Abstract
Background
JianPi QingRe HuaYu Methods (JQH) have been long used to treat chronic atrophic gastritis (CAG) and precancerous lesions of gastric cancer (PLGC). However, whether JQH can inhibit the transformation of gastritis to gastric cancer (GC) remains unclear.
Methods
Herein, we first retrieved the active ingredients and targets of JQH from the TCMSP database and the targets related to the gastric inflammation-cancer transformation from public databases. Differentially expressed genes (DEGs) related to gastric inflammation-cancer transformation were identified from the Gene Expression Omnibus (GEO) database. Then, we obtained the potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation by intersecting drugs and disease targets. The Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and protein–protein interaction (PPI) analyses of the potential therapeutic targets were conducted using R software. Next, we conducted molecular docking and in vitro experiments to validate our results.
Results
We obtained 214 potential therapeutic targets of JQH by intersecting drugs and disease targets. We found that the potential mechanisms of JQH in treating gastric inflammation-cancer transformation might be related to JAK-STAT, Wnt, p53 and VEGF signaling pathways. The molecular docking indicated that quercetin, as the main active ingredient of JQH, might inhibit gastric inflammation-cancer transformation by binding with specific receptors. Our experimental results showed that quercetin inhibited cells proliferation (P < 0.001), promoted cell apoptosis (P < 0.001), reduced the secretion of pro-inflammatory cytokines (P < 0.001) and promoted the secretion of anti-inflammatory cytokines (P < 0.001) in MNNG-induced GES-1 cells. Furthermore, quercetin inhibited cells proliferation (P < 0.001) and reduced mRNA and protein level of markers of PLGC (P < 0.001) in CDCA-induced GES-1 cells.
Conclusion
These results provide the material basis and regulatory mechanisms of JQH in treating gastric inflammation-cancer transformation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-023-04232-0.
Keywords: JianPi QingRe HuaYu Methods, Chronic atrophic gastritis, Inflammation-cancer transformation, Quercetin
Introduction
Chronic atrophic gastritis (CAG) is a common gastrointestinal disease characterized by mucosa atrophy, exposed vessels, and mucosal nodules [1]. Correa cascade proposed that CAG often develops into intestinal gastric cancer (GC) in the pathogenesis of disease evolution [2]. A recent study has suggested that Helicobacter pylori (Hp) infection plays a vital role in this process [3]. Hp eradication in patients with gastritis can prevent the occurrence of intestinal GC to some extent [4]. However, 34-54% of patients with atrophic gastritis develop GC even after Hp eradication [5]. Thus, finding effective drugs to inhibit gastric inflammation-cancer transformation is urgent.
Traditional Chinese Medicine (TCM) are complementary and alternative medicine in treating gastrointestinal diseases and has specific advantages compared to Western medicine, such as less drug dependence and side effects [6]. JianPi QingRe HuaYu, a frequently-used Traditional Chinese Medicine, is often used for treatment of CAG and precancerous lesions of gastric cancer (PLGC) in clinic [7]. JQH is composed of Astragalus membranaceus (Fisch.) Bunge (Huangqi), Atractylodes macrocephala Koidz (Baizhu), Citrus aurantium L. (Zhike), Hedyotis diffusa Willd. (Baihuasheshecao), Scutellaria barbata D. Don (Bazhilian), and Curcuma zedoaria (Christm.) Rosc. (Ezhu) (Table 1). Our clinical studies have shown that JQH could significantly ameliorate digestive tract symptoms and pathological conditions of gastric mucosal and inflammatory levels in patients with CAG or PLGC [7, 8]. In experimental animal studies, JQH improved the pathological condition of gastric mucosa and delayed the development of intestinal metaplasia or atypical hyperplasia by suppressing NF-κB pathway activation in CAG rats [9, 10]. However, whether JQH inhibits CAG from proceeding to GC (gastric inflammation-cancer transformation) remains unclear.
Table 1.
Details of the ingredients of JianPi QingRe HuaYu Methods
| Latin binomial nomenclature | Family | Medicinal parts | Chinese name |
|---|---|---|---|
| Astragalus membranaceus (Fisch.) Bunge | Leguminosae | root | Huangqi |
| Atractylodes macrocephala Koidz | Compositae | root | Baizhu |
| Citrus aurantium L | Rutaceae | fruit | Zhike |
| Hedyotis diffusa Willd | Rubiaceae | herb | Baihuasheshecao |
| Scutellaria barbata D. Don | Lamiaceae | herb | Bazhilian |
| Curcuma zedoaria (Christm.) Rosc | Zingiberaceae | root | Ezhu |
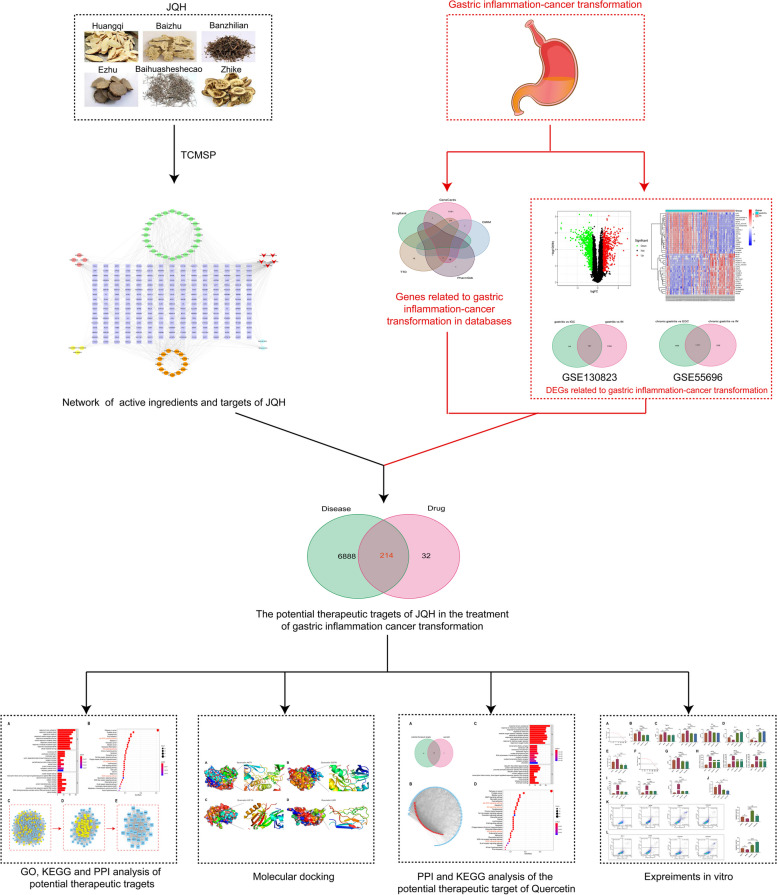
Network pharmacology has become an effective approach to investigating the mechanisms of drug therapy, screening the active ingredients, and exploring the therapeutic targets of TCM [11]. Therefore, in the present study, we used network pharmacology, bioinformatics, molecular docking, and in vitro experiments for validation to reveal the active ingredients, targets, and potential mechanisms of JQH to treat gastric inflammation-cancer transformation. The flow chart of this study is shown in Fig. 1.
Fig. 1.
Flow chart of network pharmacology and experimental validation strategies to explore JQH in treating gastric inflammation-cancer transformation
Materials and methods
Screen of JQH active ingredients and targets
We screened the JQH bioactive ingredients in the TCMSP database (https://old.tcmsp-e.com/tcmsp.php) according to the cut-off value of drug-like proprieties ≥ 018 and bioavailability ≥ 30%. The corresponding targets related to all these bioactive ingredients were also retrieved from the TCMSP database, and gene symbols were converted using the Uniport database (https://www.uniprot.org/).
Identification of gastric inflammation-cancer transformation targets by GEO database
The GSE130823 and GSE55696 datasets were retrieved from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). To identify the genes related to gastric inflammation-cancer transformation, differently expressed genes (DEGs) among different subgroups were identified based on FDR < 0.05 and |logFC|> 1. Then, the DEGs in the intersection of gastritis versus (vs.) intraepithelial neoplasia (IN) groups and gastritis vs. intestinal gastric cancer (IGC) groups in the GSE130832 dataset were selected. The DEGs obtained from chronic gastritis vs. IN groups and chronic gastritis vs. early gastric cancer (EGC) groups were also intersected in GSE55696 datasets. The intersected DEGs in GSE130823 and GSE55696 datasets were regarded as the targets for gastric inflammation-cancer transformation in the GEO database.
Collection of gastric inflammation-cancer transformation targets
We also collected targets using the keywords “atrophic gastritis”, “gastric adenocarcinoma,” and “stomach adenocarcinoma” in OMIM, TTD, DrugBank, Genecard, and PharmGkb databases. The targets obtained using the keyword “gastric adenocarcinoma” were combined with those obtained using the keyword “stomach adenocarcinoma”. Then, we intersected these combined targets with the targets identified by the keyword “atrophic gastritis” in each database. Finally, we combined the targets from the GEO and online databases using R software, and these targets were regarded as gene targets related to gastric inflammation-cancer transformation.
Potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation
The targets of gastric inflammation-cancer transformation were intersected with the targets of JQH bioactive ingredients. These genes were regarded as the potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation. This procedure was conducted using R software.
Protein–protein Interaction (PPI) network, Gene Ontology (GO), and Kyoto Encyclopedia Gene and Genomes (KEGG) analyses
The potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation were subjected to PPI network analysis by Cytoscape software. The PPI network was constructed using the CytoNCA tool in Cytoscape to identify the core genes based on specific parameters (Betweenness, Closeness, Eigenvector, and LAC) ≥ median value. To investigate the molecular mechanisms of JQH in treating gastric inflammation-cancer transformation, GO and KEGG enrichment analyses were also performed using R software.
Molecular docking
The 2D structure of active ingredients was obtained from the PubChem database. These 2D structures were converted into 3D structures using ChemBio3D software. The PDB database was used to obtain the 3D structures of core targets. The “AutoDockTool” was used to convert the 3D structure of active ingredients. The four targets were AKT1, EGFR, HIF1A and IL6R. These targets include ligand and water removal, hydrogen addition, and amino acid optimization and patching.Then, these four targets were saved as PDBQT format files and we identify the active pockets. Finally, molecular docking was conducted using “vina” software.
Identification of the key ingredients of JQH
We intersected the targets of the active ingredients of JQH with the therapeutic targets, and the ingredients with the most therapeutic targets were regarded as core JQH ingredients in the treatment of gastric inflammation-cancer transformation. Further, bioinformatics approaches were used to investigate the molecular mechanisms of these therapeutic targets related to core active ingredients.
Cell culture
Cells were cultured in RPMI-1640 medium with 10% fetal bovine serum and 50 mg/mL streptomycin, and 50 U/mL penicillin in an incubator at 37 °C and 5% CO2. Then, 20 μM N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) and 100 μM chenodeoxycholic acid (CDCA) were added to GES-1 cells for 24 h to establish an inflammatory and intestinal metaplasia model of the gastric mucosa.
Cell proliferation assay
First, cells were seeded in a 96-well microplate (6000 cells/well, 100 μL). Then, cells were routinely incubated for 24 h in a humidified incubator. After 24 h of pre-incubation, the medium was aspirated and exchanged containing celecoxib (100 μM) and/or quercetin (140 μM in MNNG-GES-1 cells, 150 μM in CDCA-GES-1 cells). Cell viability was measured using the Cell Counting Kit-8 (CCK-8) after 24 h incubation. Cells were seeded in 96-well plates, 10 μL CCK-8 solution was added to each well, and incubated at 37 °C. The optical density (OD) value of each well was measured at 450 nm.
Enzyme-Linked Immunosorbent Assay (ELISA)
After drug intervention for 24 h, cells supernatants were collected to detect the expression of TNF-α, IL-1β, IL-6 (pro-inflammatory cytokines), IL-4 and IL-10 (anti-inflammatory cytokines) by ELISA kits from Beyotime (Shanghai, China) following the manufacturer’s instructions. Similarly, cell lysate were collected to detect the expression of KLF4, MUC2 and VIL1(biomarkers of PLGC) by ELISA kits. Each well received 100 µL prepared standard. The whole plate was sealed with a plate sealing membrane and incubated at 4 ℃ overnight. The prepared 1 × wash solution was added to the plate washer, washed four times, and 300 µL wash solution was added to each well. Moreover, 100 µL prepared detection antibody (biotin-labeled antibody) was added to each well and incubated at room temperature for 1 h. Next, 100 µL HRP-streptavidin was added to each well and incubated at room temperature for 45 min. Then, 100 µL TMB chromogenic solution was added to each well and incubated at room temperature in the dark for 30 min. Finally, 50 µL of termination solution was added to each well and immediately read at 450 nm.
RT-qPCR
Total RNA from cells was isolated with the TRIzol reagent (Invitrogen, CA, USA) following the manufacturer’s instructions. One μg of total RNA was reverse transcribed using PrimeScript RT Master Mix Kit (TaKaRa Bio Inc., Kusatsu, Japan). Real-time PCR was performed on the cDNA using SYBR Green PCR Kit (TaKaRa Bio Inc.). GAPDH mRNA was used as the endogenous control. Primers are described in Table S1.
Alkaline phosphatase (ALP) activity measurement
Cells were seeded in 12-well microplates at a density of 1 × 105 cells/mL and treated with celecoxib (100 μM) or quercetin (140 μM in MNNG-GES-1 cells, 150 μM in CDCA-GES-1 cells) for 24 h before being assayed for ALP activity. After drug intervention for 24 h, cells were dissolved with non-denatured cell lysate to detect the ALP activity at 360/450 nm by QuantiFluoTM Alkaline Phosphatase Assay Kit (California, USA) following the manufacturer’s instructions.
Cell apoptosis assay
Cell apoptosis kits were purchased from Beyotime (Shanghai, China), and the experiment was conducted following the manufacturer’s instructions. Cells were centrifuged at approximately 1,000 g for 3–5 min and resuspended in phosphate-buffered saline. Next, 50,000–100,000 suspended cells were centrifuged at 1,000 g for 5 min. After discarding the supernatants, Annexin V-FITC binding solution (195 μL) was added to resuspend the cells gently. Next, Annexin V-FITC (5 μL) and propyl iodide staining solution (10 μL) were added and mixed. The solution was incubated at room temperature (20–25 ℃) for 10–20 min in the dark and analyzed using a CytoFLEX S flow cytometer (Beckman Coulter, CA, USA).
Statistical analysis
The mean ± standard deviation was used to express all data, which were subjected to normality distribution before statistical analysis. In this study, there were at least three samples in each group. Statistical analysis was conducted using SPSS version 24.0 (IBM Corp., Armonk, NY, USA). Images were plotted using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Student’s t-test and analysis of variance (ANOVA) were performed for comparisons between groups. P-values < 0.05 denoted statistically significant differences.
Results
Active ingredients and targets of JQH
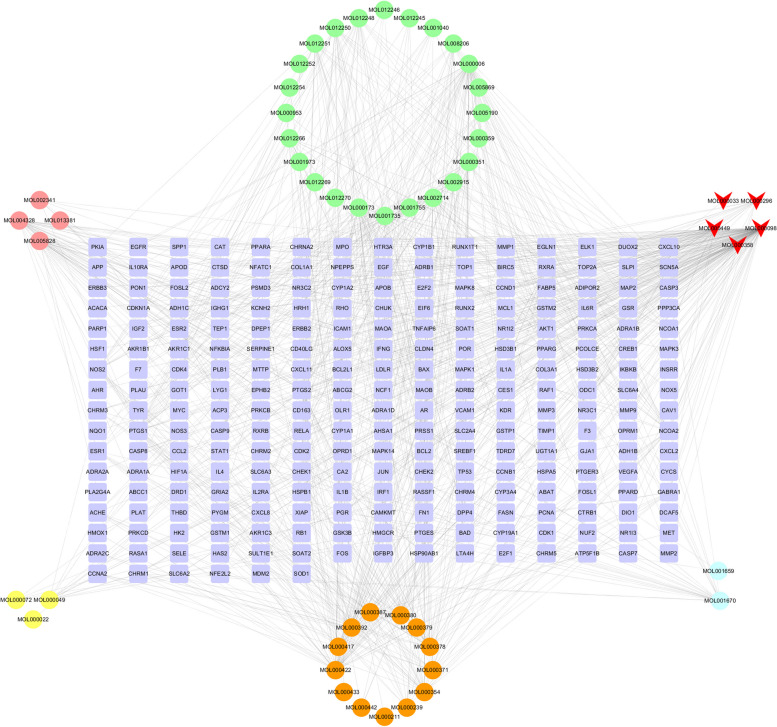
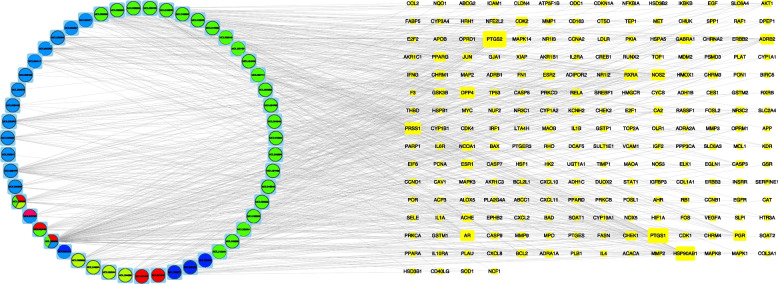
We obtained 51 active ingredients and 246 potential targets of JQH in the TCMSP database based on our filter condition (Table S2). The regulatory network between ingredients and targets of JQH was constructed using Cytoscape. The rectangles represent the potential targets, and the surrounding circle represents the active ingredients of JQH. Especially the green, pink, yellow, brown, and blue circles of Fig. 2 represent the active ingredients from Baizhilian, Zhike, Baizhu, Huangqi, and Baihuasheshecao, respectively. The red triangle represents the active ingredients from more than two herbs (Fig. 2).
Fig. 2.
Regulatory network of active ingredients and targets of JQH
Potential therapeutic targets for JQH
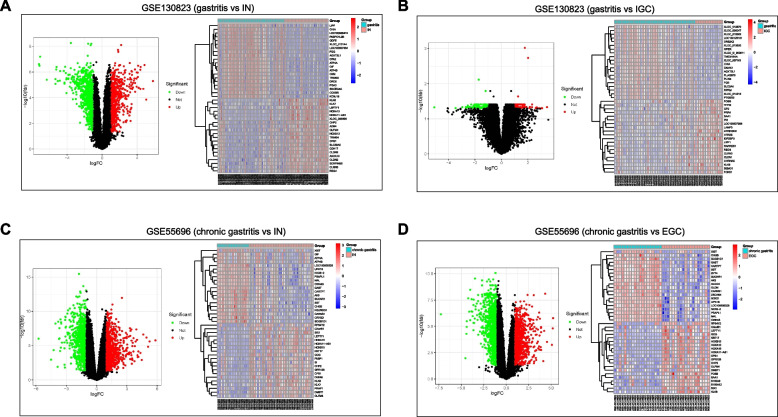
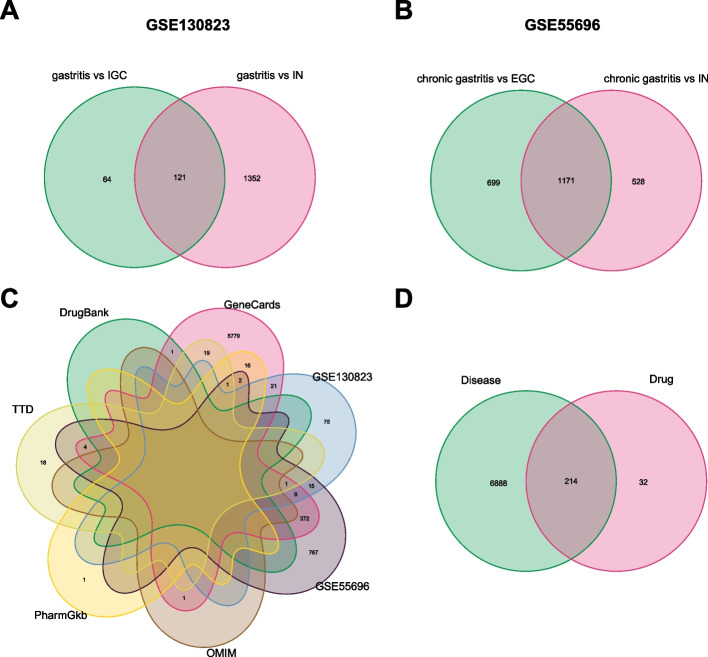
The GSE130823 dataset comprised 47 gastritis tissues, 31 IN samples, and 16 IGC samples. In the GSE130823 dataset, 1473 DEGs were detected in the IN group (Fig. 3A and Table S3) and 185 DEGs in the IGC group (Fig. 3B and Table S4) compared to the gastritis group. Then, 121 intersecting genes were obtained from gastritis vs. IN and gastritis vs. IGC subgroups (Fig. 4A). The GSE55696 dataset comprised 19 chronic gastritis tissues, 39 IN samples, and 19 EGC samples. In the GSE55696 dataset, 1699 DEGs were detected in the IN group (Fig. 3C and Table S5) and 1870 DEGs in the EGC group (Fig. 3D and Table S6) compared to the chronic gastritis group. Next, 1171 intersecting genes were obtained from chronic gastritis vs. IN and chronic gastritis vs. EGC subgroups (Fig. 4B). We found 1, 43, 1, 6226 and 20 therapeutic targets in OMIM, TTD, DrugBank, Genecard, and PharmGkb databases, respectively (Fig. 4C). A total of 7102 therapeutic targets were obtained by combining the targets from GEO and online databases (Fig. 4C). To identify the potential therapeutic targets for JQH, we intersected the 7102 targets with the targets of all active ingredients of JQH and obtained 214 repeated targets (Fig. 4D and Table S7). The regulatory network of 51 active ingredients and 214 therapeutic targets is presented in Fig. 5.
Fig. 3.
Identification of DEGs related to gastric inflammation-cancer transformation in GSE130823 and GSE55696 datasets. Volcano plot of DEGs and heatmap of the top 20 upregulated and downregulated genes in gastritis vs. IN groups (A) and gastritis vs. IGC groups (B) in the GSE130823 dataset. Volcano plot of DEGs and heatmap of the top 20 upregulated and downregulated genes in chronic gastritis vs. IN groups (C) and chronic gastritis vs. EGC groups (D) in the GSE55696 dataset
Fig. 4.
Potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation. DEGs related to gastric inflammation-cancer transformation in GSE130823 (A) and GSE55696 (B) datasets. C Identification of gastric inflammation-cancer transformation targets in public and GEO databases. D Potential therapeutic targets of JQH in treating gastric inflammation-cancer transformation
Fig. 5.
Regulatory network of 150 potential therapeutic targets and corresponding active ingredients
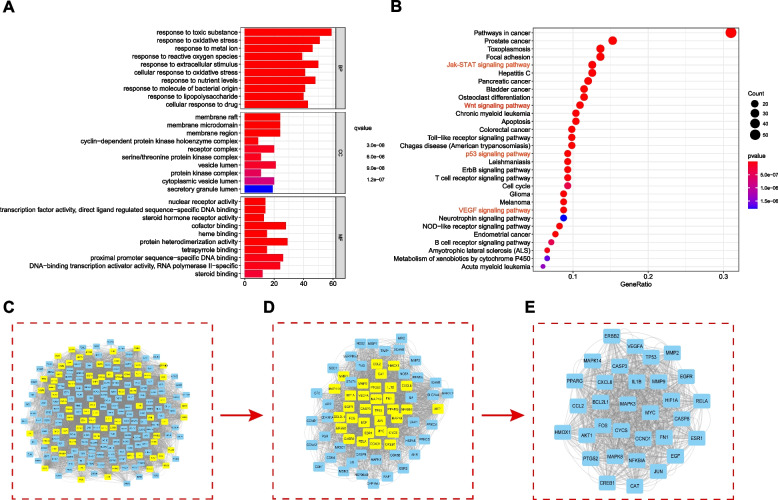
GO and KEGG enrichment analysis of therapeutic targets
To explore the potential mechanisms of JQH in treating gastric inflammation-cancer transformation, we conducted GO and KEGG enrichment analysis of the 214 therapeutic targets using R software. For GO annotation, the genes were enriched in response to lipolysaccharide, cellular response to drugs, and response to oxidative stress in Biological Process (BP); receptor complex, protein kinase complex and cyclin − dependent protein kinase holoenzyme complex in Cellular Components (CC); and nuclear receptor activity, phosphatase binding, and cofactor binding in Molecular Function (MF) (Fig. 6A). The KEGG pathways showed that the targets were enriched in JAK-STAT, Wnt, p53 and VEGF signaling pathways (Fig. 6B).
Fig. 6.
GO, KEGG, and PPI analysis of potential therapeutic targets. GO (A) and KEGG (B) enrichment analysis of potential therapeutic targets. (C-E) Core targets in the PPI network by the CytoNCA tool in Cytoscape
PPI network and identification of core genes
The PPI network of the 214 therapeutic targets was plotted using Cytoscape. The network has 214 nodes and 4046 edges in this regulatory network (Fig. 6C). The PPI network was further analyzed by the CytoNCA tool in Cytoscape to identify the core genes based on specific parameters (Betweenness, Closeness, Eigenvector, and LAC) ≥ median value, respectively. A sub-network was obtained from the genes with Betweenness > 71.42, Closeness > 0.51, Eigenvector > 0.05, and LAC > 38.21(Fig. 6D). Similarly, the genes with Betweenness, Closeness, Eigenvector, and LAC ≥ median value in the subnetwork were subjected to further analysis using the same methods. The core network comprised 32 genes with Betweenness > 15.66, Closeness > 0.74, Eigenvector > 0.12, and LAC > 73.58 (Fig. 6E). These 32 genes might be the potential core targets of JQH in treating gastric inflammation-cancer transformation.
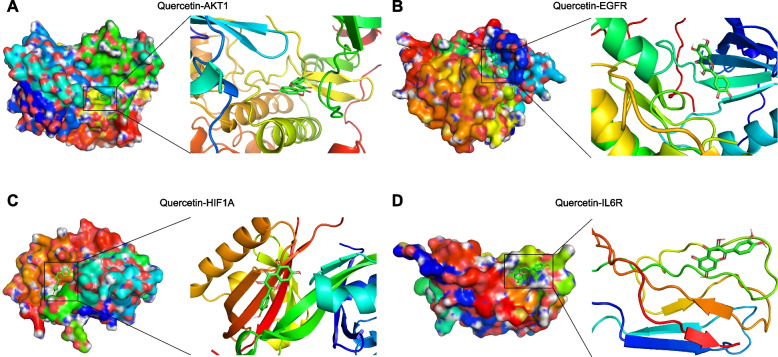
Molecular docking
Furthermore, we conducted the molecular docking of several genes related to inflammation-cancer transformation with quercetin in JQH. Four ingredient-target pairs were analyzed: quercetin-AKT1, quercetin-EGFR, quercetin-HIF1A, and quercetin-IL6R. The lowest binding energies of these compounds with their targets are presented in Fig. 7 and Table S7. The interactions between ligand and target proteins is shown in Table S8. All these results showed quercetin mostly interacted with AKT1, EGFR, HIF1A and IL6R.
Fig. 7.
Molecular docking of core targets with quercetin: quercetin to AKT1 (A), quercetin to EGFR (B), quercetin to HIF1A (C), and quercetin to IL6R (D)
Core JQH ingredients
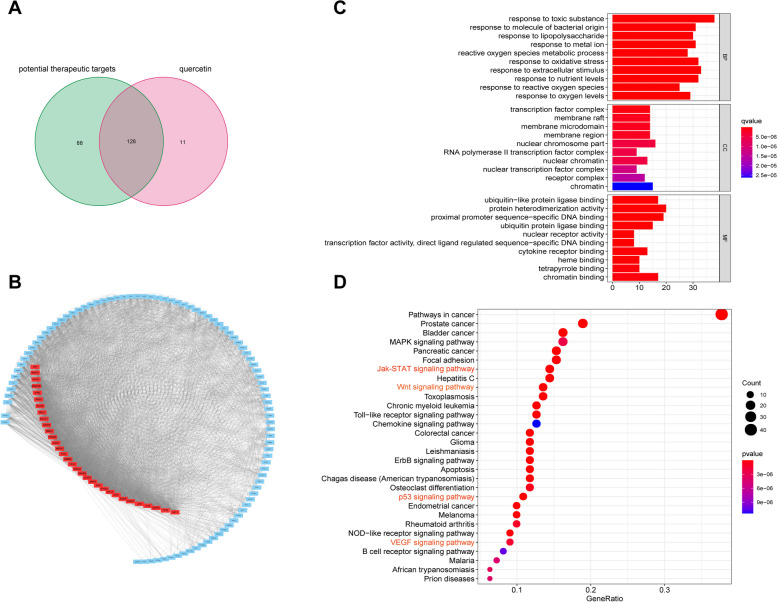
By intersecting the targets of active JQH ingredients with the 214 therapeutic targets, we determined that quercetin might be the core ingredient of JQH in the treatment of gastric inflammation-cancer transformation. We found 126 overlapped targets of quercetin and the potential therapeutic targets of gastric inflammation-cancer transformation (Fig. 8A and Table S9). The PPI analysis showed that the top 32 core genes were almost identical to those of JQH (Fig. 8B). GO annotation showed that the higher repetition rate of quercetin and JQH in BP, CC and MF items (Fig. 8C). The KEGG analysis showed that the repetition rate of quercetin and JQH pathways were nearly 90%, including JAK-STAT, Wnt, p53 and VEGF signaling pathways (Fig. 8D).
Fig. 8.
Bioinformatic analysis of quercetin’s therapeutic targets. A The intersection of quercetin and JQH therapeutic targets. B PPI analysis of 126 therapeutic targets. C GO annotation of 126 therapeutic targets. D KEGG analysis of 126 therapeutic targets
Validation with in vitro experiments
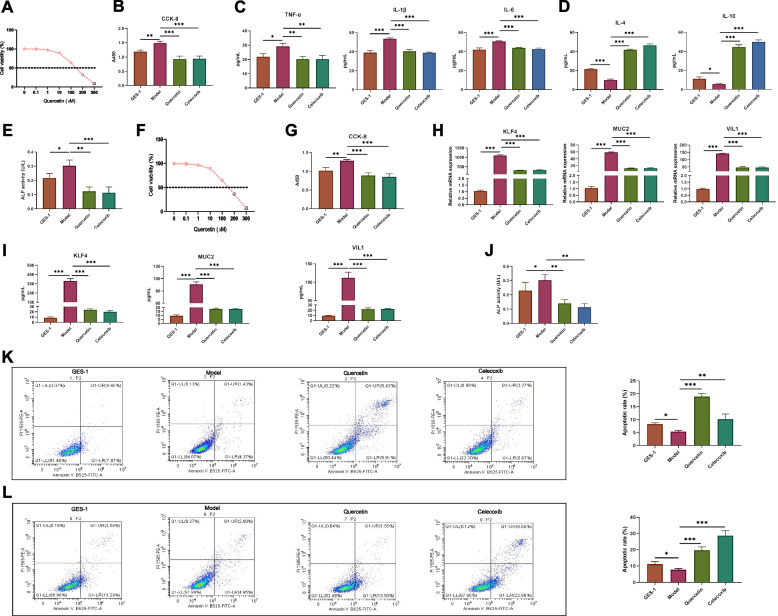
Furthermore, we found that quercetin was the active ingredient of JQH using LC-Q-TOF–MS and GC–MS [12]. Therefore, various functional experiments were conducted to validate the network pharmacology results. GES-1 cells were treated with MNNG and CDCA to construct an inflammatory [13] and intestinal metaplasia [14] cell model. Then, we detected the effect of quercetin on the proliferation of MNNG-GES-1 and CDCA-GES-1 cells in vitro. Quercetin inhibited cells proliferation with an IC50 of about 140 μM (Fig. 9A and B). MNNG was used to construct an inflammatory cell model, and specific inflammatory cytokines in cell supernatant were analyzed by ELISA. After quercetin treatment, TNF-α, IL-1β, and IL-6 levels significantly decreased compared to the model group in MNNG-GES-1 cells (PTNF-α < 0.01, PIL-1β < 0.001, PIL-6 < 0.001; Fig. 9C), and IL-4 and IL-10 levels significantly increased compared to the model group in MNNG-GES-1 cells (PIL-4 < 0.001, PIL-10 < 0.001; Fig. 9D). We also investigated whether quercetin treatment affected MNNG-GES-1 cell differentiation by detecting ALP activity. ALP is often positive in GC cells and negative in normal gastric cells [15]. ALP activity significantly decreased after quercetin treatment (P < 0.01; Fig. 9E), inducing the differentiation of GC cells. Moreover, we conducted flow cytometry analysis to investigate cell apoptosis after quercetin intervention. The quercetin intervention significantly promoted apoptosis of MNNG-GES-1 cells (P < 0.001; Fig. 9K).
Fig. 9.
Quercetin inhibited inflammation and intestinal metaplasia levels in MNNG and CDCA-induced GES-1 cells. A IC50 of MNNG-GES-1 cells after quercetin treatment for 24 h. B Cell viability of MNNG-GES-1 cells after quercetin treatment for 24 h in CCK-8 assay. C TNF-α, IL-1β, and IL-6 expression of MNNG-GES-1 cells after quercetin treatment for 24 h by ELISA. D IL-4 and IL-10 expression of MNNG-GES-1 cells after quercetin treatment for 24 h by ELISA. E ALP activity of MNNG-GES-1 cells after quercetin treatment for 24 h. F IC50 of CDCA-GES-1 cells after quercetin treatment for 24 h. G Cell viability of CDCA-GES-1 cells after quercetin treatment for 24 h in CCK-8 assay. H KLF4, MUC2, and VIL1 expression in CDCA-GES-1 cells after quercetin treatment for 24 h by qPCR. I MUC2, and VIL1 expression of CDCA-GES-1 cells after quercetin treatment for 24 h by ELISA. J ALP activity of CDCA-GES-1 cells after quercetin treatment for 24h. Apoptosis of (K) MNNG-GES-1 and (L) CDCA-GES-1 cells after quercetin treatment for 24 h by flow cytometry. The mean ± standard deviation was used to express all data, and there were at least three samples in each group. The analysis of variance (ANOVA) methods were performed for comparisons between groups. P-values < 0.05 denoted statistically significant differences. *p < 0.05, **p < 0.01, ***p < 0.001
Moreover, we found that quercetin inhibited CDCA-GES-1 cell proliferation with an IC50 of about 150 μM (Fig. 9F and G). Then, we conducted RT-qPCR to investigate the mRNA expression of several intestinal markers after quercetin intervention. The quercetin intervention significantly reduced mRNA(PKLF4 < 0.001, PMUC2 < 0.001, PVIL1 < 0.001; Fig. 9H) and protein level (PKLF4 < 0.001, PMUC2 < 0.001, PVIL1 < 0.001; Fig. 9I) of KLF4, MUC2, and VIL1 in CDCA-GES-1 cells. Similarly, after quercetin treatment, ALP activity significantly decreased in CDCA-GES-1 cells (P < 0.01; Fig. 9J). The flow cytometry analysis showed that quercetin intervention significantly promoted the apoptosis of CDCA-GES-1 cells (P < 0.001; Fig. 9K).
Discussion
JianPi QingRe HuaYu, a frequently-used Traditional Chinese Medicine, is often used for treatment of CAG and precancerous lesions of gastric cancer (PLGC) in clinic [7]. Clinical and animal studies have been conducted to investigate the correlation between JQH and CAG or PLGC. Our clinical studies showed that JQH significantly improved mental and digestive function and reduced the inflammatory level, atrophy, and intestinal metaplasia of CAG patients [8]. JQH can also reduce the intestinal metaplasia and atypical hyperplasia of PLGC patients [16]. Our animal experiments showed that JQH could improve the ultrastructure of chief and parietal cells of the gastric mucosa to a certain extent [9]. However, examining the features of multi-ingredients and targets of TCM formulations is challenging. Nevertheless, network pharmacology allows the study of the molecular features of TCM formulations.
Herein, we obtained 214 potential therapeutic targets by intersecting JQH and gastric inflammation-cancer transformation targets. The KEGG enrichment analysis showed that the potential mechanisms of JQH in the treatment of gastric inflammation-cancer transformation might be related to JAK-STAT, Wnt, p53 and VEGF signaling pathways. STAT3, a protein composed of 770 amino acids, plays a crucial role in intracellular signal transduction [17]. STAT3 signaling pathway is a "bridge" between inflammation and cancer, and is closely related to "inflammation-cancer" transformation [17]. Zhang et al. [18] found that TRIM27 mediated the activation of STAT3 through retromer-positive structures and promoted colitis cancer. Long non-coding RNA (lncRNA) FAM64A promotes Th17 differentiation and colitis-related tumor formation by positively regulating STAT3 activity [19]. Zhang et al. found [20] that CKLF1 promotes inflammatory mediated hepatocellular carcinoma formation by activating the IL6/STAT3 signaling pathway, and can block adriamycin induced apoptosis. Anther study found [21] STAT3 signaling, mediated by TFF1 silencing, promotes gastric inflammation-cancer transformation. Activation of Wnt is highly correlated with gastric inflammation-cancer transformation. For examples, activation of Wnt/β-catenin signaling pathway promotes epithelial mesenchymal transition of MNNG-GES-1 cells and gastric precancerous lesions in rats [22]. Wnt activation also accelerate the progression of atrophic gastritis, and TCM may inhibit the these effection [23, 24]. The correlation between the P53 pathway and gastritis or GC has also been explored. P53 can mediate apoptotic and gastric carcinogenesis by targeting EPSIN3 [25]. P53 degradation, induced by USF1 defect, accelerates gastric carcinogenesis when rats are infected by Helicobacter pylori [26]. VEGF is a subfamily of growth factors, specifically belonging to the platelet-derived growth factor (PDGF) family of cystine node growth factors. Activation of HIF-1α/VEGF angiogenesis pathway promotes the development of MNNG-induced atrophic gastritis [27].
Subsequently, we identified 32 core genes by PPI and CytoNCA in Cytoscape. Most genes were significantly correlated with gastric inflammation-cancer transformation, such as AKT1, EGFR, and MYC. For example, Akt can promote gastric tumorigenesis by causing PTEN deficiency [28]. AKT1, suppressed by RUNX3, inhibits gastric tumorigenesis in GC [29]. EGFR methylation might promote the transformation of chronic gastritis into gastric carcinoma [30]. Additionally, exosome-delivered EGFR promoter liver metastasis by regulating the liver microenvironment in GC [31]. Another study found [32] that EGFR degradation, induced by CMTM3, inhibited GC tumorigenesis by enhancing Rab5 activity. MYC, a classic oncogene, is significantly correlated with gastric inflammation-cancer transformation. Zheng et al. [33] found that c-MYC upregulation, induced by CHAF1A and TCF4, promotes gastric carcinogenesis. Besides, c-Myc upregulation, caused by microRNA-10b/CSMD1 axis activation, promotes the inflammation-carcinogenesis of GC [34].
Finally, the molecular docking and core ingredients analysis showed that quercetin was the core ingredient of JQH and bound well with several inflammation-cancer targets, including AKT1, EGFR, HIF1A, and IL6A. Our validated experiments indicated that quercetin inhibited cell proliferation, promoted cell apoptosis, and decreased inflammation and intestinal metaplasia levels in cell models of inflammation and intestinal metaplasia in vitro. Additionally, Zhang et al. [35] found that quercetin can ameliorate gastric inflammation by regulating p38MAPK and BCL-2 expression. Hsieh et al. demonstrated that quercetin exerts anti-inflammatory effects by inhibiting the TNF/MMP9 axis in GES-1 cells [36]. Yu et al. found [37] that quercetin can inhibit the IRF8/IFN-γ axis, reduce gastric inflammation, and enhance gastric secretory function, improving CAG induced by Hp infection. Another study [38] found that quercetin induced GC cell apoptosis and exerted potential anti-gastric cancer efficacy. Moreover, quercetin enhances the efficacy of other anti-cancer drugs in vitro and in vivo for GC [39].
However, our current study also has some limitations. First, some compounds of JQH were not well investigated, and we only explored the function of quercetin on gastric inflammation-cancer transformation. Then, we did not investigate the effects of JQH and its compounds on gastric inflammation-cancer transformation in animal experiments. We will conduct further experiments to validate the above mechanisms of JQH or its compounds.
In conclusion, we elucidated the potential molecular mechanisms of JQH in treating gastric inflammation-cancer transformation using network pharmacology, bioinformatics, and in vitro experiments for validation. Moreover, quercetin might be one of the active ingredients of JQH that exerted drug efficacy in gastric inflammation-cancer transformation. We provided robust evidence for the clinical application of JQH in gastric inflammation-cancer transformation, but further in vivo experiments are needed to validate these findings.
Supplementary Information
Additional file 1: Table S1. Sequences of primers used in the RT-qPCR.
Additional file 2: Table S2. The active ingredients and potential targets of JQH in TCMSP database.
Additional file 3: Table S3. The DEGs between IN and gastritis group In GSE130823 dataset.
Additional file 4: Table S4. The DEGs between IGC and gastritis group In GSE130823 dataset.
Additional file 5: Table S5. The DEGs between IN and chronic gastritis group in GSE55696 dataset.
Additional file 6: Table S6. The DEGs between EGC and chronic gastritis group in GSE55696 dataset.
Additional file 7: Table S7. Binding energy (kcal/mol) of quercetin target proteins.
Additional file 8: Table S8. Interaction between ligands and target proteins.
Additional file 9: Table S9. The potential therapeutic targets for JQH and Quercetin in the treatments of gastric inflammation-cancer transformation.
Acknowledgements
Thanks to everyone who contributed to this article.
Abbreviations
- JQH
JianPi QingRe HuaYu Methods
- CAG
Chronic atrophic gastritis
- PLGC
Precancerous lesions of gastric cancer
- GC
Gastric cancer
- DEGs
Differentially expressed genes
- GEO
Gene Expression Omnibus
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- PPI
Protein–protein interaction
- TCM
Traditional Chinese Medicine
- IN
Intraepithelial neoplasia
- IGC
Intestinal gastric cancer
- EGC
Early gastric cancer
- MNNG
N-methyl-N'-nitro-N-nitrosoguanidine
- CDCA
Chenodeoxycholic acid
- ELISA
Enzyme-Linked Immunosorbent Assay
- ALP
Alkaline phosphatase
- BP
Biological Process
- CC
Cellular Components
- MF
Molecular Function
Authors’ contributions
Kechao Nie contributed to the study conception and were responsible for writing the first draft of the paper; Zhihua Zheng, Xiushen Li, Yonglong Chang and FengBin Liu conducted the data analysis; Xiaoyu Wang and Kechao Nie revised the paper; All authors read and approved the final version of the manuscript.
Funding
This work were supported by the Natural Science Foundation of Hunan Province (No. 2022JJ40706), the National Natural Science Foundation of China (No. 82305207), and the Scientific Research Launch Project for new employees of the Second Xiangya Hospital of Central South University.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. GSE130823 and GSE55696 datasets used and/or analyzed during the current study are available from https://www.ncbi.nlm.nih.gov/gds/.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All author appove the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tan P, Yeoh KG. Genetics and molecular pathogenesis of gastric adenocarcinoma. Gastroenterology. 2015;149(5):1153–1162. doi: 10.1053/j.gastro.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48(13):3554–3560. [PubMed] [Google Scholar]
- 3.Collatuzzo G, Pelucchi C, Negri E, Lopez-Carrillo L, Tsugane S, Hidaka A, Shigueaki HG, Hernandez-Ramirez RU, Lopez-Cervantes M, Malekzadeh R, et al. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: a pooled analysis in the Stomach cancer Pooling (StoP) Project. Int J Cancer. 2021;149(6):1228–1238. doi: 10.1002/ijc.33678. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015;148(4):719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng W, Zhang S, Zhang Z, Xu P, Mao D, Huang S, Chen B, Zhang C, Zhang S. Jianpi Jiedu decoction, a traditional Chinese medicine formula, inhibits tumorigenesis, metastasis, and angiogenesis through the mTOR/HIF-1alpha/VEGF pathway. J Ethnopharmacol. 2018;224:140–148. doi: 10.1016/j.jep.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Hu L, Lao SX, Wang JH: Effect of “Wei Yan Xiao” on bcl-2 Oncogene mRNA Expression in Precancerous Lesion of Gastric Cancer. SH.J.TCM 2006(01):27–28.
- 8.Zhuang KH, Li HW, Liu H, Pan JL, Wen Y, Liu FB. Clinical study of Weiweiqing granule in the treatment of chronic atrophic gastritis. Modern Digest Interv. 2018;23(04):479–483. [Google Scholar]
- 9.Zhuang K, Li H, Liu F. Weiweiqing has effect on ultrastructure of gastric mucosa in rats with chronic atrophic gastritis. J New Chinese Med. 2018;50(07):4–7. [Google Scholar]
- 10.Zhuang K, Li H, Liu H, Pan J. LIU F: Effect of invigorating spleen and clearing heat and activating blood circulation on inflammatory pathway factors in rats with atrophic gastritis. Modern Digest Interv. 2018;23(02):145–148. [Google Scholar]
- 11.Wu Z, Li W, Liu G, Tang Y. Network-based methods for prediction of drug-target interactions. Front Pharmacol. 2018;9:1134. doi: 10.3389/fphar.2018.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan J, Wen S, Wen Y, Li P, Liu Y, Fu B, Wang X, Liu F. Mechanism research of weiweiqing granules for key mRNA associated with chronic inflammation transforming into gastric adenocarcinoma based on molecular docking technology. CJTCMP. 2019;34(10):4542–4546. [Google Scholar]
- 13.Liu X, Sun XH, Qi X. Creation of nitrosamine compounds MNNG induced GES-1 cell model of malignant transformation. Med J Qilu. 2013;28(02):115–116. [Google Scholar]
- 14.Li T, Guo H, Li H, Jiang Y, Zhuang K, Lei C, Wu J, Zhou H, Zhu R, Zhao X, et al. MicroRNA-92a-1-5p increases CDX2 by targeting FOXD1 in bile acids-induced gastric intestinal metaplasia. Gut. 2019;68(10):1751–1763. doi: 10.1136/gutjnl-2017-315318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, Du S, Li S. Dissecting the single-cell transcriptome network underlying gastric premalignant lesions and early gastric cancer. Cell Rep. 2019;27(6):1934–1947. doi: 10.1016/j.celrep.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 16.Lao SX, Xu XM, Zhou FS, Yu SY, Jia JG. Efficacy analysis of Weiyanxiao in the treatment of precancerous lesions of gastric cancer. Tradition Chinese Drug Res Clin Pharmacol. 1997;02:9–12. [Google Scholar]
- 17.Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. 2020;19(1):145. doi: 10.1186/s12943-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang HX, Xu ZS, Lin H, Li M, Xia T, Cui K, Wang SY, Li Y, Shu HB, Wang YY. TRIM27 mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis. Nat Commun. 2018;9(1):3441. doi: 10.1038/s41467-018-05796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu ZS, Zhang HX, Li WW, Ran Y, Liu TT, Xiong MG, Li QL, Wang SY, Wu M, Shu HB, et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc Natl Acad Sci U S A. 2019;116(21):10447–10452. doi: 10.1073/pnas.1814336116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Liu L, Zhou Y, Zhou P, Yan Q, Chen X, Ding S, Zhu F. CKLF1 enhances inflammation-mediated carcinogenesis and prevents doxorubicin-induced apoptosis via IL6/STAT3 signaling in HCC. Clin Cancer Res. 2019;25(13):4141–4154. doi: 10.1158/1078-0432.CCR-18-3510. [DOI] [PubMed] [Google Scholar]
- 21.Soutto M, Chen Z, Bhat AA, Wang L, Zhu S, Gomaa A, Bates A, Bhat NS, Peng D, Belkhiri A, et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat Commun. 2019;10(1):3039. doi: 10.1038/s41467-019-11011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao W, Wen Y, Wang J, Zhao M, Lv S, Chen N, Li Y, Wan L, Zheng Q, Mou Y, et al. Gallic acid alleviates gastric precancerous lesions through inhibition of epithelial mesenchymal transition via Wnt/beta-catenin signaling pathway. J Ethnopharmacol. 2023;302(Pt A):115885. doi: 10.1016/j.jep.2022.115885. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Xu T, Yan Z, Zheng X, Zhu F. Jian-Pi-Yi-Qi-Fang ameliorates chronic atrophic gastritis in rats through promoting the proliferation and differentiation of gastric stem cells. Ann Transl Med. 2022;10(17):932. doi: 10.21037/atm-22-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Z, Xu T, Xu Y, Chen W, An Z, Zhu F. Jianpiyiqi formula ameliorates chronic atrophic gastritis in rats by modulating the Wnt/beta-catenin signaling pathway. Exp Ther Med. 2021;22(2):878. doi: 10.3892/etm.2021.10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori J, Tanikawa C, Ohnishi N, Funauchi Y, Toyoshima O, Ueda K, Matsuda K. EPSIN 3, a novel p53 target, regulates the apoptotic pathway and gastric carcinogenesis. Neoplasia. 2017;19(3):185–195. doi: 10.1016/j.neo.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa L, Corre S, Michel V, Le Luel K, Fernandes J, Ziveri J, Jouvion G, Danckaert A, Mouchet N, Da SBD, et al. USF1 defect drives p53 degradation during Helicobacter pylori infection and accelerates gastric carcinogenesis. Gut. 2020;69(9):1582–1591. doi: 10.1136/gutjnl-2019-318640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen JX, Tong YL, Ma X, Wang RL, Li RS, Song HT, Zhao YL. Therapeutic effects and potential mechanism of dehydroevodiamine on N-methyl-N'-nitro-N-nitrosoguanidine-induced chronic atrophic gastritis. Phytomedicine. 2021;91:153619. doi: 10.1016/j.phymed.2021.153619. [DOI] [PubMed] [Google Scholar]
- 28.Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li XB, Zhang C, Yang X, Yang ZZ, Yang X. Akt-p53-miR-365-cyclin D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN deficiency. Nat Commun. 2013;4:2544. doi: 10.1038/ncomms3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin FC, Liu YP, Lai CH, Shan YS, Cheng HC, Hsu PI, Lee CH, Lee YC, Wang HY, Wang CH, et al. RUNX3-mediated transcriptional inhibition of Akt suppresses tumorigenesis of human gastric cancer cells. Oncogene. 2012;31(39):4302–4316. doi: 10.1038/onc.2011.596. [DOI] [PubMed] [Google Scholar]
- 30.Silva M, Azenha D, Pereira C, Almeida A, Balseiro S, Sampaio AM, Santos P, Carvalho L. Gastric carcinoma and chronic gastritis: epigenetic regulation of CDH1 (E-Cadherin), CDKN2A (p16INK4A), PTGS2 (COX-2) and EGFR genes through methylation. Acta Med Port. 2010;23(1):5–14. [PubMed] [Google Scholar]
- 31.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan W, Liu B, Wang X, Li T, Xue H, Mo X, Yang S, Ding S, Han W. CMTM3 decreases EGFR expression and EGF-mediated tumorigenicity by promoting Rab5 activity in gastric cancer. Cancer Lett. 2017;386:77–86. doi: 10.1016/j.canlet.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Liang X, Li S, Li T, Shang W, Ma L, Jia X, Shao W, Sun P, Chen C, et al. CHAF1A interacts with TCF4 to promote gastric carcinogenesis via upregulation of c-MYC and CCND1 expression. EBioMedicine. 2018;38:69–78. doi: 10.1016/j.ebiom.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen XL, Hong LL, Wang KL, Liu X, Wang JL, Lei L, Xu ZY, Cheng XD, Ling ZQ. Deregulation of CSMD1 targeted by microRNA-10b drives gastric cancer progression through the NF-kappaB pathway. Int J Biol Sci. 2019;15(10):2075–2086. doi: 10.7150/ijbs.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Huang J, Xie X, He Y, Mo F, Luo Z. Quercetin from polygonum capitatum protects against gastric inflammation and apoptosis associated with helicobacter pylori infection by affecting the levels of p38MAPK, BCL-2 and BAX. Molecules. 2017;22(5):744. doi: 10.3390/molecules22050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh HL, Yu MC, Cheng LC, Chu MY, Huang TH, Yeh TS, Tsai MM. Quercetin exerts anti-inflammatory effects via inhibiting tumor necrosis factor-alpha-induced matrix metalloproteinase-9 expression in normal human gastric epithelial cells. World J Gastroenterol. 2022;28(11):1139–1158. doi: 10.3748/wjg.v28.i11.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C, Lu X, Li L, Wang A. LI X: Mechanisms of quercetin-mediated IRF8/IFN-γ in improving chronic atrophic gastritis induced by Hp infection. Chin J Nosocomio. 2022;20:3073–3077. [Google Scholar]
- 38.Zhang JY, Lin MT, Zhou MJ, Yi T, Tang YN, Tang SL, Yang ZJ, Zhao ZZ, Chen HB. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules. 2015;20(6):11524–11534. doi: 10.3390/molecules200611524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei CS, Hou YC, Pai MH, Lin MT, Yeh SL. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. J Nutr Biochem. 2018;51:105–113. doi: 10.1016/j.jnutbio.2017.09.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Sequences of primers used in the RT-qPCR.
Additional file 2: Table S2. The active ingredients and potential targets of JQH in TCMSP database.
Additional file 3: Table S3. The DEGs between IN and gastritis group In GSE130823 dataset.
Additional file 4: Table S4. The DEGs between IGC and gastritis group In GSE130823 dataset.
Additional file 5: Table S5. The DEGs between IN and chronic gastritis group in GSE55696 dataset.
Additional file 6: Table S6. The DEGs between EGC and chronic gastritis group in GSE55696 dataset.
Additional file 7: Table S7. Binding energy (kcal/mol) of quercetin target proteins.
Additional file 8: Table S8. Interaction between ligands and target proteins.
Additional file 9: Table S9. The potential therapeutic targets for JQH and Quercetin in the treatments of gastric inflammation-cancer transformation.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. GSE130823 and GSE55696 datasets used and/or analyzed during the current study are available from https://www.ncbi.nlm.nih.gov/gds/.