Abstract
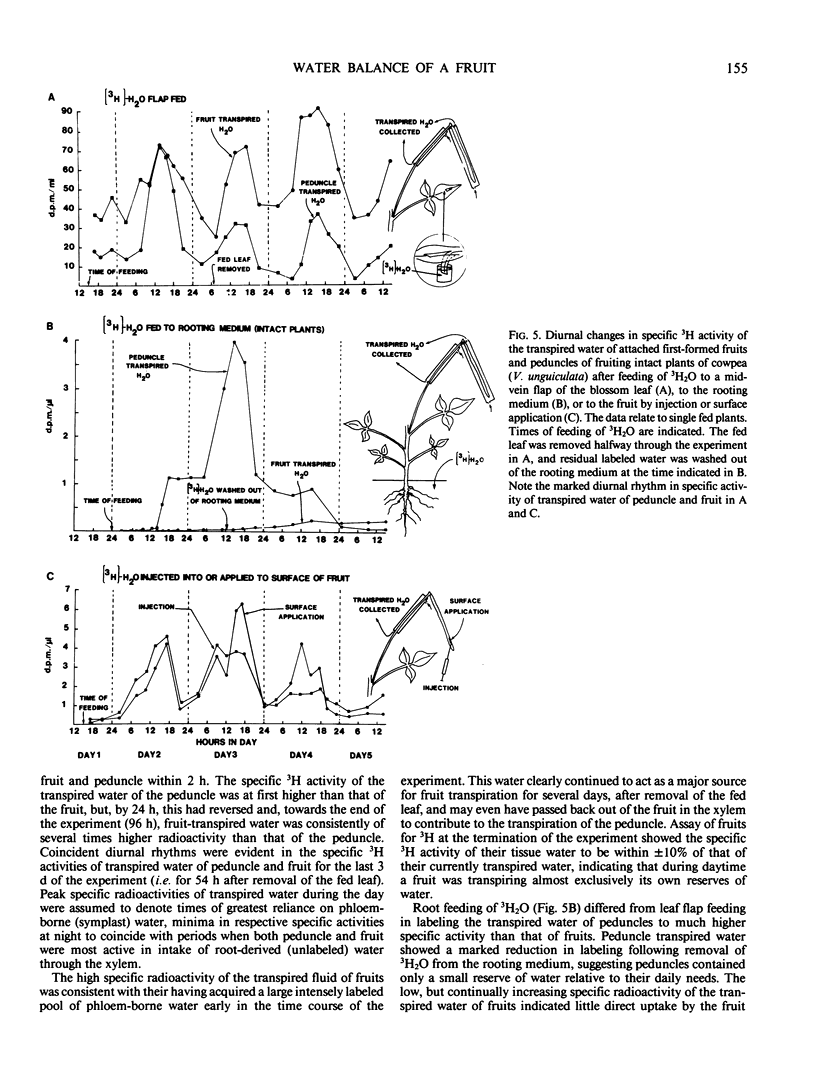
The vascular network of the cowpea (Vigna unguiculata [L.] Walp.) fruit exhibits the anatomical potential for reversible xylem flow between seeds, pod, and parent plant. Feeding of cut shoots with the apoplast marker acid fuchsin showed that fruits imported regularly via xylem at night, less frequently in early morning, and only rarely in the afternoon. The dye never entered seeds or inner dorsal pod strands connecting directly to seeds. Root feeding (early morning) of intact plants with 32PO4 or 3H2O rapidly (20 min) labeled pod walls but not seeds, consistent with uptake through xylem. Weak subsequent (4 hours) labeling of seeds suggested slow secondary exchange of label with the phloem stream to the fruit. Vein flap feeding of subtending leaves with [14C]sucrose, 3H2O, and 32PO4 labeled pod and seed intensely, indicating mass flow in phloem to the fruit. Over 90% of the 14C and 3H of fruit cryopuncture phloem sap was as sucrose and water, respectively. Specific 3H activities of transpired water collected from fruits and peduncles were assayed over 4 days after feeding 3H2O to roots, via leaf flaps, or directly to fruits. The data indicated that fruits transpired relatively less xylem-derived (apoplastic) water than did peduncles, that fruit and peduncle relied more heavily on phloem-derived (symplastic) water for transpiration in the day than at night, and that water diffusing back from the fruit was utilized in peduncle transpiration, especially during the day. The data collectively support the hypothesis of a diurnally reversing xylem flow between developing fruit and plant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clements H. F. MOVEMENT OF ORGANIC SOLUTES IN THE SAUSAGE TREE KIGELIA AFRICANA. Plant Physiol. 1940 Oct;15(4):689–700. doi: 10.1104/pp.15.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Larue T. A. Modeling C and N transport to developing soybean fruits. Plant Physiol. 1982 Nov;70(5):1290–1298. doi: 10.1104/pp.70.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Peoples M. B., Atkins C. A. Spontaneous Phloem bleeding from cryopunctured fruits of a ureide-producing legume. Plant Physiol. 1984 Mar;74(3):499–505. doi: 10.1104/pp.74.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peoples M. B., Pate J. S., Atkins C. A., Murray D. R. Economy of water, carbon, and nitrogen in the developing cowpea fruit. Plant Physiol. 1985 Jan;77(1):142–147. doi: 10.1104/pp.77.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]




