Abstract
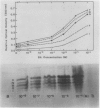
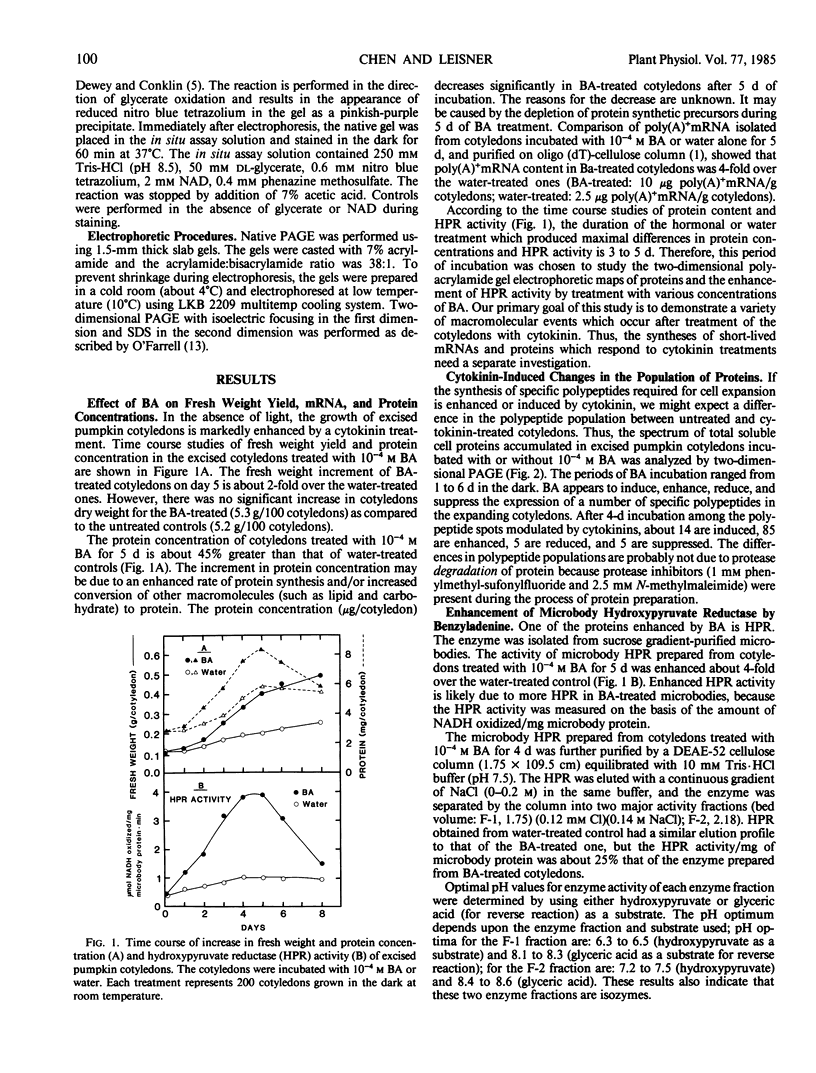
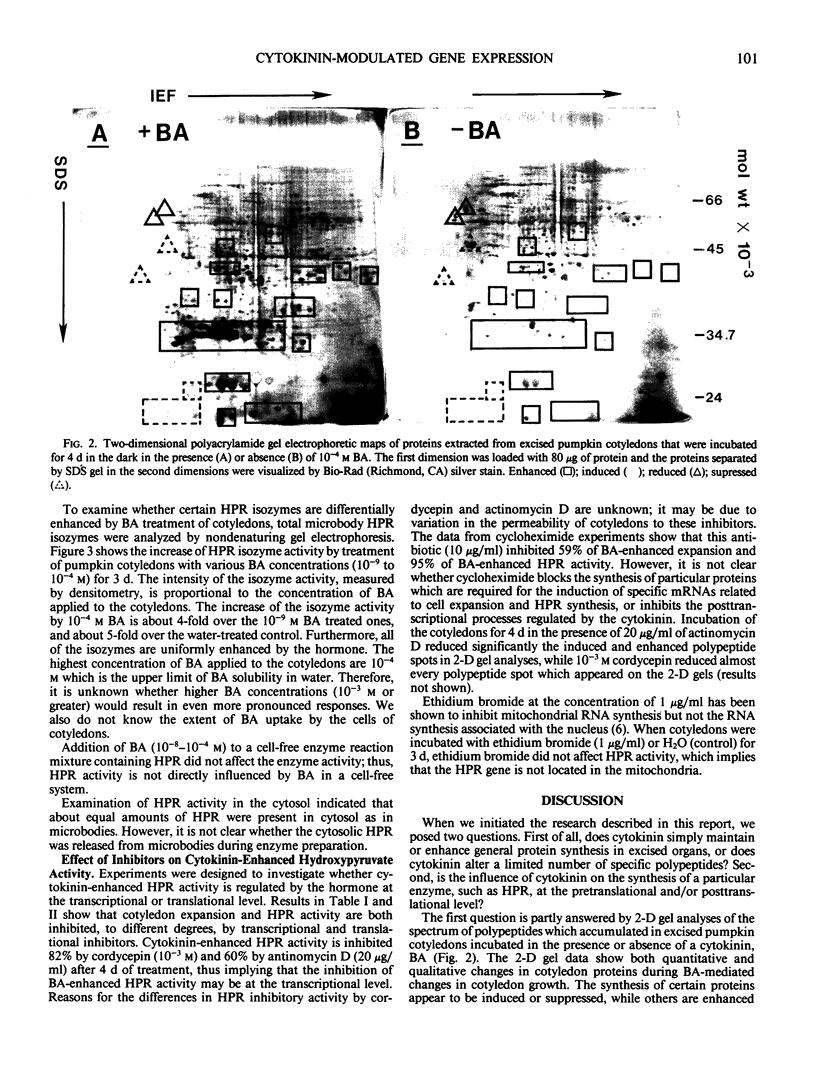
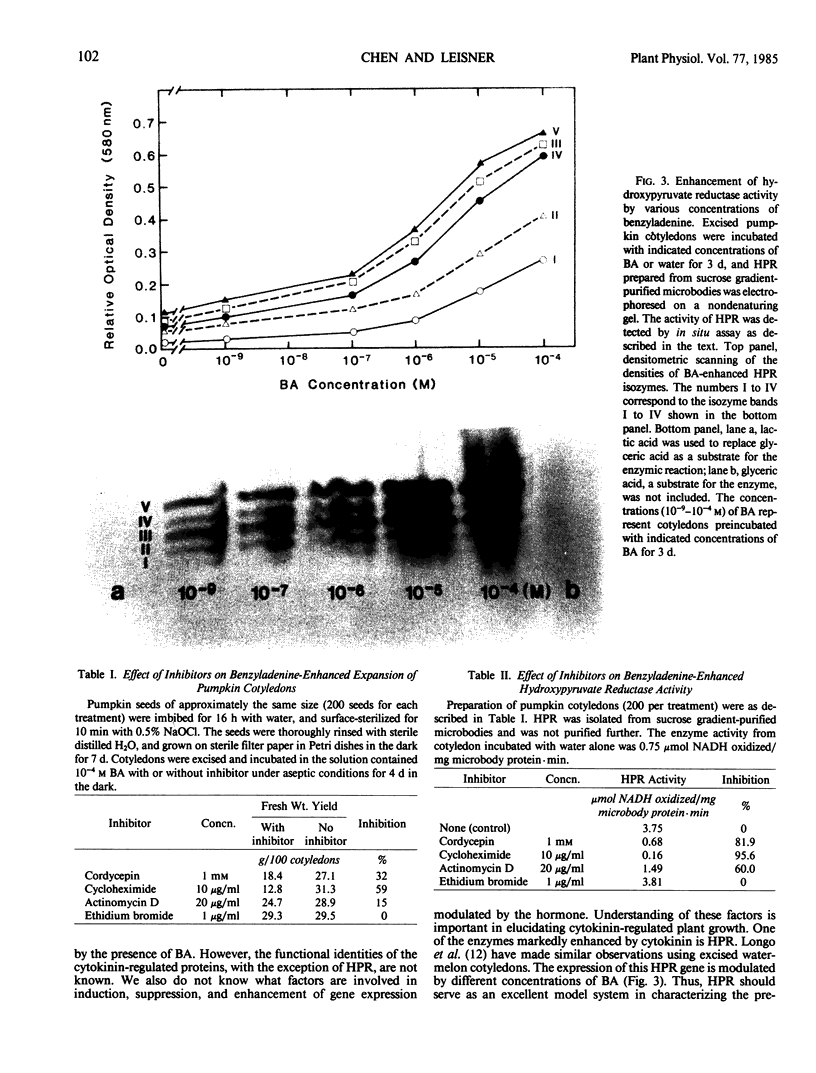
Comparison of two-dimensional polyacrylamide gel electrophoretic maps of proteins isolated from benzyladenine-treated and untreated pumpkin (Cucurbita pepo L. cv Halloween) cotyledons showed that the expression of certain proteins is enhanced, induced, or suppressed by the cytokinin treatment. The amount of poly(A)+ mRNA isolated from cotyledons incubated with 10−4 molar benzyladenine for five days was about four-fold over the water-incubated control. The activity of hydroxypyruvate reductase prepared from purified cotyledonous microbodies and analyzed by native gel electrophoresis is proportionally enhanced by sequentially higher concentrations (10−9 to 10−4 molar) of benzyladenine. Ethidium bromide (1 microgram per milliliter) did not inhibit hydroxypyruvate reductase activity; thus, the enzyme synthesis does not appear to be controlled by organelle genes. Hydroxypyruvate reductase synthesis is inhibited by cycloheximide, cordycepin, and to a certain degree by actinomycin D. These data support the view of a close association between cytokinin action and gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang A. H. Metabolism of Glycolate in Isolated Spinach Leaf Peroxisomes : KINETICS OF GLYOXYLATE, OXALATE, CARBON DIOXIDE, AND GLYCINE FORMATION. Plant Physiol. 1981 May;67(5):1003–1006. doi: 10.1104/pp.67.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEWEY M. M., CONKLIN J. L. Starch gel electrophoresis of lactic dehydrogenase from rat kidney. Proc Soc Exp Biol Med. 1960 Dec;105:492–494. doi: 10.3181/00379727-105-26153. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Mitochondrial RNA synthesis during mitosis. Science. 1970 Apr 3;168(3927):135–138. doi: 10.1126/science.168.3927.135. [DOI] [PubMed] [Google Scholar]
- Howard H. F., Witham F. H. Invertase activity and the kinetin-stimulated enlargement of detached radish cotyledons. Plant Physiol. 1983 Oct;73(2):304–308. doi: 10.1104/pp.73.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Liu K. D., Youle R. J. Organelle-specific Isozymes of Aspartate-alpha-Ketoglutarate Transaminase in Spinach Leaves. Plant Physiol. 1976 Jul;58(1):110–113. doi: 10.1104/pp.58.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff A. K., Ross C. W. Promotion of radish cotyledon enlargement and reducing sugar content by zeatin and red light. Plant Physiol. 1975 Sep;56(3):429–433. doi: 10.1104/pp.56.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Ross C. W., Robinson N. Estimation of osmotic parameters accompanying zeatin-induced growth of detached cucumber cotyledons. Plant Physiol. 1982 Dec;70(6):1634–1636. doi: 10.1104/pp.70.6.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Yamazaki R. K., Oeser A. Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J Biol Chem. 1970 Oct 10;245(19):5129–5136. [PubMed] [Google Scholar]
- Walker J. C., Key J. L. Isolation of cloned cDNAs to auxin-responsive poly(A)RNAs of elongating soybean hypocotyl. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7185–7189. doi: 10.1073/pnas.79.23.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]