Abstract
Older people often show auditory temporal processing deficits and speech-in-noise intelligibility difficulties even when their audiogram is clinically normal. The causes of such problems remain unclear. Some studies have suggested that for people with normal audiograms, age-related hearing impairments may be due to a cognitive decline, while others have suggested that they may be caused by cochlear synaptopathy. Here, we explore an alternative hypothesis, namely that age-related hearing deficits are associated with decreased inhibition. For human adults (N = 30) selected to cover a reasonably wide age range (25–59 years), with normal audiograms and normal cognitive function, we measured speech reception thresholds in noise (SRTNs) for disyllabic words, gap detection thresholds (GDTs), and frequency modulation detection thresholds (FMDTs). We also measured the rate of growth (slope) of auditory brainstem response wave-I amplitude with increasing level as an indirect indicator of cochlear synaptopathy, and the interference inhibition score in the Stroop color and word test (SCWT) as a proxy for inhibition. As expected, performance in the auditory tasks worsened (SRTNs, GDTs, and FMDTs increased), and wave-I slope and SCWT inhibition scores decreased with ageing. Importantly, SRTNs, GDTs, and FMDTs were not related to wave-I slope but worsened with decreasing SCWT inhibition. Furthermore, after partialling out the effect of SCWT inhibition, age was no longer related to SRTNs or GDTs and became less strongly related to FMDTs. Altogether, results suggest that for people with normal audiograms, age-related deficits in auditory temporal processing and speech-in-noise intelligibility are mediated by decreased inhibition rather than cochlear synaptopathy.
Keywords: ageing, speech-in-noise intelligibility, auditory temporal processing, Stroop test, auditory brainstem response
Introduction
Elderly people can show poor speech-in-noise intelligibility (CHABA, 1988; Johannesen et al., 2019; Peters et al., 1998) and auditory temporal processing deficits (Schneider & Hamstra, 1999; Lopez-Poveda et al., 2019), even when their audiometric thresholds are clinically normal (≤ 20 dB HL; American Academy of Audiology, 2015). In the United States alone, an estimated 26 million people with no hearing loss on traditional audiometric tests have hearing difficulty (Beck, 2022; Beck et al., 2018). For those people, the problem is not reduced audibility. It remains unclear, however, what aspects of ageing, independent of hearing loss, explain those age-related hearing impairments.
Ageing is associated with declining cognitive functions, such as working memory capacity, attention, and processing speed, which could impair the ability to perform some auditory tasks even when audiometric thresholds are normal (Cohen, 1987; Craik & Byrd, 1982; Füllgrabe et al., 2015; Salthouse, 1996). Ageing is also associated with a loss of cochlear afferent synapses (synaptopathy, e.g., Sergeyenko et al., 2013) and/or auditory afferent fibers (deafferentation, e.g., Makary et al., 2011). These age-related disruptions in synaptic structure and function have been observed in experimental animals (Altschuler et al., 2015; Gleich et al., 2016; Liberman et al., 2014; Möhrle et al., 2016; Sergeyenko et al., 2013; Steenken et al., 2021) as well as in human cadavers (e.g., Makary et al., 2011; Viana et al., 2015; Wu et al., 2019). They have also been reported for living humans based on analyses of the amplitude or the slope of wave-I of the auditory brainstem response (ABR), a proxy for synaptopathy and/or deafferentation (e.g., Bramhall, 2021; Johannesen et al., 2019). Synaptopathy and deafferentation can theoretically degrade the coding of temporal sound features and impair the ability to understand speech in the presence of competing sounds (e.g., Johannesen et al., 2022; Lopez-Poveda, 2014; Lopez-Poveda & Barrios, 2013). For this reason, much research is being devoted to investigating whether synaptopathy and/or deafferentation contribute to age-related hearing deficits in people with normal audiograms (e.g., Grose et al., 2019; Harris et al., 2022; Johannesen et al., 2019; reviewed by Bramhall et al., 2019). Some studies, however, do not support this idea (reviewed by Bramhall et al., 2019; Guest et al., 2016). For example, for people with normal audiograms, wave-I slope is not correlated with speech-in-noise intelligibility, or envelope or temporal fine structure sensitivity even when all those variables decline with age (Johannesen et al., 2019; Lopez-Poveda et al., 2019). Although wave-I slope could decline with age due to conditions different from synaptopathy, such as strial degeneration (Lang et al., 2010; Schmiedt et al., 2002), strial degeneration occurs only after cochlear hair cell loss (Wu et al., 2020). Therefore, this suggests that the hearing deficits of older people with normal audiograms are unrelated to synaptopathy or deafferentation.
There is accumulating evidence that age-related deficits in the inhibitory system may contribute to age-related hearing deficits in at least two ways. First, as we age, neural inhibition decreases, which likely impairs temporal processing in auditory (e.g., Caspary et al., 2008; Gleich et al., 2003; Keller et al., 2018; Khouri et al., 2011; Sturm et al., 2017; reviewed by Caspary & Llano, 2019) and other sensory domains (Hasher et al., 1991; Hasher & Zacks, 1988). Gamma-aminobutyric acid (GABA) is a prevalent inhibitory neurotransmitter in the mammalian central nervous system. In the human inferior colliculus, an important hub involved in the processing of auditory information from the inner ear to the auditory cortex, the density of GABAergic neurons is negatively correlated with age (Pal et al., 2019). The total level of GABA in the auditory cortex is associated with speech-in-noise understanding in older age (Dobri & Ross, 2021; Harris et al., 2022). Furthermore, boosting GABA through systemic application improves impaired auditory temporal resolution in older gerbils (Gleich et al., 2003). This suggests that age-related hearing deficits might be caused by, or associated with, a diminished ability of the auditory system to process temporal aspects of acoustic information and that the origin of this impairment may lie in a decrease of inhibitory function in the brain. Second, ageing is associated with a diminished capacity to regulate incoming sensory data. Thus, older adults are more susceptible to distractions (Amer et al., 2016). Moreover, the ability to selectively focus on relevant auditory stimuli seems to deteriorate with age, which may be attributed to a decrease in the brain's ability to suppress the processing of irrelevant stimuli (Alain & Woods, 1999; Stothart & Kazanina, 2016). This suggests that age-related hearing deficits may be related to an impaired ability to inhibit distraction while performing auditory tasks.
In summary, as outlined in Figure 1, for people with normal audiograms and normal cognitive function, age-related hearing deficits could be the result of degraded sound encoding in the auditory nerve (synaptopathy/deafferentation), degraded auditory temporal processing (decreased central inhibition), and/or a diminished capacity to inhibit distraction.
Figure 1.
Schematic representation of the rationale of the study. The diagram illustrates that for people with normal audiograms and normal cognitive function, age-related hearing deficits can result from cochlear synaptopathy/deafferentation and/or decreased inhibition. Synaptopathy/deafferentation would degrade sound encoding in the peripheral auditory system, whereas decreased inhibition could impair auditory temporal processing and/or the ability to suppress distraction. The associations of age with synaptopathy/deafferentation (1) and with impaired temporal processing and diminished inhibition of interference (3) have been well established by previous research (see Introduction section). Here, we investigate the relative contributions of synaptopathy/deafferentation (2) and decreased inhibition (4) to hearing deficits. The diagram also shows the variables measured here to assess each aspect.
The present study investigated if reduced inhibition may be a factor underlying age-related deficits in auditory temporal processing and speech-in-noise recognition for people with normal audiograms and normal cognitive function. Inhibition was assessed behaviorally using the Stroop color-name interference inhibition test (MacLeod, 1991; Stroop, 1935). The Stroop test is widely and primarily used to assess the ability to inhibit cognitive interference that occurs when the processing of a specific stimulus feature impedes the simultaneous processing of a second stimulus feature, for example, when naming the ink color in which a color name is printed. Stroop inhibition, however, has also been associated with GABA levels in the brain (e.g., Kühn et al., 2016; Mamiya et al., 2021; Piras et al., 2021; Sofuoglu et al., 2005). Here, the Stroop test was used to directly assess the ability to inhibit cognitive interference as well as to indirectly assess GABA levels (Figure 1). In addition to the influence of inhibition, we examined whether synaptopathy/deafferentation and/or subclinical (high-frequency) cochlear hearing loss potentially contribute to age-related hearing deficits. Based on the evidence just described, we hypothesized that for people with normal audiograms and no cognitive impairment: (1) increased age would be linked with poorer speech-in-noise intelligibility, poorer auditory temporal processing, increased synaptopathy/deafferentation, and decreased inhibition; (2) speech-in-noise intelligibility and auditory temporal processing would not be associated with synaptopathy/deafferentation; and (3) poorer speech-in-noise intelligibility and auditory temporal processing would be related to reduced inhibition.
Materials and Methods
Experimental Design
Auditory temporal processing was assessed using two measures that are well known to decline with age for people with normal audiograms: gap detection thresholds (GDTs) (e.g., Schneider & Hamstra, 1999) and frequency modulation detection thresholds (FMDTs) (e.g., He et al., 2007). GDTs and FMDTs for low modulation rates may be thought of as indicating sensitivity to acoustic envelope and temporal fine structure cues, respectively (but see Whiteford et al., 2020). Speech-in-noise intelligibility was assessed by measuring the signal-to-noise ratio (SNR in decibels) at which participants recognized 50% of spoken words masked by an international female fluctuating masker (IFFM), termed speech reception threshold in noise (SRTN). We chose this speech task because age affects word recognition more than sentence recognition, even more so when words are masked by an IFFM than by steady noise (Johannesen et al., 2019; discussed later).
We used the amplitude of the ABR wave-I and its rate of growth (slope) with increasing stimulus level as proxies for cochlear synaptopathy (e.g., Bramhall, 2021; Johannesen et al., 2019). Our analyses, however, focused on the slope because it is a differential measure, so it is theoretically less affected by individual factors such as head size, sex, electrode impedance, or audiometric threshold that can increase the intersubject variability of wave-I amplitude (Garrett & Verhulst, 2019; Johannesen et al., 2019).
By including only participants with normal audiograms (hearing thresholds ≤ 20 dB HL at all audiometric frequencies from 250 to 8000 Hz), we minimized the potential effect of damage to the inner ear on auditory perception. Nonetheless, audiometric thresholds at 12 kHz (or high-frequency thresholds, HFTs) were measured to assess the potential impact of subclinical cochlear hearing loss on both age-related hearing deficits and synaptopathy estimates (Bramhall et al., 2019). We chose 12 kHz because it seemed a reasonable compromise between detecting mild-to-moderate hearing losses in younger participants and having measurable thresholds for older participants.
Cognitive abilities are associated with both speech-in-noise perception (Akeroyd, 2008; Dryden et al., 2017; Moore et al., 2010, 2014) and performance in psychoacoustic tasks (Zhang et al., 2016). Here, cognitive abilities were assessed using the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005). To minimize the influence of cognitive deficits on the results, we excluded participants with a MoCA score < 26 (out of a maximum of 30), the normative threshold for cognitive impairment (Nasreddine et al., 2005).
Based on recent studies, we expected no association of synaptopathy (wave-I slope) with auditory temporal processing (GDTs and FMDTs) (Lopez-Poveda et al., 2019) or SRTN (Johannesen et al., 2019). In contrast, if inhibition is involved in auditory processing and/or performance (Figure 1), we would expect poorer GDTs, FMDTs, and SRTNs for participants with weak inhibition, as characterized by Stroop interference inhibition scores.
The experiments were approved by the Ethics Committee of the University of Salamanca (Spain).
Participants
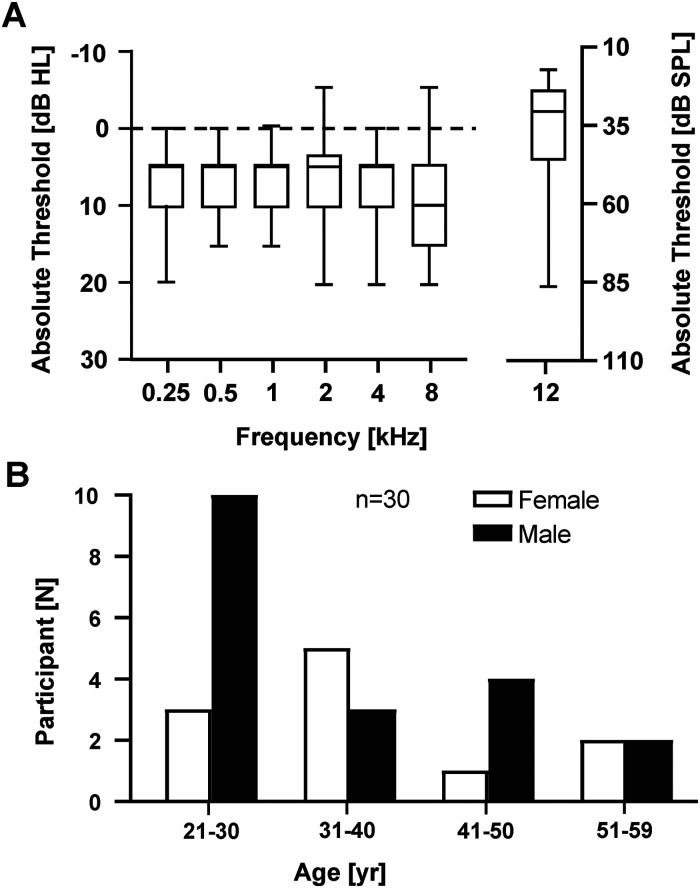
Participants were recruited through printed and electronic advertisements. The full protocol was administered to 61 volunteers with normal audiograms or mild-to-moderate hearing loss, but the analyses reported here are restricted to 30 adults (19 men) with normal audiograms (audiometric thresholds ≤20 dB HL; Figure 2A) and normal cognitive function (MoCA scores ≥ 26). Participants’ ages ranged from 21 to 59 years (mean ± SD = 35 ± 11 years; Figure 2B). They all had Spanish as their first language and at least 12 years of education. They had no history of neurological disease or cognitive impairment. Their MoCA scores (median = 27, interquartile range [IQR] = 2.25) were slightly higher than expected according to normative data (Nasreddine et al., 2005), possibly because the sample included a substantial proportion of young adults with high levels of education (i.e., master, and doctoral students). Participants were volunteers and were not paid for their time. All of them signed an informed consent to participate in the study. The standard pure-tone average (PTA) was calculated by averaging audiometric thresholds for 0.5, 1, 2, and 4 kHz and auditory stimuli were presented monaurally in the ear with the lower (better) PTA (28 right ears and two left ears).
Figure 2.
(A) Audiometric air-conduction thresholds (left panel) and high-frequency (12 kHz) thresholds (right panel) for the test ear. Note the different ordinate scales in the two panels (dB HL vs. dB SPL). Boxplots indicate the minimum threshold, first (lower) quartile, median, third (upper) quartile, and maximum threshold. (B) Distributions of age for men and women participants.
Audiometric Thresholds
Air-conduction hearing thresholds were measured at 0.25, 0.5, 1, 1.5, 2, 4, and 8 kHz using standard clinical procedures (ASHA, 2005) and equipment (audiometer: Interacoustics AD229e; headphones: Telephonics TDH-39). Stimuli were pure tones. Thresholds were measured using a 10-dB-down, 5-dB-up rule, and the threshold was defined as the level at which a tone was detected 50% of the times it was presented.
High-frequency thresholds (HFTs) were measured using custom-made software and research equipment and procedures. The stimulus was a 12 kHz pure tone, with 750 ms duration and 50 ms cosine-squared onset and offset ramps. HFTs were measured using a three-alternative forced-choice (3AFC) adaptive procedure with visual feedback. The interstimulus interval was 500 ms. The initial tone level was sufficiently high that the participant could always hear the tone. The tone level was then changed according to a one-down, one-up adaptive procedure to estimate the 50% point on the psychometric function (Levitt, 1971). The initial step size was 10 dB, which decreased to 5 dB after the first three reversals in level. The adaptive procedure continued until 12 reversals in level were measured. The threshold was calculated as the mean tone level at the last six reversals. Three threshold estimates were obtained, and their mean was taken as the HFT. Stimuli were generated digitally in Matlab (R2017b), output via an RME Fireface UCX sound card (sampling frequency of 44100 Hz, 24-bit resolution) and delivered through Etymotic ER-2 insert earphones. Calibration was performed at 1 kHz, and the obtained sensitivity was used at 12 kHz. According to the nominal calibration of the ER-2 earphones, the error introduced by this approximation was ≤ 2 dB.
Montreal Cognitive Assessment
After measuring audiometric thresholds, participants were evaluated using the MoCA for global cognitive function (Nasreddine et al., 2005). The MoCA was used because it is a screener of mild cognitive decline in several cognitive domains (Freitas et al., 2010; Nasreddine et al., 2005), including attention, concentration, executive functions, memory, language, visuoconstructional skills, conceptual thinking, calculations, and orientation, which could affect performance in the Stroop and/or auditory tests. Scores for tasks in each test domain were summed for a possible maximum score of 30 points, which would indicate no evidence of cognitive problems. The MoCA was administered by a trained experimenter, in Spanish (version 8.1), in a quiet room with a low distraction level, from a sitting position, and in bright lighting conditions. It took approximately 10 min to complete.
Stroop Color and Word Test
The Stroop Color and Word Test (SCWT) is a neuropsychological test used to assess the ability to inhibit cognitive interference (Scarpina & Tagini, 2017; Stroop, 1935). Each participant completed an SCWT (printed version) containing three conditions/cards: word card (W), color card (C), and color-word card (CW). First, the participant was asked to read aloud as quickly and accurately as possible a series of color words (red, green, or blue) printed in black on a white card for 45 s. The second part consisted of naming the ink color of nonwords (e.g., for the nonword XXXX printed in red ink, the correct answer would be red) as quickly and accurately as possible for 45 s. The third part was the interference condition, where the participant was asked to read aloud the ink color of the printed word as fast as possible for 45 s. In this condition, the participant was instructed to ignore the color names and to give the color of the ink in which the words were printed (e.g., saying “green” when the word “red” was printed in green ink). The three cards were presented to the participant in sequential order. Each condition was evaluated three times for each participant, and the mean score was taken as the final score. All conditions were run in Spanish (i.e., the words were rojo, verde, azul; Golden, 2001), in a quiet room with a low distraction level, sitting position, and in bright lighting conditions. Before running the test, it was confirmed that all participants could recognize and name the three colors.
The scoring method proposed by Golden (1978) was used. The number of items correctly named in 45 s in each of the three conditions (denoted W, C, and CW) was used to calculate a predicted CW score (Pcw) using the following formula:
| (1) |
The Pcw value was then subtracted from the number of items correctly named in the interference condition (CW) to give an interference/incongruent score (IG), that is, IG = CW – Pcw. Note that IG scores can be negative (Pcw > CW), positive (CW > Pcw), or zero (CW = Pcw), indicating worse, better than, or equal to the expected ability to inhibit interference, respectively. The higher the IG score, the better the ability to inhibit interference (Scarpina & Tagini, 2017).
Auditory Brainstem Responses
The ABRs were obtained using rarefaction clicks (duration 100 µs) with levels of 95 and 110 dB peak-to-peak equivalent sound pressure level (ppeSPL). Clicks were delivered monaurally to the test ear at a rate of 11 per second through Etymotic ER-3A insert earphones. The electrode attachment sites were cleaned with Nuprep mild abrasive gel, and the electrodes were fixed to the skin. The reference electrodes were placed on the right (A2) and left (A1) mastoids, and the active (Fz) and ground (Fpz) electrodes were placed on the forehead. The recording started only when the electrode impedance was less than 3 kΩ. Responses were acquired ipsilateral to the stimulus, amplified 100,000 times, and bandpass filtered from 100 to 3000 Hz. Noisy epochs with peak amplitude exceeding ±31 µV were excluded from the average. No additional filtering was applied after responses were acquired. At least 2000 responses were averaged for each measurement. The presence/absence of a wave-I response was determined by visual analysis of the latency and amplitude of the peak (van Zanten et al., 2022). At least two measurements were obtained for each stimulus level and the mean was taken as the final score. If the wave-I peak was challenging to identify, one or more additional measurements were obtained and included in the mean. Two experimenters independently identified the ABR peaks and troughs to verify the noted values.
As in previous studies (Johannesen et al., 2019; Johannesen & Lopez-Poveda, 2021; Mehraei et al., 2016), the ABR wave-I peak-to-trough amplitudes (in μV) for the two stimulus levels were used to calculate the slope in units of μV/dB. ABR measurements were made using an Intelligent Hearing System's Smart device (software version 5.50) in a double-wall sound-attenuating booth. Participants laid on a comfortable recliner chair and were instructed to remain as relaxed and steady as possible during the measurements. They were allowed to sleep.
Gap Detection
The task and procedure were similar to those employed by Schneider and Hamstra (1999). The task was designed to measure the shortest detectable silent interval (gap) between two sound markers. The target stimulus in the 3AFC procedure consisted of two identical pure-tone markers temporally separated by a gap. The standard intervals (no gap) consisted of an uninterrupted pure tone with the same duration and level as the target marker-gap-marker stimulus. The marker duration was held constant, and the gap duration was adapted during the experiment to measure the threshold gap duration required to discriminate the target from the standard stimuli. All stimuli had cosine onset and offset ramps with 1 ms duration. The gap was defined as the silent period between the markers. The marker duration was 50 ms. This marker duration was selected over a shorter one (e.g., 5 ms) because earlier studies have shown this marker duration to be more effective in revealing the effect of age on GDTs (Lopez-Poveda et al., 2019; Schneider & Hamstra, 1999). The frequency of the pure-tone markers was 2 kHz, and their level was 80 dB SPL. This level was 10 dB less than that used by Schneider and Hamstra (1999) to minimize activation of the stapedius reflex. A notched noise was used to mask spectral splatter during the onsets and offsets of the stimuli. The noise level was 40 dB SPL/Hz in the spectral regions 2 to 1850 Hz and 2150 to 7850 Hz and less than −20 dB SPL/Hz in the spectral region of the marker tone (1880–2120 Hz). The notched noise had 20 ms cosine ramps, started 300 ms before the marker tone, and ended 100 ms after it. The initial gap was set to 50 ms so the participant could always identify the target interval. The gap duration was then changed logarithmically following a two-down, one-up adaptive procedure to estimate the 71% point on the psychometric function (Levitt, 1971). The gap duration was initially adapted by a factor of 1.7, which was reduced to 1.3 after two reversals. The adaptive procedure continued until 10 reversals in gap value were measured. The threshold was calculated as the geometric mean gap value at the last eight reversals. An estimate was discarded if the geometric standard deviation of the last eight reversals exceeded 2.0. Three threshold estimates were obtained in this way, and their geometric mean was taken as the GDT. If the geometric standard deviation of the three estimates exceeded 2.0, a fourth estimate was obtained and included in the mean.
Frequency Modulation Detection
FMDTs were measured as described elsewhere (Johannesen et al., 2016; Strelcyk & Dau, 2009). The experiment measured the just-detectable excursion in frequency, Δf, needed to discriminate a pure tone with frequency fc from a tone modulated in frequency between fc−Δf and fc + Δf. Thresholds were measured using a 3AFC procedure. All intervals included a carrier tone with frequency fc = 1500 Hz and 750 ms duration, including 50 ms raised-cosine onset and offset ramps. In the target interval, the carrier frequency was modulated sinusoidally at a rate of 2 Hz. The tones in all intervals were also sinusoidally amplitude modulated (AM) with a modulation depth of 6 dB and with an instantaneous modulation rate that either increased or decreased linearly with time. The initial and final AM rates were each chosen randomly from the interval between 1 and 3 Hz. The use of a low rate of frequency variation (2 Hz), a low-frequency carrier (1500 Hz), and the randomized AM were intended to emphasize the dependence of the FMDT on temporal information (phase locking to the carrier) rather than on changes in the excitation patterns (Moore & Sek, 1996; Whiteford et al., 2020). The use of a carrier tone frequency of 1500 Hz, in the middle of the speech frequency range, was also intended to provide a measure of the ability to follow temporal fine structure cues in speech. Like GDTs, FMDTs were measured for a carrier level of 80 dB SPL. Δf was tracked logarithmically following a two-down, one-up adaptive procedure to estimate the 71% point on the psychometric function (Levitt, 1971). The initial value was Δf = 100 Hz. The value of Δf was changed by a factor of 2.0 until four reversals had occurred, and by a factor of 1.26 thereafter. The adaptive procedure continued until 12 reversals in Δf were measured. The threshold was calculated as the geometric mean of Δf values at the last eight reversals. A mean value was discarded if the geometric standard deviation of Δf at the last eight reversals exceeded 2.0. Three threshold estimates were obtained this way, and their geometric mean was taken as the participant's threshold. If the geometric standard deviation of the three estimates exceeded 2.0, a fourth estimate was measured and included in the mean.
Speech-In-Noise Intelligibility Test
SRTNs were measured as in Johannesen et al. (2019). Speech tokens were disyllabic words uttered by a male talker from the corpus of Cárdenas and Marrero (1994), a standard for clinical testing in Spain. The noise was the IFFM (Holube et al., 2010), which consisted of a multilingual voice signal with the spectral and temporal characteristics of a single female speaker but was unintelligible. The IFFM was based on natural recordings but was largely incomprehensible because of segmentation and remixing. To measure an SRTN, the speech level was fixed at 65 dB SPL, and the noise level varied adaptively using a one-up, one-down rule to estimate the SNR in dB at which the participant could recognize 50% of the words. The masker started 500 ms before the speech onset and ended 50 ms after the speech offset. The initial SNR was +10 dB. The step size in SNR was initially 4 dB, decreased to 2 dB after eight conditioning words, and remained at 2 dB during the test words. Twenty-five words were presented for each SRTN measurement. The SRTN was calculated as the mean SNR of the last 12 words. A measurement was discarded if the standard deviation exceeded 3 dB. Three SRTN estimates were obtained, and their mean was taken as the SRTN. If the standard deviation of the three estimates exceeded 3 dB, a fourth estimate was obtained and included in the mean.
Stimuli and Apparatus
For all psychoacoustic tasks, stimuli were generated digitally in Matlab (R2017b), output via an RME Fireface UCX sound card (sampling frequency of 44100 Hz, 24-bit resolution) and delivered to the listeners through Sennheiser HD-580 headphones. The output level was calibrated by placing the headphones on a KEMAR head (Knowles Electronics) equipped with a Zwislocki DB-100 artificial ear connected to a sound level meter (Brüel & Kjaer 2238). Calibration was performed at 1 kHz, and the obtained sensitivity was used at all other frequencies. Measurements were made in a double-wall sound-attenuating booth.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics (version 26) and GraphPad Prism (version 9). All variables except for age and HFT were normally distributed (Kolmogorov-Smirnoff test, p > .05). To confirm the typical pattern of reaction time data in Stroop tasks, scores for each pair of conditions (word reading, neutral color-naming, and incongruent color-naming) were compared using paired t-tests. Linear regression was used to investigate pairwise relationships between age, wave-I slope, SCWT inhibition score, SRTN, GDT, and FMDT. Linear regression analyses were warranted because the residuals were normally distributed (Kolmogorov-Smirnoff test, p > .05) (Andersen & Skovgaard, 2010). Partial linear regression was applied to control for the effect of SCWT inhibition on the relationship between age and SRTN, GDT, or FMDT, as well as to control for the effect of age on the relationship between SCWT inhibition and SRTN, GDT, or FMDT. Because age, SCWT, FMDT, and HFT were all co-linear, multiple linear regression was applied to examine whether the age-adjusted relationship between SCWT inhibition and FMDT remained significant after controlling for HFT. All reported significance levels (p) are for two-tailed tests.
Results
Table 1 shows means and SDs for the measured variables. As described later, values were regarded as typical for individuals with the age range, cognition state, and audiometric characteristics of the present participants.
Table 1.
Group Mean and SD for Each variable.
| Variable | Mean | SD |
|---|---|---|
| Age (years) | 35.2 | 10.7 |
| SCWT (IG) | 6.5 | 8.5 |
| SRTN (dB SNR) | −16.6 | 3.2 |
| GDT (ms) | 3.6 | 1.9 |
| FMDT (Hz) | 6.6 | 2.6 |
| Wave-I amplitude at 95 dB ppeSPL (μV) | 0.172 | 0.09 |
| Wave-I amplitude at 110 dB ppeSPL (μV) | 0.305 | 0.11 |
| Wave-I slope (μV/dB) | 0.0088 | 0.006 |
| HFT (dB SPL) | 37.4 | 18.3 |
| PTA (dB HL) | 6.8 | 3.3 |
Note. PTA was calculated by averaging the audiometric thresholds across 0.5, 1, 2, and 4 kHz. FMDT=frequency modulation detection thresholds; GDT=gap detection threshold; HFT=high-frequency threshold; PTA=pure-tone average; SCWT=Stroop Color-Word Test; SNR=signal-to-noise ratio; SRTN=speech reception threshold in noise.
Age-Related Effects
Effect of Age on High-Frequency Hearing Threshold
Hearing thresholds at 12 kHz (mean = 37.4 dB SPL, SD = 18.3; Figure 2A) were comparable to those in Shim et al. (2009) (mean = 39 dB SPL, SD = 12). The effects of age on hearing thresholds are greater at frequencies above 8 kHz than at lower frequencies (e.g., Osterhammel & Osterhammel, 1979; Wang et al., 2021). Consistent with this, HFTs tended to increase with increasing age (Figure 3A).
Figure 3.
Relationship between age and other measured variables. (A) Absolute hearing thresholds at 12 kHz (HFTs); (B) speech reception thresholds for disyllabic words masked by an international female fluctuating masker (SRTNs); (C) thresholds for detecting a silent gap (a pause) between two pure-tone markers (GDTs); (D) thresholds for detecting frequency modulation (FMDTs); (E) ABR wave-I slope; and (F) inhibition scores in the SCWT. The insets indicate the proportion of variance explained (R2) by a linear relationship and the probability (p) of a non-zero regression line slope occurring by chance. Points represent individual scores. Solid lines depict linear regression lines; dotted lines represent the regression's 5 and 95% confidence intervals. ABR=auditory brainstem response; FMDT=frequency modulation detection threshold; GDT=gap detection threshold; HFT=high-frequency threshold; SCWT=Stroop Color-Word Test; SRTN=speech reception thresholds in noise.
Speech-In-Noise Intelligibility and Age
The group mean SRTN (−16.6 dB SNR, SD = 3.2) was close to that for the normal-hearing listeners in Johannesen et al. (2019). Based on previous studies (e.g., Dubno et al., 2002; Holube et al., 2010; Johannesen et al., 2019), we expected SRTN to worsen with increasing age and the SRTN did indeed increase significantly with age (Figure 3B).
Effect of Age on GDTs
Older listeners have more difficulty than younger listeners in detecting silent gaps within speech and nonspeech stimuli (Pichora-Fuller et al., 2006). Older listeners with normal audiograms exhibit GDTs approximately twice as large as those of younger listeners (Schneider et al., 1998; Snell, 1997). Therefore, we expected GDTs to increase with increasing age (Moore et al., 1992; Schneider & Hamstra, 1999; Schneider et al., 1994; Strouse et al., 1998; Lister & Roberts, 2002; Roberts & Lister, 2004). The present group mean GDT (3.6 ms, SD = 1.9) was close to values reported elsewhere for sinusoidal markers for younger and older listeners (Schneider et al., 1994: younger: 3.8 ms, older: 6.2 ms; Strouse et al., 1998: younger: 2.8 ms, older: 6.7 ms; Schneider & Hamstra, 1999: younger: ∼2 ms, older: ∼3 ms). Moreover, consistent with expectations, GDTs increased significantly with increasing age, as shown in Figure 3C.
Effect of Age on Frequency Modulation Detection
The group mean FMDT for the present participants (6.6 Hz, SD = 2.6) was comparable to the average value (∼6 Hz) reported by Strelcyk and Dau (2009) for normal-hearing listeners aged 21 to 55 years. Consistent with the results of other studies (Grose & Mamo, 2012; He et al., 2007; Moore et al., 2019; Wallaert et al., 2016; Whiteford et al., 2017), here, FMDTs tended to increase (worsened) with increasing age, although the trend just missed statistical significance for a two-tailed test (Figure 3D). We note, however, that Schoof et al. (2014) found no significant effect of age on detecting 2-Hz FM applied to a 1000-Hz carrier.
Age-Related Changes in the Wave-I Auditory Brainstem Response
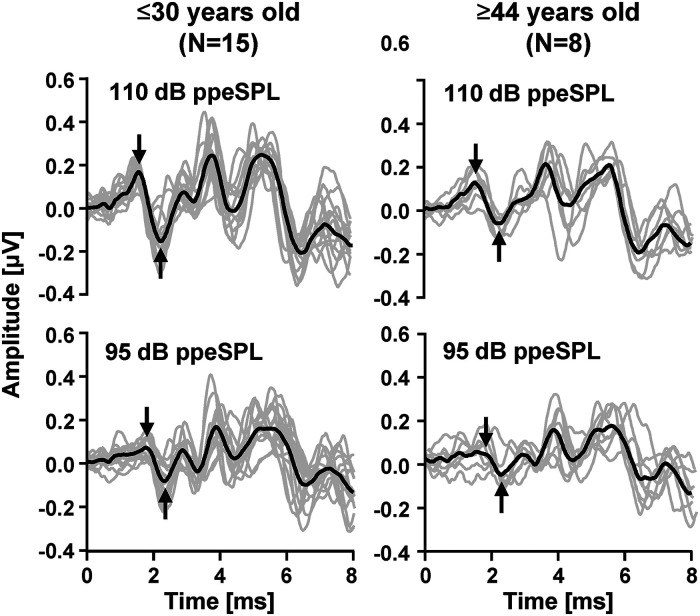
The group mean wave-I amplitudes (95 dB ppeSPL: 0.17 µV, SD = 0.09; 110 dB ppeSPL: 0.31 µV, SD = 0.11, Figure 4) agreed with previously reported values for normal-hearing listeners obtained using click stimuli (0.27 µV at 110 dB ppeSPL in Konrad-Martin et al., 2012) and were smaller than reported for tone-burst stimuli (0.41 μV at 110 dB ppeSPL in Bramhall et al., 2018). The group mean wave-I slope was 0.0088 μV/dB, which was in the range (0.0061–0.0182 μV/dB) reported by Johannesen et al. (2019); slightly higher than reported by Turner et al. (2022) (0.0063 μV/dB); and smaller than reported for normal-hearing women (0.02 μV/dB) in Guest et al. (2019).
Figure 4.
The ABR waveforms evoked by monaural click stimuli at 95 (bottom) and 110 dB ppeSPL (top), for participants aged ≤30 years (left) and ≥44 years (right). Gray and black traces illustrate individual, and group mean responses, respectively. Wave-I peak-to-peak amplitudes are indicated by pairs of arrows. ABR=Auditory brainstem response.
Based on past studies, we expected a decrease in auditory nerve output with increasing age (e.g., Bramhall, 2021; Johannesen et al., 2019; Johannesen & Lopez-Poveda, 2021; Makary et al., 2011; Sergeyenko et al., 2013; Viana et al., 2015; Wu et al., 2019). Consistent with expectations, wave-I amplitude was smaller for older than for younger listeners (Figure 4) and wave-I slope decreased with increasing age (Figure 3E).
Age-Related Alterations of Stroop Interference Inhibition
Consistent with the typical pattern of means observed on the Stroop task (Bugg et al., 2007), our participants were significantly faster at reading words (mean W = 120 words in 45 s, SD = 18) than naming colors (mean C = 81 colors in 45 s, SD = 13) [t(29) = 18.5, p < .001]. In addition, response times were significantly longer in the incongruent color-naming condition (mean CW = 55 color-words in 45 s, SD = 14) than in the neutral color-naming condition [t(29) = 25.1 p < .001]. The means of the present word, color, and color-word scores were comparable to the values reported by Connor et al. (1988) (W = 114, C = 81, CW = 50) for college students, or Doan and Swerdlow (1999) (W = 109, C = 76, CW = 45) for men and women aged 21 to 59 years.
The ability to inhibit irrelevant information has been suggested to worsen with age (Bugg et al., 2007; Hasher & Zacks, 1988; van der Elst et al., 2006), with implications across various auditory domains, including speech perception (Knight & Heinrich, 2017). Some studies have reported age-related decrements in Stroop test performance (Hartman & Hasher, 1991; Hasher & Zacks, 1988), while others did not find such effects (Graf et al., 1995). Here, the mean interference score (IG) was 6.5 ± 8.5. As shown in Figure 3F, older listeners were less able to inhibit the interference from word meaning.
The Confounding Effect of High-Frequency Hearing Loss on Wave-I Slope and Auditory Performance
Given that auditory nerve fibers innervating the cochlear base (high-frequency region) are probably the main contributors to click-evoked wave-I responses (Don & Eggermont, 1978; Eggermont & Don, 1980), we evaluated whether subclinical cochlear hearing loss, as assessed by HFT, affected our synaptopathy estimate. We did this by assessing the relationship between ABR wave-I amplitude and slope with HFTs. Based on previous studies (e.g., Bramhall et al., 2019), we expected both wave-I amplitude and slope to decrease with increasing HFT. However, the relationship between HFT and ABR wave-I amplitude (R2=.102, p = .084) and slope (R2 = .061, p = .187) was not significant. This result suggests that for the present participants, wave-I slope, our principal proxy for synaptopathy (see Methods section), was not significantly confounded by hearing loss in the extended high-frequency range.
The relationship between SRTN and HFT was not significant (R2 = .050, p = .236). This is consistent with a study that suggests that, after adjusting for age, sound energy in the high-frequency range is not functionally helpful in recognizing speech in noise (Mepani et al., 2020). However, it is inconsistent with other studies that have reported that although the effect of age is much larger than the effect of hearing status (Jain et al., 2022), speech-in-noise recognition nevertheless correlates negatively with high-frequency hearing loss (Jain et al., 2022; Zadeh et al., 2019).
Relationship Between Inhibition and Speech-In-Noise Intelligibility and Temporal Processing
There was a significant negative relationship between SCWT inhibition scores and GDTs (Figure 5A) and FMDTs (Figure 5B). Also, there was a significant negative relationship between the SCWT inhibition score and SRTN (Figure 5C). The latter finding is in line with Janse (2012), who reported that, for listeners with hearing loss, Stroop interference predicted speech processing performance in multitalker conditions over and above the effect of hearing loss.
Figure 5.
Relationship between three age-sensitive measures of hearing function (GDTs, FMDTs, or SRTNs) with SCWT inhibition score (upper panels) and ABR wave-I slope (lower panels); otherwise as Figure 3. ABR=auditory brainstem response; FMDT=frequency modulation detection threshold; GDT=gap detection threshold; SCWT=Stroop Color-Word Test; SRTN=speech reception threshold in noise.
Because age affected SCWT inhibition scores, GDTs, FMDTs, and SRTNs (Figure 3), the relationship between the three measures of auditory performance and SCWT inhibition scores could reflect the co-linearity of all four variables with age rather than the importance of inhibition for auditory temporal processing and/or speech-in-noise recognition. We applied two control analyses to clarify the importance of inhibition.
First, we investigated if decreased inhibition was necessary for the negative effect of age on auditory performance to occur. If it were, the significant relationship of age with GDTs or SRTNs should become nonsignificant after adjusting for SCWT inhibition. However, if the relationship of age with auditory performance remained significant after adjusting for SCWT scores, this would suggest that other factors hidden under the label “age” and different from SCWT inhibition affect performance. After adjusting for SCWT inhibition, age was not significantly related with SRTNs (R2 = .110 p = .079) or GDTs (R2 = .067, p = .176) (Table 2). The relationship between age and FMDTs, which just missed statistical significance (R2 = .121, p = .059), became weaker after adjusting for SCWT inhibition (R2 = .016, p = .514). This suggests that worsening of inhibition, as measured by the SCWT, is necessary to observe age-related deficits in speech-in-noise intelligibility and gap detection, and contributes to age-related deficits in frequency-modulation detection.
Table 2.
Amount of Variance (R2) Explained by Linear Regression Models Used to Investigate the Relationship of Age and SCWT Inhibition (Independent Variables) With Auditory Performance (Dependent Variables) Before and After Controlling for the Effects of SCWT Inhibition and Age, Respectively.
| Independent variable | Control variable | SRTN (dB SNR) | Dependent variable | FMDT (Hz) |
|---|---|---|---|---|
| GDT (ms) | ||||
| Age (years) | -none- | .209 p = .011 | .171, p = .023 | .121, p = .059 |
| SCWT (IG) | .110, p = .079 | .067, p = .176 | .016, p = .514 | |
| SCWT (IG) | -none- | .152, p = .033 | .183, p = .018 | .269, p = .003 |
| Age (years) | .046, p = .265 | .081, p = .134 | .181, p = .021 |
Note. Also shown is the probability of a significant linear relationship (p, two-tailed). Statistically significant relationships (p≤.05) are indicated in bold font. FMDT=frequency modulation detection threshold; GDT=gap detection threshold; SCWT=Stroop Color-Word Test; SNR=signal-to-noise ratio; SRTN=speech reception threshold in noise.
Second, we investigated if decreased inhibition per se contributes to a decline in performance in the auditory tasks. If it did, auditory performance should decrease with decreasing inhibition after adjusting for age. This was not the case for GDTs (R2 = .081, p = .134) or SRTNs (R2 = .046, p = .265) (Table 2). In other words, the significant relationship between SCWT inhibition scores and GDTs or SRTNs became nonsignificant after adjusting for age. However, the relationship between SCWT inhibition and FMDTs remained significant after adjusting for age (R2 = .181, p = .021), indicating that better FMDTs were associated with greater inhibition, regardless of age.
To assess whether the relationship between SCWT inhibition and FMDTs after adjusting for age was not due to a co-linearity of these two variables with HFTs, we performed a multiple regression analysis (Table 3). This analysis revealed that the incorporation of age (Model 2) did not improve the model (i.e., the adjusted R2 was smaller for Model 2 than Model 1), and that the addition of HFTs had a minimal effect on the model's predictive power (compare the adjusted R2 values of Model 2 and Model 3 in Table 3). We also calculated the variance inflation factor (VIF), an indicator of co-linearity (Hair et al., 2013; Kock, 2015). For all variables, VIFs were less than 4, indicating that there was no significant co-linearity between them (SCWT: Tolerance = .70, VIF = 1.43; Age: Tolerance = .41, VIF = 2.43; HFT: Tolerance = .385, VIF = 2.59) (Hair et al., 2013). In summary, this suggests that decreasing inhibition per se, regardless of age, partly accounted for the worsening in FMDTs but not in SRTNs or GDTs.
Table 3.
Multiple Linear Regression Models Used to Examine the Significance of SCWT Inhibition Scores, Age, and HFTs in Predicting FMDTs.
| Model | R² | Adj. R² | Coeff. | SE | β | p |
|---|---|---|---|---|---|---|
| Model 1 | .269 | .243 | 7.641 | .516 | <.001 | |
| SCWT | −.157 | .049 | −.519 | .003 | ||
| Model 2 | .281 | .228 | 6.478 | 1.832 | .001 | |
| SCWT | −.139 | .057 | −.458 | .021 | ||
| Age | .030 | .045 | .124 | .514 | ||
| Model 3 | .346 | .271 | 6.425 | 1.780 | .001 | |
| SCWT | −.112 | .057 | −.370 | .062 | ||
| Age | −.035 | .059 | −.148 | .556 | ||
| HFT | .058 | .036 | .413 | .118 |
Note. The table presents the amount of variance explained by the model (R2) adjusted for the number of predictors (adj. R2), the model coefficients (Coeff.), the standard error of the coefficients (SE), the standardized regression coefficients beta (β) and the probability of a significant contribution to the model for each variable (p). FMDT=frequency modulation detection threshold; GDT=gap detection threshold; HFT=high-frequency threshold; SCWT=Stroop Color-Word Test.
Relationship Between Synaptopathy and Speech-In-Noise Intelligibility and Temporal Processing
Based on previous studies (e.g., Johannesen et al., 2019; Lopez-Poveda et al., 2019), we expected wave-I slope not to be significantly related to performance in the present auditory tasks. Consistent with expectations, GDTs (R2 = .097, p = .094), FMDTs (R2 = .002, p = .835), and SRTNs (R2 = .007, p = .654) were not significantly related to wave-I slope (Figure 5D–F). If ABR wave-I slope is a proxy for synaptopathy/deafferentation, this suggests that synaptopathy may not be a significant factor in the temporal processing or speech-in-noise intelligibility deficits of older people with normal audiograms. An alternative interpretation of this result is that wave-I slope is not a good metric of synaptopathy/deafferentation. This interpretation is discussed later.
Discussion
This study investigated why older people with clinically normal hearing can nevertheless experience auditory temporal processing deficits and increased difficulties understanding speech in noise. For people with hearing loss, these difficulties have been associated with cochlear hair cell loss or damage to mechanosensory hair cell bundles (e.g., Lichtenhan et al., 2017; Lorenzi et al., 2006; Zadeh et al., 2019), as well as with a decline in central auditory processing and/or cognitive function (e.g., Cohen, 1987; Füllgrabe & Rosen, 2016; Ou & Law, 2017). Here, to separate the effects of age from the usually concomitant effects of hearing loss and/or cognitive decline, we tested participants aged 21 to 59 years, with normal audiograms (audiometric thresholds ≤ 20 dB HL), and with no sign of cognitive impairment (MoCA score ≥ 26). We examined if the age-related decline in inhibitory function, which can impair auditory temporal processing (Caspary et al., 2008; Gleich et al., 2003) as well as reduce the ability to suppress distraction (Lamers et al., 2007), and/or the age-related decline in cochlear synapses or nerve fibers (Makary et al., 2011; Sergeyenko et al., 2013; Wu et al., 2019), which can degrade the encoding of sounds in the auditory nerve (Lopez-Poveda, 2014; Lopez-Poveda & Barrios, 2013), affect auditory temporal processing and speech-in-noise intelligibility.
We found that age-related cochlear synaptopathy (as assessed by the slope of ABR wave-I) was not significantly associated with age-related deficits in temporal processing (GDTs and FMDTs) or SRTNs (Figure. 5D–F). In contrast, decline in inhibition (as assessed by the SCWT interference inhibition score) was significantly associated with worse GDTs, FMDTs, and SRTNs (Figure 5A–C). Furthermore, after partialling out the effect of inhibition, age was no longer significantly related to SRTNs or GDTs and became less strongly related to FMDTs (Table 2). The relationship between FMDTs and SCWT inhibition remained significant after partialling out the effect of age. Because all participants had audiometric thresholds within the clinically normal range, our findings support the idea that older people can suffer from listening difficulties (e.g., Craik & Byrd, 1982; Johannesen et al., 2019, 2016; Makary et al., 2011; Saunders & Haggard, 1989; Wallaert et al., 2016), and suggest that these difficulties may be more closely related to decreased inhibition than to cochlear synaptopathy.
Reduced Inhibition is Likely to Affect Speech-In-Noise Intelligibility and Auditory Temporal Processing
We found an association between decreased inhibition, as assessed by the SCWT, and worse auditory temporal processing and SRTN (Figure 5A–C). Although the SCWT measures behavioral inhibition, GABA magnetic resonance spectroscopy studies have shown that performance of the SCWT is accompanied by GABA increases in the anterior cingulate cortex (Kühn et al., 2016), the caudate nucleus in the front-striatal circuitry (Mamiya et al., 2021) and the cerebellum (Piras et al., 2021). Furthermore, Sofuoglu et al. (2005) showed that increasing synaptic GABA levels enhanced Stroop interference inhibition in a clinical study. Therefore, the present finding that subjects with an indication of weak inhibition in the SCWT also performed more poorly in auditory tasks is consistent with the accumulating evidence that central inhibition plays a key role in auditory function (reviewed by Caspary et al., 2008 and Caspary & Llano, 2019). This is in line with recent findings that age-related deficits in speech-in-noise recognition are associated with reduced GABA levels in the cortex (e.g., Dobri & Ross, 2021; Harris et al., 2022). However, it is possible that the observed relationship of SCWT inhibition with auditory performance is mediated by biological mechanisms beyond the already noted (age-related) reductions in central inhibition.
The SCWT measures the ability to inhibit interference. In speech recognition tasks, older listeners are more susceptible to distraction from competing talkers than younger listeners (Humes et al., 2006; Tun et al., 2002; Tun & Wingfield, 1999). Therefore, the relationship between SCWT inhibition and SRTNs (Figure 5C) could reflect an age-related change in the ability to inhibit distraction in the two tasks (Faßbender et al., 2023). Similarly, the relationship of SCWT inhibition with FMDTs could reflect an age-related ability to inhibit interference from the AM superimposed on the target FM, and the relationship of SCWT scores with GDTs could reflect an age-related ability to inhibit interference from the notched noise used to mask the spectral splatter caused by the gap onset/offset.
During a speech intelligibility task, a single-talker masker could compete more for the listener's attention than a non-speech masker. It might be harder for the listener to inhibit a single-talker masker than multitalker or nonspeech maskers. This notion is supported by the fact that, for people with normal audiograms, the effect of age on SRTNs is stronger for IFFM than for steady speech-shaped noise (e.g., Johannesen et al., 2019). Although the IFFM used here was mostly meaningless, it was single-talker masker. The relationship between SRTNs and SCWT inhibition (Figure 5C) might be weaker for nonspeech maskers and stronger for meaningful single-talker maskers. Also, it might be different (weaker) in listening situations where the masking of speech is less closely related to cognitive factors (Leek et al., 1991; Kidd et al., 2008).
It has been suggested that increases in SCWT interference could be due, at least in part, to factors such as a decline in attention (Lamers et al., 2007), concentration (MacLeod, 1991), or executive functions (Homack & Riccio, 2004). These cognitive deficits could slow performance in one or more Stroop conditions, potentially leading to a change in overall processing speed and test score. Because the MoCA is sensitive to mild cognitive deficits in several cognitive domains, and the present participants had MoCA scores in the normal range, it is unlikely that Stroop test scores in the present study were strongly affected by age-related cognitive impairments. With the chosen cutoff score (≥26), however, the MoCA has a sensitivity to mild cognitive impairment of 90% (Nasreddine et al., 2005). Therefore, it remains a possibility that ∼10% of the tested participants had mild cognitive impairment, and that a (minimal) part of the relationship between age or auditory performance and SCWT scores be driven by this group. It is unlikely that the age-related increase in SCWT interference was due to age-related changes in color vision (Ben-David & Schneider, 2010) because all participants were able to discriminate the three colors involved in the SCWT when presented on a white background.
Here, we have assumed that SCWT serves to directly assess cognitive inhibition as well as to indirectly assess inhibitory neurotransmission (GABA levels) (Figure 1). Previous studies have used the ratio of ABR wave V to wave I amplitude as a measure of central gain, and reported increased gain (reduced inhibition) in older adults (Grose et al., 2019; Johannesen & Lopez-Poveda, 2021; Rumschlag et al., 2022). Consistent with those studies, an exploratory analysis of the present data revealed that the wave V/I amplitude ratio increased with increasing age, although the relationship just missed statistical significance (R2 = .126, p = .054). However, the wave V/I amplitude ratio was neither related to SCWT inhibition scores (R2 = .010, p = .600) nor to GDTs (R2 = .024, p = .410), FMDTs (R2 = .023, p = .427), or SRTNs (R2 = .003, p = .775). If the wave V/I amplitude ratio is a reasonable indicator of increased gain and of decreased GABA (Chambers et al., 2016; Harris et al., 2022; Resnik & Polley, 2021), this could weaken the assumption that SCWT inhibition scores serve to indirectly assess central GABA levels. Also, it could weaken the notion that the hearing deficits of older people with normal audiograms are due to decreased GABA, and would strengthen the notion that they are due to diminished cognitive ability to inhibit interference. However, the wave V/I amplitude ratio may be an indicator for a decreased inhibition already below the level of the inferior colliculus where glycinergic inhibition is also important (Altschuler & Shore, 2010). In gerbils, a compensatory increase in amplitude of the wave IV (which is equivalent to wave V in the human ABR, see Boettcher et al., 1993) has been observed with an age-related decrease of wave I amplitude (Laumen et al., 2016). Gerbil ABR wave IV likely reflects the output of the superior olivary complex in which glycinergic inhibition provided by the medial nucleus of the trapezoid body plays a major role in setting the gain (Laumen et al., 2016; Rotschafer et al., 2015). Therefore, the wave V/I amplitude ratio may not be a good indicator for central GABAergic inhibition, which may explain the lack of a correlation between this measure and the SCWT inhibition score. Thus, it remains possible that the SCWT inhibition score provides a measure of central GABAergic inhibition as indicated by the magnetic resonance spectroscopy studies on the relation between GABA levels and SCWT performance (e.g., Kühn et al., 2016; Mamiya et al., 2021; Piras et al., 2021; Sofuoglu et al., 2005).
No Evidence that Cochlear Synaptopathy Affects Speech-In-Noise Intelligibility or Auditory Temporal Processing
Consistent with previous studies, we found the slope (and amplitude) of ABR wave-I to decrease gradually with increasing age (Figure 3E; Bramhall et al., 2019; Johannesen et al., 2019), as does the recognition of masked words (Figure 3B) and performance in auditory temporal processing tasks (Figure 3C and D; Johannesen et al., 2016; Makary et al., 2011; Schneider et al., 1994; Strelcyk & Dau, 2009). If the age-related declines in wave-I slope and performance in the present auditory tasks were both due to synaptopathy, one would expect SRTNs, GDTs, and FMDTs to increase with decreasing wave-I slope. Consistent with previous studies (e.g., Johannesen et al., 2019; Lopez-Poveda et al., 2019), this was not the case (Figure 5D–F).
The most significant contributions to click-evoked ABR wave-I responses originate from basal cochlear regions with characteristic frequencies (CFs) of 2 kHz and higher (Don & Eggermont, 1978; Eggermont & Don, 1980). Presumably, the stimuli used here to assess auditory performance excited mainly the central cochlear region (SRTN: ∼0.5–2 kHz; GDT: 2 kHz, FMDT: 1.5 kHz). Therefore, the absence of a relationship between wave-I slope and SRTNs, GDTs, or FMDTs might be related to the fact that the stimuli used in the behavioral tasks stimulate cochlear regions not directly associated with areas that generate ABR wave-I responses or not affected by synaptopathy. This, however, is unlikely because the click levels used here were high enough to stimulate the entire cochlea (He et al., 2018; Recio et al., 1998), albeit weakly and less synchronized at low CFs, and in humans (Wu et al., 2019) and gerbils (Gleich et al., 2016; Steenken et al., 2021) age-related auditory-nerve fiber and synapse loss affects all cochlear regions to some extent.
The auditory nerve comprises a heterogenous population of type I afferent fibers often classified into two subtypes: low spontaneous rate (SR) and high SR (Liberman, 1978). In some lower mammals (e.g., cat, guinea pig), low-SR fibers have higher response thresholds, wider dynamic ranges, and are more resistant (less susceptible to synaptic fatigue, i.e., vesicle depletion) to background noise than the high-SR fibers (Bharadwaj et al., 2014). In gerbil, low-SR fibers appear to be the most susceptible to effects of age, whereas high-SR fibers are generally preserved (Schmiedt et al., 1996; but see Heeringa et al., 2020). Theoretical studies have shown that high-SR fibers may be sufficient to encode the properties of sounds, at least in quiet (Johannesen et al., 2022) and that a substantial proportion of synapses/fibers would need to be lost for synaptopathy to significantly affect hearing performance (Oxenham, 2016). Although the characteristics of low-SR and high-SR fibers may be different in higher mammals, including humans (Figure 3 in Joris et al., 2011), the potential preservation of high-SR fibers during ageing for audiometrically normal listeners is another possible explanation for why SRTNs, GDTs, and FMDTs may not be severely affected by age-related cochlear synaptopathy.
Limitations
This study was an exploratory attempt to elucidate the relative contributions of reduced inhibition and cochlear synaptopathy/deafferentation to age-related hearing deficits for people with normal audiograms. One limitation is that only four out of the 30 participants had ages over 45 years (Figure 2B). This weakens the chances of finding age-related changes, including synaptopathy. Another limitation is that both inhibition and cochlear synaptopathy were assessed using proxy metrics: the SCWT inhibition score and ABR wave-I slope, respectively. While the SCWT is appropriate to directly measure the inhibition of distraction (Figure 1), the version used here did it in the visual rather than the auditory domain. Also, while GABA levels rise during the performance of the SCWT in cortical areas involved in decision making (Kühn et al., 2016), it remains an assumption that SCWT scores serve as an indirect measure of GABA levels in the central auditory system. Furthermore, inhibition is involved in sensory and cognitive stages of auditory perception (Schneider & Pichora-Fuller, 2000). While we have shown that inhibition control at the cognitive level is a significant factor in age-related hearing deficits, it is not clear whether sensory inhibition also contributes to those deficits.
Another limitation is a potential lack of sensitivity of click-evoked ABRs to synaptopathy and/or deafferentation. The net effect of strial dysfunction is the same as that of synaptopathy: fewer action potentials driving the auditory pathway (Lang et al., 2010; Schmiedt et al., 2002). Therefore, reductions of the endocochlear potential (Gratton et al., 1997; Schulte & Schmiedt, 1992) (or auditory nerve demyelination; Xing et al., 2012) may also cause reductions of wave-I amplitude and slope. Although strial degeneration is unlikely to occur for older people with normal audiograms (Wu et al., 2020), it remains possible that the age-related decline in ABR wave-I amplitude or slope reflects strial degeneration (and/or demyelination) instead of or in addition to synaptopathy or deafferentation. In other words, the shown evidence is insufficient to completely rule out a contribution of synaptopathy/deafferentation to the hearing deficits of older people with normal audiograms.
Further research is necessary to build upon these findings using more direct metrics, as well as to examine the relative contributions of sensory and cognitive inhibition to the age-related hearing deficits of adults with normal audiograms.
Acknowledgments
We thank Milagros J. Fumero for help with data collection and Almudena Eustaquio-Martín for technical help. We also thank the associate editor, Brian C. J. Moore, for useful comments.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Work supported by Junta de Castilla y León (grant SA252P20), Ministerio de Ciencia e Innovación (grant PID2019-108985GB-I00), and the European Regional Development Fund.
ORCID iD: Enrique A. Lopez-Poveda https://orcid.org/0000-0002-6886-154X
References
- Akeroyd M. A. (2008). Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol, 47(supp2), S53–S71. 10.1080/14992020802301142 [DOI] [PubMed] [Google Scholar]
- Alain C., Woods D. L. (1999). Age-related changes in processing auditory stimuli during visual attention: Evidence for deficits in inhibitory control and sensory memory. Psychology and Aging, 14(3), 507–519. 10.1037//0882-7974.14.3.507 [DOI] [PubMed] [Google Scholar]
- Altschuler R. A., Dolan D. F., Halsey K., Kanicki A., Deng N., Martin C., Eberle J., Kohrman D. C., Miller R. A., Schacht J. (2015). Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice. Neuroscience, 292, 22–33. 10.1016/j.neuroscience.2015.01.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler R. A., Shore S. E. (2010). Central auditory neurotransmitters. The Oxford Handbook of Auditory Neuroscience, 2, 65–89. 10.1093/oxfordhb/9780199233281.013.0004 [DOI] [Google Scholar]
- Amer T., Anderson J. A. E., Campbell K. L., Hasher L., Grady C. L. (2016). Age differences in the neural correlates of distraction regulation: A network interaction approach. Neuroimage, 139, 231–239. 10.1016/j.neuroimage.2016.06.036 [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology (2015). Clinical practice guidelines: Adult patients with severe-to-profound unilateral sensorineural hearing loss. Retrieved from https://www.audiology.org/wp-content/uploads/legacy/PractGuidelineAdultsPatientsWithSNHL.pdf.
- Andersen P. K., Skovgaard L. T. (2010). Regression with Linear Predictors. Springer. 10.1007/978-1-4419-7170-8 [DOI] [Google Scholar]
- ASHA (2005). American Speech-Language-Hearing Association guidelines for manual pure-tone threshold audiometry [Guidelines]. 10.1044/policy.GL2005-00014. [DOI]
- Beck D. L. (2022). The emerging relationship between cognition and audition: Why cognitive screenings are beneficial for audiology patients and why comprehensive audiometric evaluations are recommended for people with mild cognitive impairment, cognitive decline, and dementia. Journal of Otolaryngology-ENT Research, 14(1), 1–6. https://doi.or/10.15406/joentr.2022.14.00496 [Google Scholar]
- Beck D. L. Danhauer J. L. Abrams H. B. Atcherson S. R. Brown D. K. Chasin M. Clark J. G. De Placido C. Edwards B. Fabry D. A. Flexer C. Fligor B. Frazer G. Galster J. A. Gifford L. Johnson C. E. Madell J. Moore D. R. Roeser R. J., … Wolfe J. (2018). Audiologic considerations for people with normal hearing sensitivity yet hearing difficulty and/or speech-in-noise problems. The Hearing Review, 25(10), 28–38. [Google Scholar]
- Ben-David B. M., Schneider B. A. (2010). A sensory origin for color-word Stroop effects in aging: Simulating age-related changes in color-vision mimics age-related changes in Stroop. Aging, Neuropsychology, and Cognition, 17(6), 730–746. 10.1080/13825585.2010.510553 [DOI] [PubMed] [Google Scholar]
- Bharadwaj H. M., Verhulst S., Shaheen L., Liberman M. C., Shinn-Cunningham B. G. (2014). Cochlear neuropathy and the coding of supra-threshold sound. Frontiers in Systems Neuroscience, 8(26), 1–18. 10.3389/fnsys.2014.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher F. A., Mills J. H., Norton B. L. (1993). Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hearing Research, 71(1-2), 137–145. 10.1016/0378-5955(93)90029-Z [DOI] [PubMed] [Google Scholar]
- Bramhall N. F. (2021). Use of the auditory brainstem response for assessment of cochlear synaptopathy in humans. Journal of the Acoustical Society of America, 150(6), 4440–4451. 10.1121/10.0007484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N. F., Beach E. F., Epp B., le Prell C. G., Lopez-Poveda E. A., Plack C. J., Schaette R., Verhulst S., Canlon B. (2019). The search for noise-induced cochlear synaptopathy in humans: Mission impossible? Hearing Research, 377, 88–103. 10.1016/j.heares.2019.02.016 [DOI] [PubMed] [Google Scholar]
- Bramhall N. F., Konrad-Martin D., Mcmillan G. P. (2018). Tinnitus and auditory perception after a history of noise exposure: Relationship to auditory brainstem response measures. Ear and Hearing, 39(5), 881–894. 10.1097/AUD.0000000000000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg J. M., DeLosh E. L., Davalos D. B., Davis H. P. (2007). Age differences in Stroop interference: Contributions of general slowing and task-specific deficits. Aging Neuropsychol Cognit, 14(2), 155–167. 10.1080/138255891007065 [DOI] [PubMed] [Google Scholar]
- Cárdenas M. R., Marrero V. (1994). Cuaderno de Logoaudiometría: Guía de Referencia Rápida. Universidad Nacional de Educación a Distancia (UNED). ISBN: 84-362-3010-8. [Google Scholar]
- Caspary D. M., Ling L., Turner J. G., Hughes L. F. (2008). Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology, 211(Pt 11), 1781–1791. 10.1242/jeb.013581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary D. M., Llano D. A. (2019). Aging processes in the subcortical auditory system. In Kandler K. (Ed.), The Oxford Handbook of the Auditory Brainstem, Chapter 23 (pp. 639–680). Handbooks. 10.1093/oxfordhb/9780190849061.013.16 [DOI] [Google Scholar]
- CHABA (1988). Speech understanding and aging. Journal of the Acoustical Society of America, 83(3), 859–895. 10.1121/1.395965 [DOI] [PubMed] [Google Scholar]
- Chambers A. R., Resnik J., Yuan Y., Whitton J. P., Edge A. S., Liberman M. C., Polley D. B. (2016). Central gain restores auditory processing following near-complete cochlear denervation. Neuron, 89(4), 867–879. 10.1016/j.neuron.2015.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. (1987). Review article: Speech comprehension in the elderly: The effects of cognitive changes. British Journal of Audiology, 21(3), 221–226. 10.3109/03005368709076408 [DOI] [PubMed] [Google Scholar]
- Connor A., Franzen M. D., Sharp B. (1988). Effects of practice and differential instructions on Stroop performance. The International Journal of Clinical Neuropsychology, 10(1), 1–4. 10.1080/01688638808405089 [DOI] [Google Scholar]
- Craik F. I., Byrd M. (1982). Aging and cognitive deficits: The role of attentional resources. In Craik F. I. M., Trehub S. E. (Eds.), Aging and Cognitive Processes. Advances in the Study of Communication and Affect (Vol. 8, pp. 191–211). Boston, MA: Plenum. 10.1007/978-1-4684-4178-9_11. [DOI] [Google Scholar]
- Doan Q. T., Swerdlow N. R. (1999). Preliminary findings with a new Vietnamese Stroop test. Perceptual and Motor Skills, 89(1), 173–182. 10.2466/pms.1999.89.1.173 [DOI] [PubMed] [Google Scholar]
- Dobri S. G. J., Ross B. (2021). Total GABA level in human auditory cortex is associated with speech-in-noise understanding in older age. Neuroimage, 225, 117474. 10.1016/j.neuroimage.2020.117474 [DOI] [PubMed] [Google Scholar]
- Don M., Eggermont J. J. (1978). Analysis of the click-evoked brainstem potentials in man using high-pass noise masking. Journal of the Acoustical Society of America, 63(4), 1084–1092. 10.1121/1.381816 [DOI] [PubMed] [Google Scholar]
- Dryden A., Allen H. A., Henshaw H., Heinrich A. (2017). The association between cognitive performance and speech-in-noise perception for adult listeners: A systematic literature review and meta-analysis. Trends in Hearing, 21, 1–21. 10.1177/2331216517744675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno J. R., Horwitz A. R., Ahlstrom J. B. (2002). Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing. Journal of the Acoustical Society of America, 111(6), 2897–2907. 10.1121/1.1480421 [DOI] [PubMed] [Google Scholar]
- Eggermont J. J., Don M. (1980). Analysis of the click-evoked brainstem potentials in humans using high-pass noise masking. II. Effect of click intensity. Journal of the Acoustical Society of America, 68(6), 1671–1675. 10.1121/1.381816 [DOI] [PubMed] [Google Scholar]
- Faßbender K., Baumert P. M., Wintergerst M. W. M., Terheyden J. H., Aslan B., Harmening W. M., Ettinger U. (2023). GABAergic involvement in selective attention. Journal of Cognitive Neuroscience, 35(6), 976–989. 10.1162/jocn_a_01989 [DOI] [PubMed] [Google Scholar]
- Freitas S., Santana I., Simoes M. R. (2010). P2-137: The sensitivity of the MoCA and MMSE to cognitive decline: A longitudinal study. Alzheimer's & Dementia, 6, S353–S354. 10.1016/j.jalz.2010.05.1184 [DOI] [Google Scholar]
- Füllgrabe C., Moore B. C. J., Stone M. A. (2015). Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Frontiers in Aging Neuroscience, 6, 347. 10.3389/fnagi.2014.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe C., Rosen S. (2016). Investigating the role of working memory in speech-in-noise identification for listeners with normal hearing. Advances in Experimental Medicine and Biology, 894, 29–36. 10.1007/978-3-319-25474-6_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M., Verhulst S. (2019). Applicability of subcortical EEG metrics of synaptopathy to older listeners with impaired audiograms. Hearing Research, 380, 150–165. 10.1016/j.heares.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Gleich O., Hamann I., Klump G. M., Kittel M., Strutz J. (2003). Boosting GABA improves impaired auditory temporal resolution in the gerbil. Neuroreport, 14(14), 1877–1870. 10.1097/00001756-200310060-00024 [DOI] [PubMed] [Google Scholar]
- Gleich O., Semmler P., Strutz J. (2016). Behavioral auditory thresholds and loss of ribbon synapses at inner hair cells in aged gerbils. Experimental Gerontology, 84, 61–70. 10.1016/j.exger.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Golden C. (1978). Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting. [Google Scholar]
- Golden C. (2001). Test de Colores y Palabras. TEA Ediciones. ISBN: 978-84-16231-78-2. [Google Scholar]
- Graf P., Uttl B., Tuokko H. (1995). Color- and picture-word Stroop tests: Performance changes in old age. Journal of Clinical and Experimental Neuropsychology, 17(3), 390–415. 10.1080/01688639508405132 [DOI] [PubMed] [Google Scholar]
- Gratton M. A., Smyth B. J., Lam C. F., Boettcher F. A., Schmiedt R. A. (1997). Decline in the endocochlear potential corresponds to decreased Na,K-ATPase activity in the lateral wall of quiet-aged gerbils. Hearing Research, 108(1-2), 9–16. 10.1016/S0378-5955(97)00034-8 [DOI] [PubMed] [Google Scholar]
- Grose J. H., Buss E., Elmore H. (2019). Age-related changes in the auditory brainstem response and suprathreshold processing of temporal and spectral modulation. Trends in Hearing, 23, 1–11. 10.1177/2331216519839615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose J. H., Mamo S. K. (2012). Frequency modulation detection as a measure of temporal processing: Age-related monaural and binaural effects. Hearing Research, 294(1-2), 49–54. 10.1016/j.heares.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H., Munro K. J., Prendergast G., Howe S., Plack C. J. (2016). Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hearing Research, 344, 265–274. 10.1016/j.heares.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest H., Munro K. J., Prendergast G., Plack C. J. (2019). Reliability and interrelations of seven proxy measures of cochlear synaptopathy. Hearing Research, 375, 34–43. 10.1016/j.heares.2019.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair J. F., Black W., Babin B. J., Anderson R. E., Tatham R. L. (2013). Multivariate Data Analysis. Pearson Education Limited. ISBN: 129202190X, 9781292021904 [Google Scholar]
- Harris K. C., Dias J. W., McClaskey C. M., Rumschlag J., Prisciandaro J., Dubno J. R. (2022). Afferent loss, GABA, and central gain in older adults: Associations with speech recognition in noise. Journal of Neuroscience, 42(38), 7201–7212. 10.1523/JNEUROSCI.0242-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman M., Hasher L. (1991). Aging and suppression: Memory for previously relevant information. Psychology and Aging, 6(4), 587–594. 10.1037//0882-7974.6.4.587 [DOI] [PubMed] [Google Scholar]
- Hasher L., Stoltzfus E. R., Zacks R. T., Rypma B. (1991). Age and inhibition. Journal of Experimental Psychology Learning Memory and Cognition, 17(1), 163–169. 10.1037//0278-7393.17.1.163 [DOI] [PubMed] [Google Scholar]
- Hasher L., Zacks R. T. (1988). Working memory, comprehension, and aging: A review and a new view. Psychology of Learning and Motivation, 22, 193–225. 10.1016/S0079-7421(08)60041-9 [DOI] [Google Scholar]
- He N., Mills J. H., Dubno J. R. (2007). Frequency modulation detection: Effects of age, psychophysical method, and modulation waveform. Journal of the Acoustical Society of America, 122(1), 467–477. 10.1121/1.2741208 [DOI] [PubMed] [Google Scholar]
- He W., Kemp D., Ren T. (2018). Timing of the reticular lamina and basilar membrane vibration in living gerbil cochlea. eLife, 7, e37625. 10.7554/eLife.37625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa A. N., Zhang L., Ashida G., Beutelmann R., Steenken F., Köppl C. (2020). Temporal coding of single auditory nerve fibers is not degraded in aging gerbils. Journal of Neuroscience, 40(2), 343–354. 10.1523/JNEUROSCI.2784-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holube I., Fredelake S., Vlaming M., Kollmeier B. (2010). Development and analysis of an international speech test signal (ISTS). International Journal of Audiology, 49(12), 891–903. 10.3109/14992027.2010.506889 [DOI] [PubMed] [Google Scholar]
- Homack S., Riccio C. A. (2004). A meta-analysis of the sensitivity and specificity of the Stroop Color and Word Test with children. Archives of Clinical Neuropsychology, 19(6), 725–743. 10.1016/j.acn.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Humes L. E., Lee J. H., Coughlin M. P. (2006). Auditory measures of selective and divided attention in young and older adults using single-talker competition. Journal of the Acoustical Society of America, 120(5), 2926–2937. 10.1121/1.2354070 [DOI] [PubMed] [Google Scholar]
- Jain S., Narne V. K., Nataraja N. P., Madhukesh S., Kumar K., Moore B. C. J. (2022). The effect of age and hearing sensitivity at frequencies above 8 kHz on auditory stream segregation and speech perception. Journal of the Acoustical Society of America, 152(1), 716–726. 10.1121/10.0012917 [DOI] [PubMed] [Google Scholar]
- Janse E. (2012). A non-auditory measure of interference predicts distraction by competing speech in older adults. Aging, Neuropsychology, and Cognition, 19(6), 741–758. 10.1080/13825585.2011.652590 [DOI] [PubMed] [Google Scholar]
- Johannesen P. T., Buzo B. C., Lopez-Poveda E. A. (2019). Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hearing Research, 374, 35–48. 10.1016/j.heares.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Johannesen P. T., Leclère T., Wijetillake A., Segovia-Martínez M., Lopez-Poveda E. A. (2022). Modeling temporal information encoding by the population of fibers in the healthy and synaptopathic auditory nerve. Hearing Research, 426, 108621. 10.1016/j.heares.2022.108621 [DOI] [PubMed] [Google Scholar]
- Johannesen P. T., Lopez-Poveda E. A. (2021). Age-related central gain compensation for reduced auditory nerve output for people with normal audiograms, with and without tinnitus. iScience, 24(6), 1–15. 10.1016/j.isci.2021.102658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen P. T., Pérez-González P., Kalluri S., Blanco J. L., Lopez-Poveda E. A. (2016). The influence of cochlear mechanical dysfunction, temporal processing deficits, and age on the intelligibility of audible speech in noise for hearing-impaired listeners. Trends in Hearing, 20, 1–14. 10.1177/2331216516641055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris P. X., Bergevin C., Kalluri R., Mc Laughlin M., Michelet P., van der Heijden M., Shera C. A. (2011). Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in humans. Proceedings of the National Academy of Sciences, 108(42), 17516–17520. 10.1073/pnas.1105867108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C. H., Kaylegian K., Wehr M. (2018). Gap encoding by parvalbumin-expressing interneurons in auditory cortex. Journal of Neurophysiology, 120(1), 105–114. 10.1152/jn.00911.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouri L., Lesica N. A., Grothe B. (2011). Impaired auditory temporal selectivity in the inferior colliculus of aged Mongolian gerbils. Journal of Neuroscience, 31(27), 9958–9970. 10.1523/JNEUROSCI.4509-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G., Mason C. R., Richards V. M., Gallun F. J., Durlach N. I. (2008). Informational masking. In Yost W. A., Popper A. N., Fay R. R. (Eds.), Auditory Perception of Sound Sources. Springer Handbook of Auditory Research (Vol. 29, pp. 143–190). Springer. 10.1007/978-0-387-71305-2_6. [DOI] [Google Scholar]
- Knight S., Heinrich A. (2017). Different measures of auditory and visual Stroop interference and their relationship to speech intelligibility in noise. Frontiers in Psychology, 8, 230. 10.3389/fpsyg.2017.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock N. (2015). Common method bias in PLS-SEM: A full collinearity assessment approach. International Journal of e-Collaboration, 11(4), 1–10. 10.4018/ijec.2015100101 [DOI] [Google Scholar]
- Konrad-Martin D., Dille M. F., Mcmillan G., Griest S., Mcdermott D., Fausti S. A., Austin D. F. (2012). Age-related changes in the auditory brainstem response. Journal of the American Academy of Audiology, 23(1), 18–75. 10.3389/fpsyg.2017.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Schubert F., Mekle R., Wenger E., Ittermann B., Lindenberger U., Gallinat J. (2016). Neurotransmitter changes during interference task in anterior cingulate cortex: Evidence from fMRI-guided functional MRS at 3 T. Brain Structure & Function, 221(5), 2541–2551. 10.1007/s00429-015-1057-0 [DOI] [PubMed] [Google Scholar]
- Lamers M. J. M., Roelofs A., Rabeling-Keus I. M. (2007). Selective attention and response set in the stroop task. Memory & Cognition, 38(7), 893–904. 10.3758/MC.38.7.893 [DOI] [PubMed] [Google Scholar]
- Lang H., Jyothi V., Smythe N. M., Dubno J. R., Schulte B. A., Schmiedt R. A. (2010). Chronic reduction of endocochlear potential reduces auditory nerve activity: Further confirmation of an animal model of metabolic presbyacusis. Journal of the Association for Research in Otolaryngology, 11(3), 419–434. 10.1007/s10162-010-0214-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumen G., Tollin D. J., Beutelmann R., Klump G. M. (2016). Aging effects on the binaural interaction component of the auditory brainstem response in the Mongolian gerbil: Effects of interaural time and level differences. Hearing Research, 337, 46–58. 10.1016/j.heares.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek M. R., Brown M. E., Dorman M. F. (1991). Informational masking and auditory attention. Perception & Psychophysics, 50(3), 205–214. 10.3758/bf03206743 [DOI] [PubMed] [Google Scholar]
- Levitt H. (1971). Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America, 49(2, Pt 2), 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Liberman M. C. (1978). Auditory-nerve response from cats raised in a low-noise chamber. Journal of the Acoustical Society of America, 63(2), 442–455. 10.1121/1.381736 [DOI] [PubMed] [Google Scholar]
- Liberman M. C., Liberman L. D., Maison S. F. (2014). Efferent feedback slows cochlear aging. Journal of Neuroscience, 34(13), 4599–4607. 10.1523/JNEUROSCI.4923-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenhan J., Johnson T., Spankovich C., Parker M. A., Hoben R., Easow G., Pevzner S. (2017). Outer hair cell and auditory nerve function in speech recognition in quiet and in background noise. Frontiers in Neuroscience, 11, 157. 10.3389/fnins.2017.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister J. J., Roberts R. A. (2002). Effects of age and hearing loss on gap detection and the precedence effect: Narrow-band stimuli. Journal of Speech Language and Hearing Research, 48(2), 482–493. 10.1044/1092-4388(2004/071) [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda E. A. (2014). Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Frontiers in Neuroscience, 8, 348. 10.3389/fnins.2014.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda E. A., Barrios P. (2013). Perception of stochastically undersampled sound waveforms: A model of auditory deafferentation. Frontiers in Neuroscience, 7, 124. 10.3389/fnins.2013.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda E. A., Johannesen P., Buzo B. (2019). Evidence for age-related cochlear synaptopathy unconnected to auditory temporal processing deficits in humans. In: 42nd Annual Midwinter Meeting of the Association for Research in Otolaryngology. Baltimore, MD. ISSN-0742-3152. [DOI] [PubMed]
- Lorenzi C., Gilbert G., Carn H., Garnier S., Moore B. C. J. (2006). Speech perception problems of the hearing impaired reflect inability to use temporal fine structure. PNAS, 103(49), 18866–18869. 10.1073/pnas.0607364103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. M. (1991). Half a century of research on the Stroop effect: An integrative review. Psychological Bulletin, 109(2), 163–203. 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- Makary C. A., Shin J., Kujawa S. G., Liberman M. C., Merchant S. N. (2011). Age-related primary cochlear neuronal degeneration in human temporal bones. Journal of the Association for Research in Otolaryngology, 12(6), 711–717. 10.1007/s10162-011-0283-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya P. C., Richards T. L., Edden R. A. E., Lee A. K. C., Stein M. A., Kuhl P. K. (2021). Task-related excitatory/inhibitory ratios in the fronto-striatal circuitry predict attention control deficits in attention-deficit/hyperactivity disorder. medRxiv. 10.1101/2021.03.25.21254355 [DOI] [Google Scholar]
- Mehraei X. G., Hickox A. E., Bharadwaj H. M., Goldberg H., Verhulst S., Liberman M. C., Barbara X., Shinn-Cunningham G. (2016). Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. Journal of Neuroscience, 36(13), 3755–3764. 10.1523/JNEUROSCI.4460-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mepani A. M., Kirk S. A., Hancock K. E., Bennett K., de Gruttola V., Liberman M. C., Maison S. F. (2020). Middle-ear muscle reflex and word-recognition in “normal hearing” adults: Evidence for cochlear synaptopathy? Ear and Hearing, 41(1), 25–38. 10.1097/AUD.0000000000000804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrle D., Ni K., Varakina K., Bing D., Lee S. C., Zimmermann U., Knipper M., Rüttiger L. (2016). Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiology of Aging, 44, 173–184. 10.1016/j.neurobiolaging.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Peters R. W., Glasberg B. R. (1992). Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss. Journal of the Acoustical Society of America, 92(4), 1992–1932. 10.1121/1.405240 [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Sek A. (1996). Detection of frequency modulation at low modulation rates: Evidence for a mechanism based on phase locking. Journal of the Acoustical Society of America, 100(4 Pt 1), 2320–2331. 10.1121/1.417941 [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Mariathasan S., Sęk A. P. (2019). Effects of age and hearing loss on the discrimination of amplitude and frequency modulation for 2- and 10-Hz rates. Trends in Hearing, 23, 2331216519853963. 10.1177/2331216519853963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. R., Edmondson-Jones M., Dawes P., Fortnum H., McCormack A., Pierzycki R. H., Munro K. J. (2014). Relation between speech-in-noise threshold, hearing loss and cognition from 40-69 years of age. PLoS One, 9(9), e107720. 10.1371/journal.pone.0107720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. R., Ferguson M. A., Edmondson-Jones A. M., Ratib S., Riley A. (2010). Nature of auditory processing disorder in children. Pediatrics, 126(2), e382–e390. 10.1542/peds.2009-2826 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Osterhammel D., Osterhammel P. (1979). High-frequency audiometry: Age and sex variations. Scandinavian Audiology, 8(2), 73–81. 10.3109/01050397909076304 [DOI] [PubMed] [Google Scholar]
- Ou J., Law S.-P. (2017). Cognitive basis of individual differences in speech perception, production and representations: The role of domain general attentional switching. Attention, Perception & Psychophysics, 79(3), 945–963. 10.3758/s13414-017-1283-z [DOI] [PubMed] [Google Scholar]
- Oxenham A. J. (2016). Predicting the perceptual consequences of hidden hearing loss. Trends in Hearing, 20, 1–6. 10.1177/2331216516686768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal I., Paltati C. R. B., Kaur C., Saini S., Kumar P., Jacob T. G., Bhardwaj D. N., Roy T. S. (2019). Morphological and neurochemical changes in GABAergic neurons of the aging human inferior colliculus. Hearing Research, 377, 318–329. 10.1016/j.heares.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Peters R. W., Moore B. C. J., Baer T. (1998). Speech reception thresholds in noise with and without spectral and temporal dips for hearing-impaired and normally hearing people. Journal of the Acoustical Society of America, 103(1), 577–587. 10.1121/1.421128 [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller M. K., Schneider B. A., Benson N. J., Hamstra S. J., Storzer E. (2006). Effect of age on detection of gaps in speech and nonspeech markers varying in duration and spectral symmetry. Journal of the Acoustical Society of America, 119(2), 1143–1155. 10.1121/1.2149837 [DOI] [PubMed] [Google Scholar]
- Piras F., Vecchio D., Assogna F., Pellicano C., Ciullo V., Banaj N., Edden R. A. E., Pontieri F. E., Piras F., Spalletta G. (2021). Cerebellar GABA levels and cognitive interference in Parkinson’s disease and healthy comparators. Journal of Personalized Medicine, 11(1), 1–15. 10.3390/jpm11010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recio A., Rich N. C., Narayan S. S., Ruggero M. A. (1998). Basilar-membrane responses to clicks at the base of the chinchilla cochlea. Journal of the Acoustical Society of America, 103(4), 1972–1898. 10.1121/1.421377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik J., Polley D. B. (2021). Cochlear neural degeneration disrupts hearing in background noise by increasing auditory cortex internal noise. Neuron, 109(6), 984–996.e4. 10.1016/j.neuron.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. A., Lister J. J. (2004). Effects of age and hearing loss on gap detection and the precedence effect. Journal of Speech Language and Hearing Research, 47(5), 965–978. 10.1044/1092-4388(2004/071) [DOI] [PubMed] [Google Scholar]
- Rotschafer S. E., Marshak S., Cramer K. S. (2015). Deletion of Fmr1 alters function and synaptic inputs in the auditory brainstem. PLoS ONE, 10(2), e0117266. 10.1371/journal.pone.0117266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumschlag J. A., McClaskey C. M., Dias J. W., Kerouac L. B., Noble K. V., Panganiban C., Lang H., Harris K. C. (2022). Age-related central gain with degraded neural synchrony in the auditory brainstem of mice and humans. Neurobiology of Aging, 115, 50–59. 10.1016/j.neurobiolaging.2022.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. A. (1996). The processing-speed theory of adult age differences in cognition. Psychological Review, 103(3), 403–428. 10.1037/0033-295x.103.3.403 [DOI] [PubMed] [Google Scholar]
- Saunders G. H., Haggard M. P. (1989). The clinical assessment of obscure auditory dysfunction-1. Auditory and psychological factors. Ear and Hearing, 10(3), 200–208. 10.1097/00003446-198906000-00011 [DOI] [PubMed] [Google Scholar]
- Scarpina F., Tagini S. (2017). The Stroop color and word test. Frontiers in Psychology, 8, 557. 10.3389/fpsyg.2017.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt R. A., Lang H., Okamura H. O., Schulte B. A. (2002). Effects of furosemide applied chronically to the round window: A model of metabolic presbyacusis. Journal of Neuroscience, 22(21), 9643–9650. 10.1523/JNEUROSCI.22-21-09643.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt R. A., Mills J. H., Boettcher F. A. (1996). Age-related loss of activity of auditory-nerve fibers. Journal of Neurophysiology, 76(4), 2799–2803. 10.1152/jn.1996.76.4.2799 [DOI] [PubMed] [Google Scholar]
- Schneider B., Speranza F., Kathleen Pichora-Fuller M. (1998). Age-related changes in temporal resolution: Envelope and intensity effects. Canadian Journal of Experimental Psychology, 54(4), 184–191. 10.1037/h0087291 [DOI] [PubMed] [Google Scholar]
- Schneider B. A., Hamstra S. J. (1999). Gap detection thresholds as a function of tonal duration for younger and older listeners. Journal of the Acoustical Society of America, 106(1), 371–380. 10.1121/1.427062 [DOI] [PubMed] [Google Scholar]
- Schneider B. A., Pichora-Fuller K., Kowalchuk D., Lamb M. (1994). Gap detection and the precedence effect in young and old adults. Journal of the Acoustical Society of America, 95(2), 980–991. 10.1121/1.408403 [DOI] [PubMed] [Google Scholar]
- Schneider B. A., Pichora-Fuller M. K. (2000). Implications of perceptual deterioration for cognitive aging research. In The Handbook of Aging and Cognition (2nd ed., pp. 155–219). Lawrence Erlbaum Associates. ISBN: 0-8058-2966-0. [Google Scholar]
- Schoof T., Rosen S., Roberts K., Peelle J. E., Rajan R. (2014). The role of auditory and cognitive factors in understanding speech in noise by normal-hearing older listeners. Frontiers in Aging Neuroscience, 6, 307. 10.3389/fnagi.2014.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte B. A., Schmiedt R. A. (1992). Lateral wall Na, K-ATPase and endocochlear potentials decline with age in quiet-reared gerbils. Hearing Research, 61(1-2), 35–46. 10.1016/0378-5955(92)90034-K [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y., Lall K., Liberman M. C., Kujawa S. G. (2013). Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. Journal of Neuroscience, 33(34), 13686–13694. 10.1523/JNEUROSCI.1783-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H. J., Kim S. K., Park C. H., Lee S. H., Yoon S. W., Ki A. R., Chung D. H., Yeo S. G. (2009). Hearing abilities at ultra-high frequency in patients with tinnitus. Clinical and Experimental Otorhinolaryngology, 2(4), 169–174. 10.3342/ceo.2009.2.4.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. B. (1997). Age-related changes in temporal gap detection. Journal of the Acoustical Society of America, 101(4 Pt 1), 2214–2220. 10.1121/1.418205 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M., Mouratidis M., Yoo S., Culligan K., Kosten T. (2005). Effects of tiagabine in combination with intravenous nicotine in overnight abstinent smokers. Psychopharmacology (Berl), 181(3), 504–510. 10.1007/s00213-005-0010-y [DOI] [PubMed] [Google Scholar]
- Steenken F., Heeringa A. N., Beutelmann R., Zhang L., Bovee S., Klump G. M., Köppl C. (2021). Age-related decline in cochlear ribbon synapses and its relation to different metrics of auditory-nerve activity. Neurobiology of Aging, 108, 133–145. 10.1016/j.neurobiolaging.2021.08.019 [DOI] [PubMed] [Google Scholar]
- Stothart G., Kazanina N. (2016). Auditory perception in the aging brain: The role of inhibition and facilitation in early processing. Neurobiology of Aging, 47, 23–34. 10.1016/j.neurobiolaging.2016.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelcyk O., Dau T. (2009). Relations between frequency selectivity, temporal fine-structure processing, and speech reception in impaired hearing. Journal of the Acoustical Society of America, 125(5), 3328. 10.1121/1.3097469 [DOI] [PubMed] [Google Scholar]
- Stroop J. R. (1935). Studies of interference in serial verbal reactions. Journal of Experimental Psychology, 18(6), 643–662. 10.1037/h0054651 [DOI] [Google Scholar]
- Strouse A., Ashmead D. H., Ohde R. N., Grantham D. W. (1998). Temporal processing in the aging auditory system. Journal of the Acoustical Society of America, 104(4), 2385–2399. 10.1121/1.423748 [DOI] [PubMed] [Google Scholar]
- Sturm J. J., Zhang-Hooks Y.-X., Roos H., Nguyen X. T., Kandler X. K. (2017). Noise trauma-induced behavioral gap detection deficits correlate with reorganization of excitatory and inhibitory local circuits in the inferior colliculus and are prevented by acoustic enrichment. Journal of Neuroscience, 37(26), 6314–6330. 10.1523/JNEUROSCI.0602-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun P. A., O’Kane G., Wingfield A. (2002). Distraction by competing speech in young and older adult listeners. Psychology and Aging, 17(3), 453–467. 10.1037//0882-7974.17.3.453 [DOI] [PubMed] [Google Scholar]
- Tun P. A., Wingfield A. (1999). One voice too many: Adult age differences in language processing with different types of distracting sounds. Journal of Gerontology, 54B(5), 317–327. 10.1093/geronb/54b.5.p317 [DOI] [PubMed] [Google Scholar]
- Turner K., Moshtaghi O., Saez N., Richardson M., Djalilian H., Zeng F.-G., Lin H. (2022). Auditory brainstem response Wave I amplitude has limited clinical utility in diagnosing tinnitus in humans. Brain Sciences, 12(2), 1–7. 10.3390/brainsci12020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Elst W., van Boxtel M. P. J., van Breukelen G. J. P., Jolles J. (2006). The Stroop Color-Word test: Influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment, 13(1), 62–79. 10.1177/1073191105283427 [DOI] [PubMed] [Google Scholar]
- van Zanten G., Versnel H., van der Stoep N., Koopmans W., Hoetink A. (2022). Short-latency evoked potentials of the human auditory system. In Naz S. (Ed.), Auditory System: Function and Disorders (pp. 1–26). IntechOpen. 10.5772/intechopen.102039. [DOI] [Google Scholar]
- Viana L. M., O’Malley J. T., Burgess B. J., Jones D. D., Oliveira C. A. C. P., Santos F., Merchant S. N., Liberman L. D., Liberman M. C. (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research, 327, 78–88. 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallaert N., Moore B. C. J., Lorenzi C. (2016). Comparing the effects of age on amplitude modulation and frequency modulation detection. Journal of the Acoustical Society of America, 139(6), 3088–3096. 10.1121/1.4953019 [DOI] [PubMed] [Google Scholar]
- Wang M., Ai Y., Han Y., Fan Z., Shi P., Wang H. (2021). Extended high-frequency audiometry in healthy adults with different age groups. Journal of Otolaryngology - Head & Neck Surgery = Le Journal D'oto-Rhino-Laryngologie Et De Chirurgie Cervico-Faciale, 50, 1–6. 10.1186/s40463-021-00534-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford K. L., Kreft H. A., Oxenham A. J. (2017). Assessing the role of place and timing cues in coding frequency and amplitude modulation as a function of age. Journal of the Association for Research in Otolaryngology, 18(4), 619–633. 10.1007/s10162-017-0624-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford K. L., Kreft H. A., Oxenham A. J. (2020). The role of cochlear place coding in the perception of frequency modulation. eLife, 9, e58468. 10.1016/j.heares.2015.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Z., Liberman L. D., Bennett K., de Gruttola V., O’Malley J. T., Liberman M. C. (2019). Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience, 407, 8–20. 10.1016/j.neuroscience.2018.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. Z., O'Malley J. T., de Gruttola V., Liberman M. C. (2020). Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. Journal of Neuroscience, 40(33), 6357–6366. 10.1523/JNEUROSCI.0937-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y., Samuvel D. J., Stevens S. M., Dubno J. R., Schulte B. A., Lang H. (2012). Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS ONE, 7(4), e34500. 10.1371/journal.pone.0034500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadeh L. M., Silbert N. H., Sternasty K., Wet Swanepoel D., Hunter L. L., Moore D. R. (2019). Extended high-frequency hearing enhances speech perception in noise. PNAS, 116(47), 23753–23759. 10.1073/pnas.1903315116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-X., Moore D. R., Guiraud J., Molloy K., Yan T.-T., Amitay S. (2016). Auditory discrimination learning: Role of working memory. PLoS ONE, 11(1), 1–8. 10.1371/journal.pone.0147320 [DOI] [PMC free article] [PubMed] [Google Scholar]