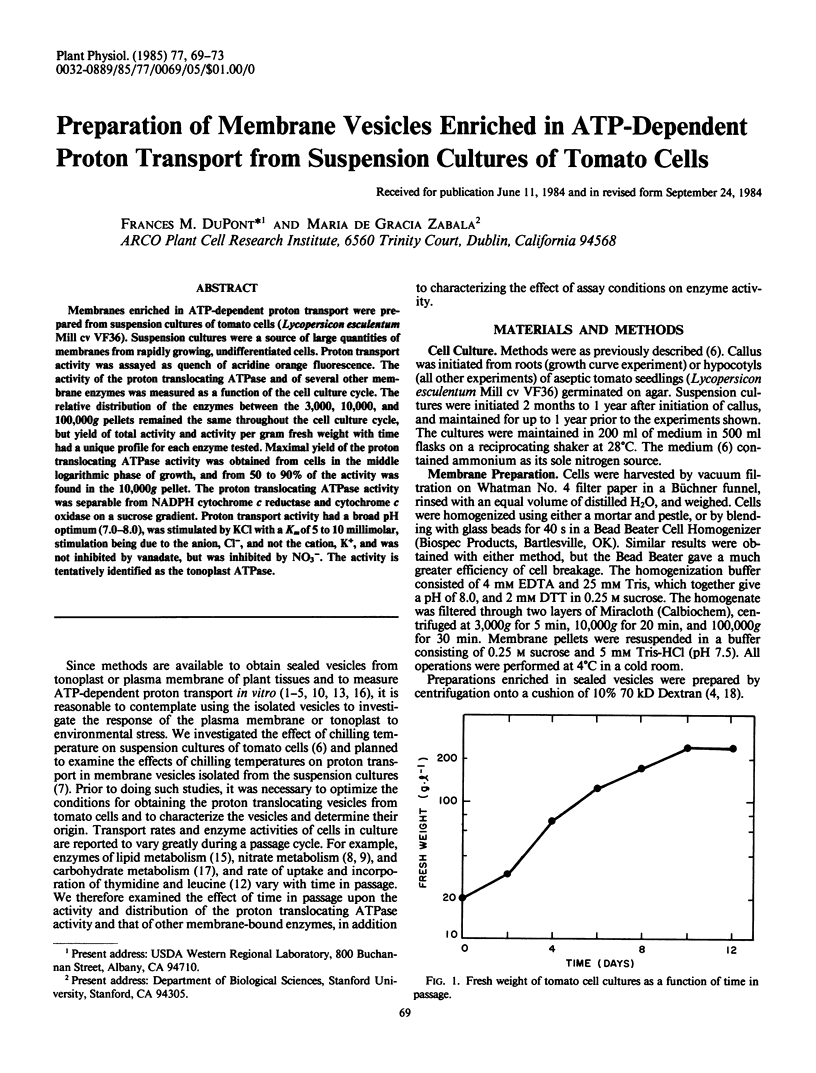
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Mudd J. B. Acclimation to low temperature by microsomal membranes from tomato cell cultures. Plant Physiol. 1985 Jan;77(1):74–78. doi: 10.1104/pp.77.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Staraci L. C., Chou B., Thomas B. R., Williams B. G., Mudd J. B. Effect of Chilling Temperatures upon Cell Cultures of Tomato. Plant Physiol. 1985 Jan;77(1):64–68. doi: 10.1104/pp.77.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelker H. C., Filner P. Regulation of nitrite reductase and its relationship to the regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1971 Oct;252(1):69–82. doi: 10.1016/0304-4165(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Moore T. S., Beevers H. Isolation and characterization of organelles from soybean suspension cultures. Plant Physiol. 1974 Feb;53(2):261–265. doi: 10.1104/pp.53.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Kinetic properties of the KCl transport at the secreting apical membrane of the oxyntic cell. J Membr Biol. 1983;71(3):195–207. doi: 10.1007/BF01875461. [DOI] [PubMed] [Google Scholar]



